Abstract
IMPORTANCE
Many established breast cancer risk factors are used in clinical risk prediction models, although the proportion of breast cancers explained by these factors is unknown.
OBJECTIVE
To determine the population-attributable risk proportion (PARP) for breast cancer associated with clinical breast cancer risk factors among premenopausal and postmenopausal women.
DESIGN, SETTING, AND PARTICIPANTS
Case-control study with 1:10 matching on age, year of risk factor assessment, and Breast Cancer Surveillance Consortium (BCSC) registry. Risk factor data were collected prospectively from January 1, 1996, through October 31, 2012, from BCSC community-based breast imaging facilities. A total of 18 437 women with invasive breast cancer or ductal carcinoma in situ were enrolled as cases and matched to 184 309 women without breast cancer, with a total of 58 146 premenopausal and 144 600 postmenopausal women enrolled in the study.
EXPOSURES
Breast Imaging Reporting and Data System (BI-RADS) breast density (heterogeneously or extremely dense vs scattered fibroglandular densities), first-degree family history of breast cancer, body mass index (>25 vs 18.5–25), history of benign breast biopsy, and nulliparity or age at first birth (≥30 years vs <30 years).
MAIN OUTCOMES AND MEASURES
Population-attributable risk proportion of breast cancer.
RESULTS
Of the 18 437 women with breast cancer, the mean (SD) age was 46.3 (3.7) years among premenopausal women and 61.7 (7.2) years among the postmenopausal women. Overall, 4747 (89.8%) premenopausal and 12 502 (95.1%) postmenopausal women with breast cancer had at least 1 breast cancer risk factor. The combined PARP of all risk factors was 52.7% (95% CI, 49.1%–56.3%) among premenopausal women and 54.7% (95% CI, 46.5%–54.7%) among postmenopausal women. Breast density was the most prevalent risk factor for both premenopausal and postmenopausal women and had the largest effect on the PARP; 39.3% (95% CI, 36.6%–42.0%) of premenopausal and 26.2% (95% CI, 24.4%–28.0%) of postmenopausal breast cancers could potentially be averted if all women with heterogeneously or extremely dense breasts shifted to scattered fibroglandular breast density. Among postmenopausal women, 22.8% (95% CI, 18.3%–27.3%) of breast cancers could potentially be averted if all overweight and obese women attained a body mass index of less than 25.
CONCLUSIONS AND RELEVANCE
Most women with breast cancer have at least 1 breast cancer risk factor routinely documented at the time of mammography, and more than half of premenopausal and postmenopausal breast cancers are explained by these factors. These easily assessed risk factors should be incorporated into risk prediction models to stratify breast cancer risk and promote risk-based screening and targeted prevention efforts.
One of the challenges in promoting the widespread utility of breast cancer risk prediction models has been the assertion that most women with a diagnosis of breast cancer have no established clinical breast cancer risk factors or are not considered to be high risk.1,2 Although it is impossible to determine the cause of breast cancer in any individual case,3 easily assessed risk factors that explain a substantial proportion of incident breast cancers can be used to stratify breast cancer risk for targeted screening4 and primary prevention5 and improve public health interventions to reduce breast cancer risk.
The population-attributable risk proportion (PARP) represents the proportion of disease cases in a population that would not have occurred in the absence of a risk factor. The PARP can be calculated for a single risk factor or combinations of risk factors and quantifies the proportion of cases averted if exposure to the risk factor was removed from the entire population, holding all other factors constant. The PARP incorporates both the prevalence of the risk factor and the magnitude of its association with disease; therefore, rare exposures with a high relative risk may explain a similar proportion of cases as common exposures with modest relative risks.
Previous studies of PARP have largely focused on quantifying the potential reductions in postmenopausal breast cancer incidence by intervening on modifiable factors.6–13 Estimates of the proportion of postmenopausal breast cancers that could be averted through lifestyle interventions range from 26%6,11 to 40.7%,12 and estimates for combinations of nonmodifiable factors range from 37.3% to 57.3%.6,12,14 To our knowledge, no studies have quantified the contributions of risk factors for pre-menopausal breast cancer, only 1 small study has included breast density as a risk factor,15 and none have examined the PARP for breast density using the Breast Imaging Reporting and Data System (BI-RADS) scale,16 which is the standard for reporting breast density in clinical practice in the United States.
We aimed to estimate the proportion of breast cancers attributable to breast cancer risk factors commonly documented in clinical practice and used in breast cancer risk prediction models, including BI-RADS breast density. We used data from a large cohort of women undergoing mammography at facilities participating in the Breast Cancer Surveillance Consortium (BCSC).
Methods
Study Population
Women with breast cancer and those serving as matched controls were selected from the BCSC, which comprises regional registries from across the United States that collect clinical characteristics and breast imaging data from community radiology facilities. Breast cancer diagnoses and tumor characteristics are obtained through linkage to pathology databases and regional Surveillance, Epidemiology, and End Results programs or state cancer registries. Each registry and the statistical coordinating center received institutional review board approval for either active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. The present study received approval from the institutional review boards of the Group Health, North Carolina, San Francisco, Vermont, and New Hampshire registries. All procedures are Health Insurance Portability and Accountability Act compliant, and all registries and the statistical coordinating center received a federal certificate of confidentiality and other protection for the identities of women, physicians, and facilities. The BCSC cohort is described in further detail elsewhere.17,18
Five BCSC registries (New Hampshire, North Carolina, San Francisco, Vermont, and Group Health) contributed data for this analysis. Eligible cases were women aged 40 to 74 years who were diagnosed with invasive breast cancer or ductal carcinoma in situ between 1996 and 2015 and with a BI-RADS breast density measure and risk factor data available within 5 years before their diagnosis. Risk factors and strength of associations with invasive cancer and ductal carcinoma in situ are similar,19,20 so both were included. Women with a history of breast cancer or missing menopausal status were excluded, as well as women with incomplete breast cancer risk factor data (Figure). We selected risk factor information associated with mammography examinations 1 year or more before diagnosis. For 4499 of 18 437 women (24.4%), risk factor information was not available more than 1 year before the diagnosis, and data from within a year of diagnosis were used. Risk factor information was collected a mean (SD) of 20.4 (15.46) months (range, <1–60 months) before breast cancer diagnosis. As a sensitivity analysis, we excluded cases with risk factor information obtained within 1 year of diagnosis and our findings remained unchanged.
Figure. Flowchart of Women in 5 Breast Cancer Surveillance Consortium (BCSC) Registries Eligible for the Study.
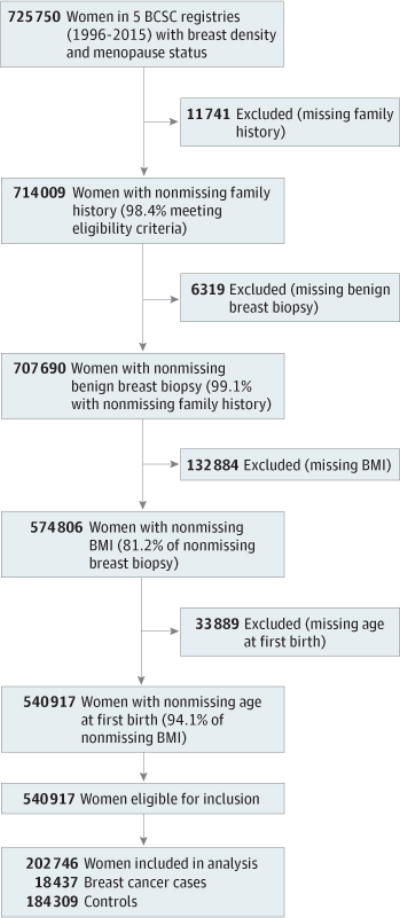
The 5 BCSC registries include New Hampshire, North Carolina, San Francisco, Vermont, and Group Health. BMI indicates body mass index.
Ten controls were matched to each breast cancer case on menopausal status, age and year of risk factor assessment, and BCSC registry data. Eligible controls had no breast cancer diagnosis between the year of risk factor assessment and the year of diagnosis of her matched case. For age and year of risk factor information, we matched to controls differing up to ±5 years, selecting controls with the closest match to the case. A total of 17 607 cases (95.5%) matched to 10 controls on age and year exactly, and 16 cases (0.09%) matched to fewer than 10 controls. A total of 18 437 women with breast cancer and 184 309 matched controls were included.
Exposure Assessment
Demographics and breast cancer risk factors were obtained through questionnaires completed at each mammography visit. Questionnaires included birth date, race, ethnicity, height, weight, first-degree family history of breast cancer, menopause status, parity, and age at first birth. Body mass index (BMI) (weight in kilograms divided by height in meters squared) was calculated as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), obesity class I (30.0–34.4), and obesity class II or III (≥35.0).21 History of benign breast biopsy was obtained by self-report and through linkages with pathology databases. The American College of Radiology’s BI-RADS system, assigned by clinical radiologists, was used to classify breast density as a, almost entirely fat; b, scattered fibroglandular densities; c, heterogeneously dense; and d, extremely dense.22
Statistical Analysis
Analyses were stratified by menopausal status. We used descriptive statistics to assess differences in demographics and clinical characteristics for cases and controls. Risk factors selected a priori for analysis were dense breasts (heterogeneously dense or extremely dense), first-degree relative with breast cancer, history of benign breast biopsy, and nulliparity or age at first birth 30 years or older. We considered BMI of 25 or more to be a risk factor only for postmenopausal breast cancer. Multivariable conditional logistic regression, stratified by matched set, was used to estimate the odds ratios and 95% CIs associated with each risk factor.
The PARP was calculated using the generalized regression-based approach described by Bruzzi et al,23 allowing for the calculation of joint PARP for combinations of risk factors. The multivariable combined PARP was measured by the equation
where pdi is the proportion of cases in stratum i of the risk factor distribution and RRi is the multivariable adjusted relative risk associated with that stratum of the risk factor. Odds ratios from the multivariable conditional logistic regression models were used as relative risk estimates.23 The PARP was calculated for individual risk factors and combinations of risk factors. For each factor, the reference level reported in Table 1 is considered the low-risk category. For combinations of factors, the PARP represents the proportion of cases eliminated in the population if everyone shifted to the referent category for all included variables. When the referent category for PARP was not the lowest level of exposure, the lowest level of exposure was assumed to remain unchanged. The 95% CIs were calculated using bootstrapping.24 All analyses were conducted in R, version 3.2.1 (R Foundation).
Table 1.
Characteristics of Women With Breast Cancer and Controls Included in the Study Population, Breast Cancer Surveillance Consortium (1996–2015)
| Characteristic | Women, No. (%) | |||
|---|---|---|---|---|
| Premenopausal | Postmenopausal | |||
| Control (n = 52 860) |
Invasive and In Situ Cancer (n = 5286) |
Control (n = 131 449) |
Invasive and In Situ Cancer (n = 13 151) |
|
| Age, y | ||||
| 40–49 | 41 120 (77.8) | 4114 (77.8) | 4711 (3.6) | 471 (3.6) |
| 50–59 | 11 740 (22.2) | 1172 (22.2) | 48 868 (37.2) | 4882 (37.1) |
| 60–69 | NA | NA | 54 153 (41.2) | 5415 (41.2) |
| 70–74 | NA | NA | 23 717 (18.0) | 2383 (18.1) |
| Race/ethnicity | ||||
| White | 40 054 (75.8) | 4091 (77.4) | 104 157 (79.2) | 10 832 (82.4) |
| Black | 1295 (2.4) | 122 (2.3) | 3323 (2.5) | 279 (2.1) |
| Asian | 5670 (10.7) | 548 (10.4) | 11 177 (8.5) | 894 (6.8) |
| Hispanic | 2719 (5.1) | 208 (3.9) | 5105 (3.9) | 395 (3.0) |
| Other/mixed | 3122 (5.9) | 317 (6.0) | 7687 (5.8) | 751 (5.7) |
| Family history of breast cancer | ||||
| No | 46 020 (87.1) | 4181 (79.1) | 109 827 (83.6) | 10 035 (76.3) |
| Yes | 6840 (12.9) | 1105 (20.9) | 21 622 (16.4) | 3116 (23.7) |
| History of benign breast biopsy | ||||
| No | 45 658 (86.4) | 4193 (79.3) | 102 741 (78.2) | 9252 (70.4) |
| Yes | 7202 (13.6) | 1093 (20.7) | 28 708 (21.8) | 3899 (29.6) |
| Age at first live birth, y | ||||
| Nulliparous | 11 729 (22.2) | 1240 (23.5) | 20 236 (15.4) | 2350 (17.9) |
| Age <30 y | 29 060 (55.0) | 2615 (49.5) | 97 101 (73.9) | 9168 (69.7) |
| Age ≥30 y | 12 071 (22.8) | 1431 (27.1) | 14 112 (10.7) | 1633 (12.4) |
| BMI | ||||
| <18.5 | 924 (1.7) | 106 (2.0) | 2223 (1.7) | 173 (1.3) |
| 18.5–24.9 | 23 739 (44.9) | 2642 (50.0) | 45 341 (34.5) | 4194 (31.9) |
| 25.0–29.9 | 15 123 (28.6) | 1456 (27.5) | 43 937 (33.4) | 4476 (34.0) |
| 30.0–34.9 | 7192 (13.6) | 616 (11.7) | 23 321 (17.7) | 2493 (19.0) |
| ≥35.0 | 5882 (11.1) | 466 (8.8) | 16 627 (12.6) | 1815 (13.8) |
| BI-RADS breast density | ||||
| Almost entirely fat (a) | 2764 (5.2) | 95 (1.8) | 16 852 (12.8) | 1014 (7.7) |
| Scattered fibroglandular densities (b) | 17 256 (32.6) | 1248 (23.6) | 62 743 (47.7) | 5749 (43.7) |
| Heterogeneously dense (c) | 24 479 (46.3) | 2803 (53.0) | 44 686 (34.0) | 5448 (41.4) |
| Extremely dense (d) | 8361 (15.8) | 1140 (21.6) | 7168 (5.5) | 940 (7.1) |
| Type of cancer | ||||
| Invasive | NA | 3890 (73.6) | NA | 10 313 (78.4) |
| In situ | 1396 (26.4) | 2838 (21.6) | ||
| No. of risk factors | ||||
| None | 9749 (18.4) | 539 (10.2) | 11 222 (8.5) | 649 (4.9) |
| 1 | 20 793 (39.3) | 1759 (33.3) | 49 661 (37.8) | 3803 (28.9) |
| 2 | 17 509 (33.1) | 2039 (38.6) | 46 076 (35.1) | 4807 (36.6) |
| 3 | 4365 (8.3) | 821 (15.5) | 19 744 (15.0) | 2914 (22.2) |
| ≥4 | 444 (0.8) | 128 (2.4) | 4746 (3.6) | 978 (7.4) |
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Results
A total of 5286 premenopausal women with breast cancer were matched to 52 860 women without breast cancer, and 13 151 postmenopausal women with breast cancer were matched to 131 449 women without breast cancer. The mean age of pre-menopausal women was 46.3 (3.7) years compared with a mean age of 61.7 (7.2) years for postmenopausal women. The sample was predominantly non-Hispanic white (>75% of women) with smaller percentages of Asian, Hispanic, and African American women. Women with breast cancer were more likely to have a first-degree family history of breast cancer, a history of benign breast biopsy, dense breasts, and an older age at first birth compared with the controls (Table 1). Postmenopausal women with breast cancer were more likely to be overweight or obese.
Overall, 89.8% (n = 4747) of premenopausal women with breast cancer and 95.1% (n = 12 502) of postmenopausal women with breast cancer had at least 1 risk factor, compared with 81.6% (n = 43 111) of premenopausal controls and 91.5% (n = 120 227) of postmenopausal controls. Most premenopausal cases (56.5% [n = 2988]) had 2 or more risk factors compared with only 42.2% (n = 22 318) of premenopausal controls. Postmenopausal women, on average, had more risk factors, with 66.2% (n = 8699) of cases having 2 or more risk factors compared with 53.7% (n = 70 566) of the control women.
First-degree family history of breast cancer, history of benign breast biopsy, dense breasts, and nulliparity or age at first birth older than 30 years were associated with an increased risk of breast cancer (Table2). Obesity was not associated with breast cancer risk among premenopausal women, but overweight and obese postmenopausal women were at a higher risk of breast cancer. This association showed a statistically significant positive association, with overweight, obesity class I, and obesity class II or III women having 1.23, 1.39, and 1.54 times the odds of breast cancer relative to normal weight women.
Table 2.
Odds Ratios and PARP of Breast Cancer Risk Factors in Women Undergoing Screening or Diagnostic Mammographya
| Characteristic | Women With Breast Cancer | |||
|---|---|---|---|---|
| Premenopausal (n = 58 146) |
Postmenopausal (n = 144 600) |
|||
| OR (95% CI)b | PARP, % (95% CI)c | OR (95% CI)b | PARP, % (95% CI)c | |
| Family history of breast cancer | ||||
| No | 1 [Reference] | 8.7 (7.3–10.1) | 1 [Reference] | 8.2 (7.2–9.1) |
| Yes | 1.71 (1.59–1.84) | 1.53 (1.46–1.60) | ||
| BMId | ||||
| Underweight | 0.93 (0.76–1.15) | NA | 0.79 (0.67–0.93) | 22.8 (18.3–27.3)e |
| Normal | 1 [Reference] | 1 [Reference] | ||
| Overweight | 0.99 (0.93–1.07) | 1.23 (1.17–1.28) | ||
| Obesity class I | 1.00 (0.91–1.10) | 1.39 (1.31–1.47) | ||
| Obesity class II or III | 1.05 (0.94–1.18) | 1.54 (1.45–1.64) | ||
| History of benign breast biopsy | ||||
| No | 1 [Reference] | 6.9 (5.5–8.4) | 1 [Reference] | 8.6 (8.0–9.2) |
| Yes | 1.50 (1.40–1.62) | 1.41 (1.35–1.47) | ||
| Age at first live birth | ||||
| Nulliparous | 1.14 (1.05–1.22) | 8.7 (4.8–12.7) | 1.20 (1.14–1.26) | 5.2 (4.2–6.2) |
| ≤30 y | 1 [Reference] | 1 [Reference] | ||
| >30 y | 1.28 (1.19–1.37) | 1.23 (1.16–1.30) | ||
| BI-RADS breast densityf | ||||
| Almost entirely fat | 0.47 (0.38–0.58) | 39.3 (36.6–42.0)g | 0.62 (0.58–0.67) | 26.2 (24.4–28.0)g |
| Scattered fibroglandular densities | 1 [Reference] | 1 [Reference] | ||
| Heterogeneously dense | 1.57 (1.46–1.69) | 1.40 (1.34–1.45) | ||
| Extremely dense | 1.81 (1.65–1.99) | 1.58 (1.46–1.71) | ||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; OR, odds ratio; PARP, population-attributable risk proportion.
Estimated by multivariable conditional logistic regression.
Odds ratios presented were adjusted for family history of breast cancer, BMI, history of benign breast biopsy, age at first live birth, and Breast Imaging Reporting and Data System (BI-RADS) breast density.
PARP calculated using multivariable adjusted ORs.
Underweight, BMI less than 18.5; normal, 18.5–24.9; overweight, 25.0–29.9; obesity class I, 30.0–34.9; and obesity class II or III, 35.0 or more.
PARP calculated for shifting everyone to normal weight and holding underweight constant.
BI-RADS classification system: a, almost entirely fat; b, scattered fibroglandular densities; c, heterogeneously dense; and d, extremely dense.22
PARP calculated for shifting the BI-RADS categories heterogeneously dense and extremely dense to scattered fibroglandular densities, and holding almost entirely fat constant.
Among premenopausal women, the largest individual PARP was for breast density, with 39.3% (95% CI, 36.6%–42.0%) of breast cancers potentially removed by reducing breast density from BI-RADS heterogeneously or extremely dense breasts to scattered fibroglandular densities. The PARP for breast density increased to 65.5% (95% CI, 60.4%–70.6%) if all premenopausal women reduced their breast density to the lowest category of almost entirely fat. A more modest reduction of all women shifting to a single lower BI-RADS category would result in a PARP of 13.4% (95% CI, 11.0%–15.8%). Among premenopausal women, the combination of first-degree family history, history of benign breast biopsy, age at first birth, and breast density had a PARP of 52.7% (95% CI, 49.1%–56.3%) (Table 3).
Table 3.
PARP for Individual Risk Factors and Combinations of Factorsa
| Risk Factor | Breast Cancer, PARP (95% CI) | |
|---|---|---|
| Premenopausal | Postmenopausal | |
| 2 Risk Factors | ||
| Family history of breast cancer, breast density | 44.6 (41.3–47.9) | 32.2 (30.0–34.4) |
| History of benign breast biopsy, family history of breast cancer | 14.8 (13.3–16.3) | 16.0 (14.8–17.1) |
| History of benign breast biopsy, breast density | 43.5 (40.3–46.7) | 32.4 (30.8–34.0) |
| Breast density, BMI | NA | 43.3 (39.3–47.3) |
| Family history of breast cancer, BMI | NA | 29.1 (26.1–32.1) |
| History of benign breast biopsy, BMI | NA | 29.5 (24.4–34.2) |
| Nulliparous or age at first birth ≥30y, BMI | NA | 26.9 (23.9–29.8) |
| Nulliparous or age at first birth ≥30 y, family history of breast cancer | 16.6 (13.5–19.8) | 13.0 (11.4–14.5) |
| Nulliparous or age at first birth ≥30 y, history of benign breast biopsy | 15.0 (12.3–17.8) | 13.3 (11.8–14.9) |
| Nulliparous or age at first birth ≥30 y, breast density | 44.6 (41.1–48.1) | 30.0 (28.1–32.0) |
| 3 Risk Factors | ||
| Family history of breast cancer, history of benign breast biopsy, breast density | 48.3 (44.6–51.9) | 37.8 (35.9–39.7) |
| Family history of breast cancer, history of benign breast biopsy, nulliparous or age at first birth ≥30 y | 22.2 (19.8–24.6) | 20.3 (18.8–21.8) |
| Family history of breast cancer, history of benign breast biopsy, BMI | NA | 35.1 (30.2–40.0) |
| Family history of breast cancer, nulliparous or age at first birth ≥30 y, breast density | 49.4 (46.2–52.5) | 35.8 (33.9–37.6) |
| Family history of breast cancer, breast density, BMI | NA | 47.9 (45.1–50.7) |
| History of benign breast biopsy, nulliparous or age at first birth ≥30 y, breast density | 48.4 (45.1–51.7) | 35.9 (34.3–37.5) |
| History of benign breast biopsy, breast density, BMI | NA | 48.0 (44.7–51.4) |
| Family history of breast cancer, nulliparous or age at first birth ≥30 y, BMI | NA | 32.8 (29.9–35.8) |
| History of benign breast biopsy, nulliparous or age at first birth ≥30 y, BMI | NA | 33.2 (28.8–37.5) |
| Breast density, BMI, nulliparous or age at first birth ≥30 y | NA | 46.2 (42.5–49.9) |
| 4 Risk Factors | ||
| Family history of breast cancer, history of benign breast biopsy, breast density, nulliparous or age at first birth ≥30 y | 52.7 (49.1–56.3) | 41.0 (39.7–42.3) |
| Family history of breast cancer, history of benign breast biopsy, breast density, BMI | NA | 52.2 (49.0–55.4) |
| History of benign breast biopsy, nulliparous or age at first birth ≥30 y, breast density, BMI | NA | 50.7 (47.9–53.6) |
| Family history of breast cancer, nulliparous or age at first birth ≥30 y, breast density, BMI | NA | 50.6 (46.5–54.7) |
| Family history of breast cancer, nulliparous or age at first birth ≥30 y, history of benign breast biopsy, BMI | NA | 38.5 (36.4–40.7) |
| 5 Risk Factors | ||
| Family history of breast cancer, history of benign breast biopsy, nulliparous or age at first birth ≥30 y, breast density, BMI | NA | 54.7 (51.6–57.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); PARP, population-attributable risk proportion.
Family history of breast cancer, first-degree family history only; breast density, heterogeneously or extremely dense breasts with scattered fibroglandular densities as reference; BMI 25 or more.
Individual PARPs for first-degree family history, age at first birth, and history of benign breast biopsy were similar for pre-menopausal and postmenopausal breast cancer. However, overweight and obesity accounted for a large proportion of postmenopausal breast cancers, with a PARP of 22.8% (95% CI, 18.3%–27.3%) if all obese and overweight women achieved a normal BMI. The estimated PARP for shifting all postmenopausal women to the BI-RADS category of almost entirely fat was 43.9% (95% CI, 39.6%–48.2%), whereas shifting only extremely or heterogeneously dense breasts to scattered fibroglandular densities was 26.2% (95% CI, 24.4%–28.0%). The PARP was 12.7% (95% CI, 11.2%–14.3%) for reductions of a single BI-RADS category. The combination of first-degree family history, history of benign breast biopsy, nulliparity or age at first birth older than 30 years, breast density (with scattered fibro-glandular densities as reference) and BMI yielded a combined postmenopausal PARP of 54.7% (95% CI, 51.6–57.8) (Table 3).
Discussion
We found that routinely collected clinical risk factors included in breast cancer risk models may explain 52.7% of pre-menopausal and 54.7% of postmenopausal breast cancers. A substantial proportion of breast cancers can be attributed to high breast density alone, suggesting that behaviors or interventions that reduce breast density have the potential to eliminate a large proportion of breast cancers in both premenopausal and postmenopausal women. These easily assessed breast cancer risk factors are highly prevalent among premenopausal and postmenopausal women with breast cancer; more than half of the breast cancer cases in the population are attributable to these factors and thus they offer promise for risk-based screening and prevention strategies.
Although breast density is a well-established, strong, and prevalent breast cancer risk factor, few studies have quantified the PARP of breast density and, to our knowledge, none have used the BI-RADS classification utilized in clinical practice. In a study of Canadian women, Boyd et al15 found a PARP of 16% if women with more than 50% breast density reduced their breast density to 50% or less,15 and this PARP was much greater (approximately 40%) for cancers detected within 12 months of a negative screening examination, reflecting the increased probability of a masking effect in dense breasts.25 We found a PARP approximately 2-fold higher than that of Boyd et al if the breast density in all women shifted to the BI-RADS scattered fibroglandular density category and the PARP was unaltered in a sensitivity analysis excluding women with breast density measured within 1 year of diagnosis. Differences between our and Boyd et al’s study may reflect distinctions between a classification of greater than 50% density using quantitative measures that include a smaller proportion of women with substantial amounts of density, whereas the qualitative BI-RADS classification of heterogeneously or extremely dense includes larger proportions of women.26 Furthermore, the use of 50% or less as a reference category is likely to attenuate the relative risk used in the PARP, since the literature suggests that women with 10% to 50% breast density have an increased breast cancer risk compared with women with less than 10% density.27
We found that reductions in breast density of a single BI-RADS category would avert 13.4% of breast cancers among premenopausal women and 12.7% among postmenopausal women; such a reduction would prevent more cases than reducing any other risk factor in this study, with the exception of BMI in postmenopausal women. Studies of longitudinal changes in BI-RADS breast density suggest that a reduction of a single BI-RADS category reduced breast cancer risk relative to density that remained stable or increased.28,29 Our results suggest that shifting the distribution of breast density down a single category would still result in a substantial reduction in breast cancers in the population. Reductions of a single BI-RADS category could potentially be achieved through increased breastfeeding, as well as primary prevention with tamoxifen citrate for those at highest risk.30–33 These interventions effectively reduce breast density, but must be carefully considered in the context of anticipated harms. Our results highlight the necessity for new approaches to reduce breast density that could be widely adopted without adverse consequences, as reductions in breast density have the potential to greatly reduce the incidence of breast cancer.
To our knowledge, no prior studies have evaluated the PARP of clinical breast cancer risk factors in combination with breast density. However, our results are broadly consistent with previous literature evaluating nonmodifiable clinical risk factors, with PARP estimates ranging from 37.2%6 to 57.3%12 combining the risk factors of age at menarche, menopause, and first full-term pregnancy; parity; family history of breast cancer; and benign breast disease. Estimates of the PARP of BMI in postmenopausal women have been disparate across studies; Barnes et al6 estimated a PARP of 2% by shifting all women to a BMI of 22.4 or lower, Mezzetti et al9 estimated a PARP of 10.2% by shifting all women to a BMI of 23.3, and 3 additional studies found PARPs of 8.0%, 9.5%, and 24.8% by shifting women to a BMI of less than 25.8,10,34 It is difficult to directly compare our results with previous findings because of different reference categories. Our finding of a PARP of 22.8% may reflect the high prevalence of overweight and obese postmenopausal women in the United States compared with studies in European populations. Our results suggest that excess bodyweight plays an important role in postmenopausal breast cancer, further reinforcing the need for weight reduction and management to prevent a substantial proportion of breast cancers. In the absence of interventions, the PARP for obesity will increase with the prevalence of obesity in the United States.35
Our study included more than 200 000 women from BCSC community breast imaging registries, which is broadly representative of the demographic composition of women in the United States18 and with clinical risk factor distributions nearly identical to the distributions estimated in the population-based National Health Interview Survey (eFigure 1 and eFigure 2 in the Supplement). The BCSC 5- and 10-year absolute risk calculator was developed within the same cohort of women,36,37 although the use of breast cancer risk models to identify women for primary and secondary preventions has been controversial, with a commonly expressed concern that most women with breast cancer have no known risk factors. We found that only 10% of premenopausal and less than 5% of postmenopausal breast cancers in our study had no clinical risk factors. The impact of assessing clinical breast cancer risk factors in combination with breast density is considerable, explaining more than half of premenopausal and postmenopausal breast cancers and identifying risk factors where targeted public health interventions would have the greatest impact. These factors represent clinically available information that can and should be used by clinicians to stratify breast cancer risk for improved risk-based screening and primary and secondary prevention efforts.4
Strengths and Limitations
Estimates of the PARP are sensitive to changes in category definitions that alter the prevalence and relative risk of the risk factor.3,38 We chose categories based on clinical relevance, but examined how robust our findings were to changes in the reference group corresponding to ideal compared with more realistic interventions to change risk factor distributions. Close attention to risk factor prevalence should be considered when applying our results to other populations. We were unable to measure other behavioral and genetic risk factors; thus, our estimated PARP likely underestimates the joint PARP of all known risk factors. Our study uses risk factor and breast density information from 1996–2015—a period when the BI-RADS density category definitions changed. Despite these changes, there is no evidence of a difference in the distribution of breast density over time in the BCSC.22 Studies have found mixed results for interrater and intrarater reliability of the BI-RADS categories39–42; however, relative risks for breast cancer are similar comparing BI-RADS with more objective quantitative density measurements.43 Most importantly, BI-RADS is presently the only measure of breast density used routinely in clinical practice; thus, using BI-RADS enhances the clinical utility of our estimates for risk stratification and screening and prevention efforts.
Our study has several strengths, including collection of clinically available breast cancer risk factors. We provide novel insights into the contributions of breast density on a population level, reinforcing existing interventions to reduce breast density among high-risk women and the need for acceptable behaviors and novel interventions to reduce risk in women at high and average risk. Finally, we provide what we believe to be the first estimate of PARP for clinical risk factors in pre-menopausal women, and our results suggest that, with the exception of BMI, the PARP of most risk factors is similar among premenopausal and postmenopausal women.44
Conclusions
In what we believe to be the largest study of PARP in US women, we found that most premenopausal and postmenopausal women with breast cancer have at least 1 breast cancer risk factor, and that breast density and clinical risk factors may explain more than half of breast cancer cases. These risk factors represent clinically available data that can and should be used to stratify risk, using established risk models that include breast density, to promote risk-based screening and targeted prevention efforts. Future research should assess whether PARP estimates differ by molecular subtypes of breast cancer, where the magnitude and direction of risk factors may differ.45–48
Supplementary Material
Key Points.
Question
What proportion of premenopausal and postmenopausal breast cancers are attributed to commonly collected clinical risk factors?
Findings
In this population-based, case-control, cohort study of 202 746 women, breast density and body mass index had the largest individual population-attributable risk proportion. Thirty-nine percent of premenopausal and 26% of postmenopausal breast cancers could be prevented if breast density in women with dense breasts was reduced to scattered fibroglandular densities on the Breast Imaging Reporting and Data System scale, and postmenopausal breast cancer incidence would be reduced by 23% if all women achieved a body mass index less than 25.
Meaning
Clinical breast cancer risk factors explain a large proportion of breast cancer incidence and should be used in the clinical setting for risk stratification and targeted screening and prevention efforts.
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health, National Cancer Institute–funded Program Project grant P01 CA154292. Data collection was additionally supported by the Breast Cancer Surveillance Consortium (BCSC) contract HHSN261201100031C, and Vermont Breast Cancer Surveillance System data collection was also supported by grant U54CA163303. The collection of cancer and vital status data used in this study was supported, in part, by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see http://breastscreening.cancer.gov/work/acknowledgement.html.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Miglioretti and Kerlikowske had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Engmann, Miglioretti, Sprague, Kerlikowske.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Engmann, Golmakani, Kerlikowske.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Golmakani.
Obtained funding: Miglioretti, Sprague, Kerlikowske.
Administrative, technical, or material support: Kerlikowske.
Study supervision: Engmann, Miglioretti, Kerlikowske.
Conflict of Interest Disclosures: None reported.
Additional Information: A description of procedures for requesting BCSC data for research purposes is provided at http://breastscreening.cancer.gov/.
Additional Contributions: We thank the BCSC investigators, participating mammography facilities, and radiologists for the data they have provided for this study.
References
- 1.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 2.Kopans DB. An open letter to panels that are deciding guidelines for breast cancer screening. Breast Cancer Res Treat. 2015;151(1):19–25. doi: 10.1007/s10549-015-3373-8. [DOI] [PubMed] [Google Scholar]
- 3.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trentham-Dietz A, Kerlikowske K, Stout NK, et al. Breast Cancer Surveillance Consortium and the Cancer Intervention and Surveillance Modeling Network Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med. 2016;165(10):700–712. doi: 10.7326/M16-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(8):604–614. doi: 10.7326/0003-4819-158-8-201304160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Barnes BB, Steindorf K, Hein R, Flesch-Janys D, Chang-Claude J. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35(4):345–352. doi: 10.1016/j.canep.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Clarke CA, Purdie DM, Glaser SL. Population attributable risk of breast cancer in white women associated with immediately modifiable risk factors. BMC Cancer. 2006;6:170. doi: 10.1186/1471-2407-6-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes J, Richardson A, Frampton C. Population attributable risks for modifiable lifestyle factors and breast cancer in New Zealand women. Intern Med J. 2013;43(11):1198–1204. doi: 10.1111/imj.12256. [DOI] [PubMed] [Google Scholar]
- 9.Mezzetti M, La Vecchia C, Decarli A, Boyle P, Talamini R, Franceschi S. Population attributable risk for breast cancer: diet, nutrition, and physical exercise. J Natl Cancer Inst. 1998;90(5):389–394. doi: 10.1093/jnci/90.5.389. [DOI] [PubMed] [Google Scholar]
- 10.van Gemert WA, Lanting CI, Goldbohm RA, et al. The proportion of postmenopausal breast cancer cases in the Netherlands attributable to lifestyle-related risk factors. Breast Cancer Res Treat. 2015;152(1):155–162. doi: 10.1007/s10549-015-3447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson LF, Page AN, Dunn NAM, Pandeya N, Protani MM, Taylor RJ. Population attributable risk of modifiable risk factors associated with invasive breast cancer in women aged 45–69 years in Queensland, Australia. Maturitas. 2013;76(4):370–376. doi: 10.1016/j.maturitas.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Sprague BL, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Hampton JM, Newcomb PA. Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am J Epidemiol. 2008;168(4):404–411. doi: 10.1093/aje/kwn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park B, Park S, Shin H-R, et al. Population attributable risks of modifiable reproductive factors for breast and ovarian cancers in Korea. BMC Cancer. 2016;16(1):5. doi: 10.1186/s12885-015-2040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87(22):1681–1685. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 15.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 16.Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255–dju255. doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;169(4):1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute Division of Cancer Control & Population Sciences. Breast Cancer Surveillance Consortium (BSCS) http://breastscreening.cancer.gov/. Updated July 26 2016. Accessed June 25, 2016.
- 19.Kerlikowske K, Barclay J, Grady D, Sickles EA, Ernster V. Comparison of risk factors for ductal carcinoma in situ and invasive breast cancer. J Natl Cancer Inst. 1997;89(1):76–82. doi: 10.1093/jnci/89.1.76. [DOI] [PubMed] [Google Scholar]
- 20.Reeves GK, Pirie K, Green J, Bull D, Beral V, Million Women Study Collaborators Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer. 2012;131(4):930–937. doi: 10.1002/ijc.26460. [DOI] [PubMed] [Google Scholar]
- 21.Expert Panel on the Identification Evaluation and Treatment of Overweight and Obesity in Adults. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 22.Sickles E, D’Orsi C, Mendelson E, Morris E. ACR BI-RADS Atlas Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 23.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122(5):904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 24.Benichou J, Gail MH. Variance calculations and confidence intervals for estimates of the attributable risk based on logistic models. Biometrics. 1990;46(4):991–1003. [PubMed] [Google Scholar]
- 25.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobbes MBI, Cleutjens JPM, Lima Passos V, et al. Density is in the eye of the beholder: visual versus semi-automated assessment of breast density on standard mammograms. Insights Imaging. 2012;3(1):91–99. doi: 10.1007/s13244-011-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5):dju078. doi: 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. National Institutes of Health Breast Cancer Surveillance Consortium Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99(5):386–395. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 29.Kerlikowske K, Gard CC, Sprague BL, Tice JA, Miglioretti DL, Breast Cancer Surveillance Consortium One vs two breast density measures to predict 5- and 10-year breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2015;24(6):889–897. doi: 10.1158/1055-9965.EPI-15-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuzick J, Warwick J, Pinney E, Warren RML, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 31.Chow CK, Venzon D, Jones EC, Premkumar A, O’Shaughnessy J, Zujewski J. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9(9):917–921. [PubMed] [Google Scholar]
- 32.Brisson J, Brisson B, Coté G, Maunsell E, Bérubé S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9(9):911–915. [PubMed] [Google Scholar]
- 33.Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8(10):863–866. [PubMed] [Google Scholar]
- 34.Ghiasvand R, Bahmanyar S, Zendehdel K, et al. Postmenopausal breast cancer in Iran; risk factors and their population attributable fractions. BMC Cancer. 2012;12:414. doi: 10.1186/1471-2407-12-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fryar D, Carroll M, Ogden C. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2009–2010. National Center of Health Statistics E-Stat. http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.htm#table3. Updated September 19, 2014. Accessed July 3, 2016.
- 36.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105(14):1043–1049. doi: 10.1093/jnci/djt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockhill B, Weinberg CR, Newman B. Population attributable fraction estimation for established breast cancer risk factors: considering the issues of high prevalence and unmodifiability. Am J Epidemiol. 1998;147(9):826–833. doi: 10.1093/oxfordjournals.aje.a009535. [DOI] [PubMed] [Google Scholar]
- 39.Ekpo EU, Ujong UP, Mello-Thoms C, McEntee MF. Assessment of interradiologist agreement regarding mammographic breast density classification using the fifth edition of the BI-RADS atlas. AJR Am J Roentgenol. 2016;206(5):1119–1123. doi: 10.2214/AJR.15.15049. [DOI] [PubMed] [Google Scholar]
- 40.Gard CC, Aiello Bowles EJ, Miglioretti DL, Taplin SH, Rutter CM. Misclassification of breast imaging reporting and data system (BI-RADS) mammographic density and implications for breast density reporting legislation. Breast J. 2015;21(5):481–489. doi: 10.1111/tbj.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spayne MC, Gard CC, Skelly J, Miglioretti DL, Vacek PM, Geller BM. Reproducibility of BI-RADS breast density measures among community radiologists: a prospective cohort study. Breast J. 2012;18(4):326–333. doi: 10.1111/j.1524-4741.2012.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprague BL, Conant EF, Onega T, et al. PROSPR Consortium Variation in mammographic breast density assessments among radiologists in clinical practice: a multicenter observational study. Ann Intern Med. 2016;165(7):457–464. doi: 10.7326/M15-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandt KR, Scott CG, Ma L, et al. Comparison of clinical and automated breast density measurements: implications for risk prediction and supplemental screening. Radiology. 2016;279(3):710–719. doi: 10.1148/radiol.2015151261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia X, Chen W, Jiaoyuan L, et al. Excess body mass index and risk of breast: a nonlinear dose-response meta-analysis of prospective studies. Sci Rep. 2014;4:1–5. doi: 10.1038/srep07480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144(1):1–10. doi: 10.1007/s10549-014-2852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 48.Kerlikowske K, Gard CC, Tice JA, Ziv E, Cummings SR, Miglioretti DL, Breast Cancer Surveillance Consortium Risk factors that increase risk of estrogen receptor–positive and –negative breast cancer. J Natl Cancer Inst. 2016;109(5):1–9. doi: 10.1093/jnci/djw276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


