Abstract
Synthetic cathinones are similar in chemical structure to amphetamines, and their behavioral effects are associated with enhanced dopaminergic signaling. The past ten years of research on the common constituent of bath salts, MDPV (the synthetic cathinone 3,4-methylenedioxypyrovalerone), has aided the understanding of how synthetic cathinones act at the dopamine (DA) transporter (DAT). Several groups have described the ability of MDPV to block the DAT with high-affinity. In this study, we demonstrate for the first time, a new mode of action of MDPV, namely its ability to promote DAT-mediated DA efflux. Using single cell amperometric assays, we determined that low concentrations of MDPV (1 nM) can cause reverse transport of DA via DAT. Notably, administration of MDPV leads to hyperlocomotion in Drosophila melanogaster. These data describe further how MDPV acts at the DAT possibly paving the way for novel treatment strategies for individuals who abuse bath salts.
INTRODUCTION
The neurotransmitter dopamine (DA) mediates behaviors relating to reward, motivation, attention, and cognition1, 2. Important to dopamine neurotransmission is the dopamine transporter (DAT). DAT is a presynaptic membrane protein responsible for the reuptake and recycling of DA following vesicular release3. Dysfunctions in DAT can lead to dopamine-associated neuropsychiatric disorders including ADHD, autism spectrum disorders, schizophrenia, and bipolar disorder4. DAT is also the target of commonly abused psychostimulants and controlled substances, namely cocaine and amphetamine (AMPH). Cocaine acts as a high-affinity antagonist of the transporter and blocks DA uptake, whereas AMPH acts as a substrate of the transporter and, through a series of intracellular mechanisms, causes DAT to reverse transport or “efflux” DA into the extracellular space5. The actions of cocaine and AMPH on the DAT are well-known to play a role in their rewarding properties and abuse potential. Thus, determining the effects of psychostimulants on DAT function is important for understanding the neural and molecular mechanisms underlying psychostimulant drug action.
In recent years, the abuse of synthetic cathinones or “bath salts” has grown to become a major world-wide health concern6. These substances are synthetic derivatives of the naturally-occurring stimulant, cathinone, found in the flowering plant Catha edulis7. The psychoactive effects of synthetic cathinones vary from the cocaine-like stimulant effects seen with 3,4-methylenedioxypyrovalerone (MDPV)8 to the MDMA-like empathogenic effects of methylone (3,4-methylenedioxymethcathinone)9. Among a number of identified biological sites, cathinones are known to target proteins that modulate dopamine neurotransmission, increasing dopaminergic signaling and associated behaviors10–15, including drug-seeking16, 17. When consumed in small doses, cathinones can lead to euphoria, alertness, increased libido, and elevated blood pressure. When consumed at higher doses, tremors, seizures, paranoia, violent behavior, psychoses, tachycardia18, delusions/hallucinations19, and death20 can occur. A recent report released by the Substance Abuse and Mental Health Services Administration (SAMHSA) showed that nearly 23,000 emergency room visits in 2011 were a result of cathinone abuse (SAMHSA 2013 Bath Salts Report). Due to the high risk associated with the use and the abuse potential of these compounds, the Drug Enforcement Administration (DEA) designated mephedrone (4-methylmethcathinone), methylone and MDPV as Schedule 1 substances under the Controlled Substances Act (DEA Drug Fact Sheet on Synthetic Cathinones). Nonetheless, illegal manufacturers continue to circumvent this ban by synthesizing "designer" substances with novel chemical structures but which produce similar psychostimulant effects21. These compounds are readily available and sold with fraudulent labels such as “plant food”, “research chemicals”, or “bath salts” at gas stations, tobacco stores, and over the Internet with a warning that the contents are not intended for human consumption. Their continued production and availability make it nearly impossible to control the exponentially rising sales and consumption of synthetic cathinones.
Despite increased data regarding the use and abuse of cathinones22, little is known about their mechanism of action. To address this issue, several research groups have begun to study the chemistry, pharmacology, and behavioral effects of various synthetic cathinones. Of these, MDPV is most commonly implicated in high-risk use18, 20, 21, 23–27. First synthesized in 1969, MDPV gained popularity much later in 2010 (2014 World Health Organization Critical Review Report on MDPV). As a highly lipophilic analogue of the synthetic cathinone pyrovalerone28, MDPV readily crosses the blood-brain barrier. Importantly, MDPV, when administered to animals exhibits striatal distribution, a brain region enriched in DA projections13. MDPV also shows high abuse potential in animal behavioral tasks12–14, 29.
Early research on MDPV demonstrated that this drug acts similarly to cocaine (a known DAT blocker), but with a 10- to 50-fold higher potency8, 30. However, increasing data suggests that there may be more to MDPV action. Work from Bauman et al. showed that after intravenous administration of MDPV, DA levels remain elevated for far longer than after cocaine administration8. In addition, MDPV administration results in long lasting cross-sensitization in mice, similar to the effects of methamphetamine16. These results suggest that MDPV, in addition to acting as a DAT blocker, may also display other modes of action. To examine further the molecular mechanisms of MDPV on the DAT, we performed amperometric studies. Specifically, to obtain greater temporal resolution, we studied MDPV action on human DAT (hDAT) by employing single cell amperometry. This assay has been previously used to discriminate AMPH versus cocaine actions in a single cell and these results have been reproduced in different model systems31. Further, we assessed MDPV-induced behaviors in Drosophila melanogaster, specifically focusing on known DAT-associated behaviors. Drosophila is a powerful genetic model for studying behaviors that are associated with DA as well as promoted by psychostimulants31–33, as several genes that regulate DA transport, synthesis, and signaling are conserved between flies and humans34.
METHODS
Drugs
(±)-3,4-Methylenedioxypyrovalerone HCl (MDPV), was synthesized in racemic form in our laboratories. Chemical and structural analysis included proton nuclear magnetic resonance, gas chromatography/mass spectrometry, thin layer chromatography, and melting point determination. All data were consistent with the expected structures. All other drugs used in this study including their salt and enantiomeric forms were as follows and purchased from Sigma-Aldrich (St. Louis, MO): Dopamine (i.e., 3-hydroxytyramine hydrochloride), D-amphetamine hemisulphate salt and Cocaine hydrochloride.
Amperometry
Chinese hamster ovary (CHO) cells stably expressing hDAT (here defined as hDAT cells) were plated at a density of ~20,000 per 35-mm culture dish. To preload cells with DA, dishes were washed with KRH assay buffer (130 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 25 mM HEPES, 1.1mM MgSO4 2.2 mM CaCl2, pH 7.4) supplemented with 10 mM dextrose, 100 μM pargyline, 1 mM tropolone, and 100 μM ascorbic acid, and incubated with 1 μM DA in KRH assay buffer for 20 minutes at 37°C. To record DA efflux, a carbon fiber electrode (ProCFE; fiber diameter of 5 μm; obtained from Dagan Corporation) juxtaposed to the plasma membrane and held at +700 mV (a potential greater than the oxidation potential of DA) was used to measure DA flux through oxidation reactions. Amperometric currents in response to the addition of 1 nM MDPV were recorded using an Axopatch 200B amplifier (Molecular Devices, Union City, CA) with a low-pass Bessel filter set at 1 kHz; traces were digitally filtered offline at 1 Hz using Clampex9 software (Molecular Devices, Union City, CA). DA efflux was quantified as the peak value of the amperometric current.
Drosophila melanogaster Behavior
To measure the locomotor response to MDPV we used the TriKinetics Drosophila Activity Monitoring (DAM) system as described in earlier studies 35, 36. Wild-type Oregon-R male flies were entrained for seven days in 12:12 h light:dark (LD) cycles at 25°C on standard cornmeal-molasses medium. On day two, flies were transferred individually to activity tubes and acclimated for a period of five days. On day seven, flies were transferred into identical activity tubes containing 20 μM MDPV or vehicle (water) in standard medium. Flies were continuously monitored for movement using activity monitors (DAM5, Trikinetics). Activity was measured as the number of times a fly crossed the infrared beam (beam crosses) per 30 minutes. Activity data was recorded for six hours after drug administration. Change in activity in response to drug treatment was reported as beam crosses normalized to average beam crosses 30 minutes prior to drug administration.
RESULTS
1 nM MDPV alone does not produce an amperometric signal
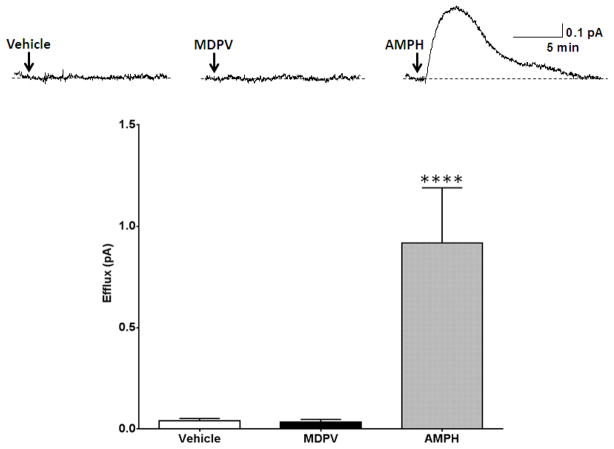
As a first control experiment, we demonstrated that hDAT cells pre-loaded with DA (see methods section) did not release DA upon application of vehicle (Figure 1, Vehicle). Next, we tested whether MDPV alone does not react at the carbon fiber electrode. At concentrations as low as 1 nM, MDPV did not elicit any amperometric current when applied to a bath chamber in the absence of hDAT cells (Figure 1, MDPV). Finally, to demonstrate that we can record DA efflux with our amperometric electrode, we show that bath application of 10 μM AMPH causes a robust DA efflux in the presence of hDAT cells (Figure 1, AMPH). We have previously shown that this AMPH-induced DA efflux is mediated by the hDAT and is cocaine-sensitive37. These control experiments were conducted to ensure that amperometry is a fitting technique to elucidate the actions of MDPV in terms of DA efflux and that MDPV at a concentration of 1 nM does not produce a non-specific amperometric signal.
Figure 1. MDPV (1 nM) does not elicit an amperometric current in the absence of hDAT cells.
Top Representative amperometric traces recorded from hDAT cells in response to application of vehicle to (Vehicle), application of 1 nM MDPV to bath in the absence of hDAT cells (MDPV), or application of 10 μM AMPH to hDAT cells (AMPH). Bottom: Quantitation of the peak amperometric current amplitude measured after vehicle or drug treatment (**** = p<0.0001 by One-way ANOVA with Dunnett’s multiple comparisons post-hoc analysis; n = 5–6).
New mode of action of MDPV
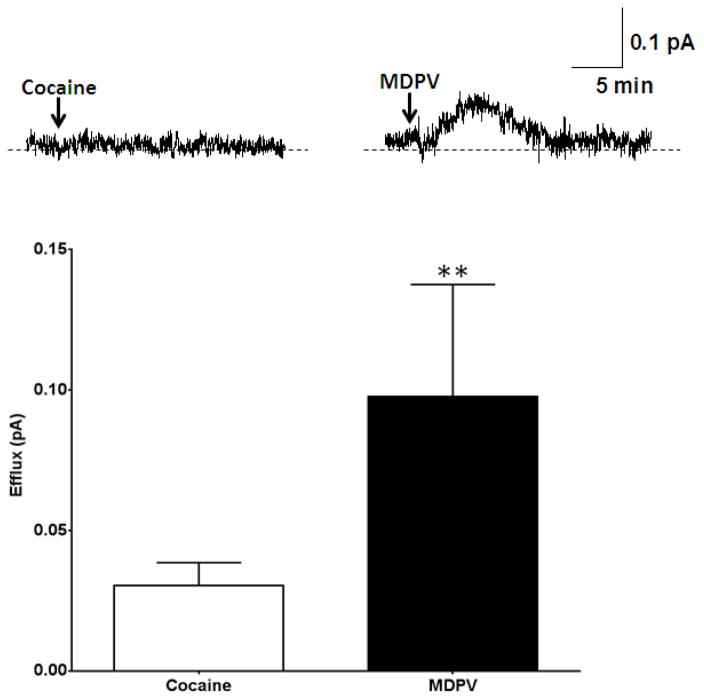
24 hours after plating, hDAT cells were preloaded with DA (see methods). Amperometric measurements were taken from individual hDAT cells after application of a low concentration of MDPV (1 nM) or cocaine (10 μM). As expected, 10 μM cocaine did not cause DA efflux as reflected by a lack of an upward deflection of the amperometric trace (Figure 2, Cocaine). These data are in agreement with previously published studies33, 37 and also establishes that there is no anomalous dopamine efflux or “leak” associated with the transporter in this in vitro system as previously shown37. Surprisingly, and in contrast to the effects of cocaine, amperometric traces recorded in response to 1 nM MDPV show a clear upward deflection of the amperometric current. This upward deflection reflects DAT-mediated DA efflux (Figure 2, MDPV). To note, hDAT cells the peak amperometric responses for MDPV were smaller than those recorded for AMPH (positive controls; compare Figure 1 (AMPH) to Figure 2 (MDPV)).
Figure 2. MDPV, but not cocaine, induces reverse transport of DA via hDAT.
Top Representative amperometric traces recorded in response to 10 μM cocaine or 1 nM MDPV in hDAT cells. Bottom: Quantitation of DA efflux measured as peak amplitude of the amperometric current after drug or vehicle treatment (** = p<0.01 by Student’s t-test; n = 5–8).
MDPV causes hyperlocomotion in flies
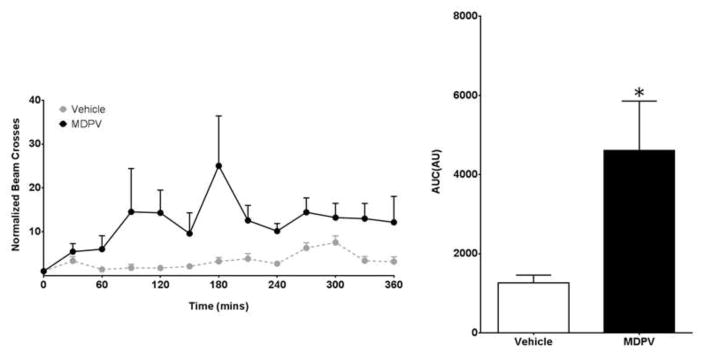
Building on our in vitro findings, we examined MDPV’s role in modifying DA-associated behaviors in Drosophila melanogaster. Wildtype flies were placed in locomotion chambers and acclimated for a period of five days. 20 μM MDPV or vehicle was administered orally via voluntary consumption. Locomotion was quantified as average beam crosses per 30 minutes normalized to pre-treatment conditions. Flies administered MDPV (n=16) show an elevated rate of locomotion compared to those administered vehicle (n=15) (Figure 3A). Cumulative beam breaks over a period of 6 hours show a greater than two-fold increase in locomotion in flies administered MDPV compared to vehicle (Figure 3B).
Figure 3. MDPV induces hyperlocomotion in flies.
(Left) Locomotion was measured by average beam crosses following 20 μM MDPV (n = 16) or vehicle (n = 15) administration. Beam crosses were normalized to pre-treatment conditions for each fly. (Right) Cumulative beam breaks were quantified for up to six hours post drug or vehicle administration. Flies exposed to MDPV displayed an increase in cumulative bream breaks compared to vehicle-controls (* = p<0.05 by Student’s t-test; n = 15–16).
DISCUSSION
DA homeostasis in the central nervous system is essential to regulating important brain functions, including reward. Synthetic cathinones disrupt normal dopaminergic neurotransmission and thus affect DA-associated behaviors. These drugs elicit behaviors indicative of enhanced dopaminergic signaling. The past ten years of research on MDPV and other synthetic cathinones demonstrate that the rewarding properties of synthetic cathinones are derived, in part, from their actions on monoamine transporters38, 39. Understanding how MDPV disrupts normal DA neurotransmission via its actions on the DAT is essential to the development of novel treatment options that can restore normal DA homeostasis in individuals that abuse MDPV and other synthetic cathinones. In this study, we aimed to reveal new modes by which low concentrations of MDPV cause an elevation in extracellular DA levels, and the behavioral consequences of its actions on DAT.
Using single cell amperometry we reveal that low concentrations of MDPV (1 nM) cause reverse transport of DA via DAT. Amperometry is a well-established paradigm that has been used by our group and others in several studies to determine different aspects of monoamine release mediated by catecholamine transporters31–33, 40. We first established that this assay is suitable for studying reverse transport of DA mediated by MDPV. We conducted an initial characterization of MDPV to demonstrate that at low concentrations, MDPV does not interact with the carbon fiber electrode to produce an artificial signal. Next, we demonstrate that in hDAT cells MDPV (1 nM) causes reverse transport of DA mediated by hDAT. To note is that high concentrations of MDPV, such as 100 nM, cause a non-specific amperometric signal (i.e. an amperometric current is recorded with MDPV in the absence of hDAT cells). These data describe for the first time a novel mode of action of MDPV at the DAT. Interestingly and importantly, previous work with hDAT has shown that higher concentrations of MDPV can block DAT function (20–30 nM)8. Taken together, these data suggest that MDPV might have multiple modes of action that are concentration-dependent, where at low concentrations MDPV works to cause DAT reverse transport, and at high concentrations MDPV primarily causes DAT blockade.
Drosophila melanogaster has been used in the past as a model organism to study the behavioral consequences of newly discovered molecular mechanisms of AMPH and cocaine41,42. In Drosophila, locomotion requires functional DA neurotransmission. Therefore to understand the significance of the actions of MDPV in vivo, in terms of changes in extracellular DA levels, we use flies as a behavioral model. Here, we show that MDPV administration leads to hyperlocomotion in Drosophila melanogaster. These data point to Drosophila melanogaster as a good animal model to further characterize in vivo the multiple actions of MDPV at the hDAT. This increase in locomotor activity has been previously documented to be associated with an increased in extracellular DA promoted by DAT blockers (e.g. cocaine) as well as DA effluxers (e.g. AMPH).
In this study, we did not determine whether the increase in Drosophila locomotion is driven by either the MDPV ability to block the hDAT, to cause DA efflux, or both. In the near future, we aim to explore these different possibilities by generating flies that are insensitive to the ability of MDPV to cause DA efflux as we have already done for AMPH31,32.
Further studying the different mode of actions of MDPV and other synthetic cathinone drugs as well as their specific behavioral consequences will not only pave the way toward better treatment strategies for those who abuse them, but also lead to better recognition/prediction of the dangers posed by novel designer cathinones that are emerging in the market today.
Highlights.
MDPV (3,4-methylenedioxypyrovalerone) is a common constituent of “bath salts”
In this study, we demonstrate for the first time, a new mode of action of MDPV
Low concentrations of MDPV cause reverse transport of dopamine via its transporter
Administration of MDPV leads to hyperlocomotion in Drosophila melanogaster
Acknowledgments
This work was supported by the NIH T-32 Training in the Pharmacological Sciences grant 4TR32GM007628-29 (JIA), University School of Nashville Summer Science Fellowship award (GG) and the National Institute on Drug Abuse grant DA35263 (AG).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Bjorklund A, Dunnett SB. Fifty years of dopamine research. Trends Neurosci. 2007;30:185–187. doi: 10.1016/j.tins.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 4.Gowrishankar R, Hahn MK, Blakely RD. Good riddance to dopamine: roles for the dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochem Int. 2014;73:42–48. doi: 10.1016/j.neuint.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 6.German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenneisen R, Fisch HU, Koelbing U, Geisshusler S, Kalix P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol. 1990;30:825–828. doi: 10.1111/j.1365-2125.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann MH, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liechti M. Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling. Swiss Med Wkly. 2015;145:w14043. doi: 10.4414/smw.2015.14043. [DOI] [PubMed] [Google Scholar]
- 10.Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen JD, et al. Locomotor Stimulant and Rewarding Effects of Inhaling Methamphetamine, MDPV, and Mephedrone via Electronic Cigarette-Type Technology. Neuropsychopharmacology. 2016;41:2759–2771. doi: 10.1038/npp.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King HE, Wetzell B, Rice KC, Riley AL. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depend. 2015;146:116–119. doi: 10.1016/j.drugalcdep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novellas J, et al. Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J Psychopharmacol. 2015;29:1209–1218. doi: 10.1177/0269881115598415. [DOI] [PubMed] [Google Scholar]
- 14.Kehr J, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic "bath salts" cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watterson LR, Watterson E, Olive MF. Abuse liability of novel 'legal high' designer stimulants: evidence from animal models. Behav Pharmacol. 2013;24:341–355. doi: 10.1097/FBP.0b013e3283641ec8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisek R, et al. Mephedrone ('bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of "bath salts" containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: "Bath Salts". Gen Hosp Psychiatry. 2011;33:525–526. doi: 10.1016/j.genhosppsych.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Wyman JF, et al. Postmortem tissue distribution of MDPV following lethal intoxication by "bath salts". J Anal Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- 21.Marusich JA, et al. Pharmacology of novel synthetic stimulants structurally related to the "bath salts" constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zawilska JB, Wojcieszak J. Designer cathinones--an emerging class of novel recreational drugs. Forensic Sci Int. 2013;231:42–53. doi: 10.1016/j.forsciint.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Coppola M, Mondola R. Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as "bath salts" or "plant food". Toxicol Lett. 2012;211:144–149. doi: 10.1016/j.toxlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive "bath salts" intoxication with methylenedioxypyrovalerone. Am J Med. 2012;125:854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Wright TH, et al. Deaths involving methylenedioxypyrovalerone (MDPV) in Upper East Tennessee. J Forensic Sci. 2013;58:1558–1562. doi: 10.1111/1556-4029.12260. [DOI] [PubMed] [Google Scholar]
- 26.Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug "bath salts" containing 3,4-Methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210:195–200. doi: 10.1016/j.forsciint.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Coppola M, Mondola R. 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett. 2012;208:12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Watterson LR, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts and components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartier E, et al. Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. EBioMedicine. 2015;2:135–146. doi: 10.1016/j.ebiom.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton PJ, et al. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol. 2014;10:582–589. doi: 10.1038/nchembio.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton PJ, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Molecular psychiatry. 2013;18:1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto S, Seto ES. Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp Anim. 2014;63:107–119. doi: 10.1538/expanim.63.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5518. pdb.prot 5518. [DOI] [PubMed] [Google Scholar]
- 36.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Mazei-Robison MS, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eshleman AJ, et al. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolanos R, Solis E, Jr, Sakloth F, De Felice LJ, Glennon RA. "Deconstruction" of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci. 2013;4:1524–1529. doi: 10.1021/cn4001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton PJ, et al. Zn(2+) reverses functional deficits in a de novo dopamine transporter variant associated with autism spectrum disorder. Molecular autism. 2015;6:8. doi: 10.1186/s13229-015-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizzo AB, et al. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freyberg Z, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nature communications. 2016;7:10652. doi: 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]