Abstract
Background
Although limited, the literature suggests alterations in activation of cognitive control regions in adults and adolescents with a history of childhood abuse. The current study examined whether such alterations are increased in the face of emotionally-distracting as compared to emotionally neutral information, and whether such alterations occur in brain regions that exert cognitive control in a more top-down sustained manner or a more bottom-up transient manner.
Methods
Participants were young adult women (ages 23–30): one group with a history of childhood physical or sexual abuse (N = 15) and one with no trauma exposure (N = 17), as assessed through the Trauma History Questionnaire and a two-stage interview adapted from the National Crime Victims Survey. Participants underwent fMRI scanning while completing hybrid block/event-related versions of a classic color-word and an emotional Stroop paradigm (threat and positive words). This paradigm allowed us to examine both sustained (activation persisting across blocks) and transient (event-specific activation) aspects of cognitive control.
Results
Women with a history of childhood abuse demonstrated decreased recruitment of frontal-parietal regions involved in cognitive control and enhanced recruitment of a ventral attention surveillance network during blocks of both versions of the Stroop task. Additionally, they had less suppression of brain regions involved in self-referential processes for threat blocks, but greater suppression of these regions for positive blocks. Severity of avoidance symptoms was associated with sustained activation in lateral prefrontal regions, whereas hyperarousal/re-experiencing symptoms were associated with sustained activity in temporal regions. No differential effects were observed for transient control.
Conclusions
Results suggest exposure to childhood abuse is associated with blunted recruitment of brain regions supporting task-set maintenance but hypervigilance for task-irrelevant information, regardless of whether distractors are emotionally neutral or emotional. Exposure to childhood abuse is also associated with less suppression of default mode brain regions associated with self-referential processing in the face of irrelevant threat information, but heightened ability to suppress similar processing for irrelevant positive information.
Keywords: Childhood abuse, Maltreatment, Trauma, fMRI, Inhibition, Cognitive control
Highlights
-
•
Examined group and individual differences in cognitive control in women exposed to child abuse
-
•
Overlapping and disparate alteration to cognitive control for emotional and neutral information
-
•
Blunted left frontal-parietal activation when ignoring salient, task-irrelevant information
-
•
Differential default mode suppression for threat and positive stimuli within and between groups
-
•
Differential top-down and bottom-up associations with specific trauma symptoms
1. Introduction
Childhood abuse (CA) is a type of traumatic experience that occurs at a time of dynamic neurobiological development and is associated with affective, cognitive, and clinical sequelae across the lifespan (Edwards et al., 2003, Hart and Rubia, 2012, Navalta et al., 2006, Pechtel and Pizzagalli, 2011, Shonk and Cicchetti, 2001, Teicher et al., 2003, Teicher and Samson, 2013, Teicher and Samson, 2016). A robust body of literature has demonstrated that children and adults with a history of childhood maltreatment demonstrate heightened attention to threatening stimuli (Dalgleish et al., 2001, Dannlowski et al., 2012, Hart and Rubia, 2012, McNally et al., 1990, Pechtel and Pizzagalli, 2011, Pine et al., 2005, Pollak et al., 2000, Pollak et al., 2005), which is associated with increased recruitment of limbic regions involved in threat responding, including the amygdala and hippocampus. Generally in a non-clinical population, heightened limbic activation can be overcome by the top-down, cognitive control influence of the frontal-parietal network (e.g., LeDoux, 2000). Cognitive control involves biasing towards task-relevant information (Banich, 2009, Miller, 2000, Miller and Cohen, 2001) and is implemented by a frontal-parietal network including the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), and posterior parietal regions (Banich et al., 2000a, Banich et al., 2000b, Botvinick et al., 2001, Brown et al., 1999, Bush et al., 1998, Carter et al., 1995, Carter et al., 1998, Milham et al., 2001). A variety of evidence suggests these cognitive control regions are altered across the lifespan in individuals with a history of childhood maltreatment (Beers and De Bellis, 2002, De Bellis, 2005, DePrince et al., 2009, Hart and Rubia, 2012, Navalta et al., 2006, Pechtel and Pizzagalli, 2011). As a result, heightened limbic activation has been shown to be coupled with decreased recruitment of prefrontal regions necessary to dampen responding (Dannlowski et al., 2012, Hart and Rubia, 2012, Herringa et al., 2013a, Herringa et al., 2013b, Pechtel and Pizzagalli, 2011, Shin and Liberzon, 2010, Tottenham et al., 2010, van Harmelen et al., 2014).
The vast majority of studies of CA have examined neural systems involved in cognitive control over emotional information as opposed to distracting, non-emotional information. There are some theoretical reasons to think that CA might lead to changes in cognitive control mechanisms more generally and not just specifically for emotional information. Cognitive control continues to develop through young adulthood because of protracted brain maturation (Gogtay et al., 2004, Paus, 2005, Sowell et al., 2003, Toga et al., 2006), which leads to differential patterns of activation during cognitive control tasks across development (Adleman et al., 2002, Andrews-Hanna et al., 2011, Luna et al., 2010). Given CA often occurs at a time when brain regions and networks related to cognitive control experience heightened neuroplasticity, it is a cognitive process that may be particularly vulnerable to CA. The only neuroimaging study to date examining cognitive control in adults with a history of childhood maltreatment regardless of the presence of psychopathology found a dose-dependent relationship between childhood maltreatment exposure and negative modulation of the dACC by left inferior frontal gyrus (IFG) during successful response inhibition (Elton et al., 2014). Alterations in activity in IFG and medial frontal regions, such as pre-supplementary motor area (pre-SMA), supplementary motor area (SMA), and ACC, have been observed during tasks requiring inhibitory control in adolescents with a history of childhood maltreatment (Lim et al., 2015, Mueller et al., 2010). Therefore, there is some limited evidence to suggest the possibility of altered recruitment of frontal cognitive control regions in individuals with a history of childhood maltreatment for non-emotional information.
The primary motivation for this study was to directly compare the degree of engagement of cognitive control regions when emotional as compared to neutral, non-emotional information must be ignored, so as to determine whether individuals with a history of CIT demonstrate specific or general deficits in cognitive control. To do so, our study compared brain activation during a classic color-word Stroop task (Stroop, 1935) to two versions of an emotional Stroop task, one with distracting stimuli that are threatening and another with distracting stimuli that are positive. The goal of the classic color word Stroop task is to identify the color in which a word is printed while ignoring the meaning of the word. Cognitive control must be exerted to engage in the less automatic process of ink color identification as compared to the more automatic process of word reading. The need for cognitive control increases when the word (e.g., “red”) and ink color (e.g., blue) are incongruent, as conflict must be resolved.
The emotional Stroop task also requires the maintenance of goal-directed behavior (i.e., ink color identification) in the face of a more automatic process (i.e., word reading). Cognitive control requirements are enhanced because the emotional word is salient and captures attention, which increases the likelihood of engaging in word reading instead of inherent sematic or response conflict (as in the Classic color-word Stroop). Thus, a question we address is whether the neural systems involved in cognitive control are altered in women with CA more generally (i.e., across both the color-word and emotional Stroop tasks) or whether such deficits occur differently in the face of emotional distraction. We know of only one prior, non-intervention related study, a PET study, in women with a history of sexual abuse that compared cognitive control during the classic and an emotional (threat) version of the Stroop (Bremner et al., 2004). That study, however, focused on group differences related to Posttraumatic Stress Disorder (PTSD) specifically. Researchers observed group differences on both the classic color word and emotional Stroop tasks, albeit in different directions (PTSD + > PTSD − vs. PTSD − > PTSD +) and in different brain regions. Nonetheless, this study points out the potential utility of investigating patterns of brain activity for both types of Stroop tasks.
A second goal of our study was to examine whether the valence of emotional information affects the engagement of neural systems involved in cognitive control in women with a history of CA. While most studies have reported alterations in processing of threatening information in individuals with a history of childhood maltreatment, some have also observed alterations in the processing of positive information as well (Dillon et al., 2009, Guyer et al., 2006, Mehta et al., 2010). Importantly, anhedonia has been identified as a particularly important clinical symptom related to psychopathology in adults with a history of childhood maltreatment (Pechtel and Pizzagalli, 2011, Teicher and Samson, 2013). Therefore, we had two conditions in our emotional Stroop task, one in which the emotionally distracting words were threat-related and another in which they were positive in valence. This design allowed us to examine whether there are alterations in cognitive control on the basis of emotional distraction in general, or whether such alterations are restricted to only distracting information that is threatening.
A third goal of the current study was to examine two well-established aspects of cognitive control, sustained and transient control, in adults with a history of CA. Sustained cognitive control involves proactively maintaining a task set, whereas transient cognitive control involves recruiting needed resources to adjust to dynamic contextual information or behavioral responses. Studies have demonstrated that the DLPFC supports initiating and maintaining task-sets while the ACC is involved more transiently, feeding information back to the DLPFC when there are dynamic changes or bolstering cognitive control in the face of poor top-down control by the DLPFC (Banich, 2009, Silton et al., 2010). Prior work with individuals with a history of childhood maltreatment has demonstrated decreased recruitment of the DLPFC and enhanced recruitment of the ACC during neutral inhibitory tasks (Bremner et al., 2004, Elton et al., 2014, Lim et al., 2015), suggesting that alterations might occur for sustained control, but that compensatory transient mechanisms may remain intact. To examine this hypothesis, we used a hybrid fMRI design with event-related trials interspersed among blocked trials of a specific task type, which allowed us to dissociate alterations in maintenance of a top-down, task-relevant attentional set (blocked trials) from transient recruitment of additional cognitive control (event-related trials) (Banich et al., 2009, Visscher et al., 2003).
2. Materials and methods
2.1. Participants
Participants were 32 young adult women between the ages of 23 and 30 who responded to flyers and electronic announcements posted at community agencies and through web-based list-serves in an urban area. Flyers and announcements did not specifically recruit individuals with a history of CA, but rather advertised a study looking at stress that was enrolling individuals with stressful life experiences as well as those without. This approach was taken in order to minimize recruitment bias. Only women were recruited for the study because of gender differences in exposure to CA and characteristics of experienced CA (Dhaliwal et al., 1996, Finkelhor et al., 1990, Goldberg and Freyd, 2006, Tolin and Foa, 2006). Participants were right-handed, native English speakers, and reported no history of brain injury, neurological disease, or psychotic symptoms, and no MR contraindications. All participants gave informed, written consent and were compensated monetarily. The study was approved by the Colorado Multiple Institutional Review Board at the University of Colorado.
Participants were categorized into one of two groups, women with a history of CA (CA group; N = 15) and women with no history of trauma (control group; N = 17). Women in the CA group experienced physical or sexual abuse prior to the age of 17 and denied any history of interpersonal trauma within the past year. Criteria for inclusion in the control group included no history of interpersonal trauma. For both the CA and control group, an individual could have experienced a potentially traumatic non-interpersonal event (e.g., car accident) as long as they did not meet DSM-IV-TR Criteria A for PTSD (American Psychiatric Association, 2000) for the non-interpersonal event.
2.2. Demographic information
Participants provided information on their age, race, occupation, and education. Current socioeconomic status (SES) was determined using education and occupation information in order to calculate the Hollingshead Index of Social Position (ISP; Hollingshead, 1975).
2.3. Measures
2.3.1. Intelligence
IQ was measured using the Wechsler Abbreviated Scale of Intelligence (WASI; Psychology Corporation, 1999) two subtest format.
2.3.2. Trauma history
A two-part approach was taken to determining the nature and extent of trauma history. First, participants were administered the Trauma History Questionnaire (THQ; Green, 1996), which is a 24-question measure that assesses for a history of various interpersonal and non-interpersonal traumas, at what ages these events occurred, and the number of incidents. It has been found to demonstrate adequate reliability and validity (Hooper et al., 2011). Endorsement of the following items prior to age 17 was considered to indicate a history of CA: forced intercourse or sex; being touched or asked to touch someone else's private parts under force of threat; any other forced and unwanted sexual contact; and/or being beaten, spanked, or shoved hard enough by a family member to cause injury. The questionnaire was administered verbally by a clinician, as opposed to self-report, so that direct follow up could be done to determine if a participant met Criteria A for PTSD for any non-interpersonal traumas.
Second, more detailed information about interpersonal trauma was obtained using a two-stage interview strategy adapted from the National Crime Victims Survey (NCVS; Fisher and Cullen, 2000). The NCVS is used annually by the U.S. Census Bureau to assess for crime, including sexual and physical assault, and has been used specifically in young adult women to assess for sexual victimization (Fisher and Cullen, 2000). In the first stage, participants were asked a series of behaviorally-defined screening questions designed to cue memory for relevant incidents. In the second stage, participants who answered “yes” to any screening questions were asked a series of detailed questions about the incident(s). This additional measure was used to allow for the opportunity to confirm that the incidents reported in the THQ were physical or sexual abuse and to provide additional details on those incidents. The initial lead behavioral questions, with subsequent follow up, used to assess childhood physical or sexual abuse included: being attacked or threatened, including attempted or complete rape, and being attacked or threatened by someone you know.
2.3.3. Clinical symptom measures
To determine the degree to which participants in the CA group also suffer from PTSD, all individuals in this group completed the Posttraumatic Diagnostic Scale (PDS; Foa et al., 1997), a 49-item self-report measure based on DSM-IV (American Psychiatric Association, 1994) criteria for PTSD. It assesses the presence of 17 possible PTSD symptoms from the three main symptom clusters (intrusive recollections, avoidance/numbing, and hyperarousal), and symptom severity separately for each cluster over the past month.
In addition, all participants completed the self-report Beck Depression Inventory - II (BDI-II; Beck et al., 1996), which provided a measure of depressive symptoms over the past two weeks.
2.4. fMRI Stroop task
While in the scanner, participants performed a manual-response version of the Stroop task (Stroop, 1935). On all trials, participants responded via a button press to the ink color in which a word was printed. There were four types of trials, defined by the type of word: incongruent, neutral, threat, and positive. Neutral trials consisted of neutral, non-color words; incongruent trials consisted of color words in a different color ink; threat words consisted of threat-specific words (e.g., abuse, hit); and positive words consisted of happy words not related to physical or emotional safety (e.g., joy, happy). The four ink colors (red, blue, green, yellow) used served as the incongruent words. Threat words were selected from a previous study using abuse-related threat words (Bremner et al., 2001). Positive words were selected from the normed words on the Affective Norms for English Words (ANEW; Bradley and Lang, 1999) and matched the threat words in arousal, to the degree possible. It was also confirmed that the valence of positive words, threat words, neutral words, and incongruent words were within positive, negative, and neutral ranges, respectively. Words across type were matched in length. Given that many of the emotional words, especially the threat words, are not used with the same frequency as color words and the neutral words, it was not possible to match words across type on frequency.
A hybrid blocked/event-related design was adapted from a previous study that allows both sustained and transient neural responses to be examined and has been shown to be sensitive to detecting differences between a clinical and control population (Banich et al., 2009). The hybrid design consisted of blocks of trials (measure of sustained activity) and event-related trials within these blocks (measure of transient activity). Each block consisted of block-specific trials (threat, positive, or incongruent) and a set of neutral frequent trials that occurred in all blocks. In all the blocks, block-specific and neutral frequent trials were presented for 2 s. There were 4 repetitions of each block type (threat, positive, incongruent) for a total of 12 Stroop blocks and 11 blocks of fixation interspersed between target blocks. Each block contained eight target trials and eight neutral frequent trials randomly distributed across the block, resulting in a total of 32 trials for each trial type. All block types, including fixation, had a 32-second duration. Block order was randomly distributed, and first block type was counter-balanced within group. There was 20 s of fixation baseline at the beginning of the scan.
2.5. Data acquisition
Functional images were acquired with a GE (Waukesha, Wisconsin) Signa 3T MRI scanner with a T2*-weighted gradient-echo, echo-planar imaging (TR = 2000 ms, TE = 32 ms, flip angle = 77°, 29 axial slices, thickness = 4 mm, gap = 0 mm, 64 m × 64 m in-plane resolution, in-plane FOV = 22 cm). A high-resolution T1-weighted SPGR anatomical scan was collected for each participant to localize functional activity (TR = 9 ms, TE = 2 ms, flip angle = 10°, 124 coronal slices, thickness = 1.7 mm, 0.87 m × 0.87 m in-plane resolution, in-plane FOV = 220 mm).
2.6. Procedures
During an initial eligibility session, a clinician administered the two-stage trauma interview, THQ, and WASI. Then, the participant completed a computerized battery of questionnaires, including the BDI-II. Women who endorsed a trauma history also completed the PDS. Other behavioral measures and questionnaires were completed, but are not reported here as they are unrelated to the hypotheses under investigation.
Participants completed a second session including MRI scanning. During the initial anatomical scan, participants completed practice trials consisting of variable length strings of X's to reinforce the color-mapping for the button box. Participants than completed the Stroop task, making responses via a button box. The Stroop task consisted of one functional scan with 400 volumes. Stimuli were programmed using E-Prime software (Psychology Software Tools, Inc.) and presented via a pair of stereoscopic, MRI-compatible goggles. Participants were given earplugs to dampen scanner noise and head movement was restricted through the use of an air pillow conformed to each participants head.
2.7. Image preprocessing
Image preprocessing was conducted with the FMIRB Software Library (FSL; http://www.fmirb.ox.ac.uk/fsl/index.html). Images were motion corrected with MCFLIRT, and brain tissue was extracted with BET to remove all non-brain tissue from the images. Prior to statistical analysis, images were spatially smoothed with a Gaussian kernel (FWHM = 8 mm), mean-based intensity normalized, high-pass temporal filtered with a cut-off period of 100 s to remove low-frequency noise, slice time corrected, and intensity-normalized to allow valid analyses across participants. Seven volumes (all fixations) were dropped from the beginning of each functional run to ensure steady-state magnetization. Only participants with < 2.5 mm of RMS movement across all 6 movement parameters were included in analyses. Data from 4 women (2 in the CA group and 2 in the control group) were not included in any analyses because of excessive movement. Participant totals and data presented throughout the manuscript do not include these women.
2.8. Imaging analyses
2.8.1. Main effects and contrasts
Statistical analyses were conducted with FMIRB's improved linear model. Analyses on the blood oxygen level dependent (BOLD) time series were run separately for each individual participant for each task, using separate blocked and event-related analyses for the Stroop task. Time series were convolved using a double-gamma hemodynamic response function. For comparisons across individuals, parameter and variance estimates for each participant were registered to Montreal Neurological Institute standard stereotaxic space (MNI152) with the two-stage registration procedure implemented in FMIRB's Linear Image Registration Tool. FMIRB's Local Analysis of Mixed Effects (FLAME1 + 2) was used to take into account that the variance of activation for the two groups might not be identical. To enable them to be more easily differentiated, blocked regressors and effects are denoted by capitalized labels (e.g., Threat), whereas event-related regressors and effects are denoted by lower-case labels (e.g., threat).
Separate GLM's were modeled for the blocked and event-related data. For the blocked analyses, separate regressors for each block type (Incongruent, Positive, and Threat) were modeled in a single GLM with the onset of each initial correct trial in a string of correct trials. Additionally, three separate regressors were modeled to account for error trials within each block type. In order to ensure that blocked effects were independent of these error trials, each blocked regressor was orthogonalized with respect to the corresponding error regressor. For the event-related analyses, seven regressors corresponding to the trial types were modeled in a single GLM: incongruent trials, neutral trials within incongruent blocks, positive trials, neutral trials within positive blocks, threat trials, neutral trials within threat blocks, and error trials. For each regressor, a double-gamma response function was convolved at the onset of each trial.
Within FLAME 1 + 2, analyses of group differences for the target conditions compared to fixation (Incongruent > Fixation, Positive > Fixation, and Threat > Fixation) and target conditions compared to each other (Incongruent > Positive, Incongruent > Threat, and vice versa) were computed using two-sample t-tests. To take advantage of spatial neighborhood information, while moderately controlling for Type I error (given the restricted sample size and a priori hypotheses), we used the following approach to examining group differences. Threshold Free Cluster Enhancement (TFCE; Smith and Nichols, 2009) was implemented in FSL randomise (Winkler et al., 2014), with permutations performed in a voxel-wise manner. TFCE transformation was applied to all voxels in the brain before permutation, and a grey matter mask applied (Woo et al., 2014). Images were thresholded at a p < 0.005 (uncorrected). This uncorrected peak threshold was selected based on evidence that permutation testing with peak thresholding up to p < 0.01 is robust to false positives (Eklund et al., 2016) and prior experience with similar sample sizes and clinical groups. Peak activation (z score) was examined separately for each condition for each group within significant clusters.
2.8.2. Conjunction analyses
Conjunction analyses were performed because certain combinations of the contrasts can be conceptualized as representing a common process or rubric, and to ensure that contrasts were not driven mainly by one condition. For example, areas of common activation across incongruent, positive, and threat trials are likely to represent regions that need to be engaged for cognitive control (regardless of the nature of the distracting information). Similarly, regions that commonly activated across positive and threat trials may represent regions that are engaged when cognitive control is required to ignore emotional information (regardless of valence). These conjunctions analyses were performed for group contrasts. For example, conjunction analyses were performed to determine if activation for the CA group was significantly higher than for the control group across trial types requiring emotional information to be ignored.
These conjunction analyses were performed in FSL using the easythresh_conj command (Nichols, 2007), which uses the minimum statistic of the conjunction null to assess for activation in each of two or more conditions and is robust even when full independence is not present (Nichols et al., 2005). For this analysis, conjunctions of significant differences in activation between the groups were assessed and thresholded at a p < 0.05 (uncorrected). A relatively liberal threshold was used for two reasons in this restricted clinical sample: 1) comparison to the conjunction null hypothesis is often overly conservative (Friston et al., 2005), and 2) we examined overlap in significant group differences as opposed to overlap across different cognitive conditions within a group, which inevitably produces more activation.
Three input maps from the blocked analyses were used to examine the overlap across the three target conditions: Incongruent > Fixation, Positive > Fixation, and Threat > Fixation. These maps were computed separately for the group comparisons of Control > CA and for CA > Control. Analogously, for the event-related data, the target trials compared to neutral trials within the target block were entered as input (e.g., incongruent > neutral, positive > neutral, threat > neutral). Two input maps were used to examine the overlap in differences between non-emotional information and both types of emotional information: Incongruent > Positive, Incongruent > Threat, and vice versa, for the comparisons of Control > CA and for CA > Control. Again, analogous input was used for target trials within the blocks (incongruent > positive, incongruent > threat, and vice versa). Regions from the conjunction analyses are only reported if they are > 10 voxels, so as to focus on meaningful results.
2.8.3. Covariate analyses
These analyses focused on the relationship of brain activation and the severity/type of symptoms within the CA group only. Whole-brain regressions were completed regressing trauma symptom severity, as measured by the PDS, on activation for contrasts of interest (Block: Incongruent > Fixation, Positive > Fixation, and Threat > Fixation; Event-related: incongruent > neutral within incongruent block, positive > neutral within positive block, and threat > neutral within threat block). Similar to the examined group contrasts, TFCE (Smith and Nichols, 2009) was implemented in FSL randomise (Winkler et al., 2014), with permutations performed in a voxel-wise manner. TFCE transformation was applied to all voxels in the brain before permutation, and a grey matter mask applied (Woo et al., 2014). Images were thresholded at a p < 0.005 (uncorrected).
Percent signal change values were then extracted from ROIs defined by significant clusters in the whole-brain regressions, in order to visually inspect the data for outliers and quantify the associations. Only clusters for which the regression results were driven by the target condition (as opposed to the fixation or neutral comparison condition) are presented. Exploratory post-hoc analyses were performed quantifying the associations while controlling for depressive symptoms. Results were relatively similar when depression was controlled. For two clusters the quantified relationship was reduced to r < 0.1 once depression was controlled and these clusters are noted in Table 4. The CA group was not large enough to examine associations between symptoms and the percent signal change from significant clusters identified in direct contrasts and conjunction analyses.
Table 4.
Whole-brain regression of trauma symptoms on activation across Stroop tasks. Correlations with avoidance symptom severity and a composite of re-experiencing and arousal symptom severity, as measured by the PDS, are presented separately for blocked and event-related activity. The z score presented represents peak activation in the cluster. Pearson correlation (r) represents the correlation between percent signal change of a 5 mm sphere around the peak coordinate for significant clusters from the whole-brain regression. All coordinates presented in MNI space. R = right. L = left. BA = Brodmann's area. I = incongruent. P = positive. T = threat.
| Region | BA | z | Voxels | x | y | z |
r (Block/trial) |
||
|---|---|---|---|---|---|---|---|---|---|
| I | P | T | |||||||
| Avoidance Symptoms | |||||||||
| Blocked activity | |||||||||
| Incongruent vs. Fixation | |||||||||
| Cerebellum (R)a | – | 2.94 | 695 | 38 | − 82 | − 38 | − 0.22 | – | – |
| Cerebellum (L) | – | 2.45 | 273 | 2 | − 92 | − 26 | − 0.53 | – | – |
| Amygdala (L) | – | 4.78 | 263 | − 18 | − 4 | − 14 | − 0.70 | – | – |
| Hippocampus (L) | – | 3.55 | 235 | − 10 | − 8 | − 22 | − 0.56 | – | – |
| Medial frontal cortex (L) | 32 | 3.80 | 170 | − 6 | 30 | − 16 | − 0.53 | – | – |
| Brain stem (R) | – | 4.27 | 145 | 24 | − 34 | − 34 | − 0.51 | – | – |
| Superior temporal gyrus (R) | 38 | 3.12 | 101 | 46 | 6 | − 14 | − 0.53 | – | – |
| Superior temporal gyrus (L) | 38 | 4.01 | 93 | − 50 | 24 | − 24 | − 0.50 | – | – |
| Middle temporal gyrus (L) | 21 | 2.78 | 88 | − 66 | − 4 | − 12 | − 0.42 | – | – |
| Postcentral gyrus (L) | 4 | 3.40 | 64 | − 68 | − 8 | 20 | − 0.42 | – | – |
| Inferior temporal gyrus (L) | 20 | 3.05 | 38 | − 56 | − 28 | − 22 | − 0.29 | – | – |
| Middle frontal gyrus (L) | 10 | 1.43 | 36 | − 30 | 44 | 20 | − 0.47 | – | – |
| Inferior temporal gyrus (L) | 20 | 1.82 | 29 | − 62 | − 54 | − 18 | − 0.52 | – | – |
| Inferior frontal gyrus/insula (L) | 45/13 | 2.29 | 28 | − 40 | 24 | − 2 | − 0.66 | – | – |
| Precentral gyrus (L) | 6 | 1.36 | 19 | − 48 | 2 | 32 | − 0.51 | – | – |
| Positive vs. Fixation | |||||||||
| Superior parietal lobule (R) | 7 | 5.29 | 19 | 28 | − 62 | 66 | – | 0.31 | – |
| Event-Related Activity | |||||||||
| positive > neutral positive | |||||||||
| Superior frontal gyrus (L) | 6 | 3.19 | 12 | − 22 | 10 | 65 | − 0.31 | ||
| threat > neutral threat | |||||||||
| Parahippocampal gyrus (R)a | 30 | 5.89 | 35 | 10 | − 36 | 6 | – | – | − 0.32 |
| Re-Experiencing and Arousal Symptoms | |||||||||
| Blocked Activity | |||||||||
| Incongruent vs. Fixation | |||||||||
| Supramarginal gyrus (L) | 40 | 1.18 | 16 | − 40 | − 46 | 36 | − 0.69 | – | – |
| Threat vs. Fixation | |||||||||
| Fusiform gyrus (R) | 20 | 2.94 | 15 | 38 | − 10 | − 26 | – | – | 0.77 |
| Positive vs. Fixation | |||||||||
| Cuneus (R) | 18 | 3.20 | 458 | 4 | − 94 | 20 | – | 0.61 | – |
| Cuneus (R) | 18 | 5.55 | 259 | 18 | − 78 | 32 | – | 0.55 | – |
| Lingual gyrus (R) | 19 | 3.52 | 208 | 16 | − 68 | − 2 | – | 0.29 | – |
| Middle temporal gyrus (R) | 21 | 2.75 | 118 | 48 | − 12 | − 18 | – | 0.26 | – |
| Cerebellum (L) | – | 2.03 | 39 | − 20 | − 86 | − 34 | – | 0.48 | – |
| Superior temporal gyrus (L) | 22 | 2.23 | 26 | − 60 | − 10 | 2 | – | 0.24 | – |
| Superior temporal gyrus (L) | 38 | 1.65 | 22 | − 40 | 0 | − 22 | – | 0.47 | – |
| Superior temporal gyrus (R) | 22 | 2.16 | 12 | 58 | − 10 | 6 | – | 0.62 | – |
| Event-Related Activity | |||||||||
| incongruent > neutral incongruent | |||||||||
| Middle frontal gyrus (L) | 8 | 3.01 | 49 | − 36 | 40 | 42 | − 0.20 | – | – |
| Middle occipital gyrus (L) | 19 | 7.07 | 25 | − 40 | − 88 | 22 | − 0.36 | – | – |
| Parahippocampal gyrus (R) | 36 | 4.50 | 13 | 16 | − 6 | − 36 | − 0.39 | – | – |
Controlling for depressive symptoms resulted in an r < 0.10.
2.9. Behavioral analyses
All statistical analyses were performed using IBM SPSS, Version 24 (2016) and used an alpha of 0.05. For demographic variables, independent samples t-tests were used for continuous variables and a chi-square test for the nominal variable of race. Accuracy and correct trial reaction time (RT) were calculated separately for the blocked and event-related Stroop data as well as for each trial type within block and across event-related trials, respectively. Interference was calculated for each condition as a percentage of RT on block-specific neutral trials (e.g., (mean target correct trial RT − mean within target block neutral correct trial RT) / mean within target neutral correct trial RT).
3. Results
3.1. Group characteristics
The CA group and control group did not differ on demographic factors or estimated IQ. Not surprisingly, the CA group reported significantly higher levels of depressive symptoms than the control group (p < 0.001). See Table 1 for group characteristics.
Table 1.
Demographic and individual differences variables. N = 32 (CA group = 15, control group = 17). Means are presented for each group, with standard deviation in parentheses. IQ = Intelligence Quotient, measured by the WASI. SES = Socioeconomic Status, measured by the Hollingshead Index of Social Position. BDI – II = total score on Beck Depression Inventory – II. PTSD criteria and symptoms were assessed using the Posttraumatic Diagnostic Scale (PDS).
| Variable | CA group | Control group |
|---|---|---|
| Age | 25.93 (2.28) | 26.00 (2.24) |
| IQ | 119.47 (7.46) | 118.41 (7.95) |
| SES | 42.80 (14.04) | 37.76 (13.93) |
| Race (% Caucasian) | 67% | 76% |
| BDI-II⁎ | 16.20 (10.64) | 4.12 (5.22) |
| PTSD | 68% | N/A |
| PTSD symptom severity: total | 16.47 (10.18) | N/A |
| PTSD symptom severity: avoidance/numbing | 7.80 (4.84) | N/A |
| PTSD symptom severity: hyperarousal | 8.73 (5.02) | N/A |
| PTSD symptom severity: re-experiencing/intrusive | 4.87 (3.56) | N/A |
p < 0.001.
Within the CA group, 19% of women reported experiencing only childhood physical abuse, 31% reported experiencing only childhood sexual abuse, and 50% reported experiencing both childhood physical and sexual abuse. Additionally, 68% of women in the CA group met criteria for PTSD (see Table 1 for symptom severity).
Investigations of symptom clusters revealed that the severity of avoidance symptoms was not significantly correlated with either the severity of re-experiencing (r = 0.41, p = 0.13) or hyperarousal symptoms (r = − 0.19, p = 0.49), but the latter two were (r = 0.69, p = 0.005). Therefore, in subsequent regressions involving symptoms, analyses were performed separately for severity of avoidance symptoms and a composite of the severity of re-experiencing and hyperarousal symptoms, which represented the average of these two scores, rather than utilizing a total symptom severity score. The correlation between total PTSD symptom severity and depressive symptom severity approached a trend (r = 0.44, p = 0.10), accounting for 19% of the variance in trauma symptoms (r2 = 0.19). We decided not to include depression as a covariate in imaging analyses because of concerns that it would remove variance associated with exposure to CA as opposed to a separate, non-overlapping depressive construct. Additionally, previous studies have demonstrated that alterations in patterns of brain activation in individuals with a history of CA may be independent of specific psychopathology (van Harmelen et al., 2013, van Harmelen et al., 2014).
3.2. Behavioral results: Stroop performance
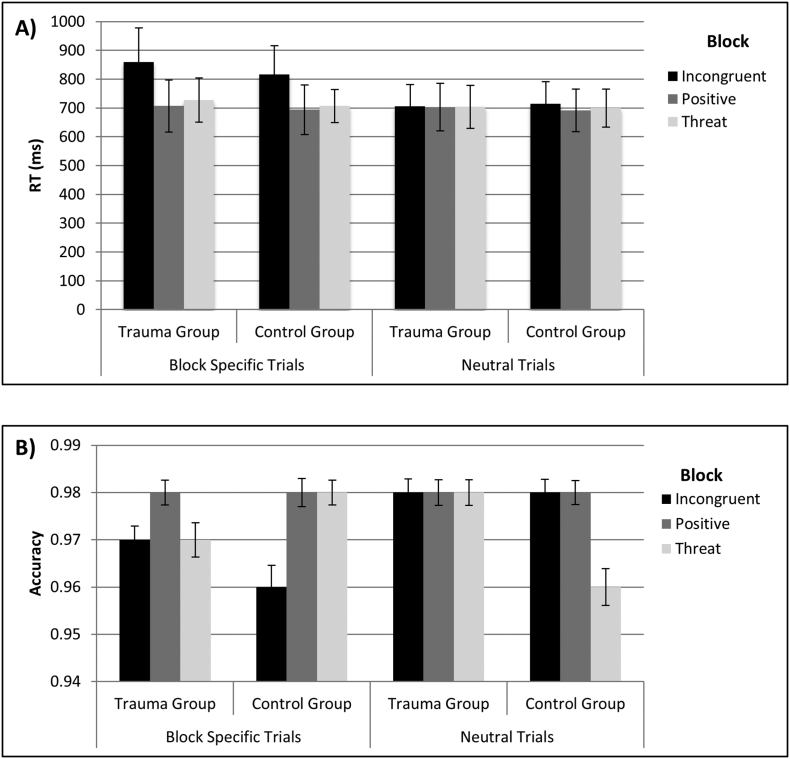
The analysis of behavior indicated that our Stroop task required cognitive control as evaluated in separate repeated measures ANOVAs with a between-subjects factor of Group (Control, CA) and within-subjects factors of Block (Incongruent, Positive, Threat) and Trial (Target, Neutral) conducted separately for correct mean RT and accuracy. For RT, as expected, there was a main effect of Trial (F(1, 29) = 56.27, p < 0.001) with participants responding slower on Target (M = 751.88, SE = 14.45) than Neutral (M = 703.20, SE = 11.99) trials (p < 0.001). There was also a main effect of Block (F(2, 28) = 32.26, p < 0.001), resulting from longer RT for incongruent (M = 774.17, SE = 15.69) than positive (M = 698.89, SE = 14.04) or threat (M = 709.57, SE = 11.86) blocks (all p's < 0.001), the latter two of which did not differ (p = 0.19). This effect of Block remained significant (F(2, 28) = 49.50, p < 0.001) when rather than using raw RT, interference scores were calculated for target trials, a percentage of RT for neutral trials within the same blocks (e.g., (incongruent RT − neutral RT within incongruent block) / (neutral RT within incongruent block)). Similar to raw RT, there was significantly more interference on the incongruent (M = 0.18, SE = 0.017) than positive (M = 0.006, SE = 0.014) or threat (M = 0.023, SE = 0.011) trials (both p's < 0.001), the latter two of which did not differ (p = 0.35).
There were no other significant effects (all p's > 0.23). Results for accuracy indicated no significant between-group, within-group, or interaction effects (all p's > 0.12). See Fig. 1 for performance data.
Fig. 1.
Behavioral performance on the hybrid Stroop task. A) Reaction time (RT) for correct trials. B) Accuracy for each trial type. Block specific trials (incongruent, positive, and threat) are shown on the left and neutral trials within a block type are on the right. RT and accuracy for the CA group and control group are shown separately. Incongruent trials and neutral trials within the incongruent block are shown in black, positive trials and neutral trials within the positive block are shown in dark grey, and threat trials and neutral trials with the threat block are shown in light grey. Error bars represent one standard deviation.
3.3. Overlapping cognitive control of emotionally neutral and emotional information
3.3.1. Sustained cognitive control (blocked analyses)
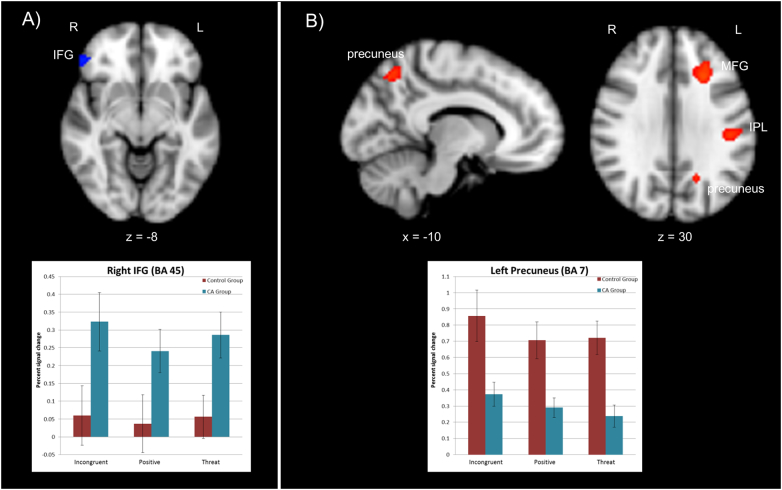
Using a conjunction analysis, we determined those brain regions that yield a significant group difference across each of the three types of blocks (i.e., Incongruent, Positive, and Threat blocks) compared to fixation, that is whenever cognitive control must be exerted. Across all three block types the following effects were observed. The CA group exhibited greater activation of rIFG, a region implicated in inhibition and contextual monitoring (Banich and Depue, 2015, Munakata et al., 2011), and the left cerebellum than the control group. Additionally, the CA group significantly deactivated left mid-DLPFC (Banich, 2009, Petrides, 2000, Petrides, 2005) and right dACC compared to no significant activation in these areas in the control group.
In contrast, the control group activated left-sided parietal regions implicated in cognitive control, including the left inferior parietal cortex and precuneus, as well as right MFG and cerebellum compared to no activation in these regions by the CA group. See Table 2 and Fig. 2. See Supplemental Table 1 for group differences for each of the blocked Stroop conditions.
Table 2.
Conjunction analyses of blocked activation. Conjunctions reflects overlap in activation across specified conditions (p < 0.05, uncorrected). Only clusters with > 10 voxels are presented. The z score presented represents peak activation in the cluster. Peak activation, presented as a z score, extracted for target conditions compared to fixation are included for all blocks. All coordinates presented in MNI space. R = right. L = left. BA = Brodmann's area. Inc. = incongruent. Post = positive. * = Group differences driven by significant deactivation.
| Region | BA | z | Voxels | x | y | z | Group |
Block/Trial |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Inc | Post | Threat | ||||||||
| Blocked Activity | ||||||||||
| Non-Emotional and Emotional | ||||||||||
| Control Group > CA Group | ||||||||||
| Middle Frontal Gyrus (L)* | 8/9 | 2.89 | 282 | − 26 | 26 | 32 | CA | − 3.66 | − 3.86 | − 3.82 |
| Control | 0.31 | − 0.12 | − 0.17 | |||||||
| Precuneus (L) | 7 | 2.73 | 235 | − 14 | − 68 | 48 | CA | 4.18 | 3.22 | 2.41 |
| Control | 6.31 | 5.77 | 5.31 | |||||||
| Inferior Parietal Lobule (L) | 40 | 2.82 | 175 | − 46 | − 24 | 24 | CA | − 0.65 | − 0.14 | − 0.19 |
| Control | 3.36 | 3.65 | 3.73 | |||||||
| Cerebellum (R) | – | 2.88 | 83 | 30 | − 44 | − 22 | CA | − 0.17 | − 0.023 | − 1.22 |
| Control | 4.44 | 3.74 | 3.40 | |||||||
| Precuneus (L) | 7 | 2.54 | 40 | − 18 | − 60 | 30 | CA | − 0.44 | 0.42 | − 1.25 |
| Control | 3.91 | 3.84 | 4.15 | |||||||
| Medial Frontal Gyrus (R)* | 32 | 2.11 | 14 | 18 | 32 | 34 | CA | − 2.92 | − 2.50 | − 3.27 |
| Control | − 0.36 | 0.61 | − 0.045 | |||||||
| Middle Frontal Gyrus (R) | 10 | 2.36 | 13 | 36 | 34 | 14 | CA | 2.90 | 2.19 | 1.41 |
| Control | 4.90 | 4.78 | 4.46 | |||||||
| Precentral Gyrus (L) | 6 | 2.23 | 12 | − 48 | − 8 | 10 | CA | − 0.13 | 0.10 | 0.26 |
| Control | 2.94 | 3.22 | 3.88 | |||||||
| CA Group > Control Group | ||||||||||
| Inferior Frontal Gyrus (R) | 45 | 2.46 | 70 | 50 | 40 | − 6 | CA | 3.44 | 3.05 | 3.85 |
| Control | 0.22 | − 0.31 | 0.063 | |||||||
| Superior Occipital Gyrus (R)* | 19 | 2.26 | 16 | 38 | − 80 | 40 | CA | 2.28 | 1.97 | 0.94 |
| Control | − 2.11 | − 1.26 | − 2.53 | |||||||
| Cerebellum (L) | – | 2.31 | 15 | − 48 | − 80 | − 24 | CA | 6.01 | 5.31 | 6.14 |
| Control | 3.76 | 3.55 | 3.74 | |||||||
| Non-Emotional > Emotional | ||||||||||
| Control Group > CA Group | ||||||||||
| Middle Frontal Gyrus (L) | 9 | 2.74 | 92 | − 56 | 20 | 24 | CA | 2.02 | 1.84 | 2.63 |
| Control | 3.89 | 2.12 | 2.75 | |||||||
| Emotional > Non-Emotional | ||||||||||
| Control Group > CA Group | ||||||||||
| Medial Frontal Gyrus (R)* | 9 | 2.56 | 45 | 8 | 48 | 28 | CA | − 0.31 | − 1.35 | − 0.89 |
| Control | − 2.31 | 0.99 | 0.68 | |||||||
| Event-Related Activity | ||||||||||
| Non-Emotional > Emotional | ||||||||||
| Control Group > CA Group | ||||||||||
| Inferior Frontal Gyrus (L) | 46 | 2.63 | 19 | − 60 | 22 | 24 | CA | 0.84 | 1.94 | 1.86 |
| Control | 3.45 | 1.62 | 1.89 | |||||||
| CA Group > Control Group | ||||||||||
| Anterior Cingulate Cortex (M)* | 24 | 2.64 | 110 | 0 | 24 | 10 | CA | − 0.32 | − 1.91 | − 1.88 |
| Control | − 2.94 | − 1.97 | − 2.81 | |||||||
| Anterior Cingulate Cortex (R) | 32 | 2.10 | 25 | 2 | 18 | 26 | CA | 0.98 | − 0.10 | 0.86 |
| Control | 0.54 | 0.48 | 1.11 | |||||||
| Middle Temporal Gyrus (L) | 21 | 2.84 | 22 | − 62 | 0 | − 28 | CA | − 0.78 | − 0.36 | − 0.75 |
| Control | − 1.78 | − 0.21 | − 0.50 | |||||||
| Brainstem (M) | 31 | 2.37 | 13 | 0 | − 44 | − 40 | CA | 3.48 | 3.17 | 3.55 |
| Control | 2.17 | 2.89 | 2.96 | |||||||
Fig. 2.
Conjunction analyses examining group differences in blocked activation across all three Stroop conditions (Incongruent, Positive, and Threat). A) Greater activation of right IFG by the CA group than control group across all three conditions, shown in blue. Graph of percent signal change by group for a 5 mm sphere around the peak of the right IFG cluster (50, 40, − 6) presented below. B) Greater activation of the left precuneus, left MFG, and right IPL by the control group than CA group across all three conditions, shown in red. Graph of percent signal change by group for a 5 mm sphere around the peak of the left precuneus cluster (− 14, − 68, 48) presented below. Error bars on graphs represent standard error. IFG = inferior frontal gyrus. IPL = inferior parietal lobule. MFG = middle frontal gyrus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.2. Transient cognitive control (event-related analyses)
An analogous conjunction analysis was performed for event-related data, examining direct overlap of activation for target (incongruent, positive, threat) trials compared to respective within-in block neutral trials. There were no significant results. See Supplemental Table 1 for group differences for each of the event-related contrasts.
3.4. Differential cognitive control of emotional vs. non-emotional information
3.4.1. Sustained cognitive control (blocked analyses)
Further conjunction analyses were performed in order to determine if the groups showed different patterns of activation when the information to be ignored was emotional, regardless of valence (Positive, Threat), compared to non-emotional (Incongruent). These analyses identified regions that showed significant differences for both the Threat vs. Incongruent as well as the Positive vs. Incongruent contrasts, and vice versa. This analysis revealed no significant regions that were more activated for the CA than control groups. However, the control group demonstrated significant and greater deactivation of a medial frontal region often associated with the default mode than the CA group during the non-emotional (Incongruent) block. See Table 2. See Supplemental Table 2 for group contrasts used in the conjunction.
3.4.2. Transient cognitive control (event-related analyses)
Analogous conjunction analyses were conducted to isolate group differences in regions activated transiently within block that were greater for blocks with emotional distractors regardless of valence as compared to non-emotional distractors (incongruent > positive, incongruent > threat). The CA group demonstrated greater activation of left IFG during the emotional than incongruent trials. In contrast, the control group demonstrated significant activation in left IFG for the incongruent trials, but less activation during the emotional trials. Furthermore, a region of the ACC, on the border of dACC and ventral ACC, was significantly deactivated during the incongruent trials (vs. neutral) by the control group but not the CA group. Of note, when viewed at a cluster level, this activation reflects a similar pattern of results, albeit left-sided rather than right-sided, observed during the blocked conjunction analyses, suggesting an overlapping pattern of group differences for sustained and transient cognitive control. Finally, the left middle temporal gyrus, a region that is involved in secondary language processing such as reading, was deactivated by the control group but not the CA group. See Table 2. See Supplemental Table 2 for group contrasts used in the conjunction.
3.5. Valence specific differences in cognitive control
3.5.1. Sustained cognitive control (blocked analyses)
Direct contrasts compared activity for the two emotional conditions (Threat > Positive, Positive > Threat). There were no regions of greater activity for the CA than control group. In contrast, the control group significantly activated right posterior cingulate cortex (PCC) during the threat block, whereas the CA group deactivated (non-significant) this region. See Table 3.
Table 3.
Group differences in activation between the emotional Stroop tasks. The z score presented represents peak activation in the cluster. Peak activation, presented as a z score, extracted for target conditions compared to fixation are included for pertinent trials. All coordinates presented in MNI space. R = right. L = left. BA = Brodmann's area. Inc. = incongruent. Post = positive.
| Region | BA | z | Voxels | x | y | z | Group |
Block/Trial |
|
|---|---|---|---|---|---|---|---|---|---|
| Post | Threat | ||||||||
| Blocked Activity | |||||||||
| Positive vs Threat | |||||||||
| Posterior Cingulate Cortex (R) | 31 | 3.15 | 10 | 18 | − 18 | 32 | CA | 0.91 | − 1.55 |
| Control | 1.23 | 3.36 | |||||||
| Event-Related Activity | |||||||||
| positive vs threat | |||||||||
| Caudate (L)/Nucleus Accumbens (R) | – | 4.43 | 903 | − 8 | 20 | 2 | CA | − 1.99 | 1.71 |
| Control | 2.57 | 0.21 | |||||||
| Middle Frontal Gyrus (R) | 10 | 4.50 | 464 | 44 | 54 | 6 | CA | 1.23 | 3.34 |
| Control | 1.91 | − 0.76 | |||||||
| Medial Frontal Gyrus (R) | 10 | 3.71 | 303 | 18 | 66 | − 10 | CA | − 0.34 | 2.14 |
| Control | 1.00 | 0.026 | |||||||
| Thalamus (L) | – | 3.62 | 125 | 0 | − 22 | 2 | CA | 0.012 | 2.94 |
| Control | 4.30 | 2.77 | |||||||
| Caudate (R) | – | 3.96 | 93 | 14 | 8 | 10 | CA | 0.26 | 2.51 |
| Control | 3.59 | 1.89 | |||||||
| Parahippocampal Gyrus (L) | 35 | 3.93 | 73 | − 18 | − 24 | − 12 | CA | 0.16 | 3.47 |
| Control | 3.68 | 2.31 | |||||||
| Superior Frontal Gyrus (R) | 9 | 3.82 | 56 | 22 | 54 | 30 | CA | − 0.25 | 4.32 |
| Control | 3.92 | 2.56 | |||||||
| Medial Frontal Gyrus (R) | 32 | 2.56 | 40 | 6 | 46 | − 10 | CA | − 5.41 | − 2.82 |
| Control | − 2.27 | − 3.70 | |||||||
| Cerebellum (R) | – | 3.32 | 40 | 16 | − 40 | − 16 | CA | − 2.89 | − 1.08 |
| Control | − 0.80 | − 3.67 | |||||||
| Superior Frontal Gyrus (L) | 8 | 3.59 | 29 | 0 | 40 | 48 | CA | − 1.34 | 2.19 |
| Control | 1.85 | 0.044 | |||||||
| Superior Frontal Gyrus (R) | 6 | 2.92 | 27 | 22 | 26 | 60 | CA | − 1.82 | − 0.023 |
| Control | 2.16 | 0.79 | |||||||
| Brain Stem | – | 2.50 | 24 | 8 | − 44 | − 38 | CA | − 0.07 | 1.30 |
| Control | 3.63 | 0.83 | |||||||
| Cerebellum (R) | – | 2.45 | 20 | 16 | − 36 | − 26 | CA | − 0.43 | − 0.50 |
| Control | 2.33 | − 2.49 | |||||||
| Thalamus (L) | – | 3.17 | 20 | − 16 | − 16 | 4 | CA | 3.37 | 6.49 |
| Control | 6.39 | 6.12 | |||||||
| Superior Frontal Gyrus (L) | 9 | 2.56 | 18 | − 18 | 60 | 26 | CA | − 0.11 | 2.60 |
| Control | 1.21 | − 0.27 | |||||||
| Superior Frontal Gyrus (R) | 8 | 3.02 | 17 | 16 | 54 | 38 | CA | − 0.79 | 4.02 |
| Control | 2.48 | 1.34 | |||||||
| Superior Frontal Gyrus (R) | 8 | 2.96 | 16 | 10 | 40 | 54 | CA | − 1.35 | 0.49 |
| Control | 1.82 | − 0.056 | |||||||
3.5.2. Transient cognitive control (event-related analyses)
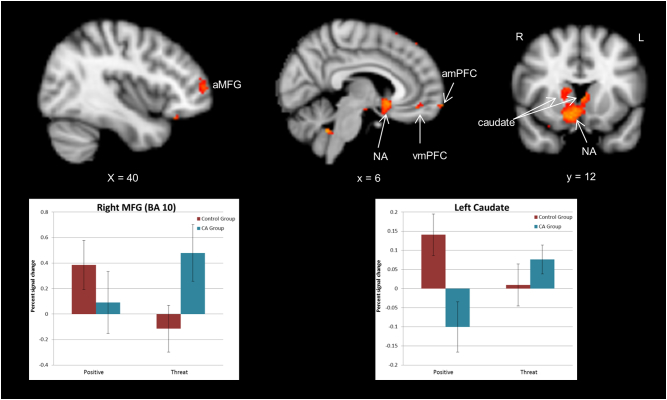
Analogous analyses for event-related contrasts indicated that the control and CA groups demonstrated differential patterns of activation for positive and threat trials (threat > positive, positive > threat) in multiple regions. The CA group uniquely activated a network of bilateral lateral and medial frontal regions, including right anterior MFG, left dmPFC and anterior mPFC, and left mid-DLPFC for the threat trials compared to positive trials, while no significant differences in threat vs. positive trials were observed for the control group. In contrast, the control group demonstrated greater activation during the positive trials than threat trials in some subcortical regions (left caudate, nucleus accumbens, and brain stem) and region of right cerebellum, which was not observed for the CA group.
In some regions, diametrically opposing patterns were observed between the groups for positive and threat trials. These included subcortical regions (left thalamus and right caudate), right-sided frontal regions (mid-DLPFC and dmPFC), and parahippocampal gyrus/hippocampus. Specifically, the CA group exhibited unique or more activation in these regions during threat compared to positive trials while the control group demonstrated unique or more activation during the positive compared to threat trials. Furthermore, contrasting patterns of significant deactivation were also seen between the groups. The CA group showed greater deactivation of right vmPFC and a region of the cerebellum during the positive than threat trials, while the control group showed greater deactivation of right vmPFC and a region of the cerebellum during the threat than positive trials. These results suggest differential neural engagement for cognitive control over threat and positive words across the groups. See Table 3 and Fig. 3.
Fig. 3.
Group differences in event-related activity between positive and threat trials. Shown in red, unique activation of right anterior MFG and anterior mPFC by the CA group compared to control group for threat trials. Also shown is unique activation of left caudate and right nucleus accumbens in the control group compared to CA group for positive trials. Lastly, greater suppression of vmPFC by the control group for threat than positive trials in contrast to greater suppression for positive than threat trials in the CA group is shown. Graph of percent signal change by group for a 5 mm sphere around the peak of two representative clusters, anterior MFG (44, 54, 6) and left caudate/right NA (− 8, 20, 2), are presented. MFG = anterior middle frontal gyrus. amPFC = anterior medial prefrontal cortex. NA = nucleus accumbens. vmPFC = ventral medial prefrontal cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Association of posttraumatic stress symptoms with cognitive control in the CA group
3.6.1. Sustained cognitive control (blocked analyses)
Avoidance symptom severity and re-experiencing/hyperarousal symptom severity were each regressed individually on blocked contrasts for each of the three target trials (Incongruent, Threat, and Positive) compared to fixation, respectively. Several regions demonstrated an association with avoidance symptoms and activation during the Incongruent block. Specifically, more severe avoidance symptoms were associated with less activation of bilateral regions in lateral temporal cortex, many of which are associated with later-stage visual processing and others of which are involved in language processing. Additionally, more severe avoidance symptoms were associated with less activation of left subcortical and medial limbic regions involved in emotion detection and regulation, including the amygdala, hippocampus, and vmPFC, as well as left-sided cognitive control regions, MFG and IFG. The only association between activation during an emotional block and avoidance symptoms was found for right precuneus activation during the Positive block, with greater activation associated with more severe avoidance symptoms. See Table 4.
A different pattern of results was observed for re-experiencing/hyperarousal symptoms. The most pronounced associations were with activation during the Positive block, as opposed to pronounced findings for avoidance symptoms during the Incongruent block. More severe re-experiencing/hyperarousal symptoms were associated with greater activation of right-sided posterior visual processing regions and bilateral lateral temporal brain regions involved in language processing. Additionally, more severe re-experiencing/hyperarousal symptoms were associated with greater activation of right parahippocampus and left cerebellum. For the Threat block, more severe re-experiencing/hyperarousal symptoms were associated with greater activation of right fusiform gyrus, a visual processing region. In contrast, more severe re-experiencing/hyperarousal symptoms were associated with greater activation of superior parietal regions during the Incongruent block. See Table 4. See Supplemental Table 3 for blocked activation during each of the Stroop conditions for the CA group.
3.6.2. Transient cognitive control (event-related analyses)
Avoidance and re-experiencing/hyperarousal symptom severity were also regressed on whole-brain event-related activation during the target trials (compared to within block neutral trials) within each of the three target blocks individually. While almost all of the associations between sustained cognitive control and avoidance symptoms were observed when emotionally neutral information needed to be ignored (Incongruent block), associations with transient cognitive control were only observed when emotional information needed to be ignored. Greater avoidance symptom severity was associated with less recruitment of left-sided frontal-parietal cognitive control regions during positive trials. Furthermore, greater avoidance symptom severity was associated with less recruitment of right parahippocampal gyrus. See Table 4.
Similarly, associations between re-experiencing/hyperarousal symptoms and brain activation during transient cognitive control demonstrated the opposite pattern of what was observed for associations with sustained cognitive control. Specifically, associations were only observed for incongruent trials (compared to within block neutral trials) and none for the emotional word conditions. Greater severity of re-experiencing/hyperarousal symptoms was associated with greater activation of left posterior DLFPC and MFG and right parahippocampal gyrus. See Table 4. There were no significant differences between target trials and within block neutral trials across conditions at the group level for the CA group; therefore, these results are not presented.
4. Discussion
Our results suggest that exposure to CA is associated with difficulties maintaining sustained cognitive control, regardless of the valence of information to be ignored and the degree of direct conflict. In contrast, alterations to transient cognitive control were primarily observed when salient emotional information had to be ignored, as compared to irrelevant and directly conflicting neutral information. Opposite patterns of activation were observed for positive and threat information in women with a history of CA compared to controls. Specifically, women with a history of CA had more difficulty transiently suppressing task-irrelevant responding to threat information than controls, but interestingly, exhibited better ability to suppress transient responding to task-irrelevant positive information. In addition, distinct neural systems were associated with the severity of avoidance and re-experiencing/hyperarousal symptoms during sustained and transient cognitive control.
4.1. Alterations to frontal-parietal cognitive control network across non-emotional and emotional contexts
Regardless of whether distracting information was emotionally neutral or emotional, women with a history of CA, as compared to controls, had reduced activation of a left-sided frontal-parietal network associated with cognitive control (Banich, 2009, Herd et al., 2006). This is consistent with previous findings of functional and structural alterations in the DLPFC and precuneus in adults with a history of childhood maltreatment (Bremner et al., 2004, De Bellis and Zisk, 2014, Teicher and Samson, 2016). Additionally, women with a history of CA show enhanced recruitment of rIFG, a region traditionally associated with inhibition (Aron et al., 2003, Aron et al., 2004, Aron et al., 2014, Depue et al., 2016, Swann et al., 2009), compared to controls. Although both children and adults with a history of childhood maltreatment demonstrate inhibitory deficits on behavioral measures (Navalta et al., 2006, Hart and Rubia, 2012, Beers and De Bellis, 2002, De Bellis, 2005, DePrince et al., 2009), results from neuroimaging studies of inhibition have been inconsistent in both children and adults (Elton et al., 2014, Lim et al., 2015, Mueller et al., 2010, Bremner et al., 2004, Carrion et al., 2008). It has been proposed that rIFG may not be specifically related to inhibition per se, but rather may support monitoring the environment in order to establish or maintain goals that are consistent with current external conditions (Banich and Depue, 2015, Chatham et al., 2012, Depue et al., 2016, Munakata et al., 2011, Swick and Chatham, 2014). As such, the rIFG activation observed during sustained cognitive control in women with a history of CA may reflect heightened reliance on external cues to facilitate maintenance of task-relevant goals, which may work in parallel with the hypervigilance often observed in individuals with a history of CA.
4.2. Alterations to sustained cognitive control networks specific to non-emotional and emotional contexts
Many classic posttraumatic stress symptoms are by definition related to emotional responding and regulation, and therefore may provide unique challenges to implementing cognitive control in emotionally arousing situations. Our contrast of emotional Stroop trials to incongruent Stroop trials provided some insight into this issue. Control participants showed greater activation of left-sided and anterior regions of DLPFC for incongruent than emotional words, a difference that was not observed for CA individuals. More specifically, while both groups showed approximately equivalent activation of these regions for the emotional Stroop trials, the control individuals had increased activation compared to the CA individuals for incongruent trials (see Table 2). Of note, the region showing this effect is a bit anterior to that which revealed group differences across all trial types, which is a region that is often associated with biasing towards task-relevant representations (Banich, 2009, Herd et al., 2006). This pattern suggests an increased area of activation for controls when incongruent stimuli are encountered as compared to emotionally distracting words. As noted in the introduction, while both incongruent and emotional Stroop trials require the prioritization of ink color identification over word reading, only incongruent trials involve conflict processing. These results could suggest women with a history of CA demonstrate alterations in the activation and suppression of brain networks critical for the resolution of conflicting environmental cues, and further supports our proposal that women with a history of CA may be overly reliant on external cues to implement cognitive control. In the context of abuse, heightened attunement to the environment and the ability to monitor the environment for cues that signal what type of goals should guide behavior in a dynamically shifting environment is likely to be highly adaptive. However, this strategy may result in an over-reliance on neural systems that monitor the environment and process environmental cues, resulting in difficulties with internally maintaining task-relevant goals and behaviors.
4.3. Alterations in transient cognitive control specific to emotional context
In contrast to alterations in sustained cognitive control discussed above, differences in activation between distracting stimuli of different emotional valences were most pronounced for transient cognitive control. Differences in activation between controls and women with a history of CA on emotional Stroop trials as a function of valence (threat, positive) were found both in regions typically activated when cognitive control is required, as well as regions typically deactivated during cognitive control, such as regions associated with the default mode network. Interestingly, women with a history of CA and controls showed opposite patterns of activation when transient control was necessary in the face of threatening as compared to positive task-irrelevant information. Specifically, when faced with distracting information that was threatening, women with a history of CA, as compared to controls, did not deactivate vmPFC and anterior mPFC, regions of the default mode implicated in self-referential thought (Andrews-Hanna et al., 2014, Buckner and Vincent, 2007, Raichle and Snyder, 2007, Raine et al., 2001) and evaluating potentially survival-salient information (Buckner and Vincent, 2007, Sheline et al., 2009), as much as when faced with distracting positive information. In contrast, controls deactivated vmPFC more when the distracting information was threatening as opposed to positive in valence. Furthermore, robust activation of subcortical regions involved in sensory gating and motivation that feed up to the cortex was observed in conjunction with less suppression of default mode regions for distracting threat information in women with a history of CA in contrast to robust activation for distracting positive information in controls. We speculate that conditions associated with less suppression of vmPFC likely reflect heightened processing of the task-irrelevant information. If so, then this pattern of results suggests that while threat words are more salient for women with a history of CA, positive words are more salient for controls. Consistent with this speculation, heightened activation of posterior medial frontal cortex, a brain area implicated in response selection (Banich, 2009), as well as that of the anterior DLPFC, implicated in inhibitory control (Banich and Depue, 2015), were observed only for threat information in women with a history of CA. Engagement of these regions suggests an increased need for cognitive control specifically on trials in which the distracting information is threatening in nature.
In sum, these results suggest that patterns of transient brain activation reflect the degree to which distracting information, whose nature cannot be predicted beforehand, interferes with cognitive control in an individual. As such, engagement of these systems varied by group (controls, women with a history of CA), depending on the valence (threat, positive) of the information. These findings are consistent with previous reports of heightened dorsal medial frontal activation (Dannlowski et al., 2012, Hart and Rubia, 2012, Pechtel and Pizzagalli, 2011) and decreased basal ganglia activity in response to reward in adults with a history of childhood maltreatment (Dillon et al., 2009). However, it is inconsistent with findings of decreased vmPFC activity specifically for threatening information in adults with a history of childhood maltreatment (Dannlowski et al., 2012, Hart and Rubia, 2012, Pechtel and Pizzagalli, 2011). This difference could be due to the fact that most prior studies compare activation to neutral as opposed to positive stimuli. Alternatively, it may reflect the mild nature of threat information in this task compared to other studies using stronger threat stimuli (e.g., pictures) and/or the fact that this sample of women with a history of CA is relatively high functioning and therefore may exhibit better executive functioning than individuals with lower functioning. In the context of abuse, moment-to-moment prioritization of threat information is likely adaptive because it has survival value. Positive information may be less salient because it is not important for survival and/or positive information may be less present in abusive contexts.
4.4. Associations between cognitive control and posttraumatic stress symptoms
Individual differences in avoidance symptoms and re-experiencing/hyperarousal symptoms among the women with CA were associated with distinct patterns of neural activation. Prior research has found that individuals often experience predominantly either avoidance or re-experiencing/hyperarousal symptoms, which have been associated with differential patterns of brain activation in trauma-exposed adults (Lanius et al., 2002, Lanius et al., 2006). We observed avoidance symptoms were primarily associated with sustained cognitive control in the face of directly conflicting emotionally neutral information. Specifically, individuals with greater avoidance symptoms exhibited less activation of semantic/language processing and late stream visual processing temporal regions when top-down control needed to be implemented in the face of external representations directly competing for processing. Concomitantly, these individuals also exhibited reduced activation of limbic regions involved in bottom-up emotional responding as well as a core default mode region, the vmPFC, when there was no emotional information present.
In contrast, severity of re-experiencing/hyperarousal symptoms was primarily associated with sustained cognitive control in the face of distracting emotional information and transient cognitive control in the face of distracting, directly conflicting emotionally neutral information. Individuals with greater re-experiencing/hyperarousal symptoms exhibited robust heightened activation across multiple early-stage, bottom-up visual processing regions when sustained cognitive control was required to ignore distracting positive information. A similar association was observed for a late-stage visual processing region when distracting threat information had to be ignored. Re-experiencing/hyperarousal symptoms were only associated with transient cognitive control in the face of directly competing information in the environment, with greater symptoms associated with more recruitment of early-stage visual processing and frontal cognitive control regions.
This pattern of results suggests that individuals with pre-dominantly avoidance symptoms may have developed strategies for pro-actively dampening task-irrelevant processing, including emotional responding, when there is direct conflict for processing representations in the environment. A prior study suggests greater recruitment of brain regions necessary for biasing towards task-relevant goals may help to buffer re-experiencing symptoms (Fonzo et al., 2016). In contrast, individuals with predominantly re-experiencing/hyperarousal symptoms rely on more transient and dynamic cognitive control in the face of environmental representations simultaneously vying for attention.
4.5. Limitations
There are some limitations to this study, the main one being our restricted sample size. Our restricted sample size informed our correction methods, both for direct group contrasts as well as conjunction analyses. As such, the results obtained should be viewed as somewhat preliminary and will require replication. Nonetheless, the study demonstrates the utility of disentangling, via a direct comparison, potential alterations of neural systems that are required to exert cognitive control in the face of distracting neutral versus emotional information, and how such systems may also vary by emotional valence. Additionally, we were not able to make direct comparisons between women with a history of CA with and without PTSD and direct comparisons in activation between women with specific symptom profiles. Future studies with a larger data set will be necessary to fully disentangle developmental from disorder-specific effects. Given the simultaneous modeling of blocked and event-related trials, it is possible that some activation from the event-related trials contributed to blocked results. However, we were able to demonstrate different patterns of alterations for blocked then event-related data, consistent with previous studies (Banich et al., 2009, Andrews-Hanna et al., 2011). By including only women with a history of CA, we were able to control for potential sex differences. On the other hand, the results may not be generalizable to male samples and future studies will be needed to further examine overlap and differences in brain regions recruited during cognitive control for males and females with a history of CA.
5. Conclusion
In summary, this study demonstrated altered activation of brain regions in women with a history of CA when cognitive control is required to ignore salient, distracting task-irrelevant information. Women with a history of CA showed blunted activation in regions of a frontal-parietal network when processing needed to be biased in a sustained manner, regardless of the emotional valence of that information. However, when more transient demands for cognitive control occurred, women with a history of CA showed increased activation in brain regions involved in control - but only when the task-irrelevant information was threat-related (as compared to positively-valenced). Furthermore, the nature of posttraumatic stress symptomatology appeared to shape neural responses to cognitive control in the face of directly competing environmental representations: individuals with elevated avoidance symptoms exhibited greater suppression of irrelevant bottom-up processing mechanisms, whereas individuals with elevated re-experiencing/hyperarousal biased processing towards task-relevant representations more reactively and transiently.
Financial disclosures
None of the authors have any financial disclosures.
Acknowledgements
We would like to thank Kathy Pearson and Kai Wang for help with data processing.
Funding: This work was supported by the National Institutes of Health [R03HD62600 (MTB) and K23MH105678 (KMS)]. The funding sources had no involvement in the study design, data collection, interpretation, or publication of this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.06.030.
Contributor Information
Kristen L. Mackiewicz Seghete, Email: mackiewi@ohsu.edu.
Roselinde H. Kaiser, Email: rhkaiser@psych.ucla.edu.
Anne P. DePrince, Email: adeprince@du.edu.
Marie T. Banich, Email: marie.banich@colorado.edu.
Appendix A. Supplementary data
Supplementary tables
References
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Fourth Edition. American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual. [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual, Fourth Edition Text Revision. [Google Scholar]
- Andrews-Hanna J.R., Mackiewicz Seghete K.L., Claus E.D., Burgess G.C., Ruzic L., Banich M.T. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Banich M.T. Executive function: the search for an integrated account. Curr. Dir. Psychol. Sci. 2009;18:89–94. [Google Scholar]
- Banich M.T., Depue B.E. Recent advances in understanding neural systems that support inhibitory control. Curr. Opin. Behav. Sci. 2015;1:17–22. [Google Scholar]
- Banich M.T., Milham M.P., Atchley R., Cohen N.J., Webb A., Wszalek T. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J. Cogn. Neurosci. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich M.T., Milham M.P., Atchley R.A., Cohen N.J., Webb A., Wszalek T. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Brain Res. Cogn. Brain Res. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Banich M.T., Burgess G.C., Depue B.E., Ruzic L., Bidwell L.C., Hitt-Laustsen S. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia. 2009;47:3095–3104. doi: 10.1016/j.neuropsychologia.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory - II. [Google Scholar]
- Beers S.R., De Bellis M.D. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. Am. J. Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. Technical Report C-1, the Center for Research in Psychophysiology. University of Florida; 1999. Affective norms for English Words (ANEW): instruction manual and affective ratings. [Google Scholar]
- Bremner J.D., Soufer R., McCarthy G., Delaney R., Staib L.H., Duncan J.S. Gender differences in cognitive and neural correlates of remembrance of emotional words. Psychopharmacol. Bull. 2001;35:55–78. [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E., Vythilingam M., Afzal N., Schmahl C., Elzinga B. Neural correlates of the classic color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Brown G.G., Kindermann S.S., Siegle G.J., Granholm E., Wong E.C., Buxton R.B. Brain activation and pupil response during covert performance of the Stroop Color Word task. J. Int. Neuropsychol. Soc. 1999;5:308–319. doi: 10.1017/s1355617799544020. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Vincent J.L. Unrest at rest: default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. (discussion 1097-9) [DOI] [PubMed] [Google Scholar]
- Bush G., Whalen P.J., Rosen B.R., Jenike M.A., McInerney S.C., Rauch S.L. The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum. Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion V.G., Garrett A., Menon V., Weems C.F., Reiss A.L. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress. Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Mintun M., Cohen J.D. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. NeuroImage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Claus E.D., Kim A., Curran T., Banich M.T., Munakata Y. Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Moradi A.R., Taghavi M.R., Neshat-Doost H.T., Yule W. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol. Med. 2001;31:541–547. doi: 10.1017/s0033291701003567. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D. The psychobiology of neglect. Child Maltreat. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Zisk A. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am. 2014;23(185–222):vii. doi: 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePrince A.P., Weinzierl K.M., Combs M.D. Executive function performance and trauma exposure in a community sample of children. Child Abuse Negl. 2009;33:353–361. doi: 10.1016/j.chiabu.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Depue B.E., Orr J.M., Smolker H.R., Naaz F., Banich M.T. The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cereb. Cortex. 2016;26:1634–1646. doi: 10.1093/cercor/bhu324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal G.K., Gauzas L., Antonowicz D.H., Ross R.R. Adult male survivors of childhood sexual abuse: prevalence, sexual abuse characteristics, and long-term effects. Clin. Psychol. Rev. 1996;16:619–639. [Google Scholar]
- Dillon D.G., Holmes A.J., Birk J.L., Brooks N., Lyons-Ruth K., Pizzagalli D.A. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards V.J., Holden G.W., Felitti V.J., Anda R.F. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am. J. Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.M., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. PNAS. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A., Tripathi S.P., Mletzko T., Young J., Cisler J.M., James G.A. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Hum. Brain Mapp. 2014;35:1654–1667. doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D., Hotaling G., Lewis I.A., Smith C. Sexual abuse in a national survey of adult men and women: prevalence, characteristics, and risk factors. Child Abuse Negl. 1990;14:19–28. doi: 10.1016/0145-2134(90)90077-7. [DOI] [PubMed] [Google Scholar]
- Fisher B.S., Cullen F.T. Measurement and Analysis of Crime and Justice. United States Department of Justice, Office of Justice Programs; Washington, DC: 2000. Measuring the sexual victimization of women: evolution, current controversies, and future research; pp. 317–390. [Google Scholar]
- Foa E.B., Cashman L., Jaycox L., Perry K. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol. Assess. 1997;9:445–451. [Google Scholar]
- Fonzo G.A., Huemer J., Etkin A. History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. J Psychiatr Res. 2016;74:45–54. doi: 10.1016/j.jpsychires.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Penny W.D., Glaser D.E. Conjunction revisited. NeuroImage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L.R., Freyd J.J. Self-reports of potentially traumatic experiences in an adult community sample: gender differences and test-retest stabilities of the items in a brief betrayal-trauma survey. J. Trauma Dissociation. 2006;7:39–63. doi: 10.1300/J229v07n03_04. [DOI] [PubMed] [Google Scholar]
- Green B. Sidran Press; Lutherville, MD: 1996. Trauma History Questionnaire. [Google Scholar]
- Guyer A.E., Kaufman J., Hodgdon H.B., Masten C.L., Jazbec S., Pine D.S. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd S.A., Banich M.T., O'Reilly R.C. Neural mechanisms of cognitive control: an integrative model of Stroop task performance and FMRI data. J. Cogn. Neurosci. 2006;18:22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Phillips M.L., Fournier J.C., Kronhaus D.M., Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol. Med. 2013;43:1533–1542. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Yale University; New Haven, CT: 1975. Four-factor Index of Social Status. [Google Scholar]
- Hooper L.M., Stockton P., Krupnick J.L., Green B.L. Development, use, and psychometric properties of the Trauma History Questionnaire. J. Loss Trauma. 2011;16:258–283. [Google Scholar]
- Lanius R.A., Williamson P.C., Boksman K., Densmore M., Gupta M., Neufeld R.W. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol. Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R., Lanius U., Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J. Psychiatr. Res. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lim L., Hart H., Mehta M.A., Simmons A., Mirza K., Rubia K. Neural correlates of error processing in young people with a history of severe childhood abuse: an fMRI study. Am. J. Psychiatry. 2015;172:892–900. doi: 10.1176/appi.ajp.2015.14081042. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally R.J., Kaspi S.P., Riemann B.C., Zeitlin S.B. Selective processing of threat cues in posttraumatic stress disorder. J. Abnorm. Psychol. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Mehta M.A., Gore-Langton E., Golembo N., Colvert E., Williams S.C., Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cogn. Neurosci. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Milham M.P., Banich M.T., Webb A., Barad V., Cohen N.J., Wszalek T. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res. Cogn. Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mueller S.C., Maheu F.S., Dozier M., Peloso E., Mandell D., Leibenluft E. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y., Herd S.A., Chatham C.H., Depue B.E., Banich M.T., O'Reilly R.C. A unified framework for inhibitory control. Trends Cogn. Sci. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalta C.P., Polcari A., Webster D.M., Boghossian A., Teicher M.H. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J. Neuropsychiatr. Clin. Neurosci. 2006;18:45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- Nichols T. Easythresh_conj - quick method of getting conjunction stats outside of Feat. 2007. https:/www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/fsl/easythresh_conj
- Nichols T., Brett M., Andersson J., Wager T., Poline J.B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp. Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. B. 2005:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Mogg K., Bradley B.P., Montgomery L., Monk C.S., McClure E. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am. J. Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Pollak S.D., Cicchetti D., Hornung K., Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol. 2000;36:679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pollak S.D., Vardi S., Putzer Bechner A.M., Curtin J.J. Physically abused children's regulation of attention in response to hostility. Child Dev. 2005;76:968–977. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation . Psychological Corporation; San Antonio, TX: 1999. WASI: Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Raichle M.E., Snyder A.Z. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. (discussion 1097-9) [DOI] [PubMed] [Google Scholar]
- Raine A., Park S., Lencz T., Bihrle S., LaCasse L., Widom C.S. Reduced right hemisphere activation in severely abused violent offenders during a working memory task: an fMRI study. Aggress. Behav. 2001;27:111–129. [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonk S.M., Cicchetti D. Maltreatment, competency deficits, and risk for academic and behavioral maladjustment. Dev. Psychol. 2001;37:3–17. [PubMed] [Google Scholar]
- Silton R.L., Heller W., Towers D.N., Engels A.S., Spielberg J.M., Edgar J.C. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. NeuroImage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Swann N., Tandon N., Canolty R., Ellmore T.M., McEvoy L.K., Dreyer S. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Chatham C.H. Ten years of inhibition revisited. Front. Hum. Neurosci. 2014;8:329. doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Polcari A., Anderson C.M., Navalta C.P., Kim D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin D.F., Foa E.B. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A.L., van Tol M.J., Demenescu L.R., van der Wee N.J., Veltman D.J., Aleman A. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 2013;8:362–369. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A.L., van Tol M.J., Dalgleish T., van der Wee N.J., Veltman D.J., Aleman A. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 2014;9:2026–2033. doi: 10.1093/scan/nsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher K.M., Miezin F.M., Kelly J.E., Buckner R.L., Donaldson D.I., McAvoy M.P. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables