Abstract
Small populations are susceptible to high genetic loads and random fluctuations in birth and death rates. While these selective forces can adversely affect their viability, small populations persist across taxa. Here, we investigate the resilience of small groups to demographic uncertainty, and specifically to fluctuations in adult sex ratio (ASR), partner availability and dispersal patterns. Using 25 years of demographic data for two Savannah Pumé groups of South American hunter–gatherers, we show that in small human populations: (i) ASRs fluctuate substantially from year to year, but do not consistently trend in a sex-biased direction; (ii) the primary driver of local variation in partner availability is stochasticity in the sex ratio at maturity; and (iii) dispersal outside of the group is an important behavioural means to mediate locally constrained mating options. To then simulate conditions under which dispersal outside of the local group may have evolved, we develop two mathematical models. Model results predict that if the ASR is biased, the globally rarer sex should disperse. The model's utility is then evaluated by applying our empirical data to this central prediction. The results are consistent with the observed hunter–gatherer pattern of variation in the sex that disperses. Together, these findings offer an alternative explanation to resource provisioning for the evolution of traits central to human sociality (e.g. flexible dispersal, bilocal post-marital residence and cooperation across local groups). We argue that in small populations, looking outside of one's local group is necessary to find a mate and that, motivated by ASR imbalance, the alliances formed to facilitate the movement of partners are an important foundation for the human-typical pattern of network formation across local groups.
This article is part of the themed issue ‘Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies’.
Keywords: dispersal, hunter–gatherers, adult sex ratio, Savannah Pumé, human evolution, sexual selection
1. Introduction
Small populations are prone to stochastic demographic processes, generating an uncertain selective landscape for individuals living within them. Compared with large groups, small populations are more vulnerable to random fluctuations in sex-biased births and deaths [1–3], and are more likely to experience shocks due to famine, disease and other exogenous sources of mortality (e.g. warfare or predation [2,4–6]). The relationship between population size and individual fitness, or the Allee effect, underscores the vulnerability of small populations to mate limitation [7–9]. In addition to these demographic uncertainties, the genetic load can be high in small populations due to inbreeding depression, recurrent deleterious mutations and the loss of adaptive variation in response to random drift [10–12]. Taken together, these are potentially strong selective forces that can adversely affect an individual's reproductive ability and a small population's long-term viability. Yet, small populations persist across animal taxa, and are prevalent among the great apes, including traditional and ancestral humans. While the potential negative genetic consequences of living in a small population are well established, relatively less is known about behavioural responses to demographic uncertainty and how these responses affect the resilience of small groups.
Across species, the adult sex ratio (ASR, usually expressed as the proportion of males in the adult population) is increasingly recognized as a key demographic factor driving behavioural variability within and between the sexes [13–16]. Because partner availability structures an individual's reproductive options, imbalances in the ratio of reproductively mature males to females are likely to impact population viability, particularly in small populations [7,17]. However, how and why the ASR varies in a population over time is understudied [15]. While the causes of ASR variation have been analysed in birds [18], little is known about these patterns in large-bodied, long-lived animals, especially humans. To address this gap, here we examine longitudinal patterns in ASR variability using data from the Savannah Pumé, hunter–gatherers of Venezuela. Our goals are to characterize ASR fluctuations in small human groups, assess the relative role of various demographic factors in these fluctuations and consider the evolutionary implications of ASR imbalance for human social organization. In doing so, our aims are to better understand the nature of demographic structure inherent to small populations and to more clearly infer the selective environment experienced by both contemporary foragers and ancestral humans.
(a). Small populations in the human past
Archaeological and genetic data, as well as behavioural reconstructions, characterize Pleistocene hunter–gatherers as living in small, mobile groups [3,19,20]. At a number of junctures in the human evolutionary past, population numbers appear to have fallen precipitously low in response to several bottleneck events [21–27]. Recent genetic research, for example, suggests that our ancestors were more endangered than gorillas and chimpanzees are today, and prior to 1.2 Mya, the global Pleistocene hominin population did not exceed 26 000 individuals, and may have dropped to as low as 18 500 [22]. Given that genetic data show a signature of bottleneck and random drift events in the past, how did we stave off the adverse consequences of living in small groups? While many reasons are likely, here we investigate whether our resilience to life in small groups can be explained by behavioural responses to uncertainty in population structure. Specifically, we evaluate the role that dispersal and partner exchange play in attenuating random fluctuations in local ASRs.
(b). Hunter–gatherer social organization
Hunter–gatherers can broadly be described as living in small multi-level societies organized in several nested levels of interaction [28–31]. These minimally include the foraging party and residential clusters of interacting families, here referred to as the band or local group [19,30–32]. Across hunter–gatherer societies, the size of local groups may range from 35 to 80 individuals [29,31,33], and vary situationally, seasonally and annually [28,30]. Composition is often variable, with band members aggregating and disaggregating in response to resource availability, labour and social needs. Local groups also interact and form larger ethno-linguistic groups or tribes. Marriage partners are commonly drawn from across this larger group. In all hunter–gatherer societies, long-term pairbonds, whether they are monogamous or polygamous, are socially recognized as marriages. Serial monogamy is common for both men and women due to divorce and remarriage, and high rates of adult mortality [34–36]. Polygamy (most often polygyny) occurs in most hunter–gatherer societies, but rates are usually low [35,37–41].
Two derived features of hunter–gatherer social organization are notable when compared with other primates. First, hunter–gatherers do not have a sex-specific dispersal pattern at sexual maturity [34,42–44]. While hunter–gatherers and early humans were traditionally described as male philopatric [45,46], a characterization that persists in some fields [47], this claim has long been disputed and is not supported empirically [48–53]. The sex that disperses varies considerably across cultures (male, female, both or neither), with many groups expressing multiple patterns simultaneously [34,41,42,54–56]. Within a local group, both males and females may marry and reside natallocally or move to live with their wife's or husband's kin, and spouses often switch the local group with whom they live several times across the duration of a marriage union [34,42,44].
This fluidity with which adult males and females move between local groups differs considerably from non-human primates, among whom dispersal is typically sex-specific [19]. While the dispersing sex varies across primate species, within species patterns are usually characterized as having little individual variation [57,58]. Sex-specific dispersal may be more predominant in some animals because selective pressures for one or the other sex to move between local groups may be prohibitive [59]. For example, among chimpanzees, males are the philopatric sex and are highly aggressive towards, and may even kill, extra-community males [60–64].
The second unusual feature about hunter–gather social organization is that individuals who disperse and marry into another group routinely maintain life-long interactions with their natal group [65]. Because these affiliations build social and cooperative ties across geographically dispersed local groups, marriage is argued to be an important foundation of human sociality [19,32,56,66]. As it relates to ASR dynamics in small groups, kin connections between groups maintain the pool from which marriage partners can be drawn for future relationships and generations [28,67,68].
(c). Resources availability versus mate availability
Explanations for why hunter–gatherers are so facultative in their dispersal patterns and why they maintain social relationships across local groups have traditionally centred on sustaining networks for the exchange of essential commodities—food, raw materials, labour and information [29,33,38,43,69–71]. Because these commodities are heterogeneously distributed, often over large geographical areas [71,72] flexibility in post-marital residence norms is hypothesized to be an effective means to reduce risk and smooth day-to-day and individual variance in food supply [34,38,39].
An unexplored explanation for hunter–gatherer intergroup social relations is related to the demography of small populations, in which random fluctuations in sex-biased births and deaths have pronounced effects on population structure [1,2]. For example, in a small group of 60 hunter–gatherers, female births may predominate for several years, no births may occur for the next few years, followed by a spike in male births. We suspect that these stochastic swings, not only in births but also mortality, have transient, yet dramatic impacts on the ASR. If so, ASR imbalances would create potential patchiness in the fitness landscape across time and place and we predict motivate facultative responses to resolve mate availability.
Because ASR dynamics have not been well characterized for small human populations, several basic questions remain unanswered. How variable are annual imbalances in ASR? What are the predominant demographic components that influence partner availability? And how do small populations respond to these fluctuations? To address these questions, we use 25 years of demographic data (1983–2007) across two bands of Pumé hunter–gatherers to examine longitudinal patterns in sex ratio imbalance and their implications for human sociality.
2. Material and methods
(a). Study population: Savannah Pumé hunter–gatherers of Venezuela
The Savannah Pumé are mobile hunter–gatherers living on the llanos of west-central Venezuela [73–78]. At the last complete census, the Savannah Pumé had a population of 670, dispersed in 24 bands over a 2800 km2 area. Because of the region's political instability, their geographical isolation and a poor terrestrial environment, the Savannah Pumé are largely buffered from outside encroachment and maintain their hunting and gathering way of life [79].
The Savannah Pumé live in small local groups and are representative of other warm-climate hunter–gatherers in many aspects of their social, married and reproductive lives (table 1). They move five to six times a year in response to changes in rainfall and the water table [73,74,76]. During the six-month dry season, food is relatively abundant and subsistence centres on aquatic resources and wild fruit. When the llanos flood during the wet season, fish are difficult to locate and the subsistence base shifts to small-bodied terrestrial game and tubers [84]. Both male- and female-foraged foods are critical to the diet and are widely shared within and across families.
Table 1.
Savannah Pumé group size, mobility and life-history traits compared with other warm-climate hunter–gatherer groups. Missing values indicate that no data are available.
| Savannah Pumé | warm-climate hunter–gatherers | |
|---|---|---|
| group size | 58±16.4 (n = 18)a | 48±45 (n = 53)b |
| moves per year | 6±1.8 | 8.75±12.8 (n = 78)b |
| age at menarchec | 12.9±1.02 (n = 16) | |
| age at marriagec♀ ♂ |
15.1±2.5 (n = 59) 18.0±4.3 (n = 51) |
|
| age at first birthc♀ ♂ |
16.0±2.5 (n = 43)d 19.5±3.4 (n = 33) |
18.4±1.7 (n = 6)b |
| completed fertility | 7.0±1.29 (n = 18)e | 5.14±1.75 (n = 28)b |
| infant mortalityf | 35% | 23%±10.2 (n = 11)b |
| life expectance at birth | 30g | 31 (n = 5)h |
| polygyny ♀ ♂ |
20%i 11%i |
24%±27 (n = 31)b 16%±16 (n = 113)b |
aAverage across 18 censuses in two bands from 1983 to 2007.
b([31], tables 1 and 2) Group size and moves per year given for Neotropical hunter–gatherers, otherwise values are for New and Old World hunter–gatherers; n, number of societies.
cAge at menarche, marriage and first birth given in years; n, number of individuals.
dThis value is from a more expanded sample than the previously reported age at first birth of 15.5 for the Savannah Pumé [80,81].
eNumber of children ever born to women aged 40 and older; n, number of individuals.
fDeaths per 1000 live births, or probability that a child will survive their first year.
gEstimate from life tables given Savannah Pumé age distribution, birth and death rates (model east level 5 life table [82]).
h([83], table 2).
iPer cent of married adults ever polygynously married.
Within the Savannah Pumé area, three geographical subregions can be identified. Most, but not all, subsistence and marriage interactions occur between bands that reside within a subregion. The two groups that are the focus of the ASR analysis live in the same subregion, which consists of five frequently interacting bands with an estimated population of 200 dispersed over a 900 km2 area. The following describes these two local groups.
Across the 25-year sample, Savannah Pumé females marry on average at age 15.1 (s.d. ± 2.5; n = 59) and males at age 18.0 (s.d. ± 4.3; n = 51; table 1). Although first marriages are often arranged by parents, young women are not obliged to accept these matches, and have autonomy about when and whom they marry. By Pumé social norms, a couple is recognized as married if they engage in conjugal relations, where upon they cohabit. Consequently, births occur within the context of marriage and coresidence, and extra-pair paternity is likely quite low. Divorce may be instigated by either spouse, and if an extramarital affair occurs, the marriage typically dissolves and the individuals remarry. Marriage to non-Pumé has not been documented.
The extreme seasonal variation in food availability and pathogen load has pronounced effects on fertility and mortality (table 1). Savannah Pumé women give birth to their first child on average at age 16.0 (s.d. ± 2.5, n = 43), with 90% of first births occurring between ages 15 and 19. While female age-at-first birth is early compared with other hunter–gatherers [31,39], it occurs at a biologically predictable age several years after menarche. Mothers who give birth in their mid- to late-teens have significantly lower infant mortality than do younger mothers, and are more likely to have an additional child over their reproductive careers [80,85]. Savannah Pumé mothers who survive their reproductive careers have on average 7.0 ± 1.29 live births (table 1) [86]. However, in this high pathogenic and seasonally food-limited environment, 35% of children born do not survive infancy, especially their first rainy season, and 45% do not survive to reproductive age (no sex-biased mortality observed, see below).
(b). Data collection
Detailed census, genealogical and reproductive history data were collected in two bands of Savannah Pumé over a 25-year period from 1983 to 2007. Both bands live in the same savannah ecology, migrated into their current location about 50 years ago, and, depending on the season and year, have foraging ranges that are within a day's walk from one another. The two bands interact frequently, have close kin ties and exchange marriage partners. The demographic data used here come from two sources: censuses that were conducted in 1983, 1986–1990 and 1992 (records obtained from R. Lizarralde and T. Gragson, portions published in [77,78]), and from censuses and reproductive histories collected by Greaves and Kramer in 1990, 1992, 1993 and 2005–2007. Both data sources are comparable—interviews were conducted in the Pumé language and recorded individual demographic information, genealogical relations, natal group affiliation, past marriages and residential locations during marriage [34,79,86].
From these data, we constructed an N×T matrix for each of the two bands, where N is the total number of individuals in the census across all years, and T the number of years included in the analysis (see electronic supplementary material, Data Format section for detail and table S1). All individuals who were living in 1983, or who were born or migrated in between 1983 and 2007, are included (n = 218; excluding infants who were born and died between censuses, which is about 30% of children born). The matrix codes for an individual's presence or absence in the population in each year, and whether a change in status occurred in a particular year (e.g. was an individual born, did they die, migrate, marry or divorce). The change in status also records the reason for a marriage's dissolution (death, divorce), whether the individual remained in their natal community or moved in or out to marry. In years when no census occurred, inferences about an individual's status could be either reliably drawn (e.g. if an individual was alive in 1983 and also in 1986 and was married to the same spouse in both years, we coded no change in status) or resolved through interview during subsequent field seasons.
(c). Analytic questions and terminology
This longitudinal matrix was used to (i) construct annual changes in the ASR, (ii) determine which demographic components (the sex composition of who ages into the mating pool, who dies and who migrates during adulthood) affect ASR imbalance from year to year, and (iii) inform two theoretic models—one explores the role that dispersal plays in buffering local groups from ASR imbalance and the other predicts when dispersal should be male- or female-biased.
Because a group's composition is affected not only by adults moving out (dispersal or emigration), but also by individuals moving in (immigration), we refer to these movements collectively as migration (i.e. migration here does not refer to mass seasonal movement of an entire group). Hereafter, we also refer to the hunter–gatherer-typical pattern of non-sex-specific post-marital residence as dual-sex dispersal, equivalent to bisexual dispersal used by biologists.
(d). Methods to calculate the adult sex ratio
The ASR is a demographic measure used across species to describe the pool of reproductive-aged individuals, and can be used to assess the relative scarcity of partners. For most animals, ASR calculations include all adults from sexual maturity to death. However, for humans, due to menopause and the age asymmetries in male and female fecundity, the age bracket to include in the ASR, while proscribed by fecundity for females, is much less straightforward for males. The appropriate male age bracket is also influenced by mating system. For instance, in monogamous societies, males and females often have isometric reproductive tenures [87,88]. However, in polygynous or serially monogamous societies, females potentially have a more limited age range of fecundity than do males, who might remain in the mating pool for longer into their adult lives. Even so, male fertility varies with age. In both developed and traditional populations, male fertility peaks during their 30s, with lower paternity probabilities at both younger and older ages [89–91]. Age variation may be due to a variety of factors: female choice, male competition, declining sexual function or the deleterious genetic effects of advanced paternal age [89,90,92,93]. The point we make here is that the ASR is sensitive to the age intervals included, particularly of males. Being broadly inclusive of the male age range has a dramatic, male-biasing influence on estimates of partner availability; however, restricting the male age range to be isometric with female fecundity likely does not capture the wider age range of males actively involved in the mating pool in polygynous mating systems.
With this in mind, we approach the question of what age interval to include in the ASR by first using data-driven criteria for fecundity indexed by when reproduction empirically begins and ends and then introducing uncertainty around this estimate. To be broadly inclusive of when most men and women begin reproducing, to age into the mating pool, we use the mean age at first birth minus one standard deviation (ages 14 and 16 for females and males, respectively). Individuals are aged out of the ASR at the average age of last birth plus one standard deviation (ages 36 and 45 for females and males, respectively). To generate confidence bands around this empirical interval, we incorporate uncertainty around the age estimates. We do so by assuming that ageing into and ageing out of the mating pool are normally distributed random variables, and include an additional year at the beginning of the interval and 3 years at the end. This is asymmetrical because, while sexual maturity has a biologically constrained lower bound (e.g. girls under the age of 13 are not considered), age at last birth is more variable for both females and males. Thus, the ASR is defined by females of ages 14 ± 1 to 36 ± 3 years, and males of ages 16 ± 1 to 45 ± 3 years (see electronic supplementary material, table S1 for ASR annual frequency distribution). With the age interval specified in this way, the ASR is estimated at time t by sampling the age distribution around ageing into and out of the mating pool using Monte Carlo integration. After drawing 1000 samples for each year, 95% confidence bands are computed (figure 1). (The sex ratio of the cohort ageing into the mating pool is similar to the sex ratio at maturity used by biologists in that both are based on age at first birth, and we use the terms interchangeably.)
Figure 1.
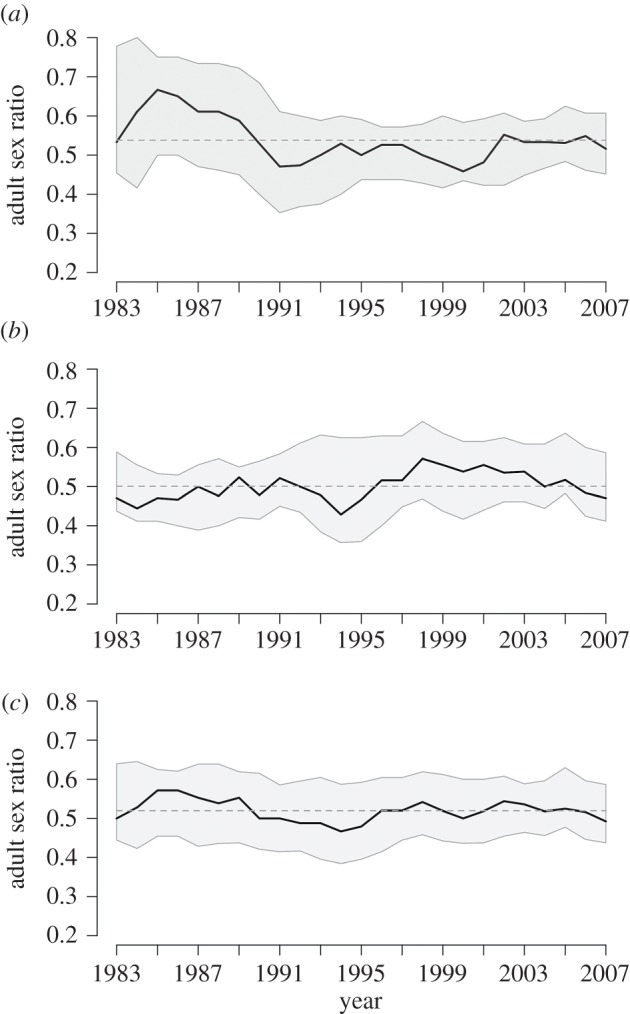
Annual ASRs in two groups of Savannah Pumé hunter–gatherers. Lines show year-to-year change in the ASR (proportion of males in the adult population defined as men of ages 16–45 and women of ages 14–36). Shaded area shows Monte Carlo-generated confidence bands for uncertainty around the ASR age interval. (a) ASR for Yaguri, which includes an average of 11.7 (±3.0) adult males and 10.2 (±3.1) females in any one year, and an average ASR of 0.54 (±0.05) across the 25-year sample. (b) ASR for Doro Aná, which includes an average of 12.5 (±2.9) adult males and 12.4 (2.5) females in any one year, and an average ASR of 0.50 (±0.04) across the 25-year sample. (c) Annual ASR for two combined groups of Savannah Pumé hunter–gatherers (a) and (b). The combined population includes an average of 24.2 (4.8) adult males and 22.5 (4.9) adult females in any one year, and an average ASR of 0.52 (±0.03) across the 25-year sample. (See electronic supplementary material, table S1 for annual counts of males and females in each band.)
Although the ASR is often stated as a ratio (males/females) in social science research, we express the ASR as the proportion of males in a population (males/(males + females)). Ratios are disproportionate in constraining the female range, while allowing the male range to be limitless, thereby artificially inflating male-biased values. (They also are not interpretable if there are no females in the population.) Consequently, we use the proportional measure, which ranges from 0 to 1.0, with 0.5 denoting a balanced distribution (50% males and 50% females) [94].
3. Results
(a). The Savannah Pumé marriage market
The 25-year census matrixes for two Savannah Pumé groups of hunter–gatherers together record 2875 person years, 91 births, 39 deaths and include 114 individuals (60 females and 54 males) who were alive and married in 1983 or since 1983 and their 160 marriages. Across these marriages, 61 changes in status were recorded; of these, 18% (n = 29) of marriages ended because of death (spouse or ego) and 20% (n = 32) terminated due to divorce. Of individuals whose first marriages dissolved because of divorce or death, most remarried (88%). Of married individuals, 62% (n = 71) were married once, and 38% (n = 43) more than once. While polygyny occurs (20% of women and 11% of men, n = 12 women and 6 men were polygynously married at some point during their lives), most marriages are monogamous, which is consistent with many hunter–gatherers [37,95]. In first marriages, 15% (n = 17) of individuals moved out of their natal band (or band in which they had lived since childhood) to marry, whereas 37% (n = 16) relocated for a subsequent marriage.
(b). Effects of birth, death and migration on adult sex ratio
Interannual fluctuations in ASR are substantial across the two groups (figure 1). The ASR varies from twice as many men as women (figure 1a, in 1985 ASR = 0.67) to a preponderance of women (figure 1b, in 1994 ASR = 0.43). These values indicate that partner availability for men and women is variable in their local group, with severe shortages in some years, not only for the incoming cohort but also for the nearly 40% of adults who reenter the marriage market due to divorce and spousal death. When uncertainty is included around the ASR age estimate (see description above), ASR fluctuations are even more dramatic in some years, from as much as four times the number of men to women (figure 1a in 1985), to nearly twice as many women as men (figure 1a in 1991).
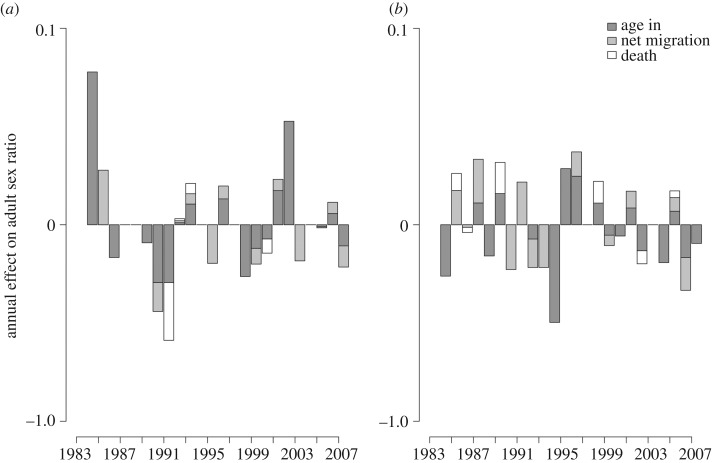
To determine which variables drive fluctuations in ASR imbalance, we disaggregate the demographic components that directly influence it over time. These variables include the sex composition of who ages into the mating pool (i.e. the sex ratio at maturation [94]), who dies and who emigrates or immigrates during adulthood. A sex-bias in any one of these variables can affect the ASR and each is an important indicator of the selective arena that individuals face. Figure 2 shows that the relative contribution of each component varies from year to year. For example, in 1984 (figure 1a), the cohort of juveniles ageing into the ASR was male-biased and produced an 8% increase in the ASR. Three years later in 1987, the cohort ageing in was female-biased, which decreased the ASR by 4%. Thus, the annual effect of a particular demographic component varies from year to year and importantly does not consistently move the ASR towards a male or female bias over time, as it does for many other animal species [17,96].
Figure 2.
Annual effect that ageing into the mating pool, net migration (immigration–emigration) and adult death have on the ASR (proportion of males in the adult population defined as men ages 16–45 and women ages 14–36) in two groups of Savannah Pumé hunter–gatherers. Shown for (a) Yaguri and (b) Doro Aná.
To then evaluate which demographic components have the greatest influence on the ASR, we calculate the relative effects that ageing into the mating pool and adult death, and net migration (immigration–emigration) have on the ASR fluctuations over time. Across both populations, mortality has the weakest effect on interannual ASR variation and net migration plays an intermediate role. The primary source of ASR imbalance is the sex-bias of the cohort ageing into the mating pool. Over the 25-year period in one hunter–gatherer band, for example, the sex ratio at maturity has 13 times the effect of mortality and three times the effect of migration on generating change in the ASR (electronic supplementary material, figure S1, left panel).
It is important to note that in the Savannah Pumé case, the sex ratio of the cohort ageing into the mating pool closely reflects the at-birth (secondary) sex ratio. Over the 25-year period, no sex differences are evident in infant or child deaths (65 males and 64 females; χ2 = 0.0078; p = 0.9298). This is consistent with ethnographic interviews, which indicate no cultural preference for boys or girls. Accordingly, because mortality from birth to maturity is not sex-biased, we infer that the sex composition at maturity is largely determined by the sex ratio at birth (children occasionally migrate in), and has the most pronounced effect on ASR imbalance.
(c). Adaptive responses to adult sex ratio imbalance
Thus far, we have shown that ASRs vary substantially across time and the stochastic yet powerful role that ageing into the mating pool has in partner availability. The noteable variability in ASRs over time raises questions about how an individual adaptively responds to sex ratio imbalance, and how this might relate to dispersal and post-marital residence patterns. A key point to highlight is that in small groups, philopatric mating options have potential costs [59]. For example, if a young Pumé woman were to reach sexual maturity during a time when available marriage partners in her group are in short supply, she could delay marriage and first reproduction. However, in the high subadult and adult mortality environments in which hunter–gatherers typically live [83], and in which the Pumé in particular live ([86], table 1), delaying marriage and reproduction even for a few years would likely have a fitness handicap [97–99]. Alternatively, she could marry polygynously. However, polygyny also has the potential cost of depressing female fitness [100–103]. Of central concern is that if she were to marry within her local group, she may have little choice but to mate with a close relative, as would be the case for males as well.
Another option for an individual facing mate scarcity is to look outside of the local group for partners. To observe the effect that this has on partner availability, we merge the two Savannah Pumé populations to create an extended pool of potential partners (figure 1c). When partner availability is pooled across groups, fluctuations in the ASR markedly decrease in amplitude. This suggests that movement between groups is an important behavioural means to mediate locally constrained mating options and smooth out partner scarcity. To then simulate conditions under which dispersal outside of the local group may have evolved, we develop two mathematical models: the first describes ‘ideal’ migration conditions and the second explores conditions under which we expect one sex or the other to disperse.
(i). Free distribution model
Because our empirical analyses suggest that group size (figure 1c compared to figures 1a and 1b) affects the magnitude of year-to-year oscillations in the ASR, we build a simple mathematical model that describes the conditions under which the ASR stabilizes and group-level mating constraints are attenuated. In constructing the model, a recursion is specified that describes the number of males (mt+1) and females (ft+1) in the next generation of a local population as a function of the number of reproductive pairs (ct), the net migration (immigration–migration) rate for men (um) and women (uf), the adult death rate by sex (dm, df) and the fraction of males (α) versus females (1 − α) born of reproductive pairs that age into the mating pool:
and
Defining the sex ratio as ASRt = mt/(mt + ft), we find that ASRt+1 − ASRt = 0 occurs when:
| 3.1 |
Result (3.1) indicates that for the ASR to stabilize, migration (either in or out of the local group) is required to offset the imbalances generated through demographically driven fluctuations in the sex composition of mortality and the cohort ageing into the mating pool. Put simply, the ASR does not change over time if the ratio of migration rates for males and females is equal to the ratio of birth to death rates for males and females. While we do not find that any specific population size generates perfect unity, ASR stability is more easily attainable at some population sizes. In larger populations, the sampling errors for ageing in (α) and death rates (dm, df) are relatively small compared with the large fluctuations that may occur due to sampling variation in small populations. Thus, in a large population, fewer individuals need to migrate to compensate for sex-biased births and deaths because they fluctuate less from year to year. However, in a small population, migration of a relatively larger percentage of individuals is necessary to correct for biologically driven sex ratio imbalances.
Although (3.1) demonstrates the conditions under which the ASR will stabilize, this model is only partially satisfying because of the simplifying assumptions of an ideal free distribution [104], which state that the movement of individuals perfectly matches partner availability and there is no cost to migrate. However, dispersing out of your local group and moving into a new group likely does have costs [59]. For example, among chimpanzees, females, who are the dispersing sex, not only lose familiarity with local resources and group members when they leave their natal group, they also suffer costs from searching for a new group and aggression from resident females when they join a new community [105–107]. Dispersal does, however, allow individuals to escape the trap of small populations. This raises a provocative question about why most non-human primates tend to be sex-specific dispersers (for exception, see [58]), while dual-sex dispersal is commonplace among hunter–gatherers. In the next step, we address migration dynamics between groups in response to partner availability and sex-biased costs to dispersal.
(ii). Dispersal cost model
Although sex-biased dispersal is a well-documented and pervasive mammalian life-history feature, why is it that in some cases males disperse whereas females do in others [59]. To address this as an evolutionary problem, we develop a theoretic approach starting with the assumption that one sex disperses with the goal to predict which sex it should be. Our question is not what the optimal ASR is for males and females. Rather, given a global ASR (across groups), our aim is to model the efficient movement of individuals between local groups to equilibrate the sex ratio across local groups. We define efficiency as the minimum number of individuals of a particular sex that have to move and be exposed to costs of migration. The model assumes that individuals will actively look for a mate, and are willing to move if there is a more favourable sex ratio elsewhere.
We propose a two-patch dispersal cost model where, in patch i, there are mi males and fi females. Dispersal may occur, such that um and uf are the net number of males and females, respectively, that move from patch 1 to patch 2. A cost function is defined such that 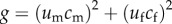 , where cm and cf are the costs incurred by males and females to move, respectively. If it is in an individual's interest to minimize the cost of dispersal while also moving to the patch with the more favourable sex ratio, then g should be minimized under the constraint that:
, where cm and cf are the costs incurred by males and females to move, respectively. If it is in an individual's interest to minimize the cost of dispersal while also moving to the patch with the more favourable sex ratio, then g should be minimized under the constraint that:
This is the condition in which the sex ratios in each of the two patches equilibrate. We can show that the fraction of migrants that are males becomes:
| 3.2 |
where R is the global ASR (the ASR combined across patch 1 and patch 2) before dispersal, R = (m1 + m2)/(m1 + f1 + m2 + f2), and k is the cost of dispersal for females relative to the cost to males for dispersal, or cf = kcm.
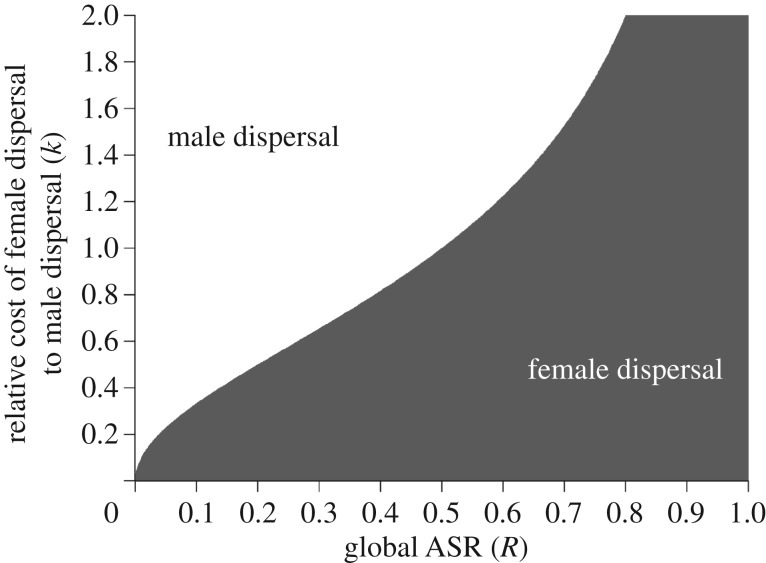
Imposing a numerical lower and upper bound on condition (3.2) at zero and one, the model predicts that the globally rarer sex disperses, except when the cost for the dispersal is especially high (figure 3). In other words, when sex ratios are male-biased, females are expected to disperse because relatively fewer of them are needed to equilibrate the ASR across populations (and vice-versa for female-biased ASRs). In sum, the model predicts the importance of ASR in determining sex-biased dispersal.
Figure 3.
Predicted sex-biased dispersal using the dispersal-cost model (result from equation (3.2)) showing regions where male or female dispersal is favoured, given sex-biased dispersal costs and the global ASR. White regions show those conditions under which male dispersal is predicted, and grey areas show those conditions under which female dispersal is predicted.
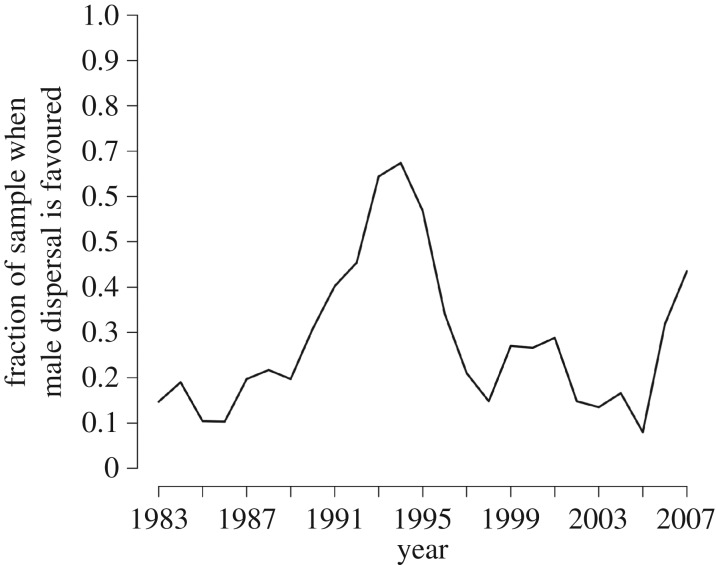
To evaluate the model's utility, its central prediction that the rarer sex should disperse is applied to the empirical ASR data as presented for the combined Savannah Pumé population data in figure 1c. We use Monte Carlo simulations to compute the fraction of time that male dispersal is favoured over female dispersal, given the ASR in a particular year (including the uncertainty around the value). Assuming male and female dispersal costs are the same (k = 1), male-biased dispersal is predicted by a larger fraction of the Monte Carlo samples in some years, while female-biased dispersal is predicted in others (figure 4). Thus, given year-to-year fluctuations in ASR and no clear directional sex-biased trend across these two local groups, a dispersal pattern that also varies is predicted. In response to fluctuating ASRs, in some years, males are predicted to disperse and in others, females are predicted to disperse. From this emerges the long-term pattern of dual-sex dispersal, which is ethnographically consistent with what both the Savannah Pumé and hunter–gatherers generally actually do.
Figure 4.
Predicted annual male and female dispersal using the dispersal-cost model and annual ASR data for two groups of Savannah Pumé hunter–gatherers. The distribution of males and female dispersal was estimated from Monte Carlo simulations using the global Savannah Pumé annual ASR data inclusive of the uncertainty around the ASR measure for a particular year (see confidence bands in figure 1c and explanation in main text).
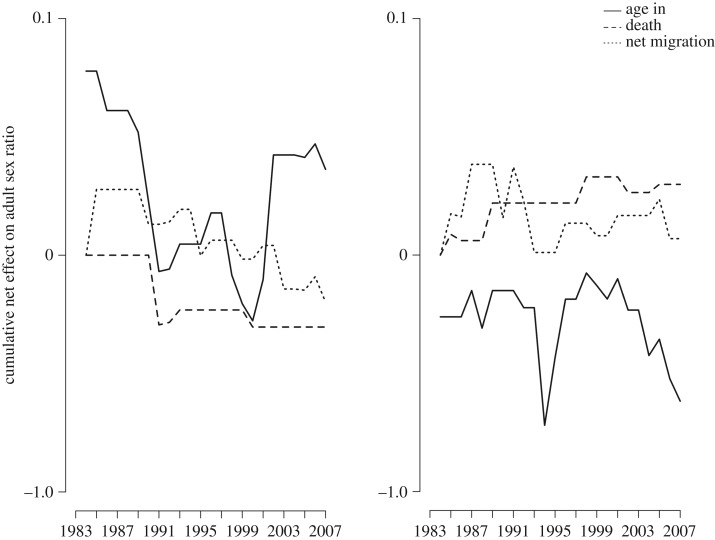
Observed migration patterns among the Savannah Pumé further corroborate the model's predictions. The cumulative effect of net migration (immigration–emigration) on the ASR over the 25-year period for both groups is near 0 (figure 5). Further, in one group (figure 5a), while cumulatively more males enter the mating pool, this is offset by a female net migration bias. The inverse is apparent in the other group (figure 5b). We infer this result to offer support that migration by both males and females serves to offset ASR imbalance over time. While preliminary, the novel insight gleaned from the dispersal cost model and its general empirical support offer a path forward for a broader examination of the evolution of sex-biased dispersal across animal taxa, and specifically to the question of whether males, females, or both are the dispersing sex.
Figure 5.
Cumulative net effects that ageing into the mating pool, adult death and net migration (immigration–emigration) have on the ASR (proportion of males in the adult population defined as men aged 16–45 and women aged 14–36) in two groups of Savannah Pumé hunter–gatherers. Shown for (a) Yaguri and (b) Doro Aná over the 25-year period.
4. Discussion
Our results are summarized by four main findings. First, interannual variation in the ASR is considerable in small populations. For a young person ageing into the mating pool, this means that reproductive options within the local group may be seriously constrained. Second, the sex ratio at maturity has the most pronounced effect on shaping ASR imbalance. While sex-biased net migration and adult mortality also play a role, fluctuation in who ages into the mating pool (and by implication the at-birth sex ratio because infant and child mortality are not sex-biased) is the primary driver of local variation in partner availability. We note that one of the only other empirical studies addressing the cause of ASR imbalance comes to a different conclusion. A phylogenetic analysis across 187 avian species found that sex-biased adult mortality was the best predictor of the ASR, more important than hatching and fledging sex ratios [18]. We suggest that the different outcomes may be due in part to the disproportionately higher mortality among female birds [108], the sex that commonly disperses. These differences in the main drivers of sex ratio imbalance suggest that the ASR is differently sensitive to demographic dynamics, socioecology and breeding systems.
Our third finding calls attention to the importance of longitudinal studies to disaggregate the causes and consequences of ASR variation. While the sex ratio at maturity is the most robust variable shaping the ASR over the 25-year period, in any one year net migration or death may be as, if not more, important (figure 2). Thus, relying on cross-sectional or a 1-year view is likely to lead to a mischaracterization of the drivers of ASR fluctuation. Only with longitudinal data did the magnitude of ASR fluctuations in small populations become apparent, highlighting it as a potentially important selective force shaping dispersal patterns. Following on from that, a longitudinal view demonstrated that what might appear to be a pattern of sex-biased dispersal in any one year was at the generational (Savannah Pumé data) or evolutionary scale (dispersal cost model) much more flexible and non-sex specific.
This leads to our fourth finding that the globally rarer sex is predicted to disperse in any particular year (or breeding season), with dual-sex dispersal emerging over the long run. This was developed theoretically and tested empirically with the Savannah Pumé data. The free distribution model illustrated that demographically driven ASR imbalances are stabilized through dispersal. When we then account for the cost to migrate, the dispersal cost model shows that the ASR is an important predictor of sex-biased dispersal, and specifically that the globally rarer sex is predicted to disperse. This central prediction was supported by observed migration patterns among the Savannah Pumé. General taxa-level trends also suggest that the globally rarer sex disperses. For example, among birds, ASRs are generally male-biased, and female dispersal prevails [109], while across mammals, ASRs are generally female-biased, and male dispersal predominates [110]. While these patterns are generalized across taxa, some research points to greater within species variation than perhaps previously expected [58,111]. This study adds to this by focusing on within population-level variation, and finding that in response to highly variable ASRs, flexible dispersal is an efficient strategy to mediate partner scarcity.
In sum, our findings draw attention to the stochastic nature of demographic structure inherent to small populations and the importance of ASR imbalance in dispersal decisions. A key point here is that ASR dynamics operate at both the local and global levels [112], which has important implications for human sociality because the movement of males and females between small local groups is characteristically fluid in hunter–gatherers. Below we pursue the role that dual-sex dispersal may have played as a strategic response to local mate unavailability in hominin evolution.
(a). Evolution of sociality in response to partner availability in small populations
Small populations experience many challenges to their long-term viability through stochastic demographic effects [2,4–6]. Beside randomly varying sex-biased births and deaths, the genetic load can be high due to inbreeding depression, recurrent deleterious mutations and the loss of adaptive variation in response to random drift [11,12]. In response to these reproductive challenges, one mechanism widely adopted across taxa is sex-biased dispersal [59,110,113–118]. Among hunter–gatherers and many other small traditional societies, however, dispersal at maturity is not sex-biased; both males and females might disperse, or remain in their natal group, and/or move several times throughout their adult lives [30,34,43,44,119–122]. This raises the question why dual-sex dispersal is the typical human pattern?
Resource temporal and spatial variation likely play a role [33,38,43,69,70,123–125], but we argue so does local partner availability. For example, in one Savannah Pumé band, there are years when men were twice as numerous as women (figure 1a). If female dispersal was the norm in this society, a male coming of age or unpartnered because of divorce or spousal death would potentially be left unable to find a mate locally. However, in the other band (figure 1b), in some years, there were many more reproductive-aged women than men, and a norm of male-biased dispersal would challenge females to find mates. Thus, under conditions where the ASR fluctuates from year to year within local groups, sex-biased dispersal is clearly a constraint in limiting individual reproductive options. By contrast, flexible dispersal patterns are more efficient in matching individuals to partners. We do not argue that humans are exceptional in this regard; however, dual-sex dispersal has received little research attention either empirically or theoretically (for exceptions, see [126,127]). While in other animals both sexes may be observed to disperse [111,114], the emphasis has been on the species-typical patterns of sex-biased dispersal.
While the ASR is critical in determining an individual's reproductive options and the likelihood of finding a mate, this topic has largely been overlooked in relationship to human social evolution. Anatomical, archaeological and molecular evidence corroborate that humans evolved in small populations and experienced population bottlenecks and near-extinction on several occasions [21,24,25]. Recent research contends that the decline and eventual extinction of Neanderthals were driven by low population-level viability due to the loss of genetic diversity coupled with a high genetic load, rather than primarily through competition with modern humans [128]. What allows modern humans to break out of the low-viability trap of living in small populations?
One way to attenuate fluctuations in ASR is to live in larger groups. However, hunter–gatherers are often inhibited from aggregating in large groups because of resource constraints [3]. Exogamy and partner exchange are a means to expand the breeding pool across multiple independent subsistence groups without having to feed a large group [19,32,43,129]. Cooperation, alliances and fluid residence across multiple local groups are unusual aspects of primate sociality, but common in humans. While some speculate that this is an ancient root of human sociality, what we do know is that modern hunter–gatherers draw marriage partners from across a larger group than the local one and are characteristically flexible in their dispersal patterns at sexual maturity [34,42,44,122].
Interannual variation in partner scarcity in small groups, we argue, favours individuals to look beyond the rim of their local group and promotes interactions between local groups and a norm-of-no-norm regarding dispersal, marital and residence patterns. Our results suggest that the establishment of cultural norms prescribing sex-biased dispersal and specific marriage rules that limit access to partners (e.g. matrilocality and patrilocality) would further intensify ASR imbalances, and thus are not likely to have developed in small, premodern populations.
5. Conclusion
An individual growing up in a small population can count on fluctuations in partner availability. When a young person comes of age or is left unpartnered because of death or divorce an appropriate mate may not be available in the local group. How modern humans stemmed the negative consequences of living in small groups and the general primate pattern of sex-biased dispersal was the central question that motivated this study. Contemporary small-scale societies show us that compared with our closest relatives, dispersal patterns at sexual maturity are highly flexible and local groups form long-term relationships that allow the flow of mating partners of both sexes. Our results offer dual-sex dispersal as an effective measure to offset the persistent, yet non-directional imbalances in ASR inherent to small human populations. These findings suggest an alternative explanation for the evolution of traits central to human sociality (e.g. flexible dispersal, friendly group relationships and cooperation across non-kin). In small populations, looking outside of one's local group is necessary to find a mate. Thus, motivated by sex ratio imbalance, alliances formed to facilitate the movement of partners may play an important role in the formation of human-typical geographically extensive social, economic and kin networks.
Supplementary Material
Acknowledgements
We are grateful to the Savanna Pumé of Doro Aná and Yaguri, who have heartily endeavoured to teach us about the complexities of their social and economic lives. We thank Russell Greaves, who has spent many years living with the Pumé collecting vital records, and Roberto Lizarralde, our Venezuelan colleague who had the foresight to meticulously collect the first indigenous Pumé censuses in 1983, 1986–1990 and 1992. We are also grateful to two anonymous reviewers for their helpful comments and to WIKO for supporting travel and providing a venue for K.L.K. to present an earlier version this paper.
Ethics
The field research performed by K.L.K. and was approved by the Institutional Review Board at Harvard University F15718-102.
Data accessibility
Data used in the analyses can be accessed via the electronic supplementary material.
Authors' contributions
K.L.K. and R.S. designed the research and wrote the paper; K.L.K. and Russell Greaves collected the data and provided background analyses and databases; A.B. conducted the matrix analyses, built and described the theoretic models. All authors edited the paper.
Competing interests
We declare we have no competing interests.
Funding
Field research was supported by the National Science Foundation (0349963 and DBS-9123875), the L.S.B. Leakey Foundation, the Milton Fund and Harvard University.
References
- 1.Lande R. 1993. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 142, 911–927. ( 10.1086/285580) [DOI] [PubMed] [Google Scholar]
- 2.May RM. 1974. Stability and complexity in model ecosystems, 2nd edn Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Wobst HM. 1974. Boundary conditions for Paleolithic social systems: a simulation approach. Am. Antiq. 39, 147–178. ( 10.2307/279579) [DOI] [Google Scholar]
- 4.Hammel EA. 2005. Demographic dynamics and kinship in anthropological populations. Proc. Natl Acad. Sci. USA 102, 2248–2253. ( 10.1073/pnas.0409762102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lande R, Engen S, Sæther B-E. 2003. Stochastic population dynamics in ecology and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Roughgarden J. 1975. A simple model for population dynamics in stochastic environments. Am. Nat. 109, 713–736. ( 10.1086/283039) [DOI] [Google Scholar]
- 7.Lee AM, Saether BE, Engen S. 2011. Demographic stochasticity, Allee effects, and extinction: the influence of mating system and sex ratio. Am. Nat. 177, 301–313. ( 10.1086/658344) [DOI] [PubMed] [Google Scholar]
- 8.Bessa-Gomes C, Legendre S, Clobert J. 2004. Allee effects, mating systems and the extinction risk in populations with two sexes. Ecol. Lett. 7, 802–812. ( 10.1111/j.1461-0248.2004.00632.x) [DOI] [Google Scholar]
- 9.Courchamp F, Berec L, Gascoigne J. 2008. Allee effects in ecology and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Whitlock MC. 2000. Fixation of new alleles and the extinction of small populations: drift load, beneficial alleles, and sexual selection. Evolution 54, 1855–1861. ( 10.1111/j.0014-3820.2000.tb01232.x) [DOI] [PubMed] [Google Scholar]
- 11.Lynch M, Gabriel W. 1990. Mutation load and the survival of small populations. Evolution 44, 1725–1737. ( 10.1111/j.1558-5646.1990.tb05244.x) [DOI] [PubMed] [Google Scholar]
- 12.Soule M. 1987. Viable populations for conservation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Kokko H, Jennions MD. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919–948. ( 10.1111/j.1420-9101.2008.01540.x) [DOI] [PubMed] [Google Scholar]
- 14.McNamara JM, Szekely T, Webb JN, Houston AI. 2000. A dynamic game theoretic model of parental care. J. Theor. Biol. 205, 605–623. ( 10.1006/jtbi.2000.2093) [DOI] [PubMed] [Google Scholar]
- 15.Székely T, Weissing FJ, Komdeur J. 2014. Adult sex ratio variation: implications for breeding system evolution. J. Evol. Biol. 27, 1500–1512. ( 10.1111/jeb.12415) [DOI] [PubMed] [Google Scholar]
- 16.Schacht R, Kramer KL, Székely T, Kappeler PM. 2017. Adult sex ratios and reproductive strategies: a critical re-examination of sex differences in human and animal societies. Phil. Trans. R. Soc. B 372, 20160309 ( 10.1098/rstb.2016.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donald PF. 2011. Lonely males and low lifetime productivity in small populations. Ibis 153, 465–467. ( 10.1111/j.1474-919X.2011.01144.x) [DOI] [Google Scholar]
- 18.Székely T, Liker A, Freckleton RP, Fichtel C, Kappeler PM. 2014. Sex-biased survival predicts adult sex ratio variation in wild birds. Proc. R. Soc. B 281, 20140342 ( 10.1098/rspb.2014.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapais B. 2008. Primeval kinship. Cambridge, MA: Harvard University Press. [Google Scholar]
- 20.Chapais B. 2013. Monogamy, strongly bonded groups, and the evolution of human social structure. Evol. Anthropol. 22, 52–65. ( 10.1002/evan.21345) [DOI] [PubMed] [Google Scholar]
- 21.Hawks J, Hunley K, Lee S-H, Wolpoff M. 2000. Population bottlenecks and Pleistocene human evolution. Mol. Biol. Evol. 17, 2–22. ( 10.1093/oxfordjournals.molbev.a026233) [DOI] [PubMed] [Google Scholar]
- 22.Huff CD, Xing J, Rogers AR, Witherspoon D, Jorde LB. 2010. Mobile elements reveal small population size in the ancient ancestors of Homo sapiens. Proc. Natl Acad. Sci. USA 107, 2147–2152. ( 10.1073/pnas.0909000107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Durbin R. 2011. Inference of human population history from individual whole-genome sequences. Nature 475, 493–496. ( 10.1038/nature10231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laval G, Patin E, Barreiro LB, Quintana-Murci L. 2010. Formulating a historical and demographic model of recent human evolution based on resequencing data from noncoding regions. PLoS ONE 5, e10284 ( 10.1371/journal.pone.0010284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmueller KE, et al. 2008. Proportionally more deleterious genetic variation in European than in African populations. Nature 451, 994–997. ( 10.1038/nature06611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reich DE, et al. 2001. Linkage disequilibrium in the human genome. Nature 411, 199–204. ( 10.1038/35075590) [DOI] [PubMed] [Google Scholar]
- 27.Posth C, et al. 2016. Pleistocene mitochondrial genomes suggest a single major dispersal of non-Africans and a late glacial population turnover in Europe. Curr. Biol. 26, 827–833. ( 10.1016/j.cub.2016.01.037) [DOI] [PubMed] [Google Scholar]
- 28.Binford LR. 2001. Constructing frames of reference. Berkeley, CA: University of California Press. [Google Scholar]
- 29.Hamilton MJ, Milne BT, Walker RS, Burger O, Brown JH. 2007. The complex structure of hunter–gatherer social networks. Proc. R. Soc. B 274, 2195–2203. ( 10.1098/rspb.2007.0564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly RL. 2013. The lifeways of hunter–gatherers. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Marlowe FW. 2005. Hunter–gatherers and human evolution. Evol. Anthropol. 14, 54–67. ( 10.1002/evan.20046) [DOI] [PubMed] [Google Scholar]
- 32.Chapais B. 2011. The evolutionary history of pair-bonding and parental collaboration. In The Oxford handbook of evolutionary family psychology (eds Salmon CA, Shackelford TK), pp. 33–50. Oxford, UK: Oxford Univeristy Press. [Google Scholar]
- 33.Gurven M. 2004. To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. 27, 543–583. ( 10.1017/S0140525X04000123) [DOI] [Google Scholar]
- 34.Kramer KL, Greaves RD. 2011. Postmarital residence and bilateral kin associations among hunter–gatherers: pume foragers living in the best of both worlds. Hum. Nat. 22, 41–63. ( 10.1007/s12110-011-9115-7) [DOI] [PubMed] [Google Scholar]
- 35.Kramer KL, Russell AF. 2015. Was monogamy a key step on the hominin road? Reevaluation of the monogamy hypothesis. Evol. Anthropol. 24, 73–83. ( 10.1002/evan.21445) [DOI] [PubMed] [Google Scholar]
- 36.Marlowe FW. 2000. Paternal investment and the human mating system. Behav. Processes 51, 45–61. ( 10.1016/S0376-6357(00)00118-2) [DOI] [PubMed] [Google Scholar]
- 37.Flinn MV, Low BS. 1986. Resource distribution, social competition and mating patterning in human societies. In Ecological aspects of social evolution: birds and mammals (eds Rubenstein BI, Wrangham RW), pp. 217–243. Princeton, NJ: Princeton University Press. [Google Scholar]
- 38.Hill K, Hurtado AM. 2009. Cooperative breeding in South American hunter–gatherers. Proc. R. Soc. B 276, 3863–3870. ( 10.1098/rspb.2009.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. ( 10.1002/1520-6505(2000)9:4%3C156::AID-EVAN5%3E3.0.CO;2-7) [DOI] [Google Scholar]
- 40.Kramer KL, Russell AF. 2014. Cooperative breeding without monogamy: human insights and animal implications. Trends Ecol. Evol. 29, 600–606. ( 10.1016/j.tree.2014.09.001) [DOI] [PubMed] [Google Scholar]
- 41.Marlowe FW. 2003. Mating systems of foragers in the standard cross-cultural sample. Cross Cult. Res. 37, 282–306. ( 10.1177/1069397103254008) [DOI] [Google Scholar]
- 42.Alvarez HP. 2000. Grandmother hypothesis and primate life histories. Am. J. Phys. Anthropol. 113, 435–450. ( 10.1002/1096-8644(200011)113:3%3C435::AID-AJPA11%3E3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 43.Hill KR, et al. 2011. Co-residence patterns in hunter–gatherer societies show unique human social structure. Science 331, 1286–1289. ( 10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 44.Marlowe F. 2004. Marital residence among foragers. Curr. Anthropol. 45, 277–284. ( 10.1086/382256) [DOI] [Google Scholar]
- 45.Service E. 1962. Primitive social organization: an evolutionary perspective. New York, NY: Random House. [Google Scholar]
- 46.Steward JH. 1955. Theory of culture C: the methodology of multilinear evolution. Urbana, IL: University of Illinois Press. [Google Scholar]
- 47.Micheletti AJC, Ruxton GD, Gardner A. 2017. Intrafamily and intragenomic conflicts in human warfare. Proc. R. Soc. B 284, 20162699 ( 10.1098/rspb.2016.2699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ember CR. 1975. Residential variation in hunter–gatherers. Cross Cult. Res. 10, 199–227. ( 10.1177/106939717501000302) [DOI] [Google Scholar]
- 49.Lee RB. 1979. The !Kung San: men, women and work in a foraging society. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Lee RB, DeVore I. 1968. Problems in the study of hunter–gatherers. In Man the hunter (eds Lee RB, DeVore I), pp. 3–12. Chicago, IL: Aldine. [Google Scholar]
- 51.Meggitt MJ. 1965. Marriage among the Walbiri of Central Australia: a statistical examination. In Aboriginal man in Australia (eds Berndt RM, Berndt CH), pp. 146–166. Sydney, Australia: Angus and Robertson. [Google Scholar]
- 52.Murdock GP. 1949. Social structure. New York, NY: Macmillan. [Google Scholar]
- 53.Turnbull CM. 1965. Wayward servants: the two worlds of African pygmies. Westport, CT: Greenwood Press. [Google Scholar]
- 54.Beckerman S, Valentine P (eds). 2002. Cultures of multiple fathers. In The theory and practice of partible paternity in lowland South America. Gainsville, FL: University of Florida Press. [Google Scholar]
- 55.Blurton JN. 2016. Demography and evolutionary ecology of Hadza hunter–gatherers. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 56.Walker R, Hill KR, Flinn MV, Ellsworth RM, Michalak P. 2011. Evolutionary history of hunter–gatherer marriage practices. PLoS ONE 6, e19066 ( 10.1371/journal.pone.0019066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthews LJ, Arnold C, Nunn CL. 2010. Niche construction and the evolution of primate sex-biased dispersal patterns. Am. J. Phys. Anthropol. 141, 166 ( 10.1002/evan.20093) [DOI] [Google Scholar]
- 58.Douadi MI, et al. 2007. Sex-biased dispersal in western lowland gorillas (Gorilla gorilla gorilla). Mol. Ecol. 16, 2247–2259. ( 10.1111/j.1365-294X.2007.03286.x) [DOI] [PubMed] [Google Scholar]
- 59.Lawson HLJ, Perrin N. 2007. Advances in our understanding of mammalian sex-biased dispersal. Mol. Ecol. 16, 1559–1578. ( 10.1111/j.1365-294X.2006.03152.x) [DOI] [PubMed] [Google Scholar]
- 60.Boesch C, Boesch H. 2000. The chimpanzees of the Tai forest. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.Goodall J. 1986. The chimpanzees of Gombe. Cambridge, MA: Harvard University Press. [Google Scholar]
- 62.Nishida T, Hiraiwa-Hasegawa M, Takahata Y. 1985. Group extinction and female transfer in wild chimpanzees in the Mahale National Park, Tanzania. Ethology 67, 284–301. [Google Scholar]
- 63.Watts DP, Mitani JC. 2001. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour 138, 299–327. ( 10.1163/15685390152032488) [DOI] [Google Scholar]
- 64.Wrangham RW. 1999. Evolution of coalitionary killing. Am. J. Phys. Anthropol. 110, 1–30. ( 10.1002/(SICI)1096-8644(1999)110:29+%3C1::AID-AJPA2%3E3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 65.Rodseth L, et al. 1991. The human community as a primate society [and comments]. Curr. Anthropol. 32, 221–254. ( 10.1086/203952) [DOI] [Google Scholar]
- 66.Grueter CC, Chapais B, Zinner D. 2012. Evolution of multilevel social systems in nonhuman primates and humans. Int. J. Primatol. 33, 1002–1037. ( 10.1007/s10764-012-9618-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gould RA. 1969. Yiwara: foragers of the Australian desert. New York City, NY: Charles Scribners and Sons. [Google Scholar]
- 68.Stewart JH. 1938. Basin-plateau aboriginal sociopolitical groups. Salt Lake City, UT: Smithsonian Institution Bureau of American Ethnology Bulletin, University of Utah Press. [Google Scholar]
- 69.Gurven M, Hill K. 2009. Why do men hunt? Curr. Anthropol. 50, 51–74. ( 10.1086/595620) [DOI] [PubMed] [Google Scholar]
- 70.Kaplan H, Hill K. 1985. Hunting ability and reproductive success among male Ache foragers: preliminary results. Curr. Anthropol. 26, 131–133. ( 10.1086/203235) [DOI] [Google Scholar]
- 71.Kramer KL, Ellison PT. 2010. Pooled energy budgets: resituating human energy allocation tradeoffs. Evol. Anthropol. 19, 136–147. ( 10.1002/evan.20265) [DOI] [Google Scholar]
- 72.Murdock GP. 1967. The ethnographic atlas. A summary. Ethnology 6, 109–236. ( 10.2307/3772751) [DOI] [Google Scholar]
- 73.Gragson TL. 1989. Allocation of time to subsistence and settlement in a Ciri Khonome Pumé Village of the Llanos of Apure, Venezuela. Pittsburgh, PA: Pennsylvania State University. [Google Scholar]
- 74.Greaves RD. 1977. Ethnoarchaeological investigation of subsistence mobility, resource targeting, and technological organization among pumé foragers of Venezuela. PhD dissertation, University of New Mexico, Albuquerque. [Google Scholar]
- 75.Greaves RD. 1997. Hunting and multifunctional use of bows and arrows: ethnoarchaeology of technological organization among Pumé Hunters of Venezuela. In Projectile technology (ed. Knecht H.), pp. 287–320. New York, NY: Plenum Press. [Google Scholar]
- 76.Greaves RD. 2006. Forager landscape use and residential organization. In Archaeology and ethnoarchaeology of mobility (eds Sellet F, Greaves RD, Yu PL), pp. 127–152. Gainesville, FL: University Press of Florida. [Google Scholar]
- 77.Oficina Central de Estadisticas e Informatica (OCEI). 1985. Censo Indígena de Venezuela. Republica de Venezuela, Presidencia de la Republica, Oficina Central de Estadisticas e Informatica. Caracas, Venezuela: Taller Gráfico de la OC.EI. [Google Scholar]
- 78.Oficina Central de Estadisticas e Informatica (OCEI). 1995. Censo Indígena de Venezuela 1992. Tomo I. Republica de Venezuela, Presidencia de la Republica, Oficina Central de Estadisticas e Informatica. Caracas, Venezuela: Taller Gráfico de la OC.EI. [Google Scholar]
- 79.Kramer KL, Greaves RD. 2016. Diversity or replace. What happens to wild foods when cultigens are introduced into hunter–gatherer diets. In Why forage? Hunters and gatherers living in the 21st century (eds Codding B, Kramer KL), pp. 15–42. Santa Fe, NM: School of Advanced Research. [Google Scholar]
- 80.Kramer KL. 2008. Early sexual maturity among Pumé foragers of Venezuela. Fitness implications of teen motherhood. Am. J. Phys. Anthropol. 136, 338–350. ( 10.1002/ajpa.20817) [DOI] [PubMed] [Google Scholar]
- 81.Kramer KL, Greaves RD. 2010. Synchrony between growth and reproductive patterns in human females: rapid juvenile growth among Pumé foragers. Am. J. Phys. Anthropol. 141, 235–244. ( 10.1002/ajpa.21139) [DOI] [PubMed] [Google Scholar]
- 82.Coale A, Demeny P. 1983. Regional model life tables and stable populations. New York, NY: Academic Press. [Google Scholar]
- 83.Gurven M, Kaplan H. 2007. Longevity among hunter–gatherers: a cross-cultural examination. Popul. Dev. Rev. 33, 321–365. ( 10.1111/j.1728-4457.2007.00171.x) [DOI] [Google Scholar]
- 84.Greaves RD, Kramer KL. 2013. Hunter–gatherer use of wild plants and domesticates: archaeological implications for mixed economies before agricultural intensification. J. Archaeol. Sci. 41, 263–271. ( 10.1016/j.jas.2013.08.014) [DOI] [Google Scholar]
- 85.Kramer KL, Greaves RD, Ellison PT. 2009. Early reproductive maturity among Pumé foragers. Implications of a pooled energy model to fast life histories. Am. J. Hum. Biol. 21, 430–437. [DOI] [PubMed] [Google Scholar]
- 86.Kramer KL, Greaves RD. 2007. Changing patterns of infant mortality and fertility among Pumé foragers and horticulturalists. Am. Anthropol. 109, 713–726. ( 10.1525/aa.2007.109.4.713) [DOI] [Google Scholar]
- 87.Schacht R, Borgerhoff MM. 2015. Sex ratio effects on reproductive strategies in humans. R. Soc. open sci. 2, 140402 ( 10.1098/rsos.140402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schacht R, Smith KR. 2017. Causes and consequences of adult sex ratio imbalance in a historical U.S. population. Phil. Trans. R. Soc. B 372, 20160314 ( 10.1098/rstb.2016.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bribiescas RG. 2006. Men. In Evolutionary and life history. Cambridge, MA: Harvard University Press. [Google Scholar]
- 90.Plas E, et al. 2000. Effects of aging on male fertility? Exp. Gerontol. 35, 543–551. ( 10.1016/S0531-5565(00)00120-0) [DOI] [PubMed] [Google Scholar]
- 91.Wood JW. 1994. Dynamics of human reproduction. New York, NY: Aldine de Gruyter. [Google Scholar]
- 92.Broer L, et al. 2013. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 21, 1163–1168. ( 10.1038/ejhg.2012.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaffe AE, Eaton WW, Straub RE, Marenco S, Weinberger DR. 2014. Paternal age, de novo mutations and schizophrenia. Mol. Psychiatry 19, 274–275. ( 10.1038/mp.2013.76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ancona S, Dénes FV, Krüger O, Székely T, Beissinger SR. 2017. Estimating adult sex ratios in nature. Phil. Trans. R. Soc. B 372, 20160313 ( 10.1098/rstb.2016.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marlowe FW, Berbesque JC. 2012. The human operational sex ratio: effects of marriage, concealed ovulation, and menopause on mate competition. J. Hum. Evol. 63, 834–842. ( 10.1016/j.jhevol.2012.09.004) [DOI] [PubMed] [Google Scholar]
- 96.Donald PF. 2007. Adult sex ratios in wild bird populations. Ibis 149, 671–692. ( 10.1111/j.1474-919X.2007.00724.x) [DOI] [Google Scholar]
- 97.Chisholm JS. 1999. Death, hope and sex: steps to an evolutionary ecology of mind and mortality. New York, NY: Cambridge University Press. [Google Scholar]
- 98.Kozlowski J. 1992. Optimal allocation to growth and reproduction: implications for age and size at maturity. Trends Evol. Ecol. 7, 15–19. ( 10.1016/0169-5347(92)90192-E) [DOI] [PubMed] [Google Scholar]
- 99.Purvis A, Harvey P. 1995. Mammal life history evolution: a comparative test of Charnov's model. J. Zool. 237, 259–283. ( 10.1111/j.1469-7998.1995.tb02762.x) [DOI] [Google Scholar]
- 100.Josephson SC. 2002. Does polygyny reduce fertility? Am. J. Hum. Biol. 14, 222–232. ( 10.1002/ajhb.10045) [DOI] [PubMed] [Google Scholar]
- 101.Pollet TV, Nettle D. 2009. Market forces affect patterns of polygyny in Uganda. Proc. Natl Acad. Sci. USA 106, 2114–2117. ( 10.1073/pnas.0810016106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strassmann B. 2000. Polygyny, family structure and child mortality: a prospective study among the Dogon of Mali. In Adaptation and human behavior: an anthropological perspective (eds Cronk L, Chagnon N, Irons W), pp. 49–67. New York, NY: Aldine de Gruyter. [Google Scholar]
- 103.Strassmann BI. 1997. Polygyny as a risk factor for child mortality among the Dogon. Curr. Anthropol. 38, 688–695. ( 10.1086/204657) [DOI] [Google Scholar]
- 104.Fretwell SD, Calver JS. 1969. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 19, 37–44. ( 10.1007/BF01601954) [DOI] [Google Scholar]
- 105.Kahlenberg SM, et al. 2008. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav. 76, 1497–1509. ( 10.1016/j.anbehav.2008.05.029) [DOI] [Google Scholar]
- 106.Pusey AE, Murray C, Wallauer W, Wilson M, Wroblewski E, Goodall J. 2008. Severe aggression among female chimpanzees at Gombe National Park, Tanzania. Int. J. Primatol. 29, 949–973. ( 10.1007/s10764-008-9281-6) [DOI] [Google Scholar]
- 107.Townsend SW, Slocombe KE, Emery TM, Zuberbhler K. 2007. Female-led infanticide in wild chimpanzees. Curr. Biol. 17, R355–R356. ( 10.1016/j.cub.2007.03.020) [DOI] [PubMed] [Google Scholar]
- 108.Johnson ML, Gaines MS. 1990. Evolution of dispersal: theoretical models and empirical tests using birds and mammals. Annu. Rev. Ecol. Syst. 21, 449–480. ( 10.1146/annurev.es.21.110190.002313) [DOI] [Google Scholar]
- 109.Komdeur J, Székely T, Long X, Kingma SA. 2017. Adult sex ratios and their implications for cooperative breeding in birds. Phil. Trans. R. Soc. B 372, 20160322 ( 10.1098/rstb.2016.0322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. ( 10.1016/S0003-3472(80)80103-5) [DOI] [Google Scholar]
- 111.Pope TR. 2000. The evolution of male philopatry in Neotropical monkeys. In Primate males: causes and consequences of variation in group composition (ed. Kappeler PM.), pp. 219–235. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 112.Kappeler PM. 2017. Sex roles and adult sex ratios: insights from mammalian biology and consequences for primate behaviour. Phil. Trans. R. Soc. B 372, 20160321 ( 10.1098/rstb.2016.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dobson FS, Jones WT. 1985. Multiple causes of dispersal. Am. Nat. 126, 855–858. ( 10.1086/284457) [DOI] [Google Scholar]
- 114.Pusey AE, Packer C. 1987. The evolution of sex-biased dispersal in lions. Behaviour 101, 275–310. ( 10.1163/156853987X00026) [DOI] [Google Scholar]
- 115.Cheney DL, Seyfarth R, Smuts B. 1986. Social relationships and social cognition in nonhuman primates. Science 234, 1361–1366. ( 10.1126/science.3538419) [DOI] [PubMed] [Google Scholar]
- 116.DiFiore A, Rendall D. 1994. Evolution of social organization: a reappraisal for primates using phylogenetic methods. Proc. Natl Acad. Sci. USA 91, 9941–9945. ( 10.1073/pnas.91.21.9941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strier KB. 1990. New World primates, new frontiers: insights from the woolly spider monkey, or muriqui (Brachyteles arachnoids). Int. J. Primatol. 11, 7–19. ( 10.1007/BF02193693) [DOI] [Google Scholar]
- 118.Strier KB. 1999. Why is female kin bonding so rare? Comparative sociality of neotropical primates. In Comparative primate socioecology (ed. Lee PC.), pp. 300–319. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 119.Blurton JN, Hawkes K, O'Connell JF. 2005. Older Hadza men and women as helpers. In Hunter–gatherer childhoods: evolutionary, developmental & cultural perspectives (eds Hewlett BS, Lamb ME), pp. 214–236. New Brunswick, NJ: Aldine/Transaction. [Google Scholar]
- 120.Marlowe FW. 2010. The Hadza: hunter–gatherers of Tanzania. Berkeley, CA: University of California Press. [Google Scholar]
- 121.Alvarez HP. 2004. Residence groups among hunter–gathers: a view of the claims and evidence for patrilocal bands. In Kinship and behavior in primates (ed. Berman CM.), pp. 420–442. Oxford, UK: Oxford University Press. [Google Scholar]
- 122.Gray JP, Costopoulos AO. 2006. On artificial trends in comparative studies using standard cross-cultural sample data: possibility and probability. Curr. Anthropol. 47, 149–151. ( 10.1086/498954) [DOI] [Google Scholar]
- 123.Kramer KL. 2005. Children's help and the pace of reproduction: cooperative breeding in humans. Evol. Anthropol. 14, 224–237. ( 10.1002/evan.20082) [DOI] [Google Scholar]
- 124.Kramer KL. 2010. Cooperative breeding and its significance to the demographic success of humans. Annu. Rev. Anthropol. 39, 414–436. ( 10.1146/annurev.anthro.012809.105054) [DOI] [Google Scholar]
- 125.Kaplan HS, et al. 2012. Risk and the evolution of human exchange. Proc. R. Soc. B 279, 20112614 ( 10.1098/rspb.2011.2614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jack KM, Isbell LA. 2009. Dispersal in primates: advancing an individualized approach: preface. Behaviour 146, 429–436. ( 10.1163/156853909X410612) [DOI] [Google Scholar]
- 127.Shultz S, Opie C, Atkinson QD. 2011. Stepwise evolution of stable sociality in primates. Nature 479, 219–224. ( 10.1038/nature10601) [DOI] [PubMed] [Google Scholar]
- 128.Castellano S, et al. 2014. Patterns of coding variation in the complete exomes of three Neandertals. Proc. Natl Acad. Sci. USA 111, 6666–6671. ( 10.1073/pnas.1405138111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chapais B. 2009. The deep structure of human society: primate origins and evolution. In Mind the gap: tracing the origins of human universals (eds Kappelar PM, Silk JB), pp. 19–51. Heidelberg, Germany: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the analyses can be accessed via the electronic supplementary material.