Abstract
Introduction
Much is to be learned about humanbreast milk (HBM)
Purpose
Extend our knowledge of HBM by investigating the role of maternal body mass index (BMI), sex and stage of lactation (month 1 vs. 6) on HBM insulin, glucose, leptin, IL-6 and TNF-α and their associations with infant body composition.
Methods
Thirty-seven exclusively breastfeeding infants (n=37; 16♀, 21♂) and their mothers (19–47 kg/m2) were studied at 1 and 6 months of lactation. Infants had body composition measured (using dual energy X-ray absorptiometry (DXA)) and HBM collected.
Results
A significant interaction between maternal BMI and infant sex on insulin levels (p = 0.0322) was observed, such that insulin was 229% higher in obese mothers nursing female infants than in normal weight mothers nursing female infants and 179% higher than obese mothers nursing male infants. For leptin, a significant association with BMI category was observed (p < 0.0001) such that overweight and obese mothers had 96.5% and 315.1% higher leptin levels than normal weight mothers, respectively. Leptin was also found to have a significant (p = 0.0004) 33.7% decrease from month 1 to month 6, controlling for BMI category and sex. A significant inverse relationship between month 1 leptin levels and infant length (p=0.0257), percent fat (p=0.0223), total fat mass (p=0.0226) and trunk fat mass (p=0.0111) at month 6 was also found. No associations or interactions were observed for glucose, TNF-α, or IL-6.
Conclusions
These data demonstrates that maternal BMI, infant sex and stage of lactation affect the compositional make-up of insulin and leptin.
Keywords: growth, body composition, inflammation, human breast milk, infant feeding
INTRODUCTION
“One of the most highly effective preventive measures a mother can make in protecting the health of her infant and herself is to breastfeed” (US Surgeon General, January 2011). Statements such as these are universally supported by the Center for Disease Control and Prevention (1), the American Academy of Pediatrics (2) and almost every governing health body concerned with maternal/infant health. Despite the extensive recommendations and purported benefits of breastfeeding, limited information beyond basic micro/macronutrient composition of human breast milk (HBM) currently exists. HBM is a highly complex, biological fluid that is dependent on a variety of factors, including timing within a given feeding, length of lactation, maternal body composition and genetic factors (3). A small body of literature also suggests that the composition of HBM may differ based on the sex of the infant (4–6). However, it is unknown whether sex differences exist in specific non-nutritive components of HBM. Furthermore, little is known about the role of non-nutritive bioactive milk components and whether they affect growth and development of the offspring in the critical first months of life. Preliminary cross-sectional evidence indicates that glucose, anabolic hormones (insulin), satiety hormones (leptin) and cytokines (IL-6 and TNF-α) found in HBM may differentially influence fat and lean body mass in infants at one-month of age (7).
The present study sought to bring clarity, while expanding our current but limited knowledge of HBM beyond that of basic macro- nutrition by investigating the following aims. One, what roles do maternal BMI and infant sex play on concentration of milk glucose, insulin, leptin, IL-6 and TNF-α? Two, what role do these non-nutritive substances play on neonatal body composition? These aims were examined in exclusively breastfed infants for the first 6-months of life from mothers varying widely in BMI (18.5 to 47.2 kg/m2) using dual-energy X-ray absorptiometry (DXA) to determine infant body composition. We hypothesized that the presence of maternal obesity creates HBM that is obesogenic, resulting in exaggerated growth and body composition abnormalities in the offspring.
METHODS
Study Overview
We conducted a longitudinal cohort study of infant- mother dyads, who were measured at month one (±5 days, 37 dyads) and again at month 6 of lactation (±5 days, 30 dyads). The overall study design and preliminary results in a subset (n=19) of these dyads were described previously (7). Briefly, a complete breast milk expression from a single breast preceded a whole-body DXA scan in the infant with both measures conducted at one and 6-months of age. The University of Oklahoma Institutional Review board approved the study. Subject consent was obtained prior to procedures with subjects generally reporting to the University of Oklahoma Health Sciences Oklahoma City campus between 8:00–10:00 am. This was done as an attempt to standardize the testing protocol and collection of breast milk.
Study Participants
Thirty-seven mother-infant dyads initially enrolled into the study with the intention of exclusively breastfeeding to 6-months (defined as no formula supplementation) with all testing occurring on the medical campus at the University of Oklahoma Health Sciences, Oklahoma City, OK. The following inclusion and exclusion criteria were used to determine participant eligibility into the study. Inclusion criteria: 1) maternal age of 18–45 years at the time of delivery, 2) gestation ≥ 37 weeks and 3) singleton birth. Exclusion criteria: 1) any tobacco use during gestation, 2) alcohol consumption defined as >one drink per week, 3) diabetes or 4) presumed or known congenital birth defects. Self-reported maternal demographic information (age, parity, pre-pregnancy weight and gestational weight gain) were collected when medical chart abstraction was unable to be performed.
Growth and Body Composition
The infant’s body weight and length were collected using standardized methods, with the infant’s nude weight and length obtained in duplicate. Total body composition was determined using a Lunar iDXA (Lunar i DXAv11-30.062) with infant whole body analysis enCore 2007 software. The room was darkened with minimal outside noise as described by our group previously with the infant wearing only a diaper while swaddled in a light receiving blanket provided by the laboratory to maximize compliance and minimize body movement (8, 9). The same person (DAF) positioned all infants and performed subsequent analyses.
Breast milk collection
Based on their discretion, mothers chose the breast they felt could provide the most complete milk expression (though the right was encouraged). Furthermore, the mother was encouraged to completely empty the entire contents of a single breast for the analyses of breast milk insulin, glucose, leptin, IL-6 and TNF-α using an electric breast pump (Medela, Inc.) provided by the investigative team. Milk fat was separated from the aqueous phase by centrifugation with the resulting skimmed milk assayed using commercially available immunoassay kits for insulin, leptin, IL-6 and TNF-α as described by our group previously (7). Glucose was measured using the glucose oxidase method (2300 STAT Plus, Yellow Springs Instruments).
Statistical analyses
To examine the relationship of infant sex and pre-gravid maternal obesity status with HBM analyte changes between 1 and 6 months, a linear model with correlated errors was used. Due to apparent non-normal distributions, a log transform was applied to insulin, leptin, IL-6, and TNF-α. In these models the repeated measures were handled with a compound symmetric correlation structure. All models contained terms for stage of lactation, pre-gravid BMI category (normal weight, overweight, and obese), and sex. Due to the exploratory nature of this study interactions between pre-gravid BMI category and infant sex were initially considered but were removed if non-significant (p>0.05). As this is an observational study of an exploratory nature, no corrections were made for multiple testing.
To compare the effects of breast milk analytes on infant body composition, general linear models were fit for the body composition measures at month 6 as the outcome and the 1 month milk analytes as predictors in separate models while controlling for the month 1 body composition value, age in days at the 6 month visit, sex, and maternal pre-gravid BMI category. All calculations were performed in SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
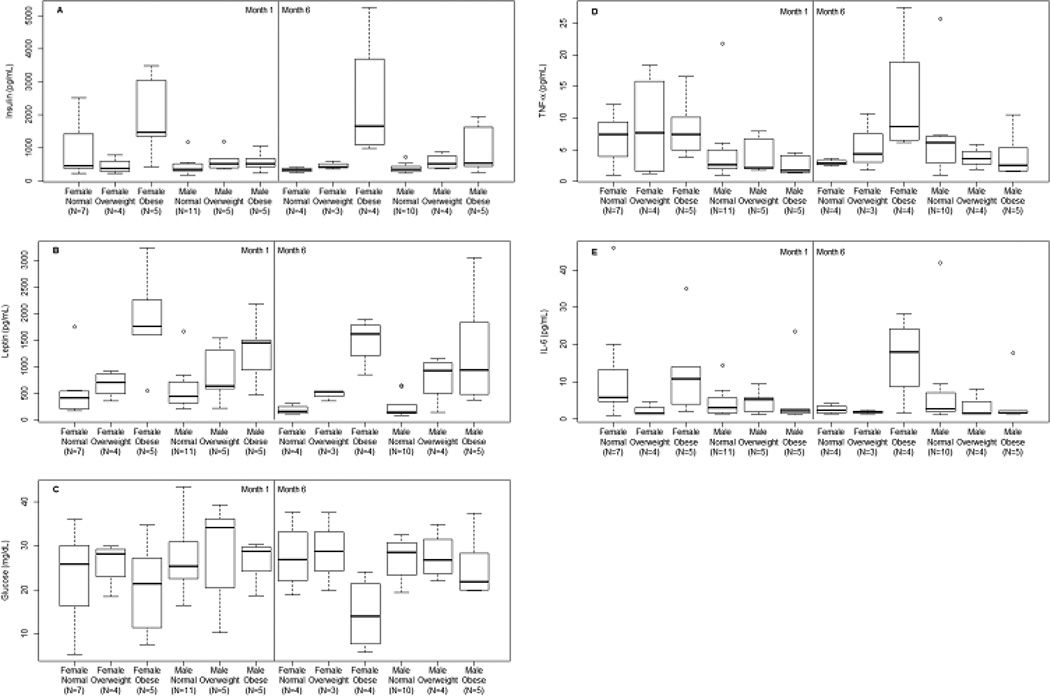
Thirty-seven mother/infant dyads enrolled into the study at one month, with complete data in thirty mother/infant dyads carried out to six-months. Infant growth and body composition along with HBM hormones and adipocytokines are presented in Table 1. To better describe the skewed distributions of HBM hormones and adipocytokines, boxplots were generated depicting unadjusted values for each HBM variable (insulin, leptin, glucose, TNF-α, and IL-6) by maternal BMI category (normal, overweight and obese), infant sex and stage of lactation (1 vs. 6-months) (Figure 1 panels A – E).
Table 1.
Infant growth, body composition and breast milk variables at 1 and 6-months postpartum
| Variable | One month (n=37) |
Six months (n=30) |
|---|---|---|
| Age (days) | 38 (36–42) | 167 (163–169) |
| Weight (grams)* | 4663.9 ± 695.0 | 7143.5 ± 1237.4 |
| Length (cm) | 55.6 ± 2.2 | 65.1 ± 2.9 |
| Weight change from birth (g/day) | 29.0 ± 10.8 | 21.2 ± 6.3 |
| Percent Fat | 24.1 ± 2.83 | 32.3 ± 3.8 |
| Total Fat Mass (grams) | 1191.1 ± 284.2 | 2423.9 ± 634.4 |
| Total Fat-Free Mass (grams) | 3720.4 ± 495.5 | 4974.1 ± 745.0 |
| Trunk Fat Mass (grams) | 408.3 ± 124.8 | 782.6 ± 309.4 |
| Glucose (mg/dL) | 25.4 ± 9.0 | 25.6 ± 7.5 |
| IL-6 (pg/mL) | 3.7 (1.7–7.1)a | 2.2 (1.6–7.0) |
| Log IL-6 | 1.4 ± 1.0a | 1.2 ± 1.0 |
| TNF-α (pg/mL) | 4.1 (1.7–7.4) | 4.8 (2.8–7.0) |
| Log TNF-α | 1.4 ± 0.9 | 1.5 ± 0.8 |
| Insulin (pg/mL) | 451 (355–918) | 426 (337–724) |
| Log Insulin | 6.3 ± 0.8 | 6.3 ± 0.7 |
| Leptin (pg/mL) | 589 (353–1450) | 426 (145–940) |
| Log Leptin | 6.5 ± 0.8 | 6.0 ± 1.0 |
Data presented as mean ± standard deviation or median (interquartile range).
Actual scale weight.
N=36 due to an IL-6 assay error.
Figure 1.
Boxplots describing the observed HBM hormone and adipocytokine levels (Panel A insulin, Panel B leptin, Panel C glucose, Panel D TNF-α and Panel E IL-6) for the combinations of pre-gravid maternal BMI category (normal, overweight and obese), infant sex (male or female) and stage of lactation (month 1 and month 6).
Overall results for pre-gravid maternal BMI, infant sex and stage of lactation on HBM hormones and adipocyokines
The results of the tests for maternal BMI category, infant sex and stage of lactation on HBM variables are reported in Table S1. We found a significant interaction between maternal BMI category and infant sex on HBM insulin levels (F2,31 = 3.85, p = 0.0322), such that the insulin levels were higher in obese mothers with female infants than other categories, controlling for stage of lactation. Interactions between maternal BMI category and infant sex on HBM leptin, glucose, TNF-α, and IL-6 were not statistically significant, therefore only aggregated results are reported. For HBM leptin, we observed a significant association (F2,33 = 15.07, p < 0.0001) between maternal BMI category and HBM leptin, such that leptin levels were 96.5% (95% CI: 13.3%, 241.0%) higher in overweight mothers versus normal weight mothers, controlling for stage of lactation and sex. Obese mothers had 315.1% higher leptin levels (95% CI: 144.1%, 605.8%) than normal weight mothers. We also observed a small but statistically significance decrease of 33.7% (95% CI: −46.1%, −18.3%) in leptin levels from month 1 to month 6, controlling for maternal BMI category and infant sex. No significant associations were observed between glucose, TNF-α, or IL-6 and the predictors: maternal BMI category, infant sex, or stage of lactation. (p > 0.0648).
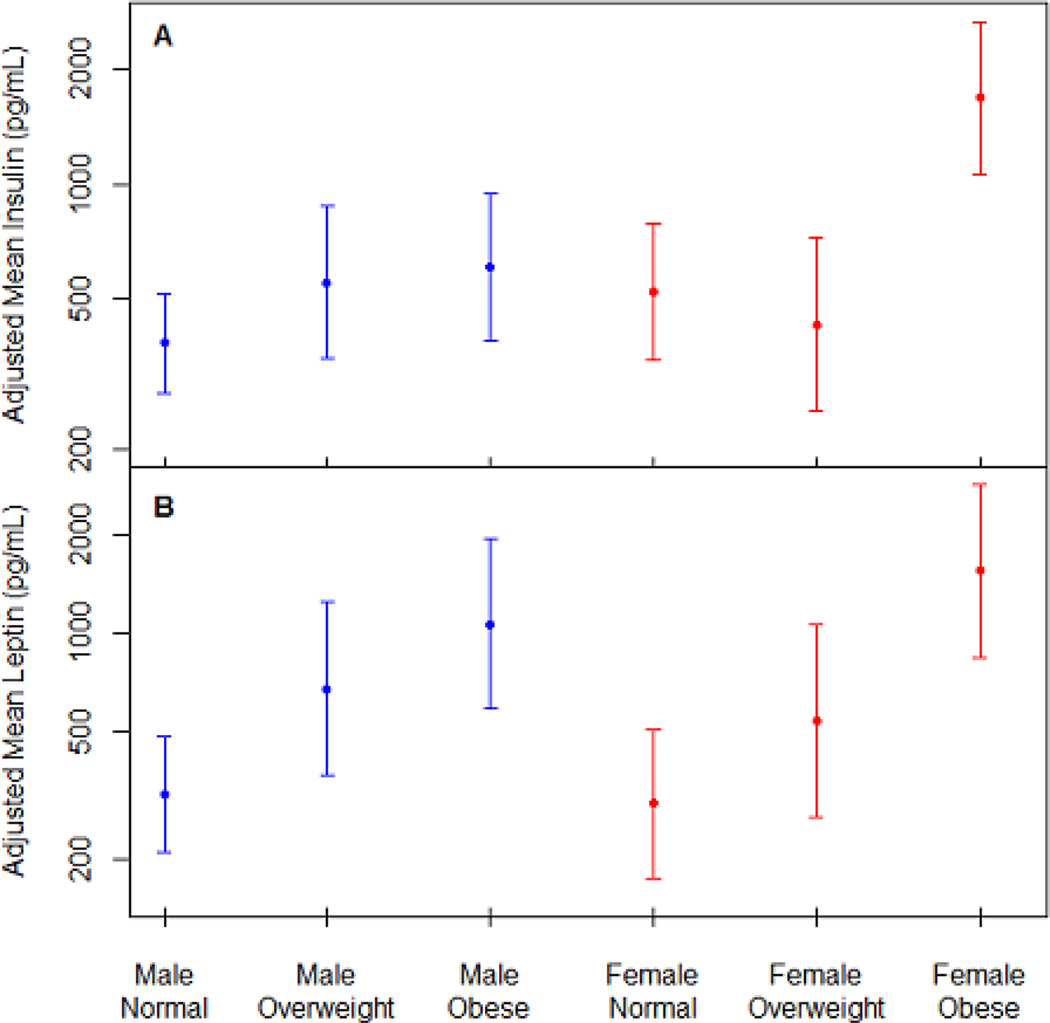
To provide further insight into the significant associations observed, Figure 2 depicts the estimated mean insulin (panel A) and leptin levels (panel B) for each combination of maternal pre-gravid BMI category and infant sex, adjusted for stage of lactation. Specifically, HBM insulin was 224.9% higher in obese mothers than in normal weight mothers with female infants (95% CI: 74.9%, 503.5%), and 178.6% higher than obese mothers with male infants (95% CI: 46.3%, 430.5%). In Panel B, a clear positive association is observed between HBP leptin and maternal BMI category in mothers of both male and female infants.
Figure 2.
Panel A estimated mean insulin (pg/mL) and Panel B estimated mean leptin (pg/mL) levels for each combination of maternal pre-gravid BMI category and infant sex (blue font males and red font females), adjusted for the month of observation.
Effects of HBM hormones and adipocytokines on infant growth and body composition
Next, we explored the associations of HBM hormone and adipocytokine levels at month 1 with infant growth (weight and length) and body composition (percent fat, total fat and fat-free mass and trunk fat mass) outcomes at month 6 (Table S2). The effects reported in Table S3 are the estimated change in growth or body composition due to the difference between a ‘high’ and ‘low’ month 1 level of HBM variables with ‘high’ and ‘low’ being the 75th and 25th percentiles of the total sample. The exact values of these interquartile ranges are given at the top of Table S3.
We found a significant association between month 1 HBM leptin levels and month 6 body length (p=0.0257), percent fat (p=0.0223), total fat mass (p=0.0226), and trunk fat mass (p=0.0111), controlling for age, sex, maternal BMI category, and month 1 value of the growth or composition measure, and a near significant association with body weight (p=0.0720). Specifically, we estimate that a 1097.7 pg/mL increase in HBM leptin was associated with a 805.1 g decrease in body weight (95% CI: −1688.3, 78.1), a 2.01 cm reduction in length (95% CI: −3.75, −0.27), a 4.41% reduction in percent fat (95% CI: −8.13, −0.69), a 613.9 g decrease in total fat mass (95% CI: −1133.5, −94.4), and a 335.0 g reduction in trunk fat mass (95% CI: −586.1, −84.0). No other statistically significant associations were observed between the other HBM variables (glucose, insulin, TNF-α, IL-6) and infant body composition measures. However, the associations observed between HBM glucose and infant length (p=0.0796), as well as HBM TNF-α and total fat mass (p=0.0843) warrant further investigation given their magnitude and the small sample size of this exploratory study.
DISCUSSION
This study examined glucose, anabolic hormones (insulin), satiety hormones (leptin) and cytokines (IL-6 and TNF-α) in HBM from women representing a wide range of body fat (18.5 to 47.2 kg/m2). A particular focus was better understanding the effect of maternal BMI, sex and stage of lactation on the compositional make-up of these substances and their subsequent associations with infant body composition during the first 6-months of life. We postulated that the imposition of maternal obesity creates HBM that is obesogenic, resulting in exaggerated growth and body composition abnormalities in the offspring. This is significant given the limited number of published studies examining the relationships between non-nutritive bioactive compounds in HBM and impact on early infant growth and body composition. A recent review by Young and colleagues enumerated gaps in our current knowledge with an important issue identified being that of the role ‘maternal obesity plays on HBM composition and the subsequent downstream effects on the infant’ (10). Understanding this potential link is important because it establishes a mechanism whereby the maternal obesity phenotype is communicated to the offspring via breast milk, resulting in excessive weight gain in the first months of life, a known risk factor for future obesity (OR 1.2 to 5.7) (11). Moreover, it represents a potentially exciting pathway by where the compositional makeup of maternal breast milk may be improved by diet, exercise or pharmacological means.
This study revealed novel and unexpected findings pertaining to HBM insulin and leptin. First, we found a significant interaction between maternal BMI and infant sex on insulin levels (p = 0.0322). Specifically, breast milk insulin was 229% higher in obese mothers nursing female infants than in normal weight mothers nursing female infants and 179% higher than obese mothers nursing male infants. Two other studies have reported positive associations between maternal BMI and HBM insulin concentrations (12, 13), while others have found no association (7, 14).
The observed interaction between maternal BMI and infant sex on insulin levels is intriguing. Differential sex-based investment in offspring may be common, particularly among mammals where reproductive success varies between males and females (15). Studies in both humans (4–6) and animals (16, 17) have identified sex-based differences in breast milk volume and macronutrient composition. For example, Powe and colleagues reported differences in energy content of HBM based on the sex of the infant, with a 25% higher energy content observed in milk for male infants as compared to female infants (4). Fujita et al., reported greater levels of milk fat in high socioeconomic status women nursing male offspring compared to women with female offspring (6). A study in rhesus macaques found that mothers of males produced higher energy density milk than for females; however, mothers of males also had lower milk yield, therefore available milk energy was the same (16). Given the focus on breast milk volume and macronutrient composition, these studies are not directly comparable with ours. Rather, the existing literature provides support for the existence of a sex-dimorphism in breast milk composition. Possible explanations for this finding include sex differences in hormones secreted by the placenta during pregnancy, which may influence developing mammary glands or other targets. Alternatively, it is possible that the sex of the infant drives milk insulin secretion through increased feeding, increased/decreased suckling pressure or some other yet known factor. Additional studies are needed with larger sample sizes to further examine potential sex differences in HBM composition.
In line with findings previously reported in the literature, we identified a significant association with BMI category and breast milk leptin (p < 0.0001), such that overweight and obese mothers had 96.5% and 315.1% higher leptin levels than normal weight mothers, respectively. A recent systematic review by Andreas et al. also reported positive associations between maternal BMI and breast milk leptin in eleven of the fifteen studies reviewed, despite variation in methodology across studies (18).
The second novel finding in this study was the significant inverse relationship observed between 1-month milk leptin levels and infant length (p=0.0257), percent fat (p=0.0223), total fat mass (p=0.0226) and trunk fat mass (p=0.0111) at 6-months. In addition, breast milk leptin decreased (p = 0.0004) 33.7% from month 1-month to 6-months, which may be attributed to the mother’s post-partum weight returning back to baseline. Existing studies in the literature have reported no associations between milk leptin and growth outcomes (19–21), while others have shown decreased BMI-for-age z-score and weight-for-length z-scores (7, 22–24). Work in HBM beyond that of basic micro- and macronutrients is still in the nascent phase, with studies just now emerging looking at low-abundant proteins in HBM with fewer yet understanding the role of maternal BMI and the subsequent impact on infant outcomes. Our present study, though still preliminary builds upon prior work from this group by doubling the number of study participants and extending the testing to 6-months (7).
An important construct in establishing plausibility of non-nutritive bioactive HBM proteins exerting an effect in the infant is having a greater understanding of digestion during infancy. In general, the newborn gut resists proteolysis of intact milk peptides by five operable pathways (25): One, though non-digestive and unmediated, paracellular diffusion (passing of molecules across the epithelium through tight intercellular junctions) represents a significant pathway for intact peptides to reach the circulation (25). Two, pancreatic enzymatic function is incomplete, partly due to immaturity and low concentrations in the maturing gut (26). Third, gastrointestinal receptors for a multitude of adipokines (such as leptin) are known to exist, suggesting direct entry into the circulation (27). Fourthly, colostrum and early milk have high concentrations of α1-antitrypsin, which binds tightly to trypsin and retards proteolysis (26, 28). Fifthly, the infant gut has a higher gastric pH (5.0) relative to adults (2.0) and resists digestion (26). Collectively, these pathways provide a viable paradigm by where intact proteins in HBM resist proteolysis indicating HBM proteins may be bioactive and exert both positive and negative effects on the infant during the first months of life.
In conclusion, our study builds upon and advances the small number of prior studies by demonstrating that HBM leptin decreases over the stage of lactation, is higher in obese mothers, and is inversely associated with infant length, %fat, total fat mass and trunk fat at 6-months of age. Further, we identified a BMI-sex interaction for milk insulin where insulin was highest in obese mother nursing female infants as compared to normal weight mothers nursing male infants. To our knowledge, this is the first time this finding has been reported. True randomized controlled breastfeeding trials are unethical and unwarranted, larger more inclusive longitudinal cohort studies are needed to confirm this finding. Though interesting, at this time, it is premature to infer causality or make overreaching statements, rather these findings provide a proof-of-principle to a much broader question, “what role do non-nutritive bio-active molecules in HBM play in infant growth and body composition, and is this association mediated by maternal BMI and potentially offspring sex?”
Our findings should not be construed to imply that obese mothers should not breastfeed, on the contrary our findings provide for a greater understanding in the role maternal obesity and other known obesogens play on HBM composition, which is an identifiable gap in the current state of the field and may help explain why some have reported breastfeeding having little impact on BMI in later childhood (29). Though preliminary, our results open up the possibility in the supposition for the efficacious delivery of maternal intervention and its subsequent signals (e.g. nutritional, metabolic, pharmacological, energy sensing) reaching the infant via breast milk and exerting a positive impact. In this scenario, breast milk is the vehicle for the interchange of signals from mother to infant that go beyond that of nutrition and non-bioactive molecules just now being discovered in HBM.
Supplementary Material
What is already known about the subject?
Human breast milk (HBM) is a highly complex, non-uniform bioactive substance that plays an important role in the long-term health and growth of infants.
Studies have focused on the macronutrient composition of HBM; however, little is known about breast milk hormones or adipocytokines.
What does this study add?
This study extends our current knowledge of HBM by investigating the role of maternal body mass index (BMI), infant sex and stage of lactation on insulin, glucose, leptin, IL-6 and TNF-α in breast milk and its association with infant body composition.
A significant interaction was observed between maternal BMI and infant sex on HBM insulin levels, where insulin was highest in obese mothers with female infants.
HBM leptin significantly decreased from one to six months, and was negatively associated with infant length, percent fat, total fat mass, and trunk fat mass at six months.
Maternal body mass index, infant sex and stage of lactation affect the compositional make-up of HBM insulin and leptin, which may in turn influence infant growth.
Acknowledgments
Preliminary data in 19 subjects irrespective of maternal BMI from this study were published previously in Pediatr Obes (7). We are indebted to the mothers for enrolling their infant into the study and for Catherine Wolf (study recruitment and testing of subjects) and April Teague (performing all breast milk analyses) for their work on the study.
FUNDING
Mead Johnson Nutrition provided financial support (DAF principal investigator) as an Independent Investigator Trial but did not have editorial control of the paper with the CMRI Metabolic Research Program and the CMRI Chickasaw Nation Endowed Research Chair in Pediatric Diabetes providing additional support. DAF and EWD were supported by NIH HD080444 with EWD also supported by NIH HD53685 and DBA by NIDDK P30DK056336. The opinions expressed are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
Footnotes
DAF, EWD, BG, MW, KW, AT and DAB have no conflicts of interest to report other than Mead Johnson Nutrition provided financial support for the project.
This trial was registered at clinicaltrials.gov as NCT02535637.
REFERENCES
- 1.Breastfeeding report card United States/2014. Atlanta, GA: 2014. p. 8. [Google Scholar]
- 2.Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 3.Stam J, Sauer PJ, Boehm G. Can we define an infant's need from the composition of human milk? Am J Clin Nutr. 2013;98(2):521S–528S. doi: 10.3945/ajcn.112.044370. [DOI] [PubMed] [Google Scholar]
- 4.Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. 2010;22:50–54. doi: 10.1002/ajhb.20941. [DOI] [PubMed] [Google Scholar]
- 5.Thakkar SK, Giuffrida F, Cristina CH, De Castro CA, Mukherjee R, Tran LA, et al. Dynamics of human milk nutrient composition of women from Singapore with a special focus on lipids. Am J Hum Biol. 2013;25:770–779. doi: 10.1002/ajhb.22446. [DOI] [PubMed] [Google Scholar]
- 6.Fujita M, Roth E, Lo YJ, Hurst C, Vollner J, Kendell A. In poor families, mothers' milk is richer for daughters than sons: a test of Trivers-Willard hypothesis in agropastoral settlements in Northern Kenya. Am J Phys Anthropol. 2012;149:52–59. doi: 10.1002/ajpa.22092. [DOI] [PubMed] [Google Scholar]
- 7.Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7:304–312. doi: 10.1111/j.2047-6310.2012.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. Body Composition at 6 Months of Life: Comparison of Air-Displacement Plethysmography and Dual Energy X-Ray Absorptiometry. Obesity. 2012;20:2302–2306. doi: 10.1038/oby.2012.102. [DOI] [PubMed] [Google Scholar]
- 9.Chandler-Laney PC, Gower BA, Fields DA. Gestational and early life influences on infant body composition at 1 year. Obesity. 2013;21:144–148. doi: 10.1002/oby.20236. [DOI] [PubMed] [Google Scholar]
- 10.Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv Nutr. 2012;3:675–686. doi: 10.3945/an.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26:19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 12.Ley SH, Hanley AJ, Sermer M, Zinman B, O'Connor DL. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am J Clin Nutr. 2012;95:867–874. doi: 10.3945/ajcn.111.028431. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja S, Boylan M, Hart S, Román-Shriver C, Spallholz J, Pence B, et al. Glucose and Insulin Levels are Increased in Obese and Overweight Mothers’ Breast-Milk. Food and Nutrition Sciences. 2011;2:201–206. [Google Scholar]
- 14.Shehadeh N, Khaesh-Goldberg E, Shamir R, Perlman R, Sujov P, Tamir A, et al. Insulin in human milk: postpartum changes and effect of gestational age. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2003;88:F214–F216. doi: 10.1136/fn.88.3.F214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 16.Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21:512–519. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- 17.Hinde K, Carpenter AJ, Clay JS, Bradford BJ. Holsteins favor heifers, not bulls: biased milk production programmed during pregnancy as a function of fetal sex. PLoS One. 2014;9:e86169. doi: 10.1371/journal.pone.0086169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreas NJ, Hyde MJ, Gale C, Parkinson JR, Jeffries S, Holmes E, et al. Effect of maternal body mass index on hormones in breast milk: a systematic review. PLoS One. 2014;9:e115043. doi: 10.1371/journal.pone.0115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uysal FK, Onal EE, Aral YZ, Adam B, Dilmen U, Ardicolu Y. Breast milk leptin: its relationship to maternal and infant adiposity. Clin Nutr. 2002;21:157–160. doi: 10.1054/clnu.2001.0525. [DOI] [PubMed] [Google Scholar]
- 20.Khodabakhshi A, Ghayour-Mobarhan M, Rooki H, Vakili R, Hashemy SI, Mirhafez SR, et al. Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. Eur J Clin Nutr. 2015;69:614–618. doi: 10.1038/ejcn.2014.205. [DOI] [PubMed] [Google Scholar]
- 21.Brunner S, Schmid D, Zang K, Much D, Knoeferl B, Kratzsch J, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10:67–73. doi: 10.1111/j.2047-6310.2014.222.x. [DOI] [PubMed] [Google Scholar]
- 22.Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity. 2006;14:1371–1377. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- 23.Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants' body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. 2011;70:633–637. doi: 10.1203/PDR.0b013e31823214ea. [DOI] [PubMed] [Google Scholar]
- 24.Quinn EA, Largado F, Borja JB, Kuzawa CW. Maternal characteristics associated with milk leptin content in a sample of Filipino women and associations with infant weight for age. J Hum Lact. 2015;31:273–281. doi: 10.1177/0890334414553247. [DOI] [PubMed] [Google Scholar]
- 25.Wada Y, Lonnerdal B. Bioactive peptides derived from human milk proteins--mechanisms of action. J Nutr Biochem. 2014;25:503–514. doi: 10.1016/j.jnutbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Lonnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr. 2010;156:S26–S30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Savino F, Fissore MF, Liguori SA, Oggero R. Can hormones contained in mothers' milk account for the beneficial effect of breast-feeding on obesity in children? Clinical endocrinology. 2009;71:757–765. doi: 10.1111/j.1365-2265.2009.03585.x. [DOI] [PubMed] [Google Scholar]
- 28.Chowanadisai W, Lonnerdal B. Alpha(1)-antitrypsin and antichymotrypsin in human milk: origin, concentrations, and stability. Am J Clin Nutr. 2002;76:828–833. doi: 10.1093/ajcn/76.4.828. [DOI] [PubMed] [Google Scholar]
- 29.Hancox RJ, Stewart AW, Braithwaite I, Beasley R, Murphy R, Mitchell EA, et al. Association between breastfeeding and body mass index at age 6 – 7 years in an international survey. Pediatr Obes. 2015;10:283–287. doi: 10.1111/ijpo.266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.