Abstract
The conjugated dienamine 4 selectively adds Piers' borane [HB(C6F5)2] to give the enamine/borane system 5, which features a boratirane structure by internal enamine carbon Lewis base to boron Lewis acid interaction. Compound 5 behaves as a C/B frustrated Lewis pair and undergoes typical addition reactions to benzaldehyde, several nitriles and to sulfur dioxide.
This article is part of the themed issue ‘Frustrated Lewis pair chemistry’.
Keywords: enamine, borane, Lewis acid, Lewis base, frustrated Lewis pair
1. Introduction
Frustrated Lewis pairs (FLPs) undergo cooperative reactions with a variety of small molecules [1–3]. Most notably, they often split dihydrogen [4–6], and many FLPs were shown to selectively add to a variety of organic and inorganic π-systems. FLPs are composed of Lewis acid/base pairs which are effectively hindered (often in an equilibrium situation) from neutralizing Lewis adduct formation. This is mostly done by attaching very bulky substituents at both the Lewis acid and the Lewis base component of the pair. While the Lewis acid is mostly boron based [1–3] (with increasing attention on alternatives such as strongly electrophilic metal [7–10] or phosphonium systems [11]) the Lewis base component is mostly a bulky phosphane or amine [1–3] and sometimes ethers have been successfully used [12–14]. In some cases, substrates containing the group 15 heteroatoms have taken a dual role in the catalytic hydrogenation process, namely as the reagent and at the same time the Lewis base [15]. It must be noted that carbon Lewis bases have seldom been used in FLP chemistry, with notable exceptions being the N-heterocyclic carbene/B(C6F5)3 and related systems described by Stephan, Tamm, and others [16–27] and Alcarazo's carbodiphosphorane/B(C6F5)3 analogues [28–32]. α-Boryl carbanions might formally be candidates for new C/B FLP developments [33,34], but usually their conjugative interaction is so pronounced that they serve as borata alkenes (with their characteristic chemistry) [35–37] rather than as FLP systems.
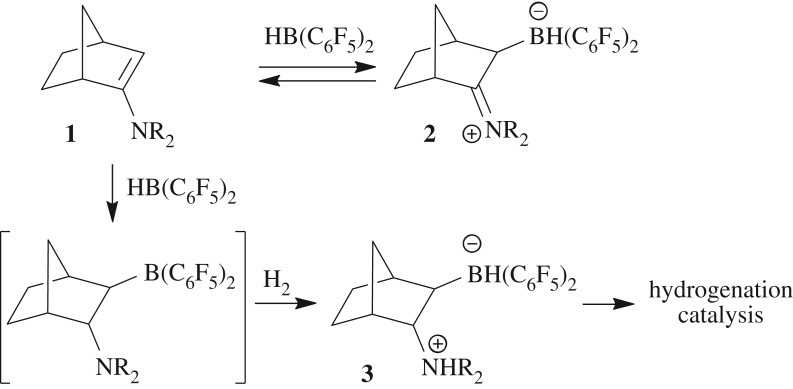
We thought that enamines might be interesting candidates for C/B FLP design and development, especially if such systems became available by a simple hydroboration route. However, there were some principal problems that needed to be overcome. We had previously shown that many enamines just form carbon/boron Lewis base/Lewis acid adducts (scheme 1); the adduct formation is often reversible and then allows simple enamine hydroboration to give N/B FLPs, a reaction that is often only detected by its subsequent H2-splitting reaction [38–41]. It requires a different substrate design to overcome this common enamine/HB(C6F5)2 behaviour. This we have done, and the first results of this new development are described below.
Scheme 1.
Trapping of the non-visible N/B FLP by the H2-splitting reaction of the respective enamine/borane adduct 2.
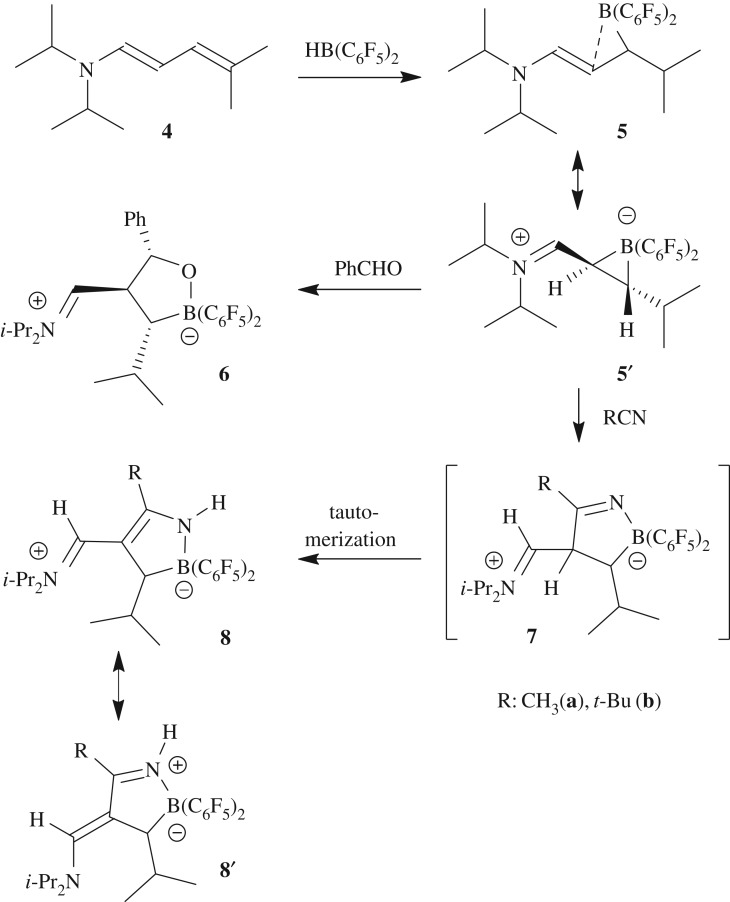
We chose the conjugated N,N-diisopropyl dienamine 4 as our substrate (see the electronic supplementary material for its synthesis). It bears a pair of geminal methyl groups at the terminal diene sp2-carbon atom and we hoped to direct the subsequent hydroboration away from the usual enamine regiochemistry. This was actually the case. We reacted a solution of the dienamine 4 in pentane with an equimolar amount of HB(C6F5)2 [42,43]. The borane does not completely dissolve in this solvent and the hydroboration reaction takes place from the suspension that is formed. After 30 min at r.t., the reaction was complete and an orange precipitate had formed. It was isolated as a solid with a 62% yield and characterized by C,H,N elemental analysis, by spectroscopy and by X-ray diffraction (single crystals were obtained at −36°C from a toluene solution layered with pentane).
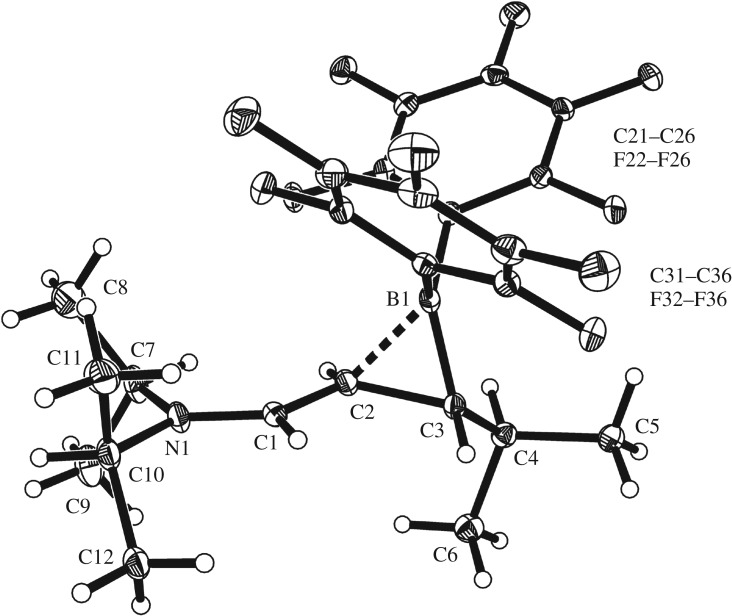
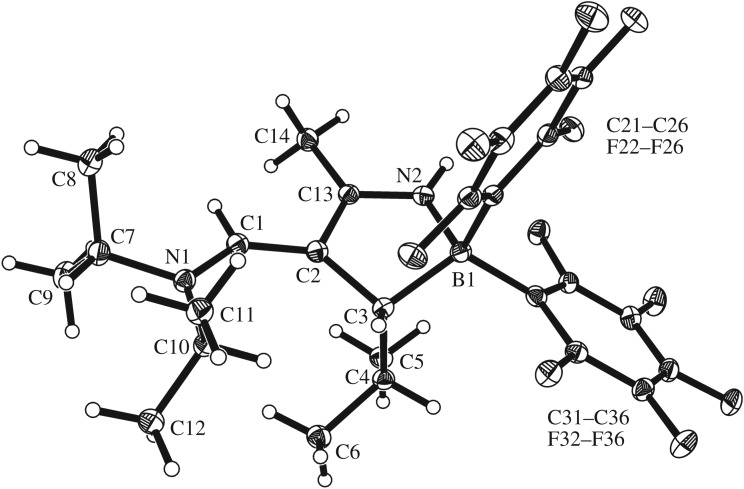
The X-ray crystal structure analysis (figure 1) shows that the hydroboration reaction of the dienamine 4 had indeed proceeded with the expected ‘inversed’ regiochemistry. The hydride had been added to the terminal dimethyl-substituted diene carbon atom C4 and, consequently, the B(C6F5)2 group was bonded to the adjacent carbon atom C3. The structure of the product can probably best be described by a resonance hybrid of the mesomeric forms 5/5′ (scheme 2). The B1–C3 bond is quite short at 1.552(2) Å (cf. B1–C21/C31: 1.610(2) Å) and the B1–C3 vector leans over towards carbon atom C2 (angle C2–C3–B1: 67.8(1)°). There is clearly a bonding interaction between B1 and C2, although it is probably weak (1.726(2) Å). We note a characteristic delocalized bond lengths pattern of the remaining 5/5′ framework (C2–C3: 1.541(2) Å, C1–C2: 1.392(2) Å, N1–C1: 1.315(2) Å). The C2–H/C3–H hydrogen atoms at the distorted boratirane [44–48] ring are trans-oriented at the framework.
Figure 1.
Molecular structure of the dienamine hydroboration product 5/5′ (thermal ellipsoids are shown with 30% probability).
Scheme 2.
Preparation of the system 5/5′ and its reactions as a C/B FLP.
The nuclear magnetic resonance (NMR) spectra of system 5/5′ in d6-benzene solution show a 11B NMR feature at δ −8.1, i.e. in the typical tetracoordinated borate range. Consequently, compound 5/5′ shows the 19F NMR resonances of a pair of diastereotopic C6F5 substituents at boron with small Δδ19Fm,p chemical shift differences of 6.4 and 5.6 ppm, respectively. The iPr2N = CH-unit shows 1H/13C NMR signals at δ 6.04/170.3 and the boratirane C2–H/C3–H 1H NMR signals occur at δ 2.31/1.87.
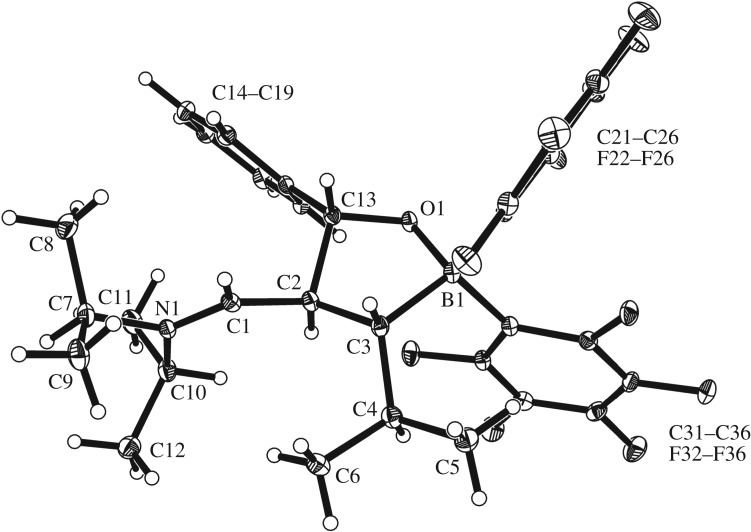
A variety of FLPs undergo 1,2-addition reactions to benzaldehyde and other organic carbonyl compounds [49–51]. Compound 5 reacts analogously. It forms the addition product 6 upon exposure to benzaldehyde at r.t. overnight. We isolated the product after work-up with a 67% yield. The X-ray crystal structure analysis shows that a five-membered heterocycle had been obtained by B–O and C–C bond formation (figure 2). It features the three large substituents in a trans-, trans-orientation, namely the phenyl group at C13, the iminium cation moiety (N1–C1: 1.291(3) Å) at C2 and the isopropyl group at C3.
Figure 2.
A projection of the molecular structure of the benzaldehyde addition product 6 to the C/B FLP (thermal ellipsoids are shown with 15% probability). Selected bond lengths (Å) and angles (°): O1–C13: 1.402(3), O1–B1: 1.488(3), N1–C1: 1.291(3), C1–C2: 1.462(3), C2–C3: 1.552(3), C2–C13: 1.572(3), C3–B1: 1.674(3), C3–C4: 1.531(4), C13–O1–B1: 109.2(2), C1–N1–C10: 124.1(2), N1–C1–C2: 129.3(2), C3–C2–C13: 101.6(2), C2–C3–B1: 100.6(2).
Compound 6 shows an iminium type 1H/13C NMR pair of signals at δ 8.52/184.2, a 11B NMR feature in the borate anion range (δ 2.2), 19F NMR signals of a pair of diastereotopic C6F5 groups at boron as well as six 1H NMR methyl signals.
The C/B FLP 5/5′ reacts in a similar way with organic nitriles. The reaction with acetonitrile is a typical example. The C/B FLP was prepared in situ by treatment of the dienylamine 4 with Piers' borane (pentane, r.t. 2 h) and then acetonitrile was added (approx. 1.1 equiv.) and the reaction mixture stirred overnight at r.t. Work-up gave the product 8a (R: CH3) with an approximately 57% yield. We assume that first the primary nitrile addition product 7a is formed, which then undergoes a tautomerization reaction under the applied reaction conditions. The X-ray crystal structure analysis of 8a shows the formation of the N-containing five-membered heterocycle (figure 3). It shows the following bond lengths of the conjugated core: N1–C1: 1.338(3) Å, C1–C2: 1.385(3) Å, C2–C13: 1.429(3) Å, and C13–N2: 1.313(3) Å, which indicates a largely delocalized structure of the central N2C3 π-system reminiscent of a Zincke-aldimine-type derivative [52].
Figure 3.
Molecular structure of compound 8a (thermal ellipsoids are shown with 30% probability).
Compound 8a/8a′ shows the 13C NMR (d8-toluene, 253 K) feature of the N1–C1 unit at δ 142.2 (1H: δ 6.58) and of the C13–N2 unit at δ 179.8 (1H: δ 7.30 NH) and it features the 11B NMR signal at δ −3.1. The C/B FLP 5/5′ reacts similarly with pivalonitrile to give the product 8b, which shows similar structural and spectroscopic parameters (see the electronic supplementary material for details).
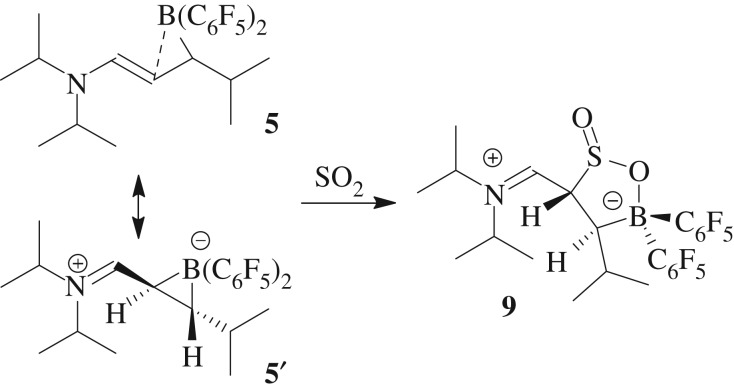
P/B FLPs are known to often typically react with simple binary main group element oxides [53,54]. Therefore, we have exposed our compound 5 to sulfur dioxide in order to find out if it undergoes a related SO2 C/B FLP addition. Actually, it does (scheme 3). Exposure of the C/B FLP system 5/5′ to SO2 (1.5 bar) in d6-benzene gave a full conversion to the C/B FLP SO2 addition product 9. Crystals suitable for crystal structure analysis were obtained from a saturated toluene solution layered with pentane at room temperature.
Scheme 3.
Reaction of the system 5/5′ with SO2.
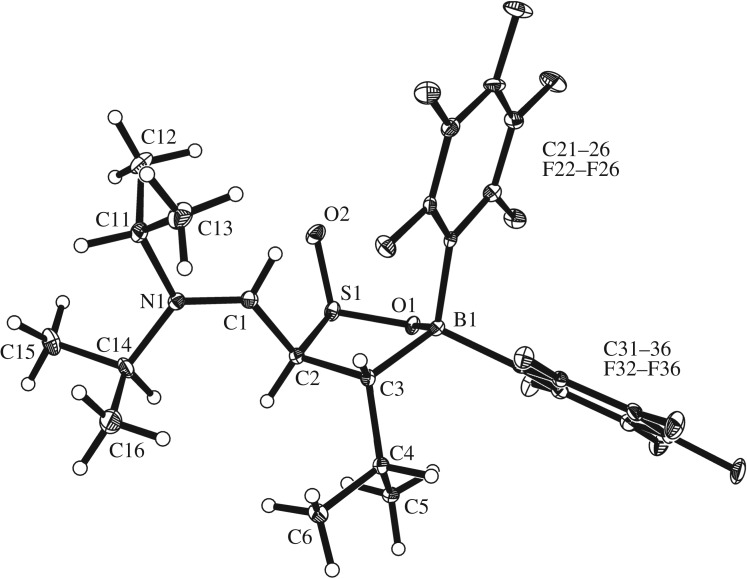
The X-ray crystal structure analysis of the C/B FLP SO2 addition product 9 shows a central five-membered heterocycle that was formed by addition of the boron Lewis acid to oxygen and the enamine carbon to sulfur (figure 4). It features an envelope-shaped conformation with boron being the tip (angle between the O1–S1–C2–C3/O1–B1–C3 planes: 144.5°). The bond angle at boron amounts to O1–B1–C3 102.1(2)°. The sulfur shows a sum of bond angles of ΣS1OOC 309.8(2)°. In compound 9, the endocyclic sulfur–oxygen bond length is 1.559(2) Å, whereas the exocyclic S=O linkage is much shorter at 1.483(2) Å. The newly introduced S=O bond is oriented cis-coplanar with the iminium substituent (N1–C1: 1.289(4) Å) at the adjacent carbon atom C2 (θ O2–S1–C2–C1: 11.4(2)°) and it is oriented trans to the isopropyl substituent at C3.
Figure 4.
A view of the molecular geometry of the C/B FLP SO2 adduct 9 (thermal ellipsoids are shown with 30% probability).
In solution (d6-benzene), we monitored the NMR signals of a single diastereomer of 9. It features the 1H NMR signals of the trio of C1–H/C2–H/C3–H hydrogen atoms at δ 7.69, 4.09 and 3.14, in addition to a total of six isopropyl CH3 doublets and the 19F NMR signals of a pair of diastereotopic C6F5 groups at boron (11B NMR: δ 9.3).
We have synthesized the enamine-derived C/B FLP system 5 by a very convenient hydroboration route, namely the reaction of the substituted conjugated dienamine 4 with Piers' borane (HB(C6F5)2). The presence of the terminal geminal dimethyl substituents directed the hydroboration reaction completely to form the C3-based borane. Characteristically, the borane Lewis acid forms a weak C···B bond with its adjacent enamine-type C(sp2) centre, giving a distorted boratirane structure. This arrangement is similar to a variety of weakly bonded P···B or N···B situations, and, consequently, our newly formed C/B FLP undergoes typical FLP addition reactions with a variety of added organic π-systems. In the case of the benzaldehyde addition reaction, a stereochemical outcome is obtained that results from a typical minimum steric interaction approach of the PhCHO reagent to the C···B boratirane unit. The trans-CH–CH arrangement of the latter is also retained to eventually yield the product 6. In the case of the nitrile addition reactions, the stereochemistry of the C/B FLP addition reaction cannot be followed due to the rapid subsequent tautomerization process giving rise to the formation of the delocalized nitrogen-containing π-system. Actually, the C/B FLP even adds to SO2, giving a typical FLP sulfur dioxide adduct. This study shows that this new type of C/B FLP is easily prepared and shows typical FLP features. We will explore its chemistry further and will see if it helps us to discover new FLP reactions.
Supplementary Material
Data accessibility
This article has no additional data.
Authors' contributions
B.W. and C.G.D. carried out the X-ray crystal structure analyses.
Competing interests
We declare we have no competing interests.
Funding
Financial support for this study by the Deutsche Forschungsgemeinschaft is gratefully acknowledged.
References
- 1.Stephan DW, Erker G (eds). 2013. Frustrated Lewis pairs I: uncovering and understanding. Topics in Current Chemistry, vol. 332 Heidelberg, Germany: Springer. [Google Scholar]
- 2.Stephan DW, Erker G (eds). 2013. Frustrated Lewis pairs II: expanding the scope. Topics in Current Chemistry, vol. 334 Heidelberg, Germany: Springer. [Google Scholar]
- 3.Stephan DW, Erker G. 2015. Frustrated Lewis pair chemistry: development and perspectives. Angew. Chem. Int. Ed. 54, 6400–6441. ( 10.1002/anie.201409800) [DOI] [PubMed] [Google Scholar]
- 4.Welch GC, Juan RRS, Masuda JD, Stephan DW. 2006. Reversible, metal-free hydrogen activation. Science 314, 1124–1126. ( 10.1126/science.1134230) [DOI] [PubMed] [Google Scholar]
- 5.Welch GC, Stephan DW. 2007. Facile heterolytic cleavage of dihydrogen by phosphines and boranes. J. Am. Chem. Soc. 129, 1880–1881. ( 10.1021/ja067961j) [DOI] [PubMed] [Google Scholar]
- 6.Spies P, Erker G, Kehr G, Bergander K, Fröhlich R, Grimme S, Stephan DW. 2007. Rapid intramolecular heterolytic dihydrogen activation by a four-membered heterocyclic phosphane-borane adduct. Chem. Commun. 2007, 5072–5074. ( 10.1039/b710475h) [DOI] [PubMed] [Google Scholar]
- 7.Chapman AM, Haddow MF, Wass DF. 2011. Frustrated Lewis pairs beyond the main group: synthesis, reactivity, and small molecule activation with cationic zirconocene-phosphinoaryloxide complexes. J. Am. Chem. Soc. 133, 18 463–18 478. ( 10.1021/ja207936p). [DOI] [PubMed] [Google Scholar]
- 8.Chapman AM, Haddow MF, Wass DF. 2011. Frustrated Lewis pairs beyond the main group: cationic zirconocene-phosphinoaryloxide complexes and their application in catalytic dehydrogenation of amine boranes. J. Am. Chem. Soc. 133, 8826–8829. ( 10.1021/ja201989c) [DOI] [PubMed] [Google Scholar]
- 9.Appelt C, Slootweg JC, Lammertsma K, Uhl W. 2012. A phosphorus/aluminum-based frustrated Lewis pair as an ion pair receptor: alkali metal hydride adducts and phase-transfer catalysis. Angew. Chem. Int. Ed. 51, 5911–5914. ( 10.1002/anie.201201855) [DOI] [PubMed] [Google Scholar]
- 10.Roters S, Appelt C, Westenberg H, Hepp A, Slootweg JC, Lammertsma K, Uhl W. 2012. Dimeric aluminum-phosphorus compounds as masked frustrated Lewis pairs for small molecule activation. Dalton Trans. 41, 9033–9045. ( 10.1039/c2dt30080j) [DOI] [PubMed] [Google Scholar]
- 11.Caputo CB, Hounjet LJ, Dobrovetsky R, Stephan DW. 2013. Lewis acidity of organo fluorophosphonium salts: hydrodefluorination by a saturated acceptor. Science 341, 1374–1377. ( 10.1126/science.1241764) [DOI] [PubMed] [Google Scholar]
- 12.Mahdi T, Stephan DW. 2014. Enabling catalytic ketone hydrogenation by frustrated Lewis pairs. J. Am. Chem. Soc. 136, 15 809–15 812. ( 10.1021/ja508829x) [DOI] [PubMed] [Google Scholar]
- 13.Scott DJ, Fuchter MJ, Ashley AE. 2014. Nonmetal catalyzed hydrogenation of carbonyl compounds. J. Am. Chem. Soc. 136, 15 813–15 816. ( 10.1021/ja5088979) [DOI] [PubMed] [Google Scholar]
- 14.Sajid M, Kehr G, Daniliuc CG, Erker G. 2014. Formylborane formation with frustrated Lewis pair templates. Angew. Chem. Int. Ed. 53, 1118–1121. ( 10.1002/anie.201307551) [DOI] [PubMed] [Google Scholar]
- 15.Chase PA, Jurca T, Stephan DW. 2008. Lewis acid-catalyzed hydrogenation: B(C6F5)3-mediated reduction of imines and nitriles with H2. Chem. Commun. 2008, 1701–1703. ( 10.1039/b718598g) [DOI] [PubMed] [Google Scholar]
- 16.Holschumacher D, Bannenberg T, Hrib CG, Jones PG, Tamm M. 2008. Heterolytic dihydrogen activation by a frustrated carbene-borane Lewis pair. Angew. Chem. Int. Ed. 47, 7428–7432. ( 10.1002/anie.200802705) [DOI] [PubMed] [Google Scholar]
- 17.Chase PA, Stephan DW.. 2008. Hydrogen and amine activation by a frustrated Lewis pair of a bulky N-heterocyclic carbene and B(C6F5)3. Angew. Chem. Int. Ed. 7, 7433–7437. ( 10.1002/anie.200802596) [DOI] [PubMed] [Google Scholar]
- 18.Chase PA, Gille AL, Gilbert TM, Stephan DW. 2009. Frustrated Lewis pairs derived from N-heterocyclic carbenes and Lewis acids. Dalton Trans. 2009, 7179–7188. ( 10.1039/B908737K) [DOI] [PubMed] [Google Scholar]
- 19.Holschumacher D, Taouss C, Bannenberg T, Hrib CG, Daniliuc CG, Jones PG, Tamm M. 2009. Dehydrogenation reactivity of a frustrated carbene-borane Lewis pair. Dalton Trans. 2009, 6927–6929. ( 10.1039/B908074K) [DOI] [PubMed] [Google Scholar]
- 20.Theuergarten E, Schluns D, Grunenberg J, Daniliuc CG, Jones PG, Tamm M. 2010. Intramolecular heterolytic dihydrogen cleavage by a bifunctional frustrated pyrazolylborane Lewis pair. Chem. Commun. 46, 8561–8563. ( 10.1039/C0CC03474F) [DOI] [PubMed] [Google Scholar]
- 21.Dureen MA, Brown CC, Stephan DW. 2010. Addition of enamines or pyrroles and B(C6F5)3 ‘frustrated Lewis pairs’ to alkynes. Organometallics 29, 6422–6432. ( 10.1021/om1008346) [DOI] [Google Scholar]
- 22.Holschumacher D, Bannenberg T, Ibrom K, Daniliuc CG, Jones PG, Tamm M. 2010. Selective heterolytic P-P bond cleavage of white phosphorus by a frustrated carbene-borane Lewis pair. Dalton Trans. 39, 10 590–10 592. ( 10.1039/C0DT01045F) [DOI] [PubMed] [Google Scholar]
- 23.Kolychev EL, Bannenberg T, Freytag M, Daniliuc CG, Jones PG, Tamm M. 2012. Reactivity of a frustrated Lewis pair and small-molecule activation by an isolable Arduengo carbene-B{3,5-(CF3)2C6H3}3 complex. Chem. Eur. J. 18, 16 938–16 946. ( 10.1002/chem.201202840) [DOI] [PubMed] [Google Scholar]
- 24.Lindqvist M, Axenov K, Nieger M, Räisänen M, Leskelä M, Repo T. 2013. Frustrated Lewis pair chemistry of chiral (+)-camphor-based aminoboranes. Chem. Eur. J. 19, 10 412–10 418. ( 10.1002/chem.201300462) [DOI] [PubMed] [Google Scholar]
- 25.Thakur A, Vardhanapu PK, Vijaykumar G, Kumar Hota P, Mandal SK. 2016. Abnormal N-heterocyclic-carbene-mediated fixation of CO2 and N2O, and the activation of tetrahydrofuran and tetrahydrothiophene under ambient conditions. Eur. J. Inorg. Chem. 2016, 913–920. ( 10.1002/ejic.201501303) [DOI] [Google Scholar]
- 26.Winkler A, Brandhorst K, Freytag M, Jones PG, Tamm M. 2016. Palladium(II) complexes with anionic N-heterocyclic carbene–borate ligands as catalysts for the amination of aryl halides. Organometallics 35, 1160–1169. ( 10.1021/acs.organomet.6b00217) [DOI] [Google Scholar]
- 27.Igarashi A, Kolychev EL, Tamm M, Nomura K. 2016. Synthesis of (imido)vanadium(V) dichloride complexes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety: new MAO-free ethylene polymerization catalysts. Organometallics 35, 1778–1784. ( 10.1021/acs.organomet.6b00200) [DOI] [Google Scholar]
- 28.Alcarazo M, Gomez C, Holle S, Goddard R. 2010. Exploring the reactivity of carbon(0)/borane-based frustrated Lewis pairs. Angew. Chem. Int. Ed. 49, 5788–5791. ( 10.1002/anie.201002119) [DOI] [PubMed] [Google Scholar]
- 29.Ines B, Patil M, Carreras J, Goddard R, Thiel W, Alcarazo M. 2011. Synthesis, structure, and reactivity of a dihydrido borenium cation. Angew. Chem. Int. Ed. 50, 8400–8403. ( 10.1002/anie.201103197) [DOI] [PubMed] [Google Scholar]
- 30.Ines B, Holle S, Goddard R, Alcarazo M. 2010. Heterolytic S-S bond cleavage by a purely carbogenic frustrated Lewis pair. Angew. Chem. Int. Ed. 49, 8389–8391. ( 10.1002/anie.201004149) [DOI] [PubMed] [Google Scholar]
- 31.Cade IA, Ingleson MJ. 2014. syn-1,2-Carboboration of alkynes with borenium cations. Chem. Eur. J. 20, 12 874–12 880. ( 10.1002/chem.201403614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGough JS, Butler SM, Cade IA, Ingleson MJ. 2016. Highly selective catalytic trans-hydroboration of alkynes mediated by borenium cations and B(C6F5)3. Chem. Sci. 7, 3384–3389. ( 10.1039/c5sc04798f) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartlett RA, Power PP. 1986. X-ray crystal-structure of the boron-stabilized carbanion—[Li(12-crown-4)2][CH2C6H2(3,5-Me2)(4-B(2,4,6-Me3C6H2)2)].Et2O—evidence for boron ylide character. Organometallics 5, 1916–1917. ( 10.1021/Om00140a032) [DOI] [Google Scholar]
- 34.Olmstead MM, Power PP, Weese KJ, Doedens RJ. 1987. Isolation and X-ray crystal-structure of the boron methylidenide ion [Mes2BCH2]-(Mes=2,4,6-Me3C6H2)—a boron carbon double bonded alkene analog. J. Am. Chem. Soc. 109, 2541–2542. ( 10.1021/Ja00242a064) [DOI] [Google Scholar]
- 35.Yu J, Kehr G, Daniliuc CG, Erker G. 2013. A unique frustrated Lewis pair pathway to remarkably stable borata-alkene systems. Eur. J. Inorg. Chem. 2013, 3312–3315. ( 10.1002/ejic.201300520). [DOI] [Google Scholar]
- 36.Möbus J, Kehr G, Daniliuc CG, Fröhlich R, Erker G. 2014. Borata-alkene derivatives conveniently made by frustrated Lewis pair chemistry. Dalton Trans. 43, 632–638. ( 10.1039/c3dt52373j) [DOI] [PubMed] [Google Scholar]
- 37.Moquist P, Chen G-Q, Muck-Lichtenfeld C, Bussmann K, Daniliuc CG, Kehr G, Erker G. 2015. Alpha-CH acidity of alkyl-B(C6F5)2 compounds—the role of stabilized borata-alkene formation in frustrated Lewis pair chemistry. Chem. Sci. 6, 816–825. ( 10.1039/c4sc01711k) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwendemann S, Fröhlich R, Kehr G, Erker G. 2011. Intramolecular frustrated N/B Lewis pairs by enamine hydroboration. Chem. Sci. 2, 1842–1849. ( 10.1039/c1sc00124h) [DOI] [Google Scholar]
- 39.Schwendemann S, Oishi S, Saito S, Fröhlich R, Kehr G, Erker G. 2013. Reaction of an ‘invisible’ frustrated N/B Lewis pair with dihydrogen. Chem. Asian J. 8, 212–217. ( 10.1002/asia.201200776) [DOI] [PubMed] [Google Scholar]
- 40.Xu B-H, Bussmann K, Fröhlich R, Daniliuc CG, Brandenburg JG, Grimme S, Kehr G, Erker G. 2013. An enamine/HB(C6F5)2 adduct as a dormant state in frustrated Lewis pair chemistry. Organometallics 32, 6745–6752. ( 10.1021/om4004225) [DOI] [Google Scholar]
- 41.Chen G-Q, Türkyilmaz F, Daniliuc CG, Bannwarth C, Grimme S, Kehr G, Erker G. 2015. Enamine/butadienylborane cycloaddition in the frustrated Lewis pair regime. Org. Biomol. Chem. 13, 10 477–10 486. ( 10.1039/c5ob01602a) [DOI] [PubMed] [Google Scholar]
- 42.Parks DJ, Spence REvH, Piers WE. 1995. Bis(pentafluorophenyl)borane—synthesis, properties, and hydroboration chemistry of a highly electrophilic borane reagent. Angew. Chem. Int. Ed. Engl. 34, 809–811. ( 10.1002/anie.199508091) [DOI] [Google Scholar]
- 43.Parks DJ, Piers WE, Yap GPA. 1998. Synthesis, properties, and hydroboration activity of the highly electrophilic borane bis(pentafluorophenyl)borane, HB(C6F5)(2). Organometallics 17, 5492–5503. ( 10.1021/Om980673e) [DOI] [Google Scholar]
- 44.Kropp M, Bhamidapaty K, Schuster GB. 1988. Boratirane—preparation and characterization of trans-1,1,2,3-tetraphenylboratirane. J. Am. Chem. Soc. 110, 6252–6254. ( 10.1021/Ja00226a054) [DOI] [PubMed] [Google Scholar]
- 45.Wilkey JD, Schuster GB. 1991. Photochemistry of tetraarylborate salts (Ar4B-)—formation of 2,5,7,7-tetraphenyl-7-boratabicyclo[4.1.0]hepta-2,4-diene (a boratanorcaradiene) by irradiation of (para-biphenylyl)triphenyl borate. J. Am. Chem. Soc. 113, 2149–2155. ( 10.1021/Ja00006a037) [DOI] [Google Scholar]
- 46.Ugolotti J, Kehr G, Fröhlich R, Grimme S, Erker G.. 2009. Nitrile insertion into a boryl-substituted five-membered zirconacycloallenoid: unexpected formation of a zwitterionic boratirane product. Chem. Commun. 2009, 6572–6573. ( 10.1039/b915924j) [DOI] [PubMed] [Google Scholar]
- 47.Zhao XX, Lough AJ, Stephan DW. 2011. Synthesis and reactivity of alkynyl-linked phosphonium borates. Chem. Eur. J. 17, 6731–6743. ( 10.1002/chem.201100203) [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Kehr G, Daniliuc CG, Bannwarth C, Grimme S, Erker G. 2015. Direct synthesis of a geminal zwitterionic phosphonium/hydridoborate system—developing an alternative tool for generating frustrated Lewis pair hydrogen activation systems. Org. Biomol. Chem. 13, 5783–5792. ( 10.1039/C5ob00634a) [DOI] [PubMed] [Google Scholar]
- 49.Mömming CM, Kehr G, Wibbeling B, Fröhlich R, Erker G. 2010. Addition reactions to the intramolecular mesityl2P-CH2-CH2-B(C6F5)2 frustrated Lewis pair. Dalton Trans. 39, 7556–7564. ( 10.1039/C0dt00015a) [DOI] [PubMed] [Google Scholar]
- 50.Ekkert O, Kehr G, Daniliuc CG, Fröhlich R, Wibbeling B, Petersen JL, Erker G. 2013. Reaction of unsaturated vicinal phosphane/borane frustrated Lewis pairs with benzaldehyde. Z. Anorg. Allg. Chem. 639, 2455–2462. ( 10.1002/zaac.201300421) [DOI] [Google Scholar]
- 51.Rosorius C, Möricke J, Wibbeling B, McQuilken AC, Warren TH, Daniliuc CG, Kehr G, Erker G. 2016. Frustrated Lewis pair chemistry derived from bulky allenyl and propargyl phosphanes. Chem. Eur. J. 22, 1103–1113. ( 10.1002/chem.201502493) [DOI] [PubMed] [Google Scholar]
- 52.Kröhnke F. 1963. Syntheses using pyridinium salts(IV). Angew. Chem. Int. Ed. Engl. 2, 380–393. ( 10.1002/anie.196303801) [DOI] [Google Scholar]
- 53.Sajid M, et al. 2013. Reactions of phosphorus/boron frustrated Lewis pairs with SO2. Chem. Sci. 4, 213–219. ( 10.1039/C2sc21161k) [DOI] [Google Scholar]
- 54.Stephan DW, Erker G. 2014. Frustrated Lewis pair chemistry of carbon, nitrogen and sulfur oxides. Chem. Sci. 5, 2625–2641. ( 10.1039/c4sc00395k) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.