Abstract
Background
Despite progress in the diagnosis and management of asthma, many patients have poorly controlled or refractory asthma (RA). The mechanism of this RA is not well understood.
Objective
We sought to explore the relationship between neutrophils and other biomarkers of RA.
Method
Sixty patients with RA, 30 patients with nonrefractory asthma (NRA), and 20 healthy subjects were enrolled. We performed a comprehensive characterization of these study subjects, which included laboratory and pulmonary function studies, chest computed tomography, and bronchoscopy with bronchoalveolar lavage (BAL). We analyzed BAL fluid and serum for a total of 244 biomolecules using a multiplex assay and correlated them with clinical and other laboratory parameters.
Results
RA was significantly different from NRA with regard to pulmonary function indices, bronchial basement membrane thickness, and BAL fluid neutrophil and lymphocyte counts but not eosinophil counts. BAL fluid neutrophil counts negatively and positively correlated with forced vital capacity and age, respectively. Of the 244 biomolecules studied, 52 and 14 biomolecules from BAL fluid and serum, respectively, were significantly different among the study groups. Thirteen of these 52 molecules correlated with BAL fluid neutrophil counts. BAL fluid from 40% of patients with RA was positive for a pathogenic microbe. Infection-negative neutrophilic RA was associated with an increase in levels of select biomarkers of inflammation in the serum, suggesting the presence of systemic inflammation.
Conclusions
RA was associated with increased numbers of neutrophils and proneutrophilic biomolecules in the airways. Subclinical infection was present in 40% of patients with RA, which likely contributed to neutrophilic inflammation. A subgroup of patients with noninfected neutrophilic RA was associated with systemic inflammation.
Key words: Refractory asthma, neutrophilic asthma, bronchoalveolar lavage, cytokines, infection
Abbreviations used: BAL, Bronchoalveolar lavage; CT, Computed tomography; Feno, Fraction of exhaled nitric oxide; FVC, Forced vital capacity; GDF-15, Growth and differentiation factor 15; GO, Gene Ontology; MMP, Matrix metalloproteinase; NAP2, Neutrophil activating peptide-2; NRA, Nonrefractory asthma; OCS, Oral corticosteroid; RA, Refractory asthma; SAA, Serum amyloid A
Graphical abstract
The majority of asthmatic patients respond well to controller medications, such as inhaled corticosteroids and long-acting β-agonists. However, a subset of patients does not respond to multiple controller medications, including omalizumab, and their asthma symptoms remain poorly controlled. This group of patients is labeled as having refractory asthma (RA) or severe asthma.1, 2, 3 These patients experience a high level of morbidity because of the disease. Many of these patients require chronic systemic steroid therapy, which results in severe side effects and adds to the morbidity. Because of heavy use of health care services (emergency department and hospital), patients with RA account for a disproportionately higher health care cost.2 It is estimated that about 3% to 10% of patients have severe RA.2, 3, 4, 5 The treatment options for RA are limited, primarily because of the lack of understanding of its mechanisms.
Asthma is a heterogeneous disease. Based on clinical and laboratory findings, asthma has been variably classified as atopic versus nonatopic asthma, eosinophilic versus neutrophilic versus noneosinophilic/nonneutrophilic asthma, obesity-associated asthma,3 asthma/chronic obstructive pulmonary disease overlap syndrome,6 TH2-high asthma,7 TH2/TH17-high asthma,8 and TH2-low asthma,7, 8 for example. Severe asthma and RA can occur within each category of patients. It is unclear whether a single or multiple mechanisms are operating in patients with severe RA. Previous studies have identified a number of factors in patients with severe asthma. Heightened activation of type 1 (TH1, the interferon pathway),9 type 2 (TH2 cells, type 2 CD8 T cells),7, 10 innate response,11 and airway neutrophilia12 have all been implicated in patients with RA. Levels of the type 3 cytokines IL-17A and IL-17F are increased in asthmatic patients.8, 13 However, they were not associated with levels of TH17 cytokines in human asthma nor were they associated with neutrophilic asthma,13, 14, 15 which is unlike what has been reported in the mouse model of asthma.16 Increased epithelial injury, smooth muscle hyperplasia and hypertrophy, and airway remodeling are some of the recognized intrinsic factors of RA.1, 2 The external factors associated with RA include heightened exposure to sensitizing allergens,17, 18 air pollution,19, 20 and infections.21, 22, 23 Heightened levels of select cytokines/inflammatory mediators were associated with many of the foregoing subgroups of RA.23, 24
Despite progress in the field, there is a need to examine the precise molecular mechanism of heterogeneity of RA that allows development of targeted therapeutic interventions. To address this matter, we studied 60 patients with RA3 and compared them with 30 patients with nonrefractory asthma (NRA) and 20 healthy control subjects. Bronchoalveolar lavage (BAL) fluid and serum were analyzed for 244 biomolecules, levels of which were then correlated with the clinical and laboratory features of asthma. The latter included pulmonary function, PC20 for methacholine, allergic sensitivity, fraction of exhaled nitric oxide (Feno), body mass index, blood eosinophil counts, total IgE levels, BAL fluid inflammatory cell counts (eosinophil, neutrophil, lymphocyte, and macrophage counts), indices of airway tissue inflammation (quantitative histologic evaluation of bronchial tissue) and basement thickness, and the results of microbiological studies with BAL, tissue biopsy, and airway brushing. The focus of this article is on the neutrophilic phenotype of RA.
Methods
Human subjects
Study subjects with RA were recruited from outpatient clinics of National Jewish Health. Patients with NRA and healthy control subjects were recruited from the community. The study protocol for bronchoscopy and BAL was approved by the institutional review board (HS#2639). Written informed consent was obtained from the study subjects. Patients were allowed to continue their routine medication. Asthmatic patients had a comprehensive phenotypical and laboratory work-up. The latter included full pulmonary function tests; methacholine tests (if FEV1 was >60% of predicted value); chest computed tomography (CT); Feno values; blood test results for eosinophils, total IgE levels, and IgE antibody levels (when appropriate); bronchoscopy with BAL; airway brushing; and endobronchial biopsy (see Fig E1, A, in this article's Online Repository at www.jacionline.org).
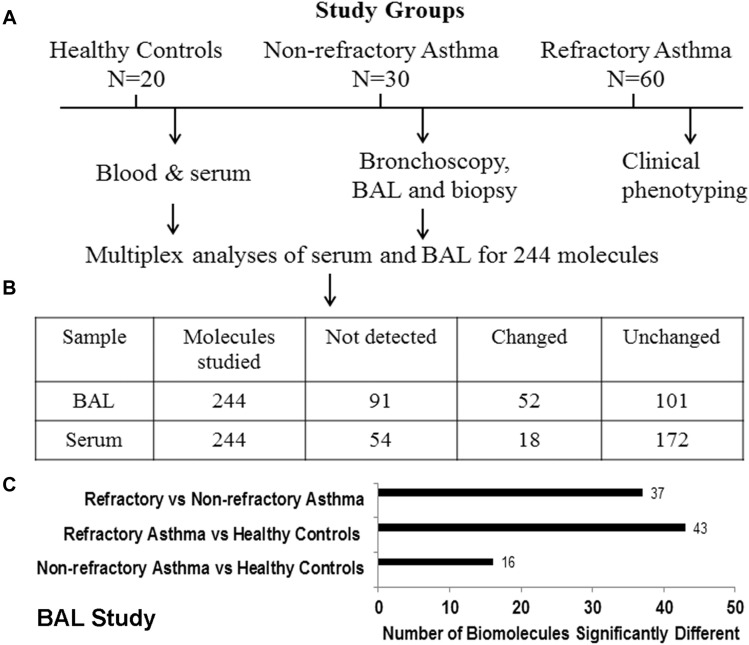
Fig E1.
Study design and overview of the results. A, Schematic presentation of study design. B, Inserted table summarizes the number of biomolecules studied, not detected, changed, and unchanged in BAL fluid and serum from the study subjects. C, Number of biomolecules in BAL fluid that were significantly (P < .05) different among study populations.
Bronchoscopy, BAL, brushing, biopsy, and other laboratory work-up
The foregoing procedures were performed, as described previously.25 BAL fluid was processed within an hour. Cells were isolated by means of centrifugation. Supernatant fluid was aliquoted into small samples and frozen at −80°C. Total BAL fluid cells and differential counts; a PCR-based respiratory viral panel (12 different respiratory viruses) with BAL cells and bronchial brushing; a PCR-based test for Mycoplasma pneumoniae and Chlamydophila pneumoniae with airway biopsy specimens, brushings, and BAL cells; BAL culture in triplicates for bacteria, Mycobacterium tuberculosis, and nontuberculous mycobacteria; and a quantitative analysis of airway tissue inflammatory cells, including CD117+ mast cells (by using immunohistochemical staining) were performed in the National Jewish Health clinical laboratory (ADx-Advanced Diagnostic Lab, National Jewish Health).
Multiplex biomolecule analyses of BAL fluid and serum
Aliquots of BAL fluid and serum were analyzed for a total 244 biomolecules (Human Discovery MAP 250+, v2) by Myriad RBM (Austin, Tex). A list of the biomolecules analyzed by using this method is presented in Table E1 in this article's Online Repository at www.jacionline.org.
Pathway analyses
We performed pathway analyses for the 36 biomolecules that distinguished RA from NRA. We used multiple software at the Enrichnet.org Web site: Gene Ontology (GO) for biological process and molecular function, Reactome, and the Kyoto Encyclopedia of Genes and Genomes.
Statistical analyses
Comparison between study groups was done with the Mann-Whitney U test. Comparison among multiple study groups was performed with the Kruskal-Wallis test followed by the Dunn test to correct for multiple comparisons. In addition, we performed the Benjamini-Hochberg test for multiple comparisons, which corrects for false discovery. The false discovery rate for this test was set at 0.1. The Pearson correlation coefficient was used to calculate correlation coefficient. Statistical analyses were performed with Prism software (GraphPad Software, La Jolla, Calif).
Results
Study populations
We recruited 20 healthy control subjects, 30 patients with well-controlled asthma, and 60 patients with RA, as defined by American Thoracic Society definitions.26 The patients with NRA did not meet the criteria for RA because their asthma symptoms were well controlled by inhaled corticosteroids and other controllers. Thirty-six percent of patients with RA (22/60) were taking systemic steroids (10-60 mg/d). Asthmatic patients with a history of current or past smoking were excluded from the study. The demographic details of the study population are shown in Table I .
Table I.
Demographic, clinical, and laboratory features of the study groups
| Variable | Healthy Control subjects (n = 20) |
Patients with NRA (n = 30) |
Patients with RA (n = 60) |
P value, all groups | P value, NRA vs RA | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | |||
| Age (y) | 33.6 ± 2 | 31 | 38 ± 2 | 35.5 | 54.5 ± 1 | 57 | <.0001 | <.0001 |
| Female/male | 13/7 | 19/11 | 36/24 | |||||
| BMI (kg/m2) | 29.7 ± 1.2 | 29.8 | 28 ± 1.1 | 27.4 | 29.7 ± 0.7 | 29.4 | .12 | .051 |
| Atopy | 50% | 66% | 88% | |||||
| FVC (L) | 4.1 ± 0.1 | 4.3 | 3.6 ± 0.1 | 3.5 | 2.7 ± 0.1 | 2.5 | <.0001 | <.0001 |
| FEV1 (L) | 3.3 ± 0.1 | 3.4 | 2.6 ± 0.1 | 2.7 | 1.8 ± 0.09 | 1.6 | <.0001 | <.0001 |
| FVC (%) | 101 ± 2.9 | 97.5 | 92 ± 2.5 | 88 | 71 ± 2 | 71 | <.0001 | <.0001 |
| FEV1 (%) | 96.5 ± 2.6 | 95 | 78 ± 3 | 74 | 60 ± 2.5 | 55 | <.0001 | <.0001 |
| Reversibility (%) | 5.2 ± 0.5 | 5.5 | 17.6 ± 2.1 | 17 | 22 ± 2.5 | 16 | <.0001 | <.0001 |
| PC20 for methacholine | 25 ± 0 | 25 | 2.5 ± 0.5 (n = 29/30) | 1 | 3.4 ± 1.1 (n = 23/60) | 1.5 | <.0001 | .19 |
| Feno | 23 ± 3 | 21 | 35 ± 3 | 28.7 | 43.8 ± 6 | 22.5 | .17 | .55 |
| IgE (kU/L) | 47 ± 9 | 33 | 333 ± 199 | 106 | 406 ± 149 | 116 | <.001 | .6 |
| Blood eosinophils/μL | 150 ± 21 | 100 | 283 ± 28 | 300 | 352 ± 43 | 300 | <.0001 | .4 |
| Blood PMNs (1000/μL) | 4.0 ± 0.2 | 3.6 | 3.6 ± 0.3 | 3.2 | 6.5 ± 0.4 | 5.1 | .0001 | .0001 |
| BAL WBCs (× 106) | 10.2 ± 0.8 | 9.4 | 8.7 ± 0.7 | 8.7 | 15.8 ± 2.7 | 8 | .6 | .5 |
| BAL eosinophils (% [total × 106]) | 0.48 ± 0.1 (0.04 ± 0.01) | 0.2 (0.022) | 1.4 ± 0.2 (0.11 ± 0.02) | 1 (0.079) | 2.6 ± 0.6 (0.32 ± 0.09) | 1 (0.042) | .07 (.19) | .3 (.6) |
| BAL PMNs (% [total × 106]) | 1.9 ± 0.2 (0.19 ± 0.02) | 1.8 (0.16) | 2.5 ± 0.4 (0.22 ± 0.05) | 2 (0.15) | 10.9 ± 2.4 (3.12 ± 1.8) | 4.5 (0.40) | .002 (.003) | .01 (.004) |
| BAL lymphocytes (% [total × 106]) | 6 ± 0.7 (0.61 ± 0.08) | 5.2 (0.45) | 6.4 ± 1.1 (0.55 ± 0.12) | 4.6 (0.30) | 11.3 ± 1.3 (1.43 ± 0.27) | 8 (0.60) | .08 (.2) | .03 (.1) |
| BAL macrophages (% [total × 106]) | 91.5 ± 0.9 (9.42 ± 0.7) | 92.8 (8.8) | 89.7 ± 1.2 (7.87 ± 0.6) | 91.8 (7.6) | 75.2 ± 2.8 (10.8 ± 1.7) | 82.5 (6.3) | .001 (.3) | .006 (0.7) |
| Biopsy eosinophils/HPF | 1.2 ± 0.6 | 0 | 6.1 ± 4.3 | 0 | 10.2 ± 3.4 | 0 | .6 | .4 |
| Biopsy PMNs/HPF | 0.7 ± 0.1 | 0.5 | 0.4 ± 0.2 | 0 | 2.3 ± 2 | 0 | .0001 | .09 |
| Biopsy CD117+ cells | 3.8 ± 1.3 | 1 | 4.9 ± 1 | 3 | 1.5 ± 0.3 | 0 | .002 | .001 |
| BM thickness | 6.4 ± 0.6 | 6 | 7.5 ± 0.6 | 7.2 | 8.8 ± 0.4 | 8 | .01 | .04 |
| Serum vitamin D | 28.4 ± 3 | 26.4 | 37.5 ± 2.6 | 34.7 | 36.5 ± 2.8 | 34.6 | .056 | .4 |
BM, Basement membrane thickness; BMI, body mass index; HPF, high-powered field; WBC, white blood cells.
Clinical and phenotypical characteristics
Healthy control subjects and patients with NRA were younger than patients with RA (Table I). Body mass index was not significantly different among the study groups. Patients with RA were more atopic (88%) than patients with NRA (66%) and healthy control subjects (55%), as judged by skin test positivity or the presence of IgE antibody against an environmental allergen. Pulmonary function indices (forced vital capacity [FVC] and FEV1) were significantly lower and reversibility was significantly higher in patients with RA compared with those with NRA. There was no difference in PC20 for methacholine between patients with RA and those with NRA. Feno values were not different among the 3 study groups. Blood eosinophil counts and total IgE levels were increased in asthmatic patients (both patients with RA and those with NRA) but were not different between patients with RA and those with NRA. Neither BAL fluid nor airway tissue eosinophil counts showed any significant difference between the NRA and RA groups. In contrast, BAL fluid and airway tissue neutrophil and BAL lymphocyte counts were increased in patients with RA. Surprisingly, numbers of tissue CD117+ cells, which mostly represent mast cells, were decreased in patients with RA. Subepithelial basement membrane thickness, a sign of airway remodeling, was significantly increased in asthmatic patients, with the greatest thickness occurring in patients with RA.
Biochemical analyses of BAL fluid
Of the 244 biomolecules assayed with the Human DiscoveryMAPs v2 multiplex assay, 66 were less than the detection limit (see Fig E1, B). One hundred one biomolecules did not show any significant difference among the study groups: healthy control subjects, patients with NRA, and patients with RA. Fifty-two biomolecules were significantly different either in multigroup comparisons or in comparisons between patients with RA and those with NRA (Table II ). Thirty-seven of these 52 biomolecules distinguished patients with RA from those with NRA (Table II and see Fig E1, C). Because BAL fluid neutrophils were one of the cell types that distinguished patients with RA from those with NRA, we focused on neutrophil-associated biomolecules for this study. We identified 17 neutrophil-associated biomolecules with levels that were significantly increased in patients with RA when compared with those in patients with NRA and healthy control subjects (Table III ). Of these 17 biomolecules, 13 showed a significant positive correlation with the BAL fluid neutrophil count (Table IV ). Four biomolecules did not show any significant correlation. It is noteworthy that levels of only one of these 13 biomolecules, CCL3 (macrophage inflammatory protein 1β), correlated with BAL fluid eosinophil counts. We classified these biomolecules based on their published cellular sources (see Table E2 in this article's Online Repository at www.jacionline.org). This classification suggests that innate immune and airway tissue cells are the primary source of RA-associated proneutrophilic biomolecules. This also implies that activation of innate immune and airway tissue cells contributes significantly to RA.
Table II.
Biomolecules that are statistically different among the study groups
| Biomolecules | Healthy subjects (n = 20) | Patients with NRA (n = 40) | P value vs healthy subjects | Patients with RA (n = 60) | P value vs healthy subjects | P value vs NRA | P value for multigroup analyses |
|---|---|---|---|---|---|---|---|
| 1. α-1 Antitrypsin∗ (μg/mL) | 0.4 | 0.7 | NS | 1.5 | .009 | .01 | .005 |
| 2. Angiogenin (ng/mL) | 0.63 | 0.6 | NS | 0.85 | .002 | .003 | .007 |
| 3. Apolipoprotein C III (μg/mL) | 0.01 | 0.018 | NS | 0.027 | .02 | NS | NS |
| 4. Apolipoprotein E (ng/mL) | 0.1 | 0.95 | .008 | 1.5 | .001 | NS | .001 |
| 5. Axl receptor (ng/mL) | 1.2 | 0.6 | <.001 | 0.39 | .001 | NS | .004 |
| 6. C3 (ng/mL) | 0.34 | 0.33 | NS | 0.66 | .04 | .03 | .03 |
| 7. CCL3 (MIP1β) (pg/mL) | 11 | 12 | NS | 23 | .007 | .002 | .0001 |
| 8. CCL5 (RANTES [pg/mL]) | 6.8 | 7.2 | NS | 12 | .009 | .03 | .01 |
| 9. CCL20 (MIP3α [pg/mL]) | 19 | 16 | NS | 31 | NS | .02 | NS |
| 10. CCL21 (pg/mL) | 3.8 | 4 | NS | 5.1 | NS | .01 | .02 |
| 11. CXCL9 (MIG [pg/mL]) | 69 | 79 | NS | 138 | NS | .02 | NS |
| 12. EGF (pg/mL) | 1.1 | 1.3 | NS | 2.6 | NS | .01 | .03 |
| 13. Ferritin (ng/mL) | 6.1 | 5.5 | NS | 9.8 | .01 | .03 | .01 |
| 14. Ficolin 3 (ng/mL) | 8.2 | 6.5 | NS | 11 | .03 | .001 | .002 |
| 15. Human epididymis protein 4 (pmol/L) | 5000 | 3820 | NS | 4980 | NS | .01 | .03 |
| 16. IL-1β (pg/mL) | 0 | 0 | NS | 0 | .07 | .01 | .01 |
| 17. IL-6 (pg/mL) | 0 | 0 | .01 | 0 | NS | .005 | .003 |
| 18. IL-8 (pg/mL) | 11 | 11 | NS | 24 | .01 | .002 | .0005 |
| 19. IL-18 (pg/mL) | 120 | 34 | .03 | 14 | .007 | .003 | .001 |
| 20. IL-22 (pg/mL) | 170 | 100 | .001 | 59 | <.001 | .001 | <.001 |
| 21. Kallikrein 7 (pg/mL) | 0 | 0 | NS | 145 | NS | .006 | .002 |
| 22. Lipocalin 2 (ng/mL) | 65 | 82 | NS | 111 | .05 | .04 | .04 |
| 23. MIF (pg/mL) | 0.39 | 1.5 | .0007 | 1.3 | .0016 | NS | .004 |
| 24. MMP3 (ng/mL) | 0 | 0 | NS | 0.01 | <.001 | <.001 | <.001 |
| 25. MMP7 (ng/mL) | 0.9 | 0.6 | .001 | 1.4 | NS | .04 | .04 |
| 26. MMP9, total (ng/mL) | 2.9 | 4.3 | NS | 20 | <.001 | <.001 | <.001 |
| 27. Myeloperoxidase (ng/mL) | 51 | 46 | NS | 133 | .0002 | <.0001 | <.0001 |
| 28. Myoglobin (ng/mL) | 0.57 | 0.94 | .03 | 0.91 | .04 | NS | NS |
| 29. NAP2 (pg/mL) | 0.41 | 0.79 | NS | 1.7 | .01 | .02 | .001 |
| 30. Neuropilin 1 (pg/mL) | 980 | 560 | .001 | 580 | <.001 | NS | .001 |
| 31. Osteoprotegerin (pmol/L) | 0 | 0 | NS | 0 | NS | .006 | .02 |
| 32. PAI-1 (pg/mL) | 42 | 41 | NS | 11 | <.001 | <.001 | <.001 |
| 33. Pepsinogen I (ng/mL) | 0.064 | 0.06 | NS | 0.093 | .007 | <.001 | .001 |
| 34. Pigment epithelium-derived factor (ng/mL) | 3.2 | 6.3 | NS | 11 | .02 | .006 | .005 |
| 35. Progranulin (ng/mL) | 13 | 5.2 | .01 | 4 | .03 | NS | .007 |
| 36. Prostasin (ng/mL) | 520 | 309 | .01 | 278 | .001 | NS | .005 |
| 37. RAGE | 0.3 | 1 | NS | 6.5 | <.001 | <.001 | <.001 |
| 38. Soluble CD25 (pg/mL) | 69 | 39 | NS | 29 | .003 | .01 | .001 |
| 39. Soluble CD40 (ng/mL) | 0.093 | 0.056 | .008 | 0.05 | .002 | NS | .03 |
| 40. SAA (ng/mL) | 0.51 | 0.57 | NS | 2 | .0002 | .001 | .003 |
| 41. Soluble ICAM (pg/mL) | 30 | 19 | .04 | 16 | .004 | NS | .02 |
| 42. Soluble IL-1 receptor (pg/mL) | 65 | 26 | .01 | 22 | .003 | NS | .008 |
| 43. Sortilin (ng/mL) | 0 | 0.05 | NS | 0.12 | .01 | .01 | .008 |
| 44. SP-D∗ (ng/mL) | 50 | 39 | NS | 35.5 | .01 | NS | .04 |
| 45. Thrombospondin 1 (ng/mL) | 0 | 1.4 | NS | 4.4 | <.001 | <.001 | <.001 |
| 46. TIMP-1 (ng/mL) | 1.3 | 1.8 | NS | 3.1 | .004 | .003 | .001 |
| 47. TNFSF12 (pg/mL) | 50 | 30 | NS | 20 | .006 | .01 | .001 |
| 48. TNFSF13 (pg/mL) | 18 | 4.3 | .002 | 1.3 | .001 | NS | .001 |
| 49. Trefoil factor 3 (μg/mL) | 0.1 | 0.22 | NS | 0.42 | .004 | .004 | .006 |
| 50. VEGF (pg/mL) | 364 | 292 | NS | 177 | .001 | NS | .008 |
| 51. Vitamin D binding protein∗ (μg/mL) | 0.34 | 0.15 | .002 | 0.2 | .01 | NS | .008 |
| 52. vWF (μg/mL) | 0.004 | 0.008 | NS | 0.012 | .003 | .002 | .009 |
EGF, Epidermal growth factor; ICAM, intercellular adhesion molecule; MIF, macrophage migration inhibitory factor; MIG, monokine induced by IFN-γ; MIP, macrophage inflammatory protein; NS, not significant; PAI-I, plasminogen activator inhibitor 1; RAGE, receptor for advanced glycosylation end products; SP-D, surfactant protein D; TIMP-1, tissue inhibitor of metalloproteinases 1; TNFSF12 and 13, tumor necrosis factor superfamily members 12 and 13; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor.
Steroid sensitive.
Table III.
Neutrophil-associated biomolecules that are statistically different among the study groups
| Biomolecules | Healthy subjects (n = 20) | Patients with NRA (n = 40) | P value vs healthy subjects | Patients with RA (n = 60) | P value vs healthy subjects | P value vs NRA | P value for multigroup analyses |
|---|---|---|---|---|---|---|---|
| 1. C3 (ng/mL) | 0.34 | 0.33 | NS | 0.66 | .06 | .03 | .03 |
| 2. CCL3 (MIP1β [pg/mL]) | 11 | 12 | NS | 23 | .007 | .002 | .0001 |
| 3. CCL20 (MIP3α [pg/mL]) | 19 | 16 | NS | 31 | NS | .02 | .05 |
| 4. EGF (pg/mL) | 1.1 | 1.3 | NS | 2.6 | NS | .01 | .03 |
| 5. Ficolin 3 (ng/mL) | 8.2 | 6.5 | NS | 11 | .03 | .001 | .002 |
| 6. IL-1β (pg/mL) | 0 | 0 | NS | 0 | .07 | .01 | .01 |
| 7. IL-6 (pg/mL) | 0 | 0 | .01 | 0 | NS | .005 | .003 |
| 8. IL-8 (pg/mL) | 11 | 11 | NS | 24 | .01 | .002 | .0005 |
| 9. Lipocalin 2 (ng/mL) | 65 | 82 | NS | 111 | .05 | .04 | .04 |
| 10. MMP7 (ng/mL) | 0.68 | 0.6 | NS | 1.6 | .002 | .0003 | .0003 |
| 11. MMP9, total (ng/mL) | 2.9 | 4.3 | NS | 20 | <.001 | <.001 | <.001 |
| 12. Myeloperoxidase (ng/mL) | 51 | 46 | NS | 133 | .0002 | <.0001 | <.0001 |
| 13. NAP2 (pg/mL) | 0.41 | 0.79 | NS | 1.7 | .01 | .02 | .001 |
| 14. RAGE | 0.3 | 1 | NS | 6.5 | <.001 | <.001 | <.001 |
| 15. SAA (ng/mL) | 0.51 | 0.57 | NS | 2 | .0002 | .001 | .003 |
| 16. Thrombospondin 1 (ng/mL) | 0 | 1.4 | NS | 4.4 | <.001 | <.001 | <.001 |
| 17. TIMP-1 (ng/mL) | 1.3 | 1.8 | NS | 3.1 | .004 | .003 | .001 |
EGF, Epidermal growth factor; MIP, macrophage inflammatory protein; NS, not significant; RAGE, receptor for advanced glycosylation end products; TIMP-1, tissue inhibitor of metalloproteinases 1.
Table IV.
Correlation of BAL fluid biomolecule level with inflammatory and other biological processes
| Biomolecules | Correlation with BAL fluid neutrophils (r) | P value | Correlation with BAL fluid eosinophils (r) | P value | Correlation with BAL fluid lymphocytes (r) | P value |
|---|---|---|---|---|---|---|
| Group 1. Biomolecules correlated with neutrophils | ||||||
| 1. Lipocalin 2 | 0.86 | <.001 | 0.13 | NS | −0.15 | NS |
| 2. SAA | 0.70 | <.001 | 0.25 | NS | 0.002 | NS |
| 3. CXCL7 (NAP2) | 0.65 | <.001 | 0.07 | NS | 0.002 | NS |
| 4. IL-8 | 0.62 | <.001 | −0.04 | NS | 0.06 | NS |
| 5. Thrombospondin 1 | 0.60 | <.001 | 0.06 | NS | 0.15 | NS |
| 6. IL-1β | 0.58 | <.001 | 0.05 | NS | 0.03 | NS |
| 7. Ficolin 3 | 0.57 | .001 | 0.18 | NS | 0.32 | .03 |
| 8. IL-6 | 0.52 | <.001 | 0.07 | NS | 0.17 | NS |
| 9. PAI | 0.46 | .003 | −0.02 | NS | −0.02 | NS |
| 10. MMP7 | 0.41 | .01 | 0.12 | NS | 0.16 | NS |
| 11. MMP9, total | 0.37 | .003 | 0.05 | NS | −0.07 | NS |
| Group 2. Biomolecules correlated with multiple inflammatory cells | ||||||
| 1. C3 | 0.48 | <.001 | 0.11 | NS | 0.45 | .002 |
| 2. CCL3 (MIP1β) | 0.36 | .006 | 0.41 | .001 | 0.19 | NS |
| Group 3. Known proneutrophilic biomolecules not correlated with neutrophils | ||||||
| 1. CCL20 | 0.12 | NS | 0.09 | NS | 0.05 | NS |
| 2. EGF | −0.01 | NS | 0.13 | NS | 0.01 | NS |
| 3. RAGE | −0.01 | NS | 0.13 | NS | 0.08 | NS |
| 4. TIMP-1 | 0.21 | NS | 0.23 | NS | 0.36 | NS |
Italicized biomolecules are also changed in the serum.
EGF, Epidermal growth factor; MIP, macrophage inflammatory protein; PAI, plasminogen activator inhibitor; RAGE, receptor for advanced glycosylation end products; TIMP-1, tissue inhibitor of metalloproteinases 1.
Biochemical analyses of serum
Of the 244 biomolecules assayed, 29 were undetectable. One hundred seventy-two biomolecules did not show any difference among the study groups. Fourteen biomolecules were significantly different among all 3 study groups and also between patients with NRA and those with RA (Table V ). Of these 14 biomolecules, 6 of them were also detected in BAL fluid and were different among the study groups. They include ficolin 3, matrix metalloproteinase (MMP) 7, thrombospondin 1, apolipoprotein E, human epididymis protein 4, and neuropilin 1.
Table V.
Serum biomolecules with levels that are statistically different among the study groups
| Biomolecules (total n = 14) | Healthy subjects (n = 20) | Patients with NRA (n = 40) | P values vs healthy subjects | Patients with RA (n = 60) | P values vs healthy subjects | P value vs NRA | P values for multigroup analyses |
|---|---|---|---|---|---|---|---|
| Proinflammatory | |||||||
| 1. CCL18 (PARC [ng/mL]) | 105 | 97 | NS | 146 | .006 | .0006 | .0006 |
| 2. Cathepsin D | 569 | 480 | .02 | 585 | NS | <.0001 | .004 |
| 3. Ficolin 3 (μg/mL) | 21.5 | 21 | NS | 29 | NS | .002 | .002 |
| 4. GDF-15 (ng/mL) | 0.33 | 0.32 | NS | 0.40 | NS | .02 | .03 |
| 5. Thrombin-activatable fibrinolysis (μg/mL) | 11.8 | 8.6 | .008 | 11 | NS | .005 | .005 |
| 6. von Willebrand factor (μg/mL) | 109.5 | 61 | .08 | 120 | NS | <.0001 | .0002 |
| 7. YKL-40 (ng/mL) | 40 | 27 | NS | 42 | NS | .003 | .008 |
| Remodeling/regeneration/angiogenesis | |||||||
| 1. MMP7 (ng/mL) | 5.7 | 4.1 | .03 | 5.1 | NS | .002 | .009 |
| 2. Tenascin C (ng/mL) | 493 | 400 | NS | 733 | .009 | .003 | .002 |
| 3. Tetranectin (μg/mL) | 22 | 20 | NS | 18 | .002 | .02 | .005 |
| 4. Thrombospondin 1 (μg/mL) | 14.7 | 12 | .01 | 14 | NS | .001 | .004 |
| Anti-inflammatory | |||||||
| 1. Apolipoprotein E (ng/mL) | 65 | 56 | NS | 65 | NS | .01 | .07 |
| 2. Human epididymis protein 4 (pmol/L) | 690 | 555 | .07 | 787 | NS | .002 | .005 |
| 3. Neuropilin 1 (pg/mL) | 248 | 177 | .005 | 214 | .003 | .006 | .003 |
Italicized variables were also altered in BAL fluid samples.
NS, Not significant; PARC, pulmonary and activation-regulated chemokine.
Next, we examined the correlation of these serum biomolecules with BAL fluid inflammatory cells (see Table E3 in this article's Online Repository at www.jacionline.org). Five biomolecules correlated with increased BAL fluid neutrophils: growth and differentiation factor 15 (GDF-15), human epididymis protein 4, MMP7, tetranectin, and von Willebrand factor. None of these biomolecules correlated with BAL fluid eosinophil or lymphocyte counts.
Microbial analyses of BAL fluid
BAL fluid from each patient was cultured in triplicate to detect pathogenic microbial organisms. The results were presented as negative or normal respiratory flora or positive for a pathogenic strain. A total of 24 of 60 patients with RA had positive results for bacteria, a virus, or both. The following bacterial strains were identified in 22 patients with RA: methicillin-sensitive Staphylococcus aureus (7 patients), methicillin-resistant Staphylococcus aureus (1 patient), Pseudomonas aeruginosa (5 patients), Haemophilus influenzae (1 patient), Haemophilus species but not influenzae (subspecies not identified, 2 patients), Haemophilus parainfluenzae (1 patient), β-hemolytic Streptococci (not group A) (1 patient), α-hemolytic streptococci (1 patient), Streptococcus pneumoniae (1 patient), Moraxella catarrhalis (1 patient), Serratia marcescens (1 patient), and Capnacytophaga species (1 patient). Note that some patients had more than 1 strain of bacteria. Two patients had positive result for viruses only (1 with respiratory syncytial virus and 1 with coronavirus OC43). Four patients with a bacterial isolate also had positive results for viruses (3 with rhinovirus and 1 with parainfluenza virus 3). It should be noted that these infections were subclinical in nature because the patients did not report symptoms of acute infection (fever, coryza, pharyngitis, or myalgia). All patients had chest CT. A summary of chest CT findings from patients with positive results for infection is shown in Table E4 in this article's Online Repository at www.jacionline.org. One patient with Pseudomonas species had consolidation; 1 patient with Serratia marcescens had infiltrates, and 3 patients had ground-glass opacity. The rest of the patients did not have any sign of acute infection.
Effect of infection on clinical and laboratory parameters of RA
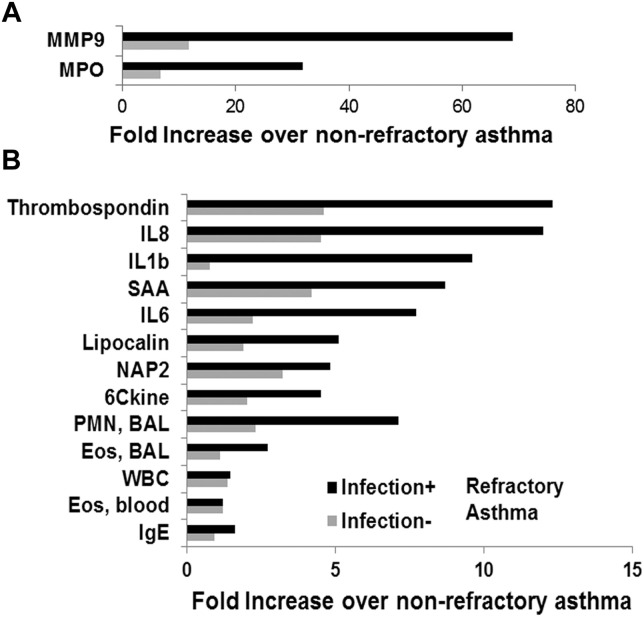
Next, we examined the effect of infection on cellular and molecular parameters in patients with RA. As anticipated, infection increased the frequency of neutrophils more than that of eosinophils in the airways when compared with that seen in patients with NRA (see Fig E1, B). This was associated with a dramatic increase in levels of the neutrophilic granular protein myeloperoxidase (see Fig E1, A) and a number of proneutrophilic biomolecules, especially MMP9, thrombospondin 1, IL-8, IL-1β, serum amyloid A (SAA), IL-6, lipocalin 2, neutrophil activating peptide-2 (NAP2), and 6 conserved cysteine containing CC-chemokine (see Fig E1, B). Although the foregoing changes were statistically significant for the groups, not all infection-positive patients with RA had increased BAL fluid neutrophil counts. Of the 24 infection-positive patients with RA, 15 had increased neutrophil frequencies (≥3%, threshold based on the normal range for the clinical laboratory) in BAL fluid, and 9 did not. Ten of these 15 infection-positive neutrophilic asthmatic patients had increased (≥1%) eosinophil frequencies in BAL fluid. Conversely, 4 of the 9 infection-positive but nonneutrophilic patients had increased eosinophil counts in BAL fluid.
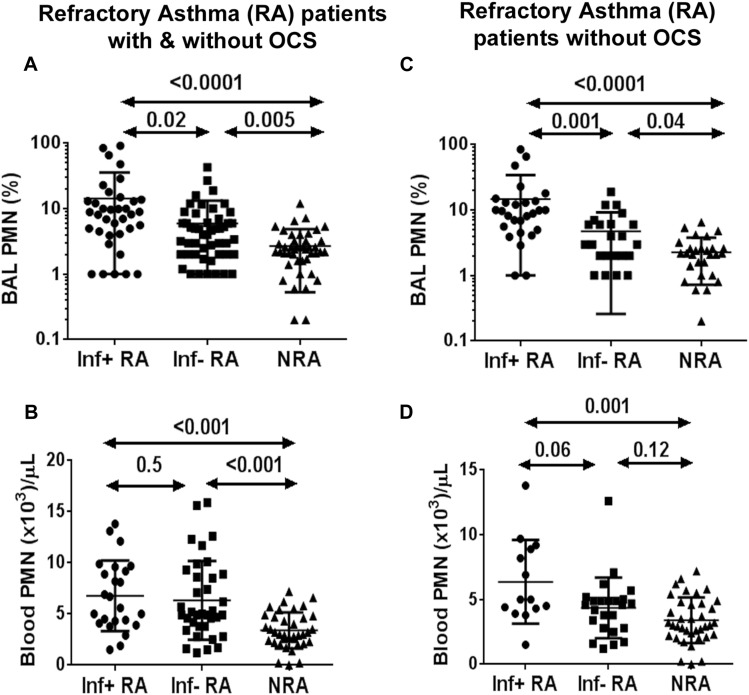
We compared BAL fluid neutrophil frequencies (as a percentage) and absolute neutrophil counts in blood among infection-positive and infection-negative patients with RA and those with NRA. A total of 22 patients with RA (10 with infection-positive RA and 12 with infection-negative RA) were receiving systemic steroids, which could have a direct effect on blood and BAL fluid neutrophil counts. For this reason, we analyzed data with and without inclusion of patients receiving oral corticosteroids (OCSs). BAL fluid neutrophil counts were increased in both infection-positive and infection-negative patients with RA regardless of their OCS use compared with healthy control subjects (Fig 1 , A). Blood neutrophil counts were also increased in both infection-positive and infection-negative patients with RA when the data were analyzed for the entire group (Fig 1, B). However, this increase was lost in the blood but not BAL fluid (Fig 1, C and D) in the infection-negative RA group when the patients receiving OCSs were excluded from analysis. The results suggest that blood neutrophilia in infection-negative patients with RA was caused by OCSs. Strikingly, the absolute neutrophil number remained increased in the infection-positive RA group, even when the patients receiving OCSs were excluded from the analysis. The results indicate that an increase in absolute neutrophil counts could serve as a blood biomarker for airway infection in patients with RA. The results also suggest that blood neutrophilia in this subgroup was due to airway infection (Fig 1, C).
Fig 1.
A-D, Comparison of blood and BAL fluid neutrophil counts among infection-positive (Inf+) and infection-negative (Inf−) patients with RA and patients with NRA, including and excluding patients with RA taking OCSs.
Asthma is a type 2 immune response disease, and eosinophils play an important role. As noted earlier, the median BAL fluid eosinophil number did not increase significantly in patients with RA compared with those with NRA. Because infection contributed to RA in a significant number of patients, we reasoned that the former might have affected the eosinophilic influx by favoring neutrophilic influx. For this reason, we analyzed BAL fluid eosinophils and neutrophils separately in infection-positive and infection-negative patients with RA and compared them with counts in patients with NRA. Median BAL eosinophil counts were 1, 1, and 1 in patients with NRA, infection-positive patients with RA, and patients with infection-negative RA, respectively. On the other hand, median BAL fluid neutrophil counts were 2.2, 8, and 3.2 in patients with NRA, infection-positive patients with RA, and infection-negative patients with RA, respectively, and the difference among the groups was statistically significant (P = .02).
Serum biomolecules that identify neutrophilic asthma in noninfected patients
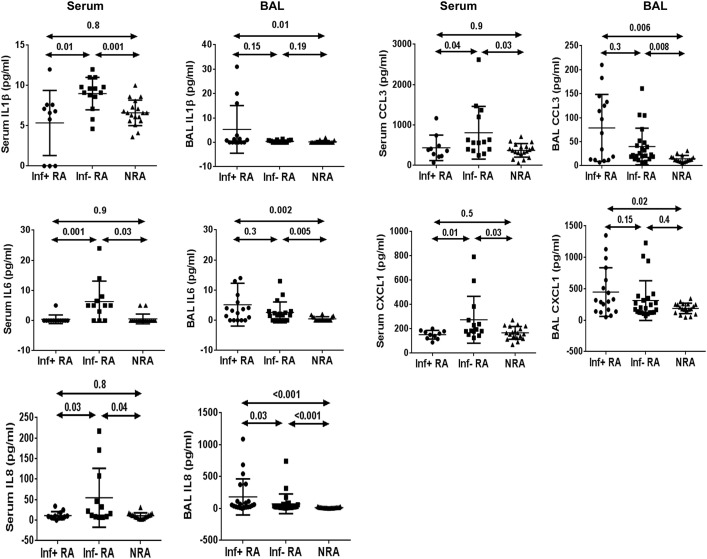
Subclinical infection was the most plausible explanation for increased airway neutrophil counts in our infection-positive patients with RA. The cause of neutrophilic asthma in infection-negative patients with RA was of major interest to us. To this goal, we compared serum proneutrophilic biomolecule levels between infection-negative and infection-positive patients with RA (Fig 2 ). IL-1β, IL-6, IL-8, CXCL1, and CCL3 levels were increased in serum from infection-negative patients with RA compared with those in infection-positive patients with RA and NRA. Interestingly, the BAL fluid concentrations of these molecules were not increased in the infection-negative patients with RA when compared with those in infection-positive patients with RA. The results raised the possibility of a systemic inflammatory process contributing to airway neutrophilia in infection-negative patients with RA.
Fig 2.
Comparison of biomolecule levels between serum and BAL fluid among infection-positive (Inf+) patients with RA, infection-negative (Inf−) patients with RA, and patients with NRA.
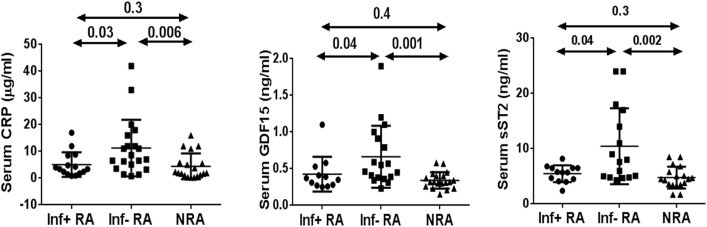
Next, we compared levels of C-reactive protein, soluble ST2 (serum stimulation 2, also known as suppressor of tumorigenicity 2), and GDF-15, 3 molecules associated with atherosclerosis, cardiovascular diseases, and systemic inflammatory diseases.27, 28 Indeed, levels of all 3 molecules were increased in infection-negative but not infection-positive patients with neutrophilic asthma (Fig 3 ). The erythrocyte sedimentation rate was similar in both groups. We were concerned about a potential role for inhaled corticosteroids in airway neutrophilia. To this goal, we compared inhaled corticosteroid treatment of patients with NRA and those with RA (see Table E5 in this article's Online Repository at www.jacionline.org). All patients with RA were receiving high-dose inhaled steroids and long-acting β-agonists. In contrast, some patients with NRA were receiving low- to medium-dose inhaled steroids. Thus it is possible that this difference in the dose of inhaled steroids contributed to airway neutrophilia.
Fig 3.
Comparison of systemic inflammatory markers in serum from infection-positive (Inf+) patients with RA, infection-negative (Inf−) patients with RA, and patients with NRA.
Clinical correlations
Next, we examined the clinical correlation of airway inflammatory cells and biomolecules. There was a positive correlation (r = 0.49, P < .001) between the age of the patient and the BAL PMN count. Age also positively correlated with FVC (r = 0.52, P < .05) and BAL fluid IL-1β levels (r = 0.67, P < .001) but not with levels of other proneutrophilic biomolecules, including IL-6 (r = 0.40) and IL-8 (r = 0.18). BAL fluid PMN counts but not BAL fluid eosinophil, lymphocyte, or blood eosinophil counts negatively correlated with FVC percentages (see Table E6 in this article's Online Repository at www.jacionline.org). CD117+ cells, which largely but not exclusively represent mast cells, did not correlate with levels of any BAL biomolecules. Levels of 2 BAL fluid biomolecules correlated with 2 pulmonary function indices. Myoglobin levels negatively correlated with FVC percentage, whereas prostasin levels negatively correlated with reversibility and positively with FEV1 percentage. We performed pathway analyses with the 36 BAL fluid biomolecules that distinguished patients with RA from those with NRA. We compared pathway results by using GO for biological process and molecular function, Reactome, and Kyoto Encyclopedia of Genes and Genomes (data not shown). The GO analysis identified the most meaningful pathways. The top pathways included neutrophil chemotaxis, chemokine receptor signaling, fibroblast growth factor stimulation, and cellular response to IL-1 (see Table E7 in this article's Online Repository at www.jacionline.org).
Discussion
We showed that RA was associated with an increase in neutrophil and lymphocyte counts in the airways. As mentioned previously, we specifically focused on neutrophilic asthma in this article. There was a concurrent increase in levels of a multitude of biomolecules known to be associated with innate immune and airway tissue responses. We identified 51 biomolecules with increased levels in asthmatic patients, and 36 of them distinguished RA from NRA. Sixteen biomolecules correlated with neutrophilic asthma. The vast majority of these biomolecules are known to be produced by macrophages and airway epithelial cells, which indirectly points to their role in patients with neutrophilic asthma. A pathway analysis identified neutrophilic influx as an important biological process in RA. Multiple previous studies demonstrated a similar role for neutrophilic inflammation in severe asthma. Our RA group resembles cluster 5 (neutrophilic) of the SARP cohort.29 In a subsequent study of SARP patients, increased neutrophil and mixed neutrophil and eosinophil counts in the sputum (clusters C and D) were associated with asthma severity.30 Our patients with RA are similar to those of cluster H (mixed neutrophilic/eosinophilic) of the multinational study by Hinks et al.31 This cluster had severe asthma, which was associated with increased IL-6, IL-8, MMP3, and MMP8 levels in sputum.
One of the weaknesses of this study is that patients with RA were significantly older than patients with NRA. RA is more prevalent in older patients,4, 5 which led to this unintended recruitment outcome. Increased age is associated with sputum neutrophilia, even in nonasthmatic subjects.32 Thus increased age could affect the quality and quantity of the mediators involved in neutrophilic asthma.
We used a commercially available multiplex assay service for measurement of biomolecules. Multiplex assays are less sensitive than other highly sensitive assays (eg, ELISA). This perhaps explains why we did not detect the typical type 2 (eg, IL-4, IL-5, and IL-13) or type 3 (IL-17) cytokines. However, the scope of biomolecules detected by using this assay is an advantage.
One outstanding issue related to the heightened production of proneutrophilic biomolecules is what induces their production in patients with RA. We observed subclinical infection (both bacterial and viral) in one third of the patients with RA. This subclinical infection could stimulate production of proneutrophilic biomolecules. We used standard microbiological cultures to detect bacteria. The sensitivity of these cultures is relatively low when compared with that of DNA-based technologies. It is possible that new technologies will identify additional patients with subclinical infections, which could explain increased airway neutrophil counts in the currently classified infection-negative patients.
The persistence of infection at a subclinical level in a subgroup of patients raises concerns for a defective immune response. This inability to clear infection could be primary or secondary to ongoing treatment with steroids (inhaled steroids in all patients and systemic steroids in 36% of patients with RA). The potential role of inhaled steroids in patients with subclinical infection cannot be addressed at this time because their discontinuation in patients with RA will be considered unethical.
We detected decreased IL-18 and IL-22 levels in patients with RA. Low IL-18 levels were previously reported in sputum from asthmatic patients and in patients during rhinovirus infection.33, 34 IL-18 and IL-22 are important for defense against infection.35, 36 Their decrease could impair clearance of infection. Macrophage scavenging of infected and dead cells is important for resolution of inflammation.
Axl receptor tyrosine kinase is specifically expressed on airway macrophages.37 We observed decreased expression of Axl receptor in BAL, which suggested a decreased expression on macrophages and, consequently, an impaired scavenger function. The latter could promote persistence of infection.
Thrombospondin 1 is a multifunctional and pleiotropic protein, levels of which are increased in patients with RA. It was recently reported to inhibit bactericidal activity of neutrophils and delay the clearance of lung infection.38 Thus we have identified a number of biomolecules that could promote persistence of subclinical infection in patients with RA.
Systemic glucocorticoids are known to induce blood and tissue neutrophilia.39 Thirty-six percent of patients with RA were taking systemic steroids. Our analysis of patients who were not taking OCSs suggested that systemic steroids contributed to blood but not airway neutrophilia in infection-negative patients with RA. Our understanding of the role of inhaled steroids in patients with airway neutrophilia is incomplete. A previous study showed that systemic but not inhaled steroids were associated with tissue neutrophilia.39 However, this study was done with a small number of patients, and the result showed a trend for increased airway neutrophil counts with inhaled steroids. Both patients with NRA and those with RA were receiving inhaled steroids in our study. However, unlike patients with RA, not all patients with NRA received maximal doses of inhaled steroids. Thus it is possible that higher doses of inhaled steroids in patients with RA contributed to some extent to airway neutrophilia. Environmental toxicants frequently induce cytokines/chemokines that can elicit low-level neutrophilic inflammation.40 There could be a cumulative effect during aging. The TH17 immune response induces neutrophilic inflammation in animal models.16 Our assay did not detect IL-17, likely because of the low sensitivity of the assay. However, the assay detected increased levels of multiple proneutrophilic biomolecules, including SAA, NAP2, thrombospondin 1, IL-6, IL-8, C3, CCL3, CCL20, epidermal growth factor, and receptor for advanced glycosylation end products. All except the last 3 significantly correlated with BAL fluid neutrophil counts. SAA induces granulocyte colony-stimulating factor and stimulates neutrophil generation.41 It also induces TH17.42 IL-6 is an inducer of TH1743 and a marker of systemic inflammation.44 NAP2, IL-8, C3, and CCL3 directly induce neutrophilic inflammation.45, 46, 47, 48 C3 also induces IL-1, IL-8, and other cytokines/chemokines, which could indirectly affect neutrophilic inflammation.48 Some of our findings on biomolecules are in agreement with those of many previous studies. Similar to our finding, IL-1β and IL-6 discriminated severe from moderate asthma in a SARP study.25 IL-8 levels have previously been reported to be increased in sputum49, 50 and BAL fluid51 from patients with RA and neutrophilic asthma.
Although the existence of neutrophilic asthma in the absence of infection has been known for many years, its mechanism is poorly understood. Our study identified a unique subgroup of infection-negative patients with neutrophilic RA, which was selectively associated with increased markers of inflammation in serum. These markers included IL-1β, IL-6, IL-8, CXCL1, and CCL3. The concomitant increase in C-reactive protein levels in the same patients suggested a systemic inflammatory process akin to what has been observed in patients with atherosclerotic cardiovascular52 and autoinflammatory53 diseases. Remarkably, this subgroup of patients also had increases in 2 additional cardiovascular risk factors: GDF-1554 and soluble ST2 (IL-33 receptor).52 We recognize that the latter is a nonspecific biomarker of systemic inflammation. Four (11%) of 36 (11%) infection-negative patients with neutrophilic RA and 5 (21%) of 24 infection-positive patients with neutrophilic RA had a history of cardiovascular disease. Therefore the frequency of existing cardiovascular disease did not explain the systemic (serum) increase in levels of these inflammatory cytokines. We also did not find any correlation of these inflammatory cytokines with other common comorbidities: diabetes and autoimmune disorders.
There are multiple known mechanisms for induction of IL-1, IL-6, IL-8, CXCL1, and CCL3. This constellation of inflammatory cytokines also constitutes the senescence-associated secretory product.55 Senescence is a biological process that defends against DNA damage–induced carcinogenesis but, by producing multiple cytokines, contributes to systemic inflammation.56 Recently, GDF-15 has been shown to induce cellular senescence.57 The correlation of neutrophilic asthma with age also raises the possibility that senescence contributed to the increased proneutrophilic cytokine profile in the serum. Additional studies will be needed to establish the relationship between cellular senescence and predisposition to neutrophilic RA.
It should be noted that 37% of infection-positive patients with RA did not have neutrophilia in BAL fluid, which further underscores the notion that this was a subclinical infection. Nearly half of them had increased eosinophil counts in BAL fluid, suggesting type 2 immunodominant asthma. Type 2 cytokines inhibit IL-8 and other proneutrophilic cytokines,58 which could explain the lack of neutrophilia. However, the absence of neutrophilia in noneosinophilic infection-positive patients with RA suggests a defective immune response or perhaps an active immune evasion by the microbial organism.
Of the 14 serum biomolecules that were different among the study groups, only 5 biomolecules overlapped with those from the BAL fluid. However, the pattern of change was different from that in BAL fluid. Four of these biomolecules showed reduced expression in patients with NRA but not in those with RA when compared with expression in healthy control subjects. The reason for this reduced expression in patients with NRA but not those with RA is not clear. Ficolin 3 is the only biomolecule in the serum that distinguished RA from NRA. Ficolin 3 is a recognition molecule of the lectin pathway of the complement system. Its deficiency is associated with increased infection.59
If subclinical infection is one of the mechanisms of RA in a subgroup of patients, its importance can be tested through targeted and bacterial strain–specific antibiotic therapy. We are aware that antibiotic therapy of patients with RA produced mixed results in the past.60, 61 The failure of antibiotic therapy in some studies could be due to inappropriate selection of study patients (eg, inclusion of patients who did not have any infection) and use of an inappropriate antibiotic.3 Note that we detected both gram-positive and gram-negative bacteria, as well as viruses, in patients with RA. The results underscore the importance of the selection of an appropriate antibiotic (targeting gram-positive vs gram-negative bacteria) and its application to a properly identified target patient population. If low-level systemic inflammation is indeed the cause of airway neutrophilia in infection-negative patients with RA, this hypothesis can be tested by using therapeutic agents targeting molecules, such as IL-1, IL-6, and IL-8. Some of these therapeutic agents are already in use in patients with rheumatologic conditions.
The number of CD117+ cells representing mostly mast cells was reduced in patients with RA. Reduced numbers of tryptase-positive cells representing mast cells in patients with severe asthma was previously reported in a SARP study.62 This was thought to be due to the loss of tryptase as a consequence of mast cell degranulation. However, unlike tryptase, CD117 is a cell-surface marker and not a granular protein and is not expected to be affected by mast cell degranulation. It is possible that the reduced CD117+ cell number is a consequence of the underlying disease process.
Clinical implications.
This study identifies airway neutrophils and proneutrophilic biomolecules as significant contributors to RA. A subgroup of neutrophilic asthma was associated with a subclinical infection. This group is likely to benefit from specific antimicrobial therapy. Neutrophilic asthma without a subclinical infection showed signs of systemic inflammation. Therapies targeting proneutrophilic biomolecules, autoinflammation, or both are likely to control asthma in this subgroup.
Acknowledgments
We acknowledge the excellent technical assistance of Christena Kolakowski and Allen Stevens.
Footnotes
Supported by National Institutes of Health grants RO1 AI091614, HL126895, AI102943, and HL126895 and a donation from Michele and Martin Cohen.
Disclosure of potential conflict of interest: R. J. Martin's institution has received a National Heart, Lung, and Blood Institute grant from AsthmaNet and a grant from MedImmune; has personally received consultancy fees from Teva, AstraZeneca, Genentech, Boehringer Ingelheim, and PMD; personally received travel expenses from the Respiratory Effectiveness Group. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Fig E2.
Fold increase in biomolecule level in patients with RA compared with those with NRA (A and B). Patients with RA were subgrouped as having infection (infection positive, black bars) or no infection (infection negative, gray bars). Results in Fig E2, A, were separated from results in Fig E2, B, because of the higher level of increase (see labels on x-axis).
Table E1.
List of biomolecules
| Analytes | Units | Myriad RBM LDD | Myriad RBM LLOQ |
|---|---|---|---|
| Adiponectin | μg/mL | 0.000086 | 0.00030 |
| Agouti-related protein (AgRP) | pg/mL | 24 | 23 |
| Aldose reductase | ng/mL | 0.41 | 0.67 |
| Alpha-1-antichymotrypsin (AACT) | μg/mL | 0.70 | 1.4 |
| Alpha-1-antitrypsin (AAT) | mg/mL | 0.000000038 | 0.000000082 |
| Alpha-1-microglobulin (A1Micro) | μg/mL | 0.000014 | 0.000025 |
| Alpha-2-macroglobulin (A2Macro) | mg/mL | 0.000098 | 0.000087 |
| Alpha-fetoprotein (AFP) | ng/mL | 0.014 | 0.11 |
| Amphiregulin (AR) | pg/mL | 131 | 199 |
| Angiogenin | ng/mL | 0.016 | 0.0081 |
| Angiopoietin 2 (ANG-2) | ng/mL | 0.048 | 0.048 |
| Angiotensin-converting enzyme (ACE) | ng/mL | 0.019 | 0.035 |
| Angiotensinogen | ng/mL | 0.051 | 0.092 |
| Apolipoprotein(a) (Lp[a]) | μg/mL | 0.00020 | 0.00091 |
| Apolipoprotein A-I (Apo A-I) | mg/mL | 0.000000016 | 0.00000006 |
| Apolipoprotein A-II (Apo A-II) | ng/mL | 0.000011 | 0.000014 |
| Apolipoprotein A-IV (Apo A-IV) | μg/mL | 0.075 | 0.16 |
| Apolipoprotein B (Apo B) | μg/mL | 0.00044 | 0.0021 |
| Apolipoprotein C-I (Apo C-I) | ng/mL | 0.0000062 | 0.000022 |
| Apolipoprotein C-III (Apo C-III) | μg/mL | 0.0000024 | 0.000011 |
| Apolipoprotein D (Apo D) | μg/mL | 0.020 | 0.037 |
| Apolipoprotein E (Apo E) | μg/mL | 0.00089 | 0.0024 |
| Apolipoprotein H (Apo H) | μg/mL | 0.000015 | 0.000062 |
| AXL receptor tyrosine kinase (AXL) | ng/mL | 0.0058 | 0.010 |
| B cell-activating factor (BAFF) | pg/mL | 1.5 | 3.7 |
| B-lymphocyte chemoattractant (BLC) | pg/mL | 9.8 | 12 |
| Beta-2-microglobulin (B2M) | μg/mL | 0.000045 | 0.000051 |
| Betacellulin (BTC) | pg/mL | 19 | 27 |
| Brain-derived neurotrophic factor (BDNF) | ng/mL | 0.0046 | 0.0072 |
| 6 Conserved cysteine containing CC-chemokine | pg/mL | 8.2 | 15 |
| C-peptide | ng/mL | 0.0041 | 0.0030 |
| C-reactive protein (CRP) | μg/mL | 0.0000034 | 0.0000078 |
| Calbindin | ng/mL | 0.53 | 0.90 |
| Cancer antigen 125 (CA-125) | U/mL | 0.45 | 0.76 |
| Cancer antigen 15-3 (CA-15-3) | U/mL | 0.055 | 0.090 |
| Cancer antigen 19-9 (CA-19-9) | U/mL | 2.1 | 1.4 |
| Cancer antigen 72-4 (CA 72-4) | U/mL | 6.5 | 4.0 |
| Carcinoembryonic antigen (CEA) | ng/mL | 0.028 | 0.037 |
| Cathepsin D | ng/mL | 0.23 | 0.18 |
| CD5 antigen-like (CD5L) | ng/mL | 0.0056 | 0.0081 |
| CD40 antigen (CD40) | ng/mL | 0.0036 | 0.0031 |
| CD40 ligand (CD40L) | ng/mL | 0.0037 | 0.0033 |
| Cellular fibronectin (cFib) | μg/mL | 0.0089 | 0.042 |
| Chemokine CC4 (HCC-4) | ng/mL | 0.0066 | 0.0095 |
| Chromogranin-A (CgA) | ng/mL | 5.0 | 2.6 |
| Ciliary neurotrophic factor (CNTF) | pg/mL | 4.5 | 19 |
| Clusterin (CLU) | μg/mL | 0.0013 | 0.0024 |
| Collagen IV | ng/mL | 1.2 | 1.9 |
| Complement C3 (C3) | mg/mL | 0.000000016 | 0.0000001 |
| Complement factor H–related protein 1 (CFHR1) | μg/mL | 0.00076 | 0.0011 |
| Cortisol (Cortisol) | ng/mL | 1.2 | 2.0 |
| Creatine kinase-MB (CK-MB) | ng/mL | 0.035 | 0.070 |
| Cystatin-C | ng/mL | 0.011 | 0.0075 |
| E-selectin | ng/mL | 0.033 | 0.041 |
| EN-RAGE | ng/mL | 0.0036 | 0.0076 |
| Endoglin | ng/mL | 0.0023 | 0.0041 |
| Endostatin | ng/mL | 0.014 | 0.054 |
| Eotaxin-1 | pg/mL | 1.9 | 29 |
| Eotaxin-2 | pg/mL | 4.7 | 7.6 |
| Eotaxin-3 | pg/mL | 16 | 31 |
| Epidermal growth factor (EGF) | pg/mL | 0.78 | 5.8 |
| Epidermal growth factor receptor (EGFR) | ng/mL | 0.031 | 0.058 |
| Epiregulin (EPR) | pg/mL | 3.7 | 14 |
| Epithelial cell adhesion molecule (EpCam) | pg/mL | 16 | 80 |
| Epithelial-derived neutrophil-activating protein 78 (ENA-78) | ng/mL | 0.0030 | 0.017 |
| Ezrin | ng/mL | 2.5 | 3.6 |
| Factor VII | ng/mL | 1.2 | 0.77 |
| Fas ligand (FasL) | pg/mL | 2.6 | 5.3 |
| FASLG receptor (FAS) | ng/mL | 0.47 | 1.2 |
| Fatty acid–binding protein, adipocyte (FABP, adipocyte) | ng/mL | 0.023 | 0.049 |
| Fatty acid–binding protein, heart (FABP, heart) | ng/mL | 1.0 | 3.0 |
| Fatty acid–binding protein, liver (FABP, liver) | ng/mL | 2.8 | 4.8 |
| Ferritin (FRTN) | ng/mL | 0.013 | 0.021 |
| Fetuin-A | μg/mL | 0.00034 | 0.00062 |
| Fibrinogen | mg/mL | 0.00000022 | 0.00000024 |
| Fibroblast growth factor 4 (FGF-4) | pg/mL | 123 | 87 |
| Fibroblast growth factor basic (FGF-basic) | pg/mL | 5.9 | 6.9 |
| Fibulin-1C (Fib-1C) | μg/mL | 0.00015 | 0.00015 |
| Follicle-stimulating hormone (FSH) | mIU/mL | 0.11 | 0.13 |
| Galectin-3 | ng/mL | 0.071 | 0.074 |
| Gelsolin | μg/mL | 0.00034 | 0.00072 |
| Glucagon | pg/mL | 31 | 64 |
| Glucagon-like peptide 1, active (GLP-1 active) | pg/mL | 1.4 | 2.6 |
| Glucagon-like peptide 1, total (GLP-1 total) | pg/mL | 0.89 | 1.1 |
| Glucose-6-phosphate isomerase (G6PI) | ng/mL | 0.062 | 0.12 |
| Glutathione S-transferase α (GST-α) | ng/mL | 0.29 | 0.59 |
| Glutathione S-transferase Mu 1 (GST-M1) | ng/mL | 0.58 | 1.3 |
| Granulocyte colony-stimulating factor (G-CSF) | pg/mL | 0.73 | 1.1 |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) | pg/mL | 3.2 | 18 |
| Growth hormone (GH) | ng/mL | 0.0054 | 0.030 |
| Growth-regulated α protein (GRO-α) | pg/mL | 0.36 | 0.94 |
| Haptoglobin | mg/mL | 0.00000012 | 0.00000032 |
| HE4 | pmol/L | 16 | 14 |
| Heat shock protein 60 (HSP-60) | ng/mL | 5.3 | 7.4 |
| Heparin-binding EGF-like growth factor (HB-EGF) | pg/mL | 9.2 | 43 |
| Hepatocyte growth factor (HGF) | ng/mL | 0.33 | 0.20 |
| Hepatocyte growth factor receptor (HGF receptor) | ng/mL | 0.055 | 0.079 |
| Hepsin | pg/mL | 15 | 14 |
| Human chorionic gonadotropin β (hCG) | mIU/mL | 0.24 | 0.45 |
| Human epidermal growth factor receptor 2 (HER-2) | ng/mL | 0.0029 | 0.0060 |
| IgA | mg/mL | 0.000000035 | 0.00000028 |
| IgE | μ/mL | 0.43 | 3.6 |
| IgM | mg/mL | 0.00000036 | 0.00000047 |
| Insulin | mg/mL | 0.0035 | 0.043 |
| Insulin-like growth factor-binding protein 1 (IGFBP-1) | ng/mL | 0.11 | 0.19 |
| Insulin-like growth factor-binding protein 2 (IGFBP-2) | ng/mL | 0.57 | 0.48 |
| Insulin-like growth factor-binding protein 3 (IGFBP-3) | ng/mL | 1.2 | 3.2 |
| Insulin-like growth factor binding protein 4 (IGFBP4) | ng/mL | 0.19 | 0.30 |
| Insulin-like growth factor binding protein 5 (IGFBP5) | ng/mL | 1.3 | 1.9 |
| Insulin-like growth factor binding protein 6 (IGFBP6) | ng/mL | 0.65 | 0.77 |
| Intercellular adhesion molecule 1 (ICAM-1) | ng/mL | 0.35 | 0.26 |
| IFN-γ | pg/mL | 0.49 | 0.30 |
| IFN-γ induced protein 10 (IP-10) | pg/mL | 2.9 | 25 |
| Interferon-inducible T-cell α chemoattractant (ITAC) | pg/mL | 4.0 | 4.2 |
| IL-1α | ng/mL | 0.00030 | 0.00040 |
| IL-1β | pg/mL | 0.30 | 0.57 |
| IL-1 receptor antagonist (IL-1ra) | pg/mL | 9.9 | 19 |
| IL-2 | pg/mL | 1.0 | 1.7 |
| IL-2 receptor α (IL-2 receptor α) | pg/mL | 25 | 18 |
| IL-3 | ng/mL | 0.0011 | 0.0032 |
| IL-4 | pg/mL | 4.4 | 5.9 |
| IL-5 | pg/mL | 1.0 | 2.6 |
| IL-6 | pg/mL | 0.84 | 2.2 |
| IL-6 receptor (IL-6r) | ng/mL | 0.0027 | 0.0054 |
| IL-6 receptor subunit β (IL-6Rβ) | ng/mL | 0.023 | 0.049 |
| IL-7 | pg/mL | 2.1 | 1.8 |
| IL-8 | pg/mL | 0.63 | 0.79 |
| IL-10 | pg/mL | 1.1 | 1.4 |
| IL-12 subunit p40 (IL-12p40) | ng/mL | 0.016 | 0.055 |
| IL-12 subunit p70 (IL-12p70) | pg/mL | 5.9 | 9.7 |
| IL-13 | pg/mL | 0.63 | 1.2 |
| IL-15 | ng/mL | 0.12 | 0.079 |
| IL-16 | pg/mL | 4.3 | 17 |
| IL-17 | pg/mL | 1.2 | 0.99 |
| IL-18 | pg/mL | 3.7 | 8.3 |
| IL-23 | ng/mL | 0.12 | 0.080 |
| Kallikrein 5 | ng/mL | 0.045 | 0.14 |
| Kallikrein-7 (KLK-7) | pg/mL | 90 | 67 |
| Kidney injury molecule-1 (KIM-1) | ng/mL | 0.0071 | 0.0030 |
| Lactoylglutathione lyase (LGL) | ng/mL | 0.039 | 0.18 |
| Latency-associated peptide of transforming growth factor β1 (LAP TGF-β1) | ng/mL | 0.0092 | 0.015 |
| Lectin-like oxidized LDL receptor 1 (LOX-1) | ng/mL | 0.16 | 0.18 |
| Leptin | ng/mL | 0.011 | 0.027 |
| Luteinizing hormone (LH) | mIU/mL | 0.042 | 0.15 |
| Macrophage colony-stimulating factor 1 (M-CSF) | ng/mL | 0.035 | 0.053 |
| Macrophage-derived chemokine (MDC) | pg/mL | 1.2 | 3.7 |
| Macrophage inflammatory protein-1 α (MIP1α) | pg/mL | 8.2 | 8.4 |
| Macrophage inflammatory protein-1 β (MIP1β) | pg/mL | 4.0 | 6.2 |
| Macrophage inflammatory protein-3 γ (MIP3α) | pg/mL | 2.5 | 6.0 |
| Macrophage inflammatory protein 3β (MIP3β) | pg/mL | 3.2 | 7.8 |
| Macrophage migration inhibitory factor (MIF) | ng/mL | 0.0015 | 0.0020 |
| Macrophage-stimulating protein (MSP) | ng/mL | 0.037 | 0.051 |
| Malondialdehyde-modified low-density lipoprotein (MDA-LDL) | ng/mL | 25 | 49 |
| Maspin | pg/mL | 466 | 353 |
| Matrix metalloproteinase-1 (MMP-1) | ng/mL | 0.12 | 0.18 |
| Matrix metalloproteinase-2 (MMP-2) | ng/mL | 1.3 | 0.87 |
| Matrix metalloproteinase-3 (MMP-3) | ng/mL | 0.0066 | 0.012 |
| Matrix metalloproteinase-7 (MMP-7) | ng/mL | 0.017 | 0.0088 |
| Matrix metalloproteinase-9 (MMP-9) | ng/mL | 5.0 | 7.9 |
| Matrix metalloproteinase-9, total (MMP-9, total) | ng/mL | 0.26 | 0.34 |
| Matrix metalloproteinase-10 (MMP-10) | ng/mL | 0.0027 | 0.0054 |
| Mesothelin (MSLN) | nmol/L | 0.32 | 0.31 |
| MHC class I chain–related protein A (MICA) | pg/mL | 9.2 | 15 |
| Monocyte chemotactic protein 1 (MCP-1) | pg/mL | 4.6 | 9.0 |
| Monocyte chemotactic protein 2 (MCP-2) | pg/mL | 1.1 | 1.3 |
| Monocyte chemotactic protein 3 (MCP-3) | pg/mL | 0.45 | 1.9 |
| Monocyte chemotactic protein 4 (MCP-4) | pg/mL | 131 | 89 |
| Monokine induced by IFN-γ (MIG) | pg/mL | 10 | 22 |
| Myeloid progenitor inhibitory factor 1 (MPIF-1) | ng/mL | 0.0052 | 0.028 |
| Myeloperoxidase (MPO) | ng/mL | 3.2 | 3.7 |
| Myoglobin | ng/mL | 0.011 | 0.040 |
| N-terminal prohormone of brain natriuretic peptide (NT proBNP) | pg/mL | 11 | 15 |
| Nerve growth factor β (NGF-β) | ng/mL | 0.0099 | 0.016 |
| Neuron-specific enolase (NSE) | ng/mL | 0.030 | 0.035 |
| Neuronal cell adhesion molecule (Nr-CAM) | ng/mL | 0.044 | 0.040 |
| Neuropilin-1 | ng/mL | 0.00077 | 0.0017 |
| Neutrophil gelatinase-associated lipocalin (NGAL) | ng/mL | 0.013 | 0.058 |
| Osteopontin | ng/mL | 0.30 | 0.62 |
| Osteoprotegerin (OPG) | pmol/L | 0.075 | 0.17 |
| Pancreatic polypeptide (PPP) | pg/mL | 0.11 | 0.96 |
| Pepsinogen I (PGI) | ng/mL | 0.029 | 0.029 |
| Peptide YY (PYY) | pg/mL | 31 | 26 |
| Phosphoserine aminotransferase (PSAT) | ng/mL | 0.067 | 0.12 |
| Placenta growth factor (PLGF) | pg/mL | 3.7 | 11 |
| Plasminogen activator inhibitor 1 (PAI-1) | ng/mL | 0.0079 | 0.016 |
| Platelet-derived growth factor BB (PDGF-BB) | pg/mL | 31 | 144 |
| Progesterone | ng/mL | 0.45 | 0.65 |
| Proinsulin, intact | pmol/L | 1.4 | 0.73 |
| Proinsulin, total | pmol/L | 7.9 | 6.7 |
| Prolactin (PRL) | ng/mL | 0.013 | 0.017 |
| Prostasin | ng/mL | 0.28 | 0.33 |
| Prostate-specific antigen, free (PSA-f) | ng/mL | 0.00053 | 0.0027 |
| Protein S100-A4 (S100-A4) | ng/mL | 1.0 | 1.9 |
| Pulmonary and activation-regulated chemokine (PARC) | ng/mL | 1.2 | 1.2 |
| Receptor for advanced glycosylation end products (RAGE) | ng/mL | 0.034 | 0.069 |
| Receptor tyrosine-protein kinase erbB-3 (ErbB3) | ng/mL | 0.0086 | 0.0065 |
| Resistin | ng/mL | 0.0012 | 0.0052 |
| S100 calcium-binding protein B (S100-B) | ng/mL | 0.076 | 0.099 |
| Serotransferrin (transferrin) | mg/dL | 0.0000069 | 0.000047 |
| Serum amyloid P-component (SAP) | μg/mL | 0.000011 | 0.000014 |
| Sex hormone-binding globulin (SHBG) | nmol/L | 0.00038 | 0.0010 |
| Sortilin | ng/mL | 0.046 | 0.044 |
| Squamous cell carcinoma antigen-1 (SCCA-1) | ng/mL | 0.053 | 0.22 |
| Stem cell factor (SCF) | pg/mL | 18 | 23 |
| Stromal cell–derived factor-1 (SDF-1) | pg/mL | 8.7 | 13 |
| Superoxide dismutase 1, soluble (SOD-1) | ng/mL | 0.032 | 0.024 |
| T cell–specific protein RANTES (RANTES) | ng/mL | 0.00018 | 0.0010 |
| T lymphocyte–secreted protein I-309 (I-309) | pg/mL | 7.0 | 11 |
| Tamm-Horsfall urinary glycoprotein (THP) | μg/mL | 0.000045 | 0.00011 |
| Tenascin-C (TN-C) | ng/mL | 5.7 | 9.7 |
| Testosterone, Total | ng/mL | 0.061 | 0.10 |
| Tetranectin | μg/mL | 0.00093 | 0.0025 |
| Thrombomodulin (TM) | ng/mL | 0.017 | 0.049 |
| Thrombospondin 1 | ng/mL | 0.12 | 0.35 |
| Thyroglobulin (TG) | ng/mL | 1.8 | 2.8 |
| Thyroid-stimulating hormone (TSH) | uIU/mL | 0.0036 | 0.0077 |
| Thyroxine-binding globulin (TBG) | μg/mL | 0.000019 | 0.000035 |
| Tissue inhibitor of metalloproteinases 1 (TIMP-1) | ng/mL | 0.010 | 0.016 |
| Tissue-type plasminogen activator (tPA) | ng/mL | 0.0075 | 0.089 |
| TNF-related apoptosis-inducing ligand receptor 3 (TRAIL-R3) | ng/mL | 0.034 | 0.19 |
| TGF-α | pg/mL | 4.6 | 17 |
| TGF-β3 | pg/mL | 4.2 | 21 |
| Transthyretin (TTR) | mg/dL | 0.00000095 | 0.000004 |
| Trefoil factor 3 (TFF3) | μg/mL | 0.00005 | 0.00010 |
| TNF-α | pg/mL | 2.9 | 4.5 |
| TNF-β | pg/mL | 2.3 | 1.9 |
| TNF receptor I (TNF RI) | pg/mL | 5.9 | 7.6 |
| TNF receptor 2 (TNFR2) | ng/mL | 0.0011 | 0.0057 |
| Tyrosine kinase with immunoglobulin and EGF homology domains 2 (TIE-2) | ng/mL | 0.021 | 0.036 |
| Urokinase-type plasminogen activator (uPA) | pg/mL | 8.1 | 23 |
| Urokinase-type plasminogen activator receptor (uPAR) | ng/mL | 0.034 | 0.059 |
| Vascular cell adhesion molecule 1 (VCAM-1) | ng/mL | 0.0090 | 0.057 |
| Vascular endothelial growth factor (VEGF) | pg/mL | 12 | 6.8 |
| Vascular endothelial growth factor B (VEGF-B) | ng/mL | 2.7 | 2.3 |
| Vascular endothelial growth factor C (VEGF-C) | ng/mL | 0.19 | 1.1 |
| Vascular endothelial growth factor D (VEGF-D) | pg/mL | 100 | 100 |
| Vascular endothelial growth factor receptor 1 (VEGFR-1) | pg/mL | 29 | 91 |
| Vascular endothelial growth factor receptor 2 (VEGFR-2) | ng/mL | 0.0074 | 0.12 |
| Vascular endothelial growth factor receptor 3 (VEGFR-3) | ng/mL | 0.26 | 1.1 |
| Vitamin D–binding protein (VDBP) | μg/mL | 0.00002 | 0.000028 |
| Vitamin K–dependent protein S (VKDPS) | μg/mL | 0.000021 | 0.000022 |
| Vitronectin | μg/mL | 0.013 | 0.029 |
| von Willebrand factor (vWF) | μg/mL | 0.0016 | 0.0034 |
| YKL-40 | ng/mL | 0.0040 | 0.0073 |
Least detectable dose (LDD) was determined as the mean + 3 SDs of 20 blank readings. Results less than the LDD will be more variable than results greater than the LDD. Lower limit of quantitation (LLOQ) was the lowest concentration of an analyte in a sample that can be reliably detected and at which the total error meets the laboratory's requirements for accuracy. Myriad RBM's requirement for accuracy is the concentration of an analyte at which the coefficient of variation of replicate standard samples is 30%.
MIP, Macrophage inflammatory protein; ND, not detected.
Table E2.
Predominant cellular sources of biomolecules
| Biomolecule | Cellular source | Biomolecule | Cellular source |
|---|---|---|---|
| Innate immune cell–derived biomolecules | Mixed cell–derived biomolecules | ||
| 1. C3 | M, DC | 1. IL-1β | Most cell types |
| 2. CCL3 (MIP1β) | M | 2. IL-6 | M, DC, Ep |
| 3. CXCL7 (NAP2) | P, M | 3. IL-8 | Ep, M, En, F, SM |
| 4. Ficolin-3 | M | 4. Lipocalin 2 | N, Ep |
| 5. MMP9, total | N | 5. MMP7 | N, Ep |
| 6. PAI | M, Ma, Ep | ||
| Airway tissue–derived biomolecules | 7. SAA | M, Ep | |
| 1. Thrombospondin 1 | F | ||
DC, Dendritic cell; En, endothelial cell; Ep, epithelial cell; F, fibroblast; M, macrophage/monocyte; Ma, mast cell; MIP, macrophage inflammatory protein; N, neutrophil; P, platelet; PAI, plasminogen activator inhibitor; SM, smooth muscle.
Table E3.
Correlation of serum biomolecule levels with inflammatory cell counts
| Variables | Correlation with BAL fluid neutrophil counts (r) | P value | Correlation with BAL fluid eosinophil counts (r) | P value | Correlation with BAL fluid lymphocyte counts (r) | P value |
|---|---|---|---|---|---|---|
| Serum biomolecule levels associated with neutrophil counts | ||||||
| 1. GDF-15 | 0.44 | .003 | −0.1 | NS | −0.18 | NS |
| 2. Human Epididymis protein 4 | 0.43 | .004 | 0.29 | .05 | −0.05 | NS |
| 3. MMP7 | 0.35 | .02 | −0.06 | NS | −0.25 | NS |
| 4. Tetranectin | −0.35 | .02 | 0.02 | NS | 0.06 | NS |
| 5. von Willebrand factor | 0.34 | .02 | 0.08 | NS | 0.03 | NS |
NS, Not significant.
Table E4.
Chest CT findings in infection-positive patients with RA
| Identifier | Description of chest CT findings |
|---|---|
| 001R | Bronchial wall thickening |
| 003R | Bronchiolitis, consistent with aspiration, ground glass, consistent with asthma, consistent with reflux, esophageal thickening |
| 004R | Bronchial wall thickening, consistent with asthma, bronchiectasis, infiltrates, hiatal hernia, esophageal thickening, consistent with reflux, air trapping |
| 005R | Bronchial wall thickening, consistent with aspiration, consistent with asthma, tracheobronchomalacia, pulmonary hypertension, consistent with reflux, esophageal thickening, hiatal hernia |
| 006R | Bronchial wall thickening, consistent with aspiration, consistent with asthma, dysmotility, consistent with reflux |
| 008R | Tracheobronchomalacia, bronchial wall thickening, consistent with asthma, consistent with aspiration, dysmotility, consistent with reflux |
| 011R | Bronchial wall thickening, bronchiectasis, air trapping, hiatal hernia, dysmotility |
| 015R | Hiatal hernia, esophageal thickening, bronchial wall thickening, air trapping, consistent with reflux |
| 012R | Bronchiectasis, bronchial wall thickening, consistent with asthma, scarring, hiatal hernia, dysmotility |
| 020R | Bronchial wall thickening, ground glass, esophageal thickening, hiatal hernia, dysmotility, consistent with reflux |
| 021R | Bronchial wall thickening, consistent with asthma, consistent with aspiration, hiatal hernia, consistent with reflux, esophageal thickening |
| 030R | Bronchial wall thickening, hiatal hernia, esophageal thickening |
| 033R | Hiatal hernia, bronchiectasis, consistent with aspiration, bronchial wall thickening, air trapping |
| 035R | Bronchial wall thickening, consistent with asthma, esophageal thickening, dysmotility, consistent with reflux, air trapping, tracheobronchomalacia |
| 018R | Air trapping, bronchial wall thickening, consolidation, hiatal hernia, esophageal thickening, bronchiectasis, consistent with reflux |
| 047R | Tracheobronchomalacia, consistent with reflux, consistent with aspiration, consistent with asthma, bronchial wall thickening, air trapping, esophageal thickening |
| 048R | Bronchial wall thickening, atelectasis |
| 049R | Hiatal hernia, bronchial wall thickening, scarring, granuloma, air trapping, tracheobronchomalacia |
| 053R | Bronchial wall thickening, atelectasis, air trapping, consistent with asthma |
| 055R | Bronchial wall thickening, scarring, ground glass, consistent with reflux, hiatal hernia, consistent with asthma, consistent with aspiration |
| 061R | Bronchial wall thickening, atelectasis, scarring, air trapping, consistent with asthma |
| 062R | Esophageal thickening, bronchial wall thickening, atelectasis, scarring, air trapping, consistent with reflux, dysmotility, consistent with aspiration |
| 063R | Esophageal thickening, bronchial wall thickening, air trapping, consistent with asthma |
| 068R | Bronchial wall thickening, atelectasis, scarring, air trapping, tracheobronchomalacia, dysmotility |
Table E5.
List of medications
| Patient ID | Asthma medications (ICS, LABA, oral steroid and SABA) | Oral steroid | Medication by pharmacologic class | Others |
|---|---|---|---|---|
| 002NRA | Advair 250/50 bid, Xopenex prn | No | ||
| 003NRA | Symbicort 160/4.5 2 puffs bid, albuterol prn | No | ||
| 004NRA | Proventil prn, Advair HFA 230/12 bid | No | ||
| 005NRA | Alvesco 80 μg qd, Albuterol prn | No | ||
| 007NRA | Alvesco 80 μg 2 puffs qd, Albuterol MDI prn | No | ||
| 008NRA | Xopenex prn, Advair 100/50 | No | ||
| 009NRA | Advair 250/50 bid | No | ||
| 010NRA | Flovent 220 bid, albuterol prn | No | ||
| 011NRA | Dulera 100/5 bid, albuterol prn | No | ||
| 012NRA | QVAR 80 bid, albuterol prn | No | ||
| 013NRA | Flovent 44 2 puffs bid | No | ||
| 014NRA | Alvesco 160; Xopenex prn | No | ||
| 015NRA | QVAR 80 2 puffs/d | No | ||
| 016NRA | QVAR 80 2 puffs/bid; albuterol prn | No | ||
| 017NRA | Flovent 220 bid | No | ||
| 018NRA | Flovent 220 bid, albuterol prn | No | ||
| 022NRA | Dulera 100/5 bid | No | ||
| 024NRA | QVAR 2 puffs bid | No | ||
| 026NRA | Advair 100/50 bid | No | ||
| 027NRA | Alvesco 160 2 puffs/d; Xopenex prn | No | ||
| 028NRA | Flovent 110 2 puffs/d, albuterol prn | No | ||
| 029NRA | Advair 250/50 bid; ProAir prn | No | ||
| 030NRA | Advair 250/50 bid; albuterol once/wk | No | ||
| 036NRA | QVAR 80 2 puffs/d; albuterol prn | No | ||
| 039NRA | Pulmicort 180 μg/d, ProAir prn | No | ||
| 040NRA | Dulera 100/50 2 puffs bid; Ventolin prn | No | ||
| 042NRA | QVAR 80 2 puffs/d; Proventil prn | No | ||
| 044NRA | Advair 100/50 bid | No | ||
| 046NRA | Advair 100/50, Proventil prn | No | ||
| 049NRA | QVAR 80 bid | No | ||
| 001R | Advair 500/50 bid, monthly prednisone tapers, QVAR 160 μg bid | Yes | ||
| 002R | Spiriva 18 μg/d, Symbicort 160/4.5 2× bid, albuterol prn, Singulair 10 mg/d, QVAR 80 μg 4× bid | No | LAMA, ICS, LTRA | |
| 003R | Dulera 200/5.0 2× bid, fluticasone 220 2× bid, Xopenex prn | No | ICS | |
| 004R | Prednisone 40 mg/d; Symbicort 160 2× bid; Combivent 2 puffs prn; DuoNeb prn | Yes | LAMA | |
| 005R | Advair HFA 230/21 2 puffs bid; albuterol prn, prednisone, Singulair 10 mg qd, Proventil | Yes | LTRA | |
| 006R | Dulera 200 two puffs bid; albuterol neb prn, Acapella device | No | ||
| 007R | 40 mg prednisone daily, Advair 500/50 bid, Singulair 10 mg/d | Yes | LTRA | |
| 008R | Symbicort 160/4.5 2 puffs bid; QVAR 4 puffs bid; Proventil prn | No | ICS | |
| 009R | Advair 500/50 bid, QVAR 80 2× bid, Singulair 10 mg | No | ICS, LTRA | |
| 010R | Advair 250/50 bid; Singulair 10 mg/d; ProAir prn | No | LTRA | |
| 011R | Advair 250/50 bid, Ventolin 2× bid | No | ||
| 012R | Advair 500/50 bid, Singulair 10 mg qd, Spiriva once daily, Xopenex neb prn | No | LAMA, LTRA | |
| 013R | Advair 500/50 bid, Flovent 110 twice bid, Singulair 10 mg/d, Ventolin prn | No | ICS, LTRA | |
| 015R | Advair HFA 230/21 2 puffs bid, prednisone 60 mg qd, Ventolin prn, Combivent 18-103 prn | Yes | LAMA | |
| 016R | Xolair, Symbicort 160/4.5 two puffs bid, Spiriva qd, theophylline 900 mg qd, albuterol neb prn | No | LAMA, theophylline, Xolair | |
| 017R | Symbicort 160/4.5 2 puffs bid; Alvesco 160 2 puffs bid; Xopenex prn | No | ICS | |
| 018R | Zyflo CR 1200 mg bid; Advair HFA 230/21 two puffs bid | No | Zileuton | |
| 020R | Prednisone 60 mg qd; Singulair 10mg qd; Advair 500/50 bid | Yes | LTRA | |
| 021R | Albuterol HFA prn; Asmanex 220 μg bid; prednisone 15 mg qd; Serevent bid; Zyflo 600 mg qd | Yes | Zileuton | |
| 022R | Dulera 200/5.0 2 puffs bid, Pulmicort 180 μg 2 puffs bid, Singulair 10 mg qd, Ventolin prn | No | ICS, LTRA | |
| 023R | Advair 500/50 bid, Ventolin 2 puffs every 4 h, DuoNeb prn | No | ||
| 024R | Theophylline 300 mg 3×/d; albuterol neb prn; Ventolin HFA prn; Symbicort 160-4.5 μg 2 puffs bid; Spiriva HandiHaler 18 μg qd; Alvesco 160 μg 2 puffs bid; Accolate 20 mg bid; prednisone 2 mg/d | Yes | LAMA, LTRA, Theophylline | |
| 025R | Advair 500/50 bid, ProAir HFA prn | No | ||
| 026R | Singulair 10 mg, Daliresp 500 mg, Advair 500/50 bid; Alvesco 160 bid; prednisone taper prn on monthly basis, Proventil MDI prn; albuterol neb prn; Xolair | Yes | LTRA, roflumilast, Xolair | |
| 028R | Prednisone 20 mg bid; Symbicort 160/4.5 two puffs bid and QVAR 80 μg 2-4 puffs BID; albuterol HFA or neb PRN | Yes | ||
| 029R | 20 mg prednisone; theophylline 300 mg 3×/d; Spiriva neb prn every 2-4 h; albuterol every 2-4 h; Zyflo 600 mg bid; Advair 230/21 two puffs bid; Xolair 150 mg every 2 mo | Yes | LAMA, LTRA, theophylline, Xolair, zileuton | |
| 030R | ProAir HFA; prednisone taper ongoing; Dulera 200-5 2 puffs bid | Yes | ||
| 031R | 30 mg Prednisone; albuterol/ipratropium neb bid; Dulera 200/5 two puffs bid; Albuterol prn; Xolair | Yes | LAMA, Xolair | |
| 032R | Advair 230/21 two puffs BID | No | ||
| 033R | Symbicort 160/4.5 two puffs BID; DuoNeb | No | ||
| 034R | Advair 500/50 BID; ProAir 2 puffs prn; QVAR 80 three puffs bid | No | ICS | |
| 035R | Spiriva 18 μg/d; Flovent HFA 220 μg 2 puffs bid; Ventolin and Xopenex prn | No | LAMA | |
| 036R | Advair 500/50 with QVAR 80 two puffs bid | No | ||
| 037R | 10 mg Prednisone/d; Advair 500/50 bid; Pro-Air prn | Yes | ||
| 038R | Albuterol HFA prn; Proventil HFA 108 prn; Pulmicort 180 2 puffs bid; QVAR 2 puffs bid | No | ICS | |
| 039R | Symbicort 160/4.5 two puffs bid; QVAR 2 puffs bid; ProAir and albuterol neb prn; Zyrtec 10 mg | No | ICS | |
| 041R | Albuterol neb prn; Albuterol HFA prn; Dulera AERO 2 puffs bid; Prednisone 10 mg every other day | Yes | ||
| 042R | 5 mg/d Prednisone, Advair 500/50 bid; Spiriva 18 μg/d; albuterol neb daily prn | Yes | LAMA | |
| 043R | Symbicort 160 two puffs bid; Flovent 110 two puffs once/day; Xopenex neb BID; Spiriva qd | No | ICS, LAMA | |
| 046R | Symbicort 160/4.5 two puffs bid; prednisone 20 mg every other day | Yes | ||
| 047R | Ipratropium-albuterol for neb prn; Advair HFA AERO | No | LAMA | |
| 048R | Albuterol prn; Dulera 200-5 two puffs bid | No | ||
| 049R | Advair 500/50 bid; Prednisone 10 mg 3×/wk; albuterol prn; Xolair; ProAir HFA 2 puffs every 4-6 h | Yes | Xolair | |
| 050R | Dulera 100/5 two puffs bid; QVAR 80 two puffs bid; Zyflo 1200 mg bid; prednisone 20 mg qd; nasal saline washes | Yes | ||
| 051R | Advair 500/50 BID | No | ||
| 052R | Dulera 200/5 2 puffs bid; Ventolin prn; Zyrtec 10-20 mg; Nasonex prn; Albuterol neb prn | No | ||
| 053R | Advair HFA 230 two puffs bid; fluticasone nasal spray, theophylline 300 bid, albuterol prn | No | ||
| 054R | Symbicort 160/4.5 two puffs bid; QVAR 80 μg 3 puffs bid; montelukast 10 mg; Allegra 180 mg; Fluticasone 50 μg; Ventolin prn; NSW | No | ||
| 055R | Albuterol nebs/MDI prn; Brovana 15 μg neb bid; budesonide 1000 mg neb bid; Pulmicort; Omeprazole 40 mg bid; ranitidine | No | ||
| 056R | Albuterol neb tid; Symbicort 160/4.5 two puffs bid, Singulair 10 mg; saline nasal rinse with budesonide 0.5 mg twice a day | No | ||
| 057R | Albuterol nebulizer treatment every 4 h; Dulera 200/5 two puffs bid; Asmanex to 2 puffs bid, ProAir prn; Spiriva daily; Flonase; Singulair | No | ||
| 058R | Dulera 200-5MCG/ACT 2 puffs bis; ProAir inhaler prn; Singulair 10 mg; Zyrtec; Zetonna; QVAR 2 puffs bid, Nexium 40 mg | No | Xolair, Zetonna | |
| 059R | Prednisone 10 mg/d, ProAir HFA prn; Dulera 200/5 2p bid; DuoNeb prn | Yes | ||
| 060R | Xopenex and Atrovent nebulizers prn; Symbicort 160 two puffs bid | No | ||
| 061R | Singulair; albuterol nebulizer prn; Advair 500/50 bid, Cetirizine prn, Flonase | No | ||
| 062R | Advair 500 μg bid, albuterol MDI/neb | No | ||
| 063R | Alvesco, methylprednisolone 4 mg qd, Prilosec, Qnasl, Spiriva, Symbicort 160, Theophylline 300 mg bid, Ventolin prn, Zyrtec, Nasacort, Patanase nasal solution | Yes | Theophylline | |
| 066R | Pulmicort 180 μg 2 puffs bid, Symbicort 160/4.5 two puffs bid, theophylline, DuoNeb | No | Theophylline | |
| 067R | QVAR 80 μg 2 puffs twice daily; Symbicort 160/4.5 two puffs twice daily; albuterol nebs prn; Singulair 10 mg/d | No | Flonase prn; NSW prn | Lipitor, Lisinopril |
| 068R | Albuterol inhaler/neb prn, Symbicort 160 two puffs bid, QVAR 80 two puffs bid, Zyrtec | Yes |
bid, Twice daily; HFA, hydrofluoroalkane; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; MDI, metered-dose inhaler; prn, as necessary; qd, one daily; tid, 3 times daily.
Table E6.
Correlations among clinical and laboratory variables
| Variables | Correlation FEV1 (% [r]) | P value | Correlation with FVC (% [r]) | P value | Correlation with reversibility (r) | P value |
|---|---|---|---|---|---|---|
| Inflammatory cells | ||||||
| PMN, BAL fluid | −0.16 | NS | −0.32 | .01 | 0.15 | NS |
| Eosinophils, BAL fluid | −0.23 | NS | −0.20 | NS | 0.15 | NS |
| Lymphocytes, BAL fluid | 0.03 | NS | 0.05 | NS | −0.15 | NS |
| Eosinophils, blood | 0.02 | NS | 0.04 | NS | 0.06 | NS |
| BAL biomolecules | ||||||
| Myoglobin | −0.24 | .05 | −0.27 | .03 | 0.12 | NS |
| Prostasin | 0.27 | .03 | 0.15 | NS | −0.27 | .04 |
Table E7.
GO (biological process) pathway analysis
| Annotation (pathway/process) | XD score | Fisher q value | Gene set size | Pathway size | Overlap size |
|---|---|---|---|---|---|
| GO:0090023∼positive regulation of neutrophil chemotaxis | 0.5976409 | 0.58840 | 1 | 15 | 1 |
| GO:0044344∼cellular response to fibroblast growth factor stimulus | 0.5976409 | 0.58840 | 1 | 15 | 1 |
| GO:0050930∼induction of positive chemotaxis | 0.5270527 | 0.58840 | 1 | 17 | 1 |
| GO:0048566∼embryonic digestive tract development | 0.4476409 | 0.58840 | 1 | 20 | 1 |
| GO:0031623∼receptor internalization | 0.3190695 | 0.58840 | 1 | 28 | 1 |
| GO:0071347∼cellular response to IL-1 | 0.2976409 | 0.58840 | 1 | 30 | 1 |
References
- 1.Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.Bahadori K., Doyle-Waters M.M., Marra C., Lynd L., Alasaly K., Swiston J. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 4.Hekking P.P., Wener R.R., Amelink M., Zwinderman A.H., Bouvy M.L., Bel E.H. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Gibson P.G., McDonald V.M. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70:683–691. doi: 10.1136/thoraxjnl-2014-206740. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff P.G., Modrek B., Choy D.F., Jia G., Abbas A.R., Ellwanger A. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvin C., Zafar I., Good J., Rollins D., Christianson C., Gorska M.M. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–1186. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raundhal M., Morse C., Khare A., Oriss T.B., Milosevic J., Trudeau J. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelfand E.W., Alam R. The other side of asthma: Steroid-refractory disease in the absence of TH2-mediated inflammation. J Allergy Clin Immunol. 2015;135:1196–1198. doi: 10.1016/j.jaci.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 10.DeKruyff R.H., Yu S., Kim H.Y., Umetsu D.T. Innate immunity in the lung regulates the development of asthma. Immunol Rev. 2014;260:235–248. doi: 10.1111/imr.12187. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel S.E., Schwartz L.B., Langmack E.L., Halliday J.L., Trudeau J.B., Gibbs R.L. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 12.Doe C., Bafadhel M., Siddiqui S., Desai D., Mistry V., Rugman P. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinks T., Zhou X., Staples K., Dimitrov B., Manta A., Petrossian T. Multidimensional endotypes of asthma: topological data analysis of cross-sectional clinical, pathological, and immunological data. Lancet. 2015;385(suppl 1):S42. doi: 10.1016/S0140-6736(15)60357-9. [DOI] [PubMed] [Google Scholar]
- 14.Hinks T.S., Zhou X., Staples K.J., Dimitrov B.D., Manta A., Petrossian T. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136:323–333. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinley L., Alcorn J.F., Peterson A., Dupont R.B., Kapadia S., Logar A. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutsen A.P., Bush R.K., Demain J.G., Denning D.W., Dixit A., Fairs A. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–291. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 17.O'Hollaren M.T., Yunginger J.W., Offord K.P., Somers M.J., O'Connell E.J., Ballard D.J. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 18.Brandt E.B., Kovacic M.B., Lee G.B., Gibson A.M., Acciani T.H., Le Cras T.D. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204.e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly F.J., Fussell J.C. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnston S.L., Martin R.J. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med. 2005;172:1078–1089. doi: 10.1164/rccm.200412-1743PP. [DOI] [PubMed] [Google Scholar]
- 21.Johnston S.L., Blasi F., Black P.N., Martin R.J., Farrell D.J., Nieman R.B. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 22.Gern J.E. Virus/allergen interaction in asthma exacerbation. Ann Am Thorac Soc. 2015;12(suppl 2):S137–S143. doi: 10.1513/AnnalsATS.201503-153AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasier A.R., Victor S., Ju H., Busse W.W., Curran-Everett D., Bleecker E. Predicting intermediate phenotypes in asthma using bronchoalveolar lavage-derived cytokines. Clin Transl Sci. 2010;3:147–157. doi: 10.1111/j.1752-8062.2010.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick A.M., Higgins M., Holguin F., Brown L.A., Teague W.G. National Institutes of Health/National Heart, Lung, and Blood Institute's Severe Asthma Research Program. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857.e18. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good J.T., Jr., Kolakowski C.A., Groshong S.D., Murphy J.R., Martin R.J. Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy. Chest. 2012;141:599–606. doi: 10.1378/chest.11-0741. [DOI] [PubMed] [Google Scholar]
- 26.The ATS report on the criteria–American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2005;173:149–160. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 27.Andersson C., Enserro D., Sullivan L., Wang T.J., Januzzi J.L., Jr., Benjamin E.J. Relations of circulating GDF-15, soluble ST2, and troponin-I concentrations with vascular function in the community: the Framingham Heart Study. Atherosclerosis. 2016;248:245–251. doi: 10.1016/j.atherosclerosis.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown D.A., Breit S.N., Buring J., Fairlie W.D., Bauskin A.R., Liu T. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 29.Moore W.C., Meyers D.A., Wenzel S.E., Teague W.G., Li H., Li X. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore W.C., Hastie A.T., Li X., Li H., Busse W.W., Jarjour N.N. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinks T.S., Brown T., Lau L.C., Rupani H., Barber C., Elliott S. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138:61–75. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks C.R., Gibson P.G., Douwes J., Van Dalen C.J., Simpson J.L. Relationship between airway neutrophilia and ageing in asthmatics and non-asthmatics. Respirology. 2013;18:857–865. doi: 10.1111/resp.12079. [DOI] [PubMed] [Google Scholar]
- 33.Rovina N., Dima E., Bakakos P., Tseliou E., Kontogianni K., Papiris S. Low interleukin (IL)-18 levels in sputum supernatants of patients with severe refractory asthma. Respir Med. 2015;109:580–587. doi: 10.1016/j.rmed.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Ketelut-Carneiro N., Silva G.K., Rocha F.A., Milanezi C.M., Cavalcanti-Neto F.F., Zamboni D.S. IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J Immunol. 2015;194:4507–4517. doi: 10.4049/jimmunol.1402321. [DOI] [PubMed] [Google Scholar]
- 35.Jackson D.J., Glanville N., Trujillo-Torralbo M.B., Shamji B.W., Del-Rosario J., Mallia P. Interleukin-18 is associated with protection against rhinovirus-induced colds and asthma exacerbations. Clin Infect Dis. 2015;60:1528–1531. doi: 10.1093/cid/civ062. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz M., Eidenschenk C., Ota N., Wong K., Lohmann U., Kühl A.A. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 2015;42:321–331. doi: 10.1016/j.immuni.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Fujimori T., Grabiec A.M., Kaur M., Bell T.J., Fujino N., Cook P.C. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunol. 2015;8:1021–1030. doi: 10.1038/mi.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Olonisakin T.F., Xiong Z., Hulver M., Sayeed S., Yu M.T. Thrombospondin-1 restrains neutrophil granule serine protease function and regulates the innate immune response during Klebsiella pneumonia infection. Mucosal Immunol. 2015;8:896–905. doi: 10.1038/mi.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen L.T., Lim S., Oates T., Chung K.F. Increase in airway neutrophils after oral but not inhaled corticosteroid therapy in mild asthma. Respir Med. 2005;99:200–207. doi: 10.1016/j.rmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Thompson A.M., Zanobetti A., Silverman F., Schwartz J., Coull B., Urch B. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect. 2010;118:120–124. doi: 10.1289/ehp.0900550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He R.L., Zhou J., Hanson C.Z., Chen J., Cheng N., Ye R.D. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood. 2009;113:429–437. doi: 10.1182/blood-2008-03-139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ather J.L., Ckless K., Martin R., Foley K.L., Suratt B.T., Boyson J.E. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187:64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zielinski C.E., Mele F., Aschenbrenner D., Jarrossay D., Ronchi F., Gattorno M. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 44.McGeough M.D., Pena C.A., Mueller J.L., Pociask D.A., Broderick L., Hoffman H.M. Cutting edge: IL-6 is a marker of inflammation with no direct role in inflammasome-mediated mouse models. J Immunol. 2012;189:2707–2711. doi: 10.4049/jimmunol.1101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghasemzadeh M., Kaplan Z.S., Alwis I., Schoenwaelder S.M., Ashworth K.J., Westein E. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121:4555–4566. doi: 10.1182/blood-2012-09-459636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michalec L., Choudhury B.K., Postlethwait E., Wild J.S., Alam R., Lett-Brown M. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168:846–852. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 47.Ottonello L., Montecucco F., Bertolotto M., Arduino N., Mancini M., Corcione A. CCL3 (MIP-1alpha) induces in vitro migration of GM-CSF-primed human neutrophils via CCR5-dependent activation of ERK 1/2. Cell Signal. 2005;17:355–363. doi: 10.1016/j.cellsig.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Di Paolo N.C., Baldwin L.K., Irons E.E., Papayannopoulou T., Tomlinson S., Shayakhmetov D.M. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014;10:e1004035. doi: 10.1371/journal.ppat.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson J.L., Grissell T.V., Douwes J., Scott R.J., Boyle M.J., Gibson P.G. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green B.J., Wiriyachaiporn S., Grainge C., Rogers G.B., Kehagia V., Lau L. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9:e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosoki K., Ying S., Corrigan C., Qi H., Kurosky A., Jennings K. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One. 2015;10:e0126035. doi: 10.1371/journal.pone.0126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soeki T., Sata M. Inflammatory biomarkers and atherosclerosis. Int Heart J. 2016;57:134–139. doi: 10.1536/ihj.15-346. [DOI] [PubMed] [Google Scholar]
- 53.de Jesus A.A., Canna S.W., Liu Y., Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–874. doi: 10.1146/annurev-immunol-032414-112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kempf T., von Haehling S., Peter T., Allhoff T., Cicoira M., Doehner W. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 55.Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodier F., Coppé J.P., Patil C.K., Hoeijmakers W.A., Muñoz D.P., Raza S.R. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park H., Kim C.H., Jeong J.H., Park M., Kim K.S. GDF15 contributes to radiation-induced senescence through the ROS-mediated p16 pathway in human endothelial cells. Oncotarget. 2016;7:9634–9644. doi: 10.18632/oncotarget.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choy D.F., Hart K.M., Borthwick L.A., Shikotra A., Nagarkar D.R., Siddiqui S. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 59.Munthe-Fog L., Hummelshøj T., Honoré C., Madsen H.O., Permin H., Garred P. Immunodeficiency associated with FCN3 mutation and ficolin-3 deficiency. N Engl J Med. 2009;360:2637–2644. doi: 10.1056/NEJMoa0900381. [DOI] [PubMed] [Google Scholar]
- 60.Wong E.H., Porter J.D., Edwards M.R., Johnston S.L. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657–670. doi: 10.1016/S2213-2600(14)70107-9. [DOI] [PubMed] [Google Scholar]
- 61.Sutherland E.R., King T.S., Icitovic N., Ameredes B.T., Bleecker E., Boushey H.A. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126:747–753. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balzar S., Fajt M.L., Comhair S.A., Erzurum S.C., Bleecker E., Busse W.W. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]