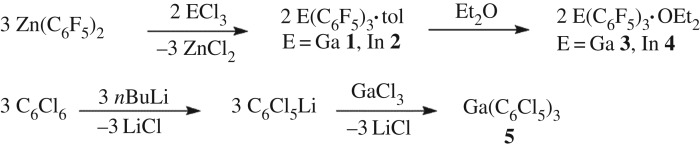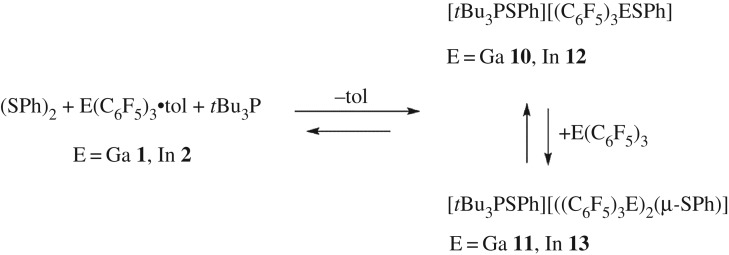Abstract
The Lewis acids Ga(C6F5)3, In(C6F5)3 and Ga(C6Cl5)3 are prepared and their Lewis acidity has been probed experimentally and computationally. The species Ga(C6F5)3 and In(C6F5)3 in conjunction with phosphine donors are shown to heterolytically split H2 and catalyse the hydrogenation of an imine. In addition, frustrated Lewis pairs (FLPs) derived from Ga(C6F5)3 and In(C6F5)3 and phosphines react with diphenyldisulfide to phosphoniumgallates or indates of the form [tBu3PSPh][PhSE(C6F5)3] and [tBu3PSPh][(μ-SPh)(E(C6F5)3)2] (E = Ga, In). The potential of the FLPs based on Ga(C6F5)3, In(C6F5)3 and Ga(C6Cl5)3 and phosphines is also shown in reactions with phenylacetylene to give pure or mixtures of the products [tBu3PH][PhCCE(C6X5)3] and R3P(Ph)C=C(H)E(C6X5)3. A number of these species are crystallographically characterized. The implications for the use of these species in FLP chemistry are considered.
This article is part of the themed issue ‘Frustrated Lewis pair chemistry’.
Keywords: gallium, indium, frustrated Lewis pair chemistry, hydrogenation
1. Introduction
While classic Lewis acids and Lewis bases usually form strong adducts [1], in 2006, we disclosed that the intramolecular Lewis acid/Lewis base pair (Mes2P)C6F4(B(C6F5)2) cleanly cleaves the dihydrogen molecule under mild conditions [2]. Subsequently, we showed this ability to heterolytically split H2 could be generalized to combinations of phosphines and boranes where steric demands precluded dative bond formation [3]. This finding swiftly led to the discovery of the first metal-free hydrogenation catalysts that involve main group species activating H2 and delivering it to a variety of unsaturated organic substrates [4–10].
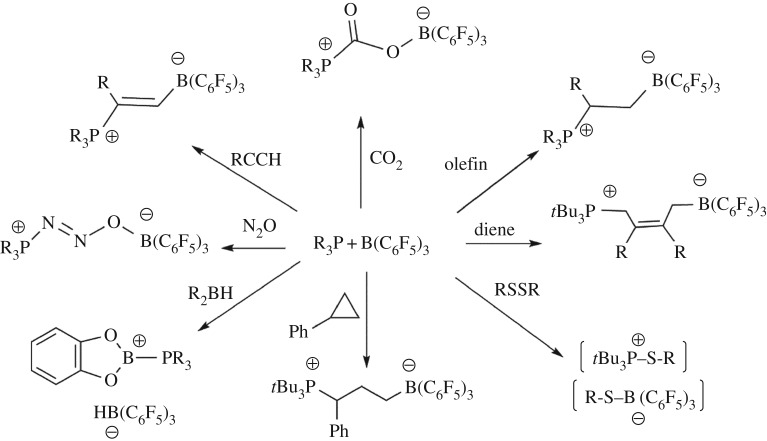
In the intervening decade, these findings have garnered considerable attention, and indeed, frustrated Lewis pair (FLP) chemistry has broadened dramatically [8–10]. Indeed, a broad range of polar and non-polar unsaturated substrates have been shown to undergo FLP hydrogenations. Moreover, asymmetric FLP catalysts have been developed leading to highly selective metal-free hydrogenations [11–18]. FLPs have been shown to react with a variety of other small molecule substrates, including olefins and alkynes, CO2, N2O, SO2, RNSO and NO [8,10,19,20]. Recently, this unique reactivity has been extended to C–H bond activation as well as to provide new strategies for organic and radical chemistry [21]. The concept of FLP chemistry has also been applied in transition-metal chemistry, applied to develop synthetic models for hydrogenases, unveiled new strategies for polymer syntheses and found analogies in the mechanisms of surface reaction chemistry [8] (scheme 1).
Scheme 1.
Representative reactivity of phosphine/borane FLPs with small molecules.
While much of the above FLP chemistry exploits boron-based Lewis acids, the Al-based Lewis acid Al(C6F5)3 has also been explored in FLP reactions with alkynes [22], olefins [23–25], H2 [26], CO2 [27–30] and N2O [31]. At the same time, the Fontaine [32] and Uhl [33–37] groups have explored the FLP chemistry of intramolecular Al/P systems, of the form R2PCH2AlMe2 and 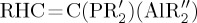 , respectively.
, respectively.
Ga and In Lewis acids have been used as homogeneous catalysts in a number of organic transformations [38]. For example, gallium triflate catalyses Friedel–Crafts alkylations and acylations, epoxyolefin cyclizations, Mukaiyama aldol condensations and ketone reductions [38–46]. Indium(III) Lewis acids have also been shown to effectively promote Diels–Alder reactions between various dienes and dienophiles in water, as well as Michael reactions between amines and α,β-ethylenic compounds [47]. Despite these applications of gallium and indium Lewis acids, the application of these heavier group 13 reagents in FLP chemistry has received less attention [48–50]. In such efforts, Gandon and co-workers [48] have exploited Ga species to effect the hydroarylation and hydrogenation of olefins, while Aldridge and co-workers [49] have described the catalytic reduction in CO2. In related work, the Ozin group [51] has recently described the activation of H2 and CO2 at the surface of heterogeneous nanocrystalline hydroxylated indium oxide (In2O3−x(OH)y) as a catalyst [51–53]. These authors propose that the activation of these molecules occurs via an FLP-like mechanism, in which proximal surface separated Lewis acidic indium and Lewis basic InOH sites act to activate H2. These advances notwithstanding, the paucity of gallium and indium applications in FLP chemistry has inspired us to probe the Lewis acidity of gallium and indium derivatives with perhalogenated substituents. In addition, we have investigated the FLP reactivity of these species in the reactions with H2, disulfides and alkynes.
2. Results and discussion
The Lewis acids E(C6F5)3•tol (E = Ga 1, In 2) and E(C6F5)3•OEt2 (E = Ga 3, In 4) were prepared using a modification of a patented preparation [54] (scheme 2). This involved the reaction of Zn(C6F5)2•tol with GaCl3 or InCl3. This afforded the direct isolation of 1 and 2 while recrystallization of the product from ether afforded 3 and 4. In the case of 2, the nature of the toluene adduct was confirmed crystallographically (figure 1). The structural data affirm an η1-interaction with toluene with an In–C distance of 2.634(2) Å. The In–C bond lengths to the C6F5 rings were found to range from 2.155(2) to 2.168(2) Å. The geometry at indium is pseudo-tetrahedral with C–In–C angles of 119.46(7)°, 110.80(7)° and 115.94(7)° between the aryl rings, while the C–In–C angles between the aryl rings and the coordinated toluene carbon atom are 105.84(7)°, 101.65(7)° and 100.06(7)°. These data are similar to those previously reported for the Al analogue [55].
Scheme 2.
Synthesis of 1–5.
Figure 1.
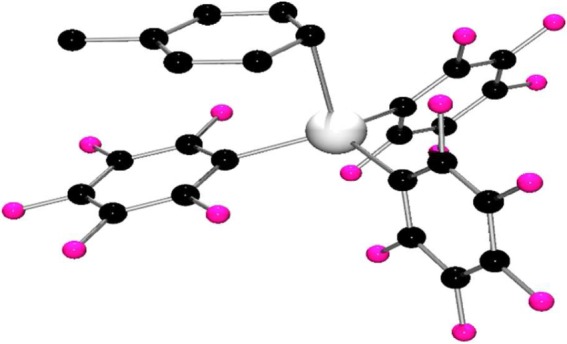
Molecular structure of 2. H-atoms have been omitted for clarity. C, black; In, grey; F, pink.
Recrystallization of 1 from diethyl ether afforded crystals of the corresponding ether adduct Ga(C6F5)3•OEt2 3. An X-ray crystallographic study of 3 reveals a structure that, as expected, shows a pseudo-tetrahedral geometry at gallium, with a Ga–O bond length of 2.018(2) Å (figure 2).
Figure 2.
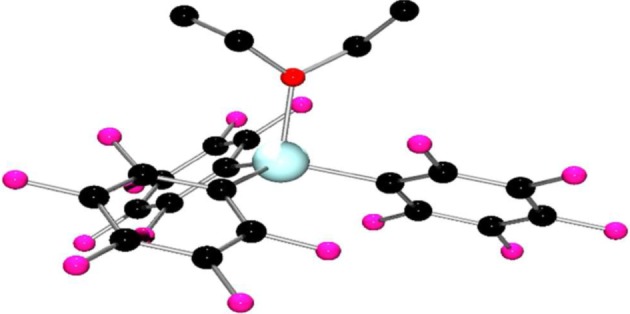
Molecular structure of 3. H-atoms have been omitted for clarity. C, black; Ga, turquoise; O, red; F, pink.
The species Ga(C6Cl5)3 5 was prepared in a fashion analogous to that described by Ashley et al. [56] for the preparation of the boron analogue. Treatment of C6Cl6 with nBuLi in n-hexane was followed by addition to a solution of GaCl3 at −78°C. Warming to room temperature and stirring overnight followed by workup and Soxhlet extraction afforded 5 in 86% yield (scheme 2). Electron ionization mass spectrometric data for 5 were consistent with the constitution of this species although it was shown to decompose at 280°C. This compound proved challenging to characterize spectroscopically due to the quadrupolar nature of Cl atoms; nonetheless, its subsequent reactivity provided further support for its formulation (vide infra).
Efforts to garner some information about the relative Lewis acidity were undertaken by employing the Gutmann–Beckett method [57,58]. When a 1 : 1 ratio of 1 and Et3PO were mixed in dichloromethane, the 31P NMR resonance of coordinated Et3PO was shifted 17.9 ppm downfield of free Et3PO. The analogous reaction with 2 and Et3PO resulted in a downfield shift of 18.7 ppm in the 31P NMR spectrum. Both values are smaller than those seen for the corresponding experiment using B(C6F5)3 (Δδ = 26.6 ppm). This is consistent with the diminished Lewis acidity of 1 and 2 in comparison to B(C6F5)3 [59]. Applying the same test to 5 was unsuccessful as a very weak adduct was formed with Et3PO as evidenced by the observation of only a very broad resonance in the 31P NMR spectrum. Similarly, Ashley et al. [56] reported that B(C6Cl5)3 did not form an adduct with Et3PO.
To further address the issue of relative Lewis acidity, the fluoride ion affinities (FIAs) were computed at the B3LYP/Def2TZVP(GD3BJ) level of theory [60–62] (table 1). For the series of species E(C6F5)3, the computed FIAs are consistent with the greater Lewis acidity of the Al species and a decrease for the series of Al, Ga and In species. The perchlorinated species is computed to have a significantly lower FIA. It is important to note that these calculations reflect the electronic features at Ga and fail to account for the increased steric demands of the C6Cl5 rings.
Table 1.
Computed fluoride ion affinities.
| Lewis acid | FIA (kJ mol−1) |
|---|---|
| B(C6F5)3 | 426 |
| Al(C6F5)3 | 535 |
| Ga(C6F5)3 | 445 |
| In(C6F5)3 | 413 |
| Ga(C6Cl5)3 | 385 |
Compounds 1 and 2 form adducts with the tertiary phosphine tBu3P. In the case of 1 and tBu3P, storage of the mixture at −35°C resulted in precipitation of a white solid, 6. The 19F NMR spectrum of this product showed a decreased gap between the resonances attributed to the meta and para fluorine atoms, which is indicative of four rather than three coordinate group 13 Lewis acid centres which contain C6F5 ligands. The 31P NMR spectrum displayed a signal at 58.6 ppm. These data support the formulation of the product 6 as the adduct (tBu3P)Ga(C6F5)3. Crystals of 6 suitable for X-ray diffraction were grown by vapour diffusion of n-pentane into a toluene solution (figure 3). X-ray crystallographic data confirmed the proposed formulation and revealed a Ga–P bond length of 2.635(4) Å. This is significantly longer than the typical covalent Ga–P bond distances of approximately 2.4 Å [63].
Figure 3.
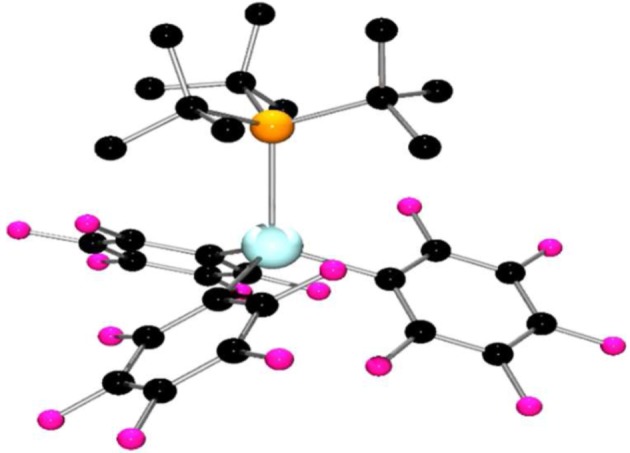
Molecular structure of 6. H-atoms have been omitted for clarity. C, black; P, orange; Ga, turquoise; F, pink.
The analogous reaction of 2 with tBu3P afforded a clear colourless solution that showed 19F resonances consistent with the quaternization of the indium centre, while the 31P NMR spectrum showed a shift in the resonance to 67.1 from 62.0 ppm for the free tBu3P. These data confirmed the formation of the product (tBu3P)In(C6F5)3 7. While this species is observable in solution, attempts to isolate it afforded consistently impure product.
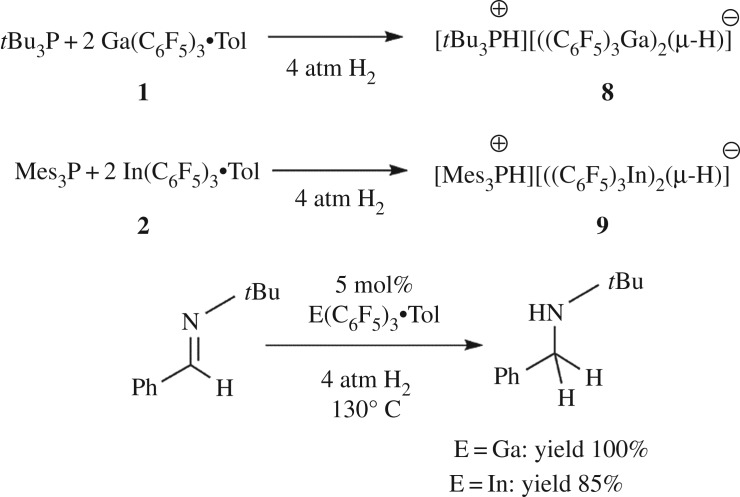
The quintessential reaction of FLPs is the reaction of such combination of Lewis acid and base with H2 [3]. Exploring this reactivity with 1 and 2 was thus undertaken. The reaction of 1 with tBu3P in a 2 : 1 ratio in toluene under 4 atm of H2 afforded the species [tBu3PH][(Ga(C6F5)3)2(μ-H)] 8 (scheme 3). The 1H NMR spectrum shows a doublet with a coupling constant of 427 Hz at 5.02 ppm, and the 31P NMR spectrum displayed the corresponding doublet at 60.8 ppm, consistent with the protonated phosphonium cation. The 19F NMR spectrum showed three resonances at −122.9, −155.9 and −163.7 ppm, corresponding to the C6F5 rings, consistent with the formation of a four coordinate gallium centre. In this case, the bridging hydride was observed at 3.70 ppm as a broad singlet, presumably as a result of the quadrupolar gallium nuclei (69Ga and 71Ga, I = 3/2). Elemental analysis and X-ray crystallography confirmed the formation of 8 (figure 4). The structure is directly analogous to the aluminium analogue [26], with a Ga–H–Ga angle of 175(2)° and an average Ga–H distance of 1.68(1) Å in the anion.
Scheme 3.
Stoichiometric/catalytic reactions of 1 or 2 with H2.
Figure 4.
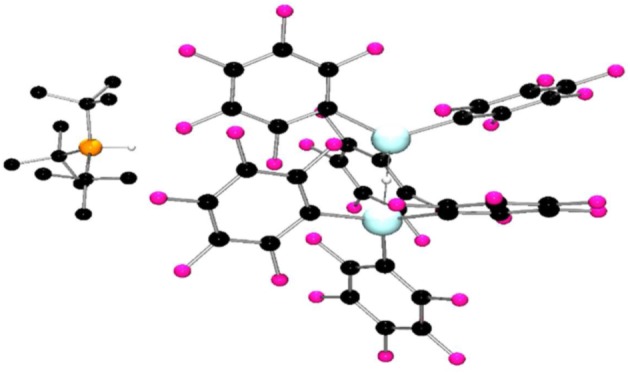
Molecular structure of 8. H-atoms have been omitted for clarity. C, black; P, orange; Ga, turquoise; H, grey; F, pink.
In the case of 2, addition of 1 equivalent of Mes3P in toluene under 4 atm of H2 at 25°C resulted in complete consumption of In(C6F5)3. The 31P NMR spectrum revealed the presence of two approximately equimolar species in solution: one new species exhibiting a doublet at −27.4 ppm with a coupling constant of 478 Hz, and a second resonance attributed to free Mes3P at −36.5 ppm. The corresponding 1H NMR spectrum showed a doublet at 8.24 ppm with a coupling constant of 478 Hz, characteristic of the [Mes3PH]+ cation. The 19F NMR spectrum showed three resonances at −117.4, −155.9 and −162.5 ppm, consistent with the formation of a four coordinate indium centre. Interestingly, when a 1 : 2 ratio of Mes3P and In(C6F5)3•tol was used, complete consumption of both starting materials was observed. These results were consistent with the formulation of 9 as [Mes3PH][(In(C6F5)3)2(μ-H)] (scheme 3). The bridging hydride was observed in the 1H NMR spectrum at 4.82 ppm as a broad singlet, presumably because of the proximity to 113In and 115In, both of which are quadrupolar with a nuclear spin of 9/2.
This demonstration of the activation of H2 probed questions about the potential utility of 1 and 2 in catalytic hydrogenation. To this end, attempts were undertaken to hydrogenate N-benzylidene-tert-butylamine (tBuN═CHPh). A bromobenzene solution of 1 or 2 was added to 20 equivalents of imine substrate. To the resulting solutions, 4 atm of H2 was added and the mixtures were heated at 130°C overnight. 1H NMR data revealed that 1 catalysed the complete reduction in the imine to the amine, while 2 afforded 85% conversion.
Analogous participation in the heterolytic activation of disulfides has been previously reported for FLPs derived from phosphine and B(C6F5)3. In similar reactions, 1 or 2 were combined with diphenyl disulfide and tBu3P. The 19F NMR spectrum showed resonances attributable to two species suggesting the formation of the anions in [tBu3PSPh][PhSE(C6F5)3] (E = Ga 10, In 12) and [tBu3PSPh][(μ-SPh)(E(C6F5)3)2] (E = Ga 11, In 13) (scheme 4) in ratios of 1 : 0.34 and 1 : 0.22, respectively. In the reaction of In(C6F5)3, the 31P NMR data showed a single resonance at 84.6 ppm attributable to the cation [tBu3PSPh] [64]. Measuring the 19F spectrum at 60°C shows that the two sets of peaks from the two species coalesce into a single resonance, while at −60°C, the ratio of the indium products was altered to 1 : 0.45. These data suggest the presence of equilibria between these anions. Varying the ratios of 2 to diphenyl disulfide and tBu3P to 0.5 : 1 : 1 showed that the resonances attributable to 12 dominated, while the peaks attributed to 13 were enhanced when five equivalents of 2 was used instead. These data stand in contrast with the corresponding reaction of B(C6F5)3 that yields only [tBu3PSPh][PhSB(C6F5)3] [64].
Scheme 4.
Reactions of 1 or 2 with diphenyl disulfide.
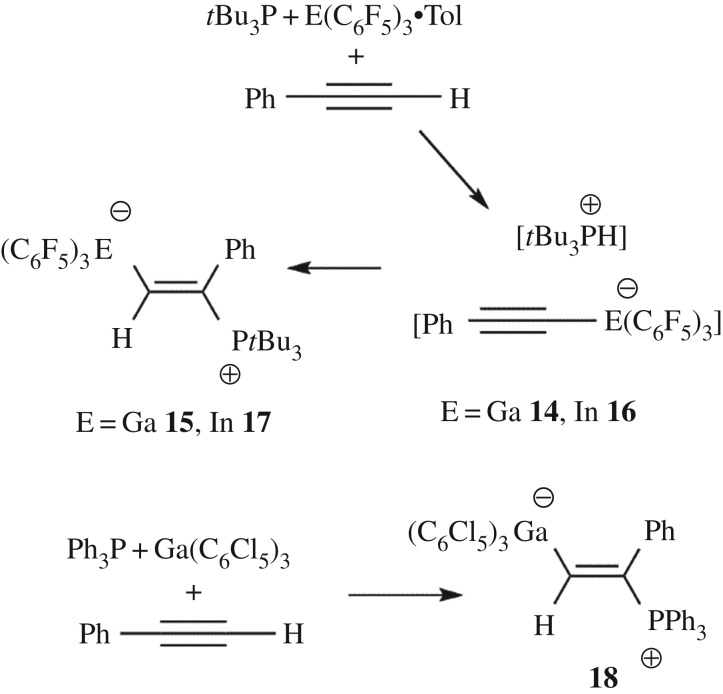
A classical reaction of FLPs involves either FLP addition to a terminal acetylene or deprotonation of the alkyne to give the phosphonium alkynylborate. The analogous reaction of 1 with an equimolar amount of tBu3P and phenylacetylene in toluene resulted in the formation of an oil at room temperature after 15 min. The 31P NMR spectrum indicated the presence of a minor amount of (tBu3P)Ga(C6F5)3 adduct as evidenced by the signal at 58.8 ppm. In addition, a major species 14 gave rise to a doublet at 59.9 ppm with 1JPH = 429 Hz attributable to the cation [tBu3PH]. Meanwhile, a new singlet peak appeared at 40.7 ppm in the 31P NMR spectrum attributable to a new species 15. This latter peak correlated to a doublet (J = 30 Hz) in the 1H NMR spectrum. This latter observation is reminiscent of the previous report [22] describing the reaction of Al(C6F5)3, (o-tol)3P and phenylacetylene which was shown to give the addition of the FLP to the alkyne affording the zwitterionic product (o-tol)3P(Ph)C═C(H)Al(C6F5)3, which gave a doublet in the 1H NMR at 8.05 ppm with 3JPH = 43 Hz [22]. Thus, compounds 14 and 15 are proposed to be the analogous species [tBu3PH][PhCCGa(C6F5)3] and tBu3P(Ph)C=C(H)Ga(C6F5)3, respectively (scheme 5). On warming the mixture to 40°C overnight, partial conversion of 14 to 15 was observed as evidenced by the 31P NMR spectra. These data suggest that 14 is the kinetic product and this slowly transforms to the thermodynamic product 15. The formulation of compound 15 was confirmed by single-crystal X-ray diffraction (figure 5). The structure shows addition of phosphine and gallium to the alkyne in an E-fashion, with phosphine adding to the substituted carbon of the alkyne. The phenyl ring is oriented parallel to one of the C6F5 rings with a distance of 3.6 Å, suggesting π–π stacking interaction between the two rings. The trans-orientation of the Ga and P fragments stands in contrast with the cis-addition previously observed by the Uhl group in [PhCH=C(P(C6H3Me2)2)(EtBu2)(PhCH=CH)] (E = Al [33], Ga [50]).
Scheme 5.
Reactions of 1 or 2 with phosphine and alkyne.
Figure 5.
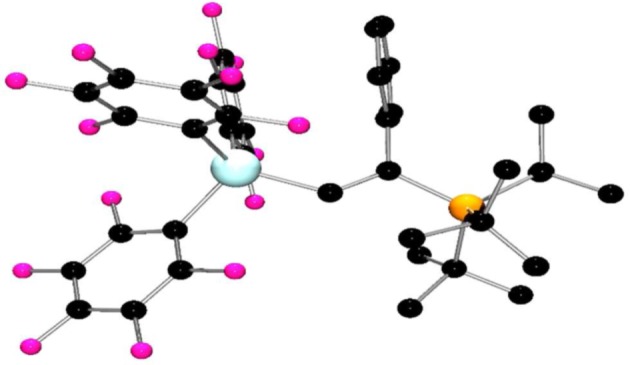
Molecular structure of 15. H-atoms have been omitted for clarity. C, black; P, orange; Ga, turquoise; F, pink.
In the analogous reaction of 2 with tBu3P and phenylacetylene, stirring for 20 min at room temperature and removing all the volatiles afforded a white solid. The 1H NMR spectrum revealed a characteristic doublet at 3.64 ppm (1JPH = 432 Hz) and the 31P NMR spectrum also contained a doublet at 57.9 ppm (1JPH = 432 Hz). The 19F NMR spectrum showed three resonances at −116.3, −157.0 and −162.1 ppm, consistent with the formation of a four coordinate indium centre. These results suggest the formation of the simple deprotonation [tBu3PH][PhCCIn(C6F5)3] 16 (scheme 5). However, another set of 19F resonances was also observed, suggesting the formation of the addition product tBu3P(Ph)C=C(H)In(C6F5)3 17. This addition product is formed typically in about 15–20% of the total yield. While separation of these products proved challenging, crystals of 17 were obtained from a solution in an NMR tube. The subsequent crystallographic study confirmed the formulation of 17 (figure 6). The structure of 17 is analogous to 15 with an In–C distance of 2.204(4) Å and a new P–C bond length of 1.850(4) Å. It is interesting to note that the initially formed ratios of 14 : 15 and 16 : 17 are 1 : 1.5 and 1 : 4.5, respectively, reflecting the greater Lewis acidity of 1 over 2.
Figure 6.
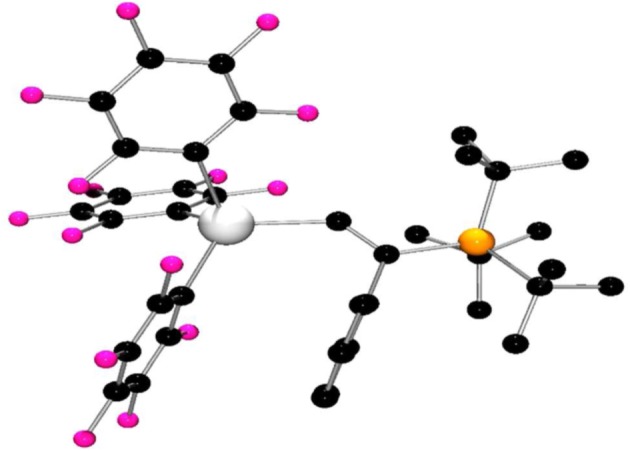
Molecular structure of 17. H-atoms have been omitted for clarity. C, black; P, orange; In, grey; F, pink.
In our efforts to examine the reactivity of 5, reactions with phosphine and H2 were challenged by product solubility difficulties. However, the reaction of 5 with PPh3 and phenylacetylene at room temperature gave a yellow solution, from which crystals were isolated in 30% yield. The 1H NMR spectrum showed a doublet at 8.85 ppm with 3JPH = 41 Hz and a 31P{1H} signal at 20.2 ppm. Mass spectral data showed a peak corresponding to the mass ion consistent with the formulation of 18 as Ph3P(Ph)C=C(H)Ga(C6Cl5)3 (figure 7). This was further supported by the determination of the crystal structure of 18, which showed the addition of phosphine and gallium to the alkyne in an analogous fashion to 15 and 17. The newly formed Ga–C bond to the linker was found to be 2.020(3) Å, while the P–C bond to the olefin carbon is 1.810(3) Å.
Figure 7.
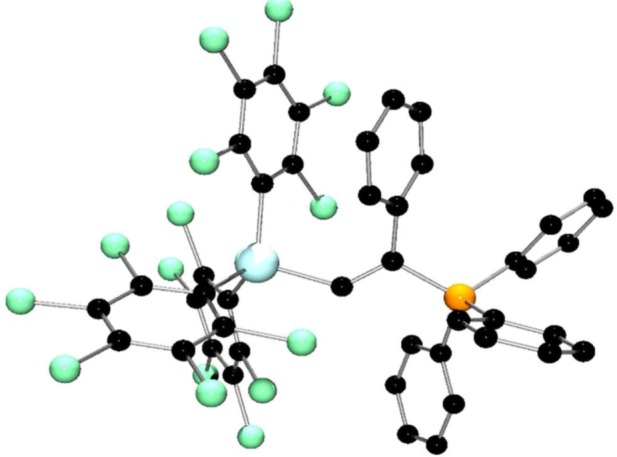
Molecular structure of 18. H-atoms have been omitted for clarity. C, black; P, orange; Ga, turquoise; Cl, green.
It is noteworthy that combinations of B(C6F5)3 and Al(C6F5)3 and tBu3P in reactions with phenylacetylene lead exclusively to the deprotonation products [tBu3PH][PhCCB(C6F5)3] and [tBu3PH][PhCCAl(C6F5)3]. A proposed mechanism for these reactions [65] involves an initial π-interaction of the alkyne with the Lewis acid. This prompts increased acidity of the alkynyl proton. The present results imply the decreased Lewis acidity of the gallium and indium Lewis acids prompting competitive addition of phosphine to the β-carbon. In addition, the longer Ga–C and In–C bond lengths diminish the steric congestion about the alkyne, allowing addition to compete with deprotonation.
3. Conclusion
The Lewis acids Ga(C6F5)3, In(C6F5)3 and Ga(C6Cl5)3 were prepared and shown to exhibit lesser Lewis acidity than the boron and aluminium species B(C6F5)3 and Al(C6F5)3. Nonetheless, these species participate in FLP chemistry in conjunction with phosphine donors. These species are shown to heterolytically split H2, catalyse the hydrogenation of an imine, effect the heterolytic cleavage of diphenyldisulfide and effect reactions with alkynes. Interestingly, the larger size of gallium and indium results in hydride- and thiolate-bridged anions, while the lower Lewis acidity prompts the formation of mixtures of deprotonation and addition products in the reactions with phenylacetylene. These data extend the range of Lewis acids that effect FLP chemistry and current efforts are directed at exploring further aspects of gallium and indium species in hydrogenation chemistry.
Supplementary Material
Data accessibility
Experimental and spectroscopic data have been deposited as electronic supplementary material. Crystallographic data are deposited in the CCDC (1535322–1535327 and 1535221).
Authors' contributions
J.R., M.X. and J.P. performed the synthetic chemistry described herein. J.P. also performed the computational work, while A.E.W. assisted with X-ray crystallography. J.P., M.X., W.U. and D.W.S. wrote and edited the manuscript. W.U. and D.W.S. were the research directors.
Competing interests
We declare we have no competing interests.
Funding
NSERC of Canada is thanked for financial support and the award of a Canada Research Chair to D.W.S. In addition, D.W.S. is grateful for the award of an Einstein Fellowship by the Einstein Foundation. We thank the Deutsche Forschungsgemeinschaft (IRTG 2027) for generous financial support.
References
- 1.Lewis GN. 1923. Valence and the structure of atoms and molecules. New York, NY: Chemical Catalogue Company, Inc. [Google Scholar]
- 2.Welch GC, Juan RRS, Masuda JD, Stephan DW. 2006. Reversible, metal-free hydrogen activation. Science 314, 1124–1126. ( 10.1126/science.1134230) [DOI] [PubMed] [Google Scholar]
- 3.Welch GC, Stephan DW. 2007. Facile heterolytic cleavage of dihydrogen by phosphines and boranes. J. Am. Chem. Soc. 129, 1880–1881. ( 10.1021/Ja067961j) [DOI] [PubMed] [Google Scholar]
- 4.Chase PA, Welch GC, Jurca T, Stephan DW. 2007. Metal-free catalytic hydrogenation. Angew. Chem. Int. Ed. 46, 8050–8053. ( 10.1002/anie.200702908) [DOI] [PubMed] [Google Scholar]
- 5.Mahdi T, Stephan DW. 2014. Enabling catalytic ketone hydrogenation by frustrated Lewis pairs. J. Am. Chem. Soc. 136, 15 809–15 812. ( 10.1021/ja508829x) [DOI] [PubMed] [Google Scholar]
- 6.Hounjet LJ, Stephan DW. 2014. Hydrogenation by frustrated Lewis pairs: main group alternatives to transition metal catalysts? Org. Process Res. Dev. 18, 385–391. ( 10.1021/Op400315m) [DOI] [Google Scholar]
- 7.Stephan DW. 2012. ‘Frustrated Lewis pair’ hydrogenations. Org. Biomol. Chem. 10, 5740–5746. ( 10.1039/C2OB25339A) [DOI] [PubMed] [Google Scholar]
- 8.Stephan DW. 2016. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 ( 10.1126/science.aaf7229) [DOI] [PubMed] [Google Scholar]
- 9.Stephan DW, Erker G. 2015. Frustrated Lewis pair chemistry: development and perspectives. Angew. Chem. Int. Ed. 54, 6400–6441. ( 10.1002/anie.201409800) [DOI] [PubMed] [Google Scholar]
- 10.Stephan DW, Erker G. 2010. Frustrated Lewis pairs: metal-free hydrogen activation and more. Angew. Chem. Int. Ed. 49, 46–76. ( 10.1002/anie.200903708) [DOI] [PubMed] [Google Scholar]
- 11.Liu YB, Du HF. 2013. Metal-free borane-catalyzed highly stereoselective hydrogenation of pyridines. J. Am. Chem. Soc. 135, 12 968–12 971. ( 10.1021/ja406761j) [DOI] [PubMed] [Google Scholar]
- 12.Liu YB, Du HF. 2013. Chiral dienes as ‘ligands’ for borane-catalyzed metal-free asymmetric hydrogenation of imines. J. Am. Chem. Soc. 135, 6810–6813. ( 10.1021/ja4025808) [DOI] [PubMed] [Google Scholar]
- 13.Liu YB, Du HF. 2014. Frustrated Lewis pair catalyzed asymmetric hydrogenation. Acta Chim. Sin. 72, 771–777. ( 10.6023/A14040344) [DOI] [Google Scholar]
- 14.Wang G, Chen C, Du TQ, Zhong WH. 2014. Metal-free catalytic hydrogenation of imines with recyclable [2.2]paracyclophane-derived frustrated Lewis pairs catalysts. Adv. Synth. Catal. 356, 1747–1752. ( 10.1002/adsc.201301007) [DOI] [Google Scholar]
- 15.Zhu XX, Du HF. 2015. A chiral borane catalyzed asymmetric hydrosilylation of imines. Org. Biomol. Chem. 13, 1013–1016. ( 10.1039/c4ob02419b) [DOI] [PubMed] [Google Scholar]
- 16.Ren X, Du H. 2016. Chiral frustrated Lewis pairs catalyzed highly enantioselective hydrosilylations of 1,2-dicarbonyl compounds. J. Am. Chem. Soc. 138, 810–813. ( 10.1021/jacs.5b13104) [DOI] [PubMed] [Google Scholar]
- 17.Chen DJ, Klankermayer J. 2013. Frustrated Lewis pairs: from dihydrogen activation to asymmetric catalysis. Top. Curr. Chem. 334, 1–26. ( 10.1007/128_2012_402) [DOI] [PubMed] [Google Scholar]
- 18.Lam J, Gunther BAR, Farrell JM, Eisenberger P, Bestvater BP, Newman PD, Melen RL, Crudden CM, Stephan DW. 2016. Chiral carbene–borane adducts: precursors for borenium catalysts for asymmetric FLP hydrogenations. Dalton Trans. 45, 15 303–15 316. ( 10.1039/c6dt02202b) [DOI] [PubMed] [Google Scholar]
- 19.Stephan DW. 2015. Frustrated Lewis pairs. J. Am. Chem. Soc. 137, 10 018–10 032. ( 10.1021/jacs.5b06794) [DOI] [PubMed] [Google Scholar]
- 20.Stephan DW. 2015. Frustrated Lewis pairs: from concept to catalysis. Acc. Chem. Res. 48, 306–316. ( 10.1021/ar500375j) [DOI] [PubMed] [Google Scholar]
- 21.Legare MA, Courtemanche MA, Rochette E, Fontaine FG. 2015. Metal-free catalytic C-H bond activation and borylation of heteroarenes. Science 349, 513–516. ( 10.1126/science.aab3591) [DOI] [PubMed] [Google Scholar]
- 22.Dureen MA, Stephan DW. 2009. Terminal alkyne activation by frustrated and classical Lewis acid/phosphine pairs. J. Am. Chem. Soc. 131, 8396–8398. ( 10.1021/ja903650w) [DOI] [PubMed] [Google Scholar]
- 23.Ménard G, Stephan DW. 2012. C-H activation of isobutylene using frustrated Lewis pairs: aluminum and boron σ-allyl complexes. Angew. Chem. Int. Ed. 51, 4409–4412. ( 10.1002/anie.201200328) [DOI] [PubMed] [Google Scholar]
- 24.Ménard G, Tran L, McCahill JSJ, Lough AJ, Stephan DW. 2013. Contrasting the reactivity of ethylene and propylene with P/Al and P/B frustrated Lewis pairs. Organometallics 32, 6759–6763. ( 10.1021/Om400222w) [DOI] [Google Scholar]
- 25.Ménard G, Tran L, Stephan DW. 2013. Activation of H2 using P/Al based frustrated Lewis pairs and reactions with olefins. Dalton Trans. 42, 13 685–13 691. ( 10.1039/C3dt51739j) [DOI] [PubMed] [Google Scholar]
- 26.Ménard G, Stephan DW. 2012. H2 activation and hydride transfer to olefins by Al(C6F5)3-based frustrated Lewis pairs. Angew. Chem. Int. Ed. 51, 8272–8275. ( 10.1002/anie.201203362) [DOI] [PubMed] [Google Scholar]
- 27.Ménard G, Stephan DW. 2010. Room temperature reduction of CO2 to methanol by Al-based frustrated Lewis pairs and ammonia borane. J. Am. Chem. Soc. 132, 1796–1797. ( 10.1021/Ja9104792) [DOI] [PubMed] [Google Scholar]
- 28.Ménard G, Stephan DW. 2013. CO2 reduction via aluminum complexes of ammonia boranes. Dalton Trans. 42, 5447–5453. ( 10.1039/C3dt00098b) [DOI] [PubMed] [Google Scholar]
- 29.Ménard G, Stephan DW. 2011. Stoichiometric reduction of CO2 to CO by aluminum-based frustrated Lewis pairs. Angew. Chem. Int. Ed. 50, 8396–8399. ( 10.1002/anie.201103600) [DOI] [PubMed] [Google Scholar]
- 30.Ménard G, Gilbert TM, Hatnean JA, Kraft A, Krossing I, Stephan DW. 2013. Stoichiometric reduction of CO2 to CO by phosphine/AlX3-based frustrated Lewis pairs. Organometallics 32, 4416–4422. ( 10.1021/Om400619y) [DOI] [Google Scholar]
- 31.Otten E, Neu RC, Stephan DW. 2009. Complexation of nitrous oxide by frustrated Lewis pairs. J. Am. Chem. Soc. 131, 9918–9919. ( 10.1021/Ja904377v) [DOI] [PubMed] [Google Scholar]
- 32.Boudreau J, Courtemanche MA, Fontaine FG. 2011. Reactivity of Lewis pairs (R2PCH2AlMe2)2 with carbon dioxide. Chem. Commun. 47, 11 131–11 133. ( 10.1039/C1cc14641f) [DOI] [PubMed] [Google Scholar]
- 33.Appelt C, Westenberg H, Bertini F, Ehlers AW, Slootweg JC, Lammertsma K, Uhl W. 2011. Geminal phosphorus/aluminum-based frustrated Lewis pairs: C-H versus C identical with C activation and CO2 fixation. Angew. Chem. Int. Ed. 50, 3925–3928. ( 10.1002/anie.201006901) [DOI] [PubMed] [Google Scholar]
- 34.Appelt C, Slootweg J, Lammertsma K, Uhl W. 2012. A phosphorus/aluminum-based frustrated Lewis pair as an ion pair receptor: alkali metal hydride adducts and phase-transfer catalysis. Angew. Chem. Int. Ed. 51, 5911–5914. ( 10.1002/anie.201201855) [DOI] [PubMed] [Google Scholar]
- 35.Roters S, Appelt C, Westenberg H, Hepp A, Slootweg J, Lammertsma K, Uhl W. 2012. Dimeric aluminum–phosphorus compounds as masked frustrated Lewis pairs for small molecule activation. Dalton Trans. 41, 9033–9045. ( 10.1039/c2dt30080j) [DOI] [PubMed] [Google Scholar]
- 36.Uhl W, Appelt C. 2013. Reactions of an Al–P-based frustrated Lewis pair with carbonyl compounds: dynamic coordination of benzaldehyde, activation of benzoyl chloride, and Al–C bond cleavage with benzamide. Organometallics 32, 5008–5014. ( 10.1021/Om400620h) [DOI] [Google Scholar]
- 37.Uhl W, Appelt C, Backs J, Westenberg H, Wollschlager A, Tannert J. 2014. Al/P-based frustrated Lewis pairs: limitations of their synthesis by hydroalumination and formation of dialkylaluminum hydride adducts. Organometallics 33, 1212–1217. ( 10.1021/Om4012246) [DOI] [Google Scholar]
- 38.Prakash GKS, Mathew T, Olah GA. 2012. Gallium(III) triflate: an efficient and a sustainable Lewis acid catalyst for organic synthetic transformations. Acc. Chem. Res. 45, 565–577. ( 10.1021/ar2002039) [DOI] [PubMed] [Google Scholar]
- 39.Prakash GKS, Do C, Mathew T, Olah GA. 2011. Reduction of carbonyl to methylene: organosilane-Ga(OTf)3 as an efficient reductant system. Catal. Lett. 141, 507–511. ( 10.1007/s10562-011-0551-0) [DOI] [Google Scholar]
- 40.Prakash GKS, Yan P, Török B, Bucsi I, Tanaka M, Olah GA. 2003. Gallium(III) trifluormethanesulfonate: a water-tolerant, reusable Lewis acid catalyst for Friedel–Crafts reactions. Catal. Lett. 85, 1–6. ( 10.1023/a:1022133227407) [DOI] [Google Scholar]
- 41.Boumizane K, Herzog-Cance MH, Jones DJ, Pascal JL, Potier J, Roziere J. 1991. Synthesis, vibrational spectroscopy and EXAFS analysis of some divalent and trivalent trifluoromethanesulphonato complexes. Polyhedron 10, 2757–2769. ( 10.1016/S0277-5387(00)86177-X) [DOI] [Google Scholar]
- 42.Kobayashi S, Komoto I, Matsuo J-I. 2001. Catalytic Friedel–Crafts acylation of aniline derivatives. Adv. Synth. Catal. 343, 71–74. ( 10.1002/1615-4169(20010129)343:1) [DOI] [Google Scholar]
- 43.Kobayashi S, Ogawa C. 2006. New entries to water-compatible Lewis acids. Chem. Eur. J. 12, 5954–5960. ( 10.1002/chem.200600385) [DOI] [PubMed] [Google Scholar]
- 44.Laali KK, Sarca VD, Okazaki T, Brock A, Der P. 2005. Triflic acid-catalyzed adamantylation of aromatics in [BMIM][OTf] ionic liquid; synthetic scope and mechanistic insight. Org. Biomol. Chem. 3, 1034–1042. ( 10.1039/B416997B) [DOI] [PubMed] [Google Scholar]
- 45.Lacey JR, Anzalone PW, Duncan CM, Hackert MJ, Mohan RS. 2005. A study of epoxyolefin cyclizations catalyzed by bismuth trifluoromethanesulfonate and other metal triflates. Tetrahedron Lett. 46, 8507–8511. ( 10.1016/j.tetlet.2005.10.013) [DOI] [Google Scholar]
- 46.Olah GA, Farooq O, Farnia SMF, Olah JA. 1988. Friedel–Crafts chemistry. 11. Boron, aluminum, and gallium tris(trifluoromethanesulfonate) (triflate): effective new Friedel–Crafts catalysts. J. Am. Chem. Soc. 110, 2560–2565. ( 10.1021/ja00216a032) [DOI] [Google Scholar]
- 47.Loh T-P, Wei L-L. 1998. Indium trichloride-catalyzed conjugate addition of amines to α,β-ethylenic compounds in water. Synlett 1998, 975–976. ( 10.1055/s-1998-1845) [DOI] [Google Scholar]
- 48.Michelet B, Bour C, Gandon V. 2014. Gallium-assisted transfer hydrogenation of alkenes. Chem. Eur. J. 20, 14 488–14 492. ( 10.1002/chem.201404139) [DOI] [PubMed] [Google Scholar]
- 49.Abdalla JAB, Riddlestone IM, Tirfoin R, Aldridge S. 2015. Cooperative bond activation and catalytic reduction of carbon dioxide at a group 13 metal center. Angew. Chem. Int. Ed. 54, 5098–5102. ( 10.1002/anie.201500570) [DOI] [PubMed] [Google Scholar]
- 50.Backs J, Lange M, Possart J, Wollschlager A, Muck-Lichtenfeld C, Uhl W.. 2017. Facile modulation of FLP properties: a phosphinylvinyl Grignard reagent and Ga/P- and In/P2-based frustrated Lewis pairs. Angew. Chem. Int. Ed. 56, 3094–3097. ( 10.1002/anie.201612485) [DOI] [PubMed] [Google Scholar]
- 51.Ghuman KK, Hoch LB, Szymanski P, Loh JY, Kherani NP, El-Sayed MA, Ozin GA, Singh CV. 2016. Photoexcited surface frustrated Lewis pairs for heterogeneous photocatalytic CO2 reduction. J. Am. Chem. Soc. 138, 1206–1214. ( 10.1021/jacs.5b10179) [DOI] [PubMed] [Google Scholar]
- 52.Ghoussoub M, Yadav S, Ghuman KK, Ozin GA, Singh CV. 2016. Metadynamics-biased ab initio molecular dynamics study of heterogeneous CO2 reduction via surface frustrated Lewis pairs. ACS Catal. 6, 7109–7117. ( 10.1021/acscatal.6b01545) [DOI] [Google Scholar]
- 53.Ghuman KK, Wood TE, Hoch LB, Mims CA, Ozin GA, Singh CV. 2015. Illuminating CO2 reduction on frustrated Lewis pair surfaces: investigating the role of surface hydroxides and oxygen vacancies on nanocrystalline In2O3-x(OH)y. Phys. Chem. Chem. Phys. 17, 14 623–14 635. ( 10.1039/c5cp02613j) [DOI] [PubMed] [Google Scholar]
- 54.Lewis SP. 2009. (Pentafluorophenyl) group 11 and 12 metal compounds, processes for preparing (pentafluorophenyl) group 11 and 12 metal compounds, and uses thereof. US Patent no. US7585991 B2.
- 55.Hair GS, Cowley AH, Jones RA, McBurnett BG, Voigt A. 1999. Arene complexes of Al(C6F5)3. Relationship to a déjà vu silylium ion. J. Am. Chem. Soc. 121, 4922–4923. ( 10.1021/ja9904336) [DOI] [Google Scholar]
- 56.Ashley AE, Herrington TJ, Wildgoose GG, Zaher H, Thompson AL, Rees NH, Kramer T, O'Hare D. 2011. Separating electrophilicity and Lewis acidity: the synthesis, characterization, and electrochemistry of the electron deficient tris(aryl)boranes B(C6F5)3-n(C6Cl5)n (n = 1–3). J. Am. Chem. Soc. 133, 14 727–14 740. ( 10.1021/ja205037t) [DOI] [PubMed] [Google Scholar]
- 57.Gutmann V. 1976. Solvent effects on the reactivities of organometallic compounds. Coord. Chem. Rev. 18, 225–255. ( 10.1016/S0010-8545(00)82045-7) [DOI] [Google Scholar]
- 58.Mayer U, Gutmann V, Gerger W. 1975. The acceptor number—a quantitative empirical parameter for the electrophilic properties of solvents. Monatsh. Chem./Chem. Mon. 106, 1235–1257. ( 10.1007/bf00913599) [DOI] [Google Scholar]
- 59.Caputo CB, Hounjet LJ, Dobrovetsky R, Stephan DW. 2013. Lewis acidity of organofluorophosphonium salts: hydrodefluorination by a saturated acceptor. Science 341, 1374–1377. ( 10.1126/science.1241764) [DOI] [PubMed] [Google Scholar]
- 60.Krishnan R, Binkley JS, Seeger R, Pople JA. 1980. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654. ( 10.1063/1.438955) [DOI] [Google Scholar]
- 61.McLean AD, Chandler GS.. 1980. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 72, 5639–5648. ( 10.1063/1.438980) [DOI] [Google Scholar]
- 62.Peverati R, Truhlar DG. 2011. Improving the accuracy of hybrid meta-GGA density functionals by range separation. J. Phys. Chem. Lett. 2, 2810–2817. ( 10.1021/jz201170d) [DOI] [Google Scholar]
- 63.Atwood DA, Atwood VO, Cowley AH, Gobran HR, Jones RA, Smeal TM, Carrano CJ. 1993. Dimeric gallium and indium dialkylphosphido complexes with unusual group 13–15 stoichiometries. Organometallics 12, 3517–3521. ( 10.1021/om00033a024) [DOI] [Google Scholar]
- 64.Dureen MA, Welch GC, Gilbert TM, Stephan DW. 2009. Heterolytic cleavage of disulfides by frustrated Lewis pairs. Inorg. Chem. 48, 9910–9917. ( 10.1021/Ic901590s) [DOI] [PubMed] [Google Scholar]
- 65.Fukazawa A, Yamada H, Yamaguchi S. 2008. Phosphonium- and borate-bridged zwitterionic ladder stilbene and its extended analogues. Angew. Chem. Int. Ed. 47, 5582–5585. ( 10.1002/anie.200801834) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental and spectroscopic data have been deposited as electronic supplementary material. Crystallographic data are deposited in the CCDC (1535322–1535327 and 1535221).