Abstract
Sarcoid like reaction is a well-known entity that occurs as a consequence to several malignancies or their therapies. Immunotherapy has gained a lot of interest in the past few years and has recently gained approval as first line therapy in multiple advanced stage malignancies. Pneumonitis has been described as complication of such therapy. Granulomatous inflammation has been only rarely reported subsequent to immunotherapy. We describe a case of granulomatous inflammation reactivation affecting the lungs in a patient previously exposed to Pembrolizumab and have evidence of a distant granulomatous infection. We discuss potential mechanisms of the inflammation and assert the importance of immunosuppression in controlling the dis-inhibited immune system.
Keywords: Drug induced sarcoid like reaction, Granulomatosis reactivation, PD-1 inhibitors side effect
1. Introduction
Two drug antibodies targeting the programmed cell death 1 (PD-1) pathway are currently approved by the US food and drug administration for treatment of patients with advanced malignancies [1]. Systemic toxicities associated with their use such as pyrexia, chills, generalized fatigue, insulin depended diabetes, dermatitis, alveolitis and infusion reaction have been commonly reported [2]. Most of these complications are thought related to autoimmune phenomena. Only few cases have been reported a link of pulmonary sarcoidosis with this class of medications.
2. Case report
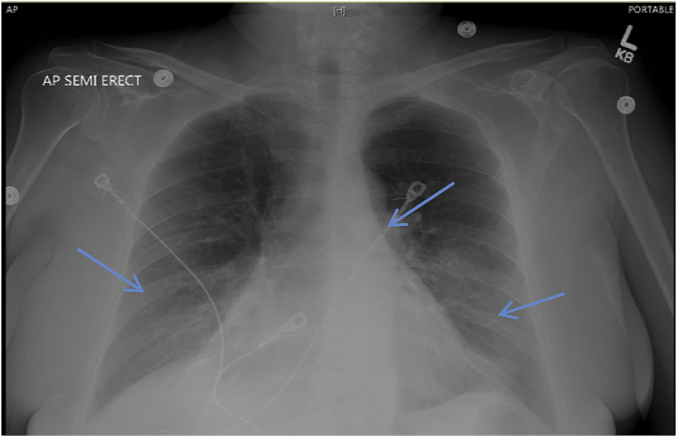
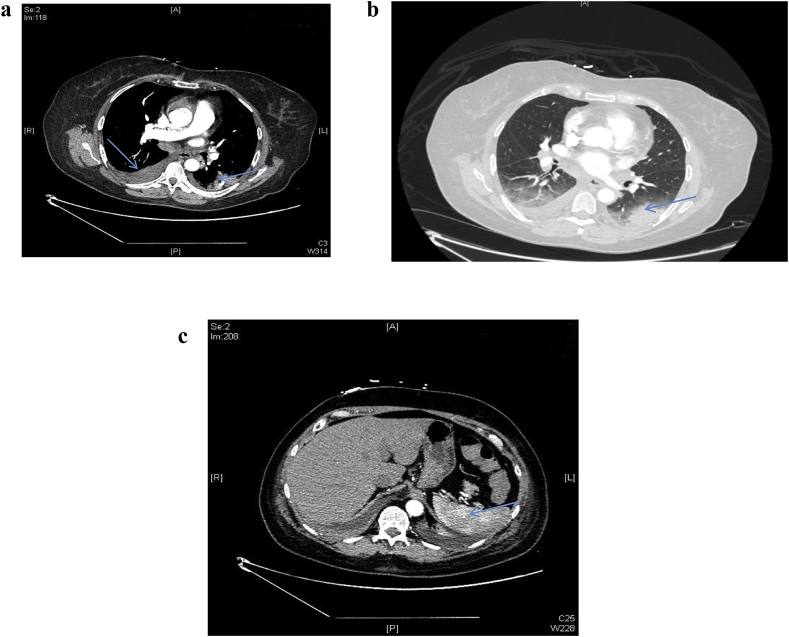
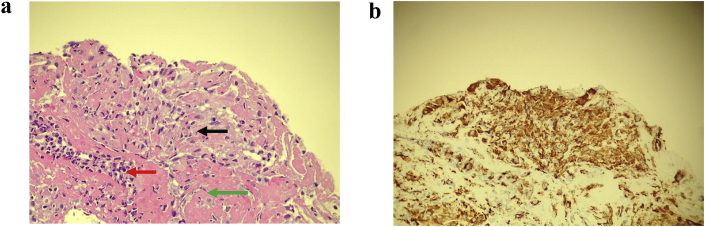
Our patient is a 65-year-old white female. Her past medical history is significant for stage III, T3B, N2A superficial Melanoma on the left buttock. This was treated with surgical removal of the tumor with the ipsilateral inguinal lymph node (LN). She has evidence of old granulomatous disease in the form of bilateral granulomas some of which where calcified along with hilar lymph node calcifications and splenic calcifications. Pembrolizumab was used as part of a trial for one year. She presented to the walk in clinic with worsening productive cough of white sputum with neither fever nor hemoptysis. Chest x ray showed possible pulmonary metastasis versus pneumonia (Fig. 1). She was admitted for investigations of the abnormalities. Vital signs and physical exam were normal except for decreased air entry on the right lower zone. Complete blood count showed chronic leukocytosis and mild anemia. Chemistry panel was significant for hypomagnesaemia, hypokalemia, and hypoalbuminemia. Patient was started on antibiotics for possible pneumonia. Blood culture, mycoplasma DNA, legionella urine antigen, acid-fast bacilli (AFB) and sputum culture came back negative. Chest computed tomography (CT) scan obtained due to worsening hypoxemia needing 5 L of oxygen flow per minute showed lower lobes consolidation, and bilateral hilar and mediastinal lymphadenopathy. Multiple lucencies surrounding the splenic calcifications were noted (Fig. 2a, b, c). An immune reaction to chemotherapy was considered and patient consented to bronchoscopy with endobronchial ultrasound (EBUS) guided biopsy of the lymph nodes for suspicion of sarcoidal reaction. White light bronchoscopy did not show any endobronchial lesions or anomalies. Bronchoalveolar lavage (BAL) and transbronchial parenchymal biopsies were obtained from the right lower lobe. The patient developed severe bronchospasm that precluded sampling the lymph nodes. The patient was started on high dose intravenous steroids and showed dramatic clinical improvement. Biopsy result showed chronic granulomatous inflammation with histocytes with no malignancy identified (Fig. 3a, b).
Fig. 1.
Chest x ray showing bilateral infiltrate and hilar adenopathy.
Fig. 2.
a: CT chest shows left lower lobe infiltrate (arrow) with right pleural effusion. b; CT chest showing the left lower lobe infiltrate. c: CT abdomen shows heterogeneity of the spleen due to granulomatous disease (arrow).
Fig. 3.
A. Hematoxylin & eosin stain (400×) Histiocytes (black arrow), neutrophils, lymphocytes, histiocytes (red arrow), and fibrin (green arrow) Figure 3b CD68 immunohistochemical antibody, DAB chromogen (400×) Stain (brown color) highlights histiocytes.
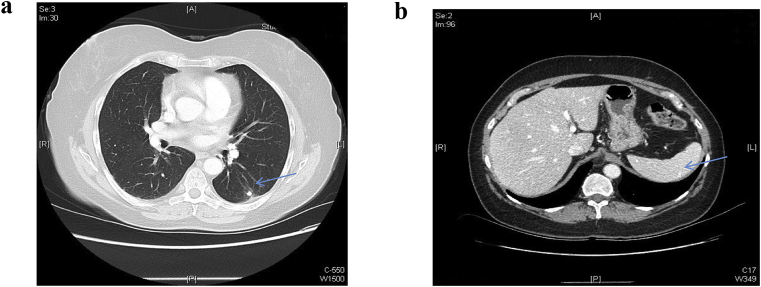
Given the lack of evidence for infection, and the bilateral hilar and mediastinal adenoapthy along with the granulomatous inflammation obtained from the biopsy, the patient was diagnosed with pulmonary sarcoidal reaction secondary to pembrolizumab. The patient was discharged on 40 mg daily oral steroids. One month follow up CT scan chest was done showed resolution of the pleural effusion and near complete resolution of the lower zone air space disease (Fig. 4 A, B). Due to the long lasting effect of PD-1 pathway inhibition the patient is being evaluated for a prolonged and slow taper of oral steroids to prevent recurrence.
Fig. 4.
a: follow up chest CT shows resolution of the left lower lobe infiltrate, resolution of the left pleural effusion, notice the calcification of the left lower lobe (arrow). Figure 4b: follow up CT abdomen after Pembrolizumab has been stopped and patient has been shows remnant splenic calcification (arrow).
3. Discussion
Immunotherapy for cancer treatment has been well established through multiple clinical trials for various malignancies [1], [2]. Given their relatively young age in clinical practice, experience regarding the complications and side effects of this relatively new therapy in everyday practice is not well known. Multiple diseases that are considered related to heightened autoimmunity have been reported after their use [3]. For instance, Type I Diabetes Mellitus, exacerbation of psoriasis, vitiligo, and interstitial nephritis to name a few. Sarcoidosis, whether de novo or as reactivation of an existing disease, has only been reported twice after the use of PD-1 inhibitors pathway [4], [5].
Non-caseating Granulomatous inflammation is considered a type IV immune reaction [6]. It forms as the activated T lymphocytes, induced by a foreign antigen, secrete a specific set of interleukins and chemo attractants [6]. Sarcoid like reaction is a term used to describe granulomatous inflammation found in the setting of active cancer or after the delivery of effective chemotherapy [7]. Sarcoid like reactions has been confused with recurrent metastatic disease due to high PET avidity of the lymph nodes involved. Of Interest is that sarcoid like reactions are asymptomatic and are only diagnosed incidentally during follow-ups for cancer therapy [7]. The exact mechanism of sarcoidal reaction is not really known. It is likely different depending on the type of malignancy treated and the type of cancer treatment being implemented. For instance, granulomatous inflammation associated with active cancer could be an attempt of the body to control the malignancy and “wall it in”. The same inflammation after treatment of lymphoma might be seen as a reconstitution of normal immunity [8]. Following cancer therapy, the sarcoidal reaction could be a response of the body to the cancer antigens that were secreted in the blood or as a side effect of the chemotherapy causing damage to certain tissue inducing the granulomatous inflammation. Other therapies like interferon and IL-2 can induce such a sarcoidal reaction due to their direct effect on activating lymphocytes and macrophages [9].
The case of PD-1 pathway inhibition is somewhat different. Preclinical studies have shown that PD-1 blockade reinvigorated exhausted T-cell that has been suppressed by repeated exposure to the PD-1 ligand. The lifting of inhibition induced by these biologics restores T-cell cytotoxic and other immune functions [10]. Blockade of the PD1 pathway by Pembrolizumab resulted in a reproducible enhancement of both proliferation and IFN-γ release in the mixed lymphocyte reaction. It is well known that the secretion of interferon gamma is essential for the formation of granulomatous disease even in the absence of intact T cells [9]. This data indicates that a blockade of PD1, increases IFN-γ secretion from memory T cells, and could explain a sarcoid-like reaction.
In our case, the granulomatous inflammation was only noted in the pneumonia-like infiltrate in the right lower lobe. It is unlikely for Sarcoidosis to result in such a pneumonic infiltrate. The presence of mediastinal and hilar lymphadenopathy is classic for sarcoidosis [11]. The lymph nodes could not biopsied, however, due to the complication during bronchoscopy. It is possible that the lymphadenopathy could have been reactive to the parenchymal inflammation rather than true involvement with the disease process <. Infectious etiologies are unlikely given the negative cultures for fungal or bacterial or mycobacterial elements. One more note to make is that our patient had evidence of granulomatous inflammation in the form of a calcified pulmonary granuloma. This is a very common finding in regions of Ohio and Mississippi river valleys [12], [13]. The patient responded immediately after the first dose of steroids and her hypoxia reversed without oxygen on the 4th day after steroid initiation. The important difference in our case from the known sarcoidal reaction, is the fact that this form of inflammation was very symptomatic and life threatening inducing hypoxic respiratory failure. The presentation with a pneumonic type of inflammation and pleural effusion was highly atypical for sarcoidosis. Based on these factors, we speculate that our patient suffered from a granulomatous reaction. As noted previously, this patient likely had previously suppressed T lymphocytes based on prior exposure to a foreign antigen, resulting in the calcified granulomas. We suggest that exposure to Pembrolizumab, lifted the inhibition of these suppressed T lymphocytes resulting in reactivation of the underlying disease process. Suppressing these lymphocytes again by the use of steroids has restored part of their inhibition/tolerance. This remains a speculation but the fact that the inflammation was centered on the calcifications (healed granuloma) in both lungs and spleen makes that speculation a very valid one.
4. Conclusion
In line with the increased use of anti-PD1 agents, it appears to be important for clinicians to be aware of this new potential immune-mediated toxic effect. Therapy with PD-1 inhibitors can awaken the memory T-cells against previous targets for which tolerance has developed including prior granulomatous disease.
References
- 1.Whiteside T.L., Demaria S., Rodriguez-Ruiz M.E., Zarour H.M., Melero I. Emerging opportunities and challenges in cancer immunotherapy. Clin. Cancer Res. 2016;22(8):1845–1855. doi: 10.1158/1078-0432.CCR-16-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber J.S., Yang J.C., Atkins M.B., Disis M.L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidoo J., Page D.B., Li B.T., Connell L.C., Schindler K., Lacouture M.E., …, Wolchok J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. Okamoto, Masahide et al. “Fulminant Type 1 Diabetes Mellitus with Anti-programmed Cell death-1 Therapy.” Journal of Diabetes Investigation 7.6(2016): 915–918. PMC. Web. 30 Jan. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuss J.E., Kunk P.R., Stowman A.M., Gru A.A., Slingluff C.L., Jr., Gaughan E.M. Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: a case report & review of the literature. J. Immunother. Cancer. 2016;4:94. doi: 10.1186/s40425-016-0199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmermans W.M., van Laar J.A., van Hagen P.M., van Zelm M.C. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin. Transl. Immunol. 2016;5(12):e118. doi: 10.1038/cti.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt B.M., Vallieres E., Buduhan G. Sarcoidosis as a benign cause of lymphadenopathy in cancer patients. Am. J. Surg. 2009;197:629–632. doi: 10.1016/j.amjsurg.2009.01.004. discussion 632. [DOI] [PubMed] [Google Scholar]
- 8.Ishida M., Hodohara K., Furuya A., Okuno H., Yoshii M., Horinouchi A., …, Yoshida T. Sarcoidal granulomas in the mediastinal lymph nodes after treatment for marginal zone lymphoma of the esophagus: report of a case with review of the concept of the sarcoidosis-lymphoma syndrome. Int. J. Clin. Exp. Pathol. 2014;7(7):4428–4432. [PMC free article] [PubMed] [Google Scholar]
- 9.Saidha S., Sotirchos E.S., Eckstein C. Etiology of sarcoidosis: does infection play a role? Yale J. Biol. Med. 2012;85(1):133–141. [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Weiping, Wolchok Jedd D., Chen Lieping. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers and combinations. Sci. Transl. Med. 2016;8:328. doi: 10.1126/scitranslmed.aad7118. 328rv4. PMC. Web. 30 Jan. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marginal zone lymphoma of the esophagus: report of a case with review of the concept of the sarcoidosis-lymphoma syndrome. Int. J. Clin. Exp. Pathol., 7(7), 4428–4432. [PMC free article] [PubMed]
- 12.Koyama T., Ueda H., Togashi K., Umeoka S., Kataoka M., Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004;24(1):87–104. doi: 10.1148/rg.241035076. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto K., Kase M., Nagatomo A., Kunikane H., Okamoto H., Watanabe K. Pulmonary histoplasmosis exhibiting solitary pulmonary nodule resected by thoracoscopic surgery: a case report and review of the Japanese literature. Nihon Kokyuki Gakkai Zasshi. 1999;37(11):909–914. [PubMed] [Google Scholar]