Abstract
Theoretical models and empirical studies in various taxa have identified important links between variation in sex roles and the number of adult males and females (adult sex ratio (ASR)) in a population. In this review, I examine these relationships in non-human primates. Because most existing theoretical models of the evolution of sex roles focus on the evolutionary origins of sex-biased behaviour, they offer only a general scaffold for predicting variation in sex roles among and within species. I argue that studies examining sex role variation at these more specific levels need to take social organization into account to identify meaningful levels for the measurement of ASR and to account for the fact that ASR and sex roles mutually influence each other. Moreover, taxon-specific life-history traits can constrain sex role flexibility and impact the operational sex ratio (OSR) by specifying the minimum length of female time outs from reproduction. Using examples from the primate literature, I highlight practical problems in estimating ASR and OSR. I then argue that interspecific variation in the occurrence of indirect forms of paternal care might indeed be linked to variation in ASR. Some studies also indicate that female aggression and bonding, as well as components of inter-sexual relationships, are sensitive to variation in ASR. Thus, links between primate sex roles and sex ratios merit further study, and such studies could prompt the development of more specific theoretical models that make realistic assumptions about taxon-specific life history and social organization.
This article is part of the themed issue ‘Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies’.
Keywords: adult sex ratio, life history, social organization, sexual selection, parental care, sex roles
It is now clear that relationships between relative gamete size, the evolution of parental care, OSRs, the relative intensity of competition, and the extent of selectivity in the two sexes are not as straightforward as was originally supposed. Tim Clutton-Brock [1, p. 1882]
1. Introduction
Sex differences in behaviour are ubiquitous among sexually reproducing animals. Many of these sex differences can be ultimately related to sex roles, defined as differences between males and females in the intensity of reproductive competition with members of the same sex, in how choosy they are in selecting mates and in the nature and extent of parental care they exhibit [2]. A frequently observed pattern across vertebrates, characterized by caring females and competing males, has been referred to as ‘conventional’ sex roles. These widespread sex differences in behaviour have ultimately been traced back to differences in gamete size, i.e. anisogamy [3,4], but exactly how anisogamy shapes sex roles has recently been questioned [5]. However, denying any effects of anisogamy for sex roles, and attributing all observed variability among species to chance and idiosyncratic, environment-driven factors [6,7] or doubting the existence of sex roles altogether [8–10], are positions that cannot be easily reconciled with the fact that sexually reproducing animals exhibit stunning diversity in mating systems [11–13] and patterns of parental care [14,15]. Nonetheless, this criticism has been helpful in focusing conceptual and empirical attention on the flexibility of sex roles and the causes of variation within and across species [16], suggesting that sex differences in gamete size create important initial asymmetries for the evolution of sex roles, but that anisogamy alone does not guarantee uniformly stereotypical sex roles. Understanding how additional factors, including sexual conflict, are impacting sex-specific selection to shape species-specific sex roles is therefore one of the current frontiers in sexual selection research.
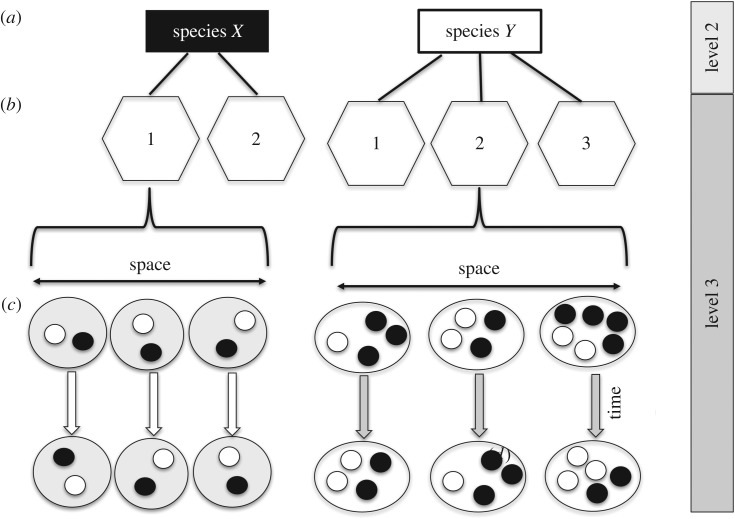
Because of complex interactions between various factors, predicting the actual pattern of sex roles in a given (set of) species is not straightforward. A number of influential models have illuminated the initial evolution of sex roles, in particular the origins of sex differences in parental care [5,17–19]. These models indicated that anisogamy only sets the stage for various trade-offs and feedbacks among multiple other factors, including sexual selection, female polyandry and the number of adult males and females [5,16]. The proportion of males in the adult population (adult sex ratio (ASR)), in particular, has emerged as a key predictor variable from these models, and it also plays an important role in theoretical models, such as biological market models [20], that focus on proximate determinants of sex-specific behaviour. However, existing ‘origin (or level 1) models’ address neither subsequent variation in sex roles among closely related taxa with shared life histories and ecologies (level 2), nor do they make specific predictions for phenotypically plastic responses in sex roles to changes in environmental variables within species (level 3) (figure 1).
Figure 1.
Levels of analysis of ASR variation. Species (a) consist of different populations (b), each of which is made up of multiple social units (pairs in Species X and groups in Species Y) with variable numbers of adult females (black circles) and males (white circles). Social units change in adult sex ratio also over time (c) as a result of births, deaths, immigration and emigration. For different analytical purposes, it is useful to distinguish between intra-specific variation (level 3), interspecific variation (level 2) and theoretical models that explore the evolutionary origins of sex roles (level 1).
According to the origin models of sex role evolution [5,17,18], variation in the number of adult males and females is a variable that should affect the evolution of sex differences in parental care, but the magnitude in biases of ASR variation, its determinants, its consequences for parental and reproductive strategies as well as potential feedback between ASR and sex roles are only beginning to be empirically explored [21,22]. Importantly, most empirical studies of the causes and consequences of ASR variation on sex roles have focused on explaining variation among and within species, i.e. at taxon-specific levels (i.e. levels 2 and 3) for which explicit predictive models are not yet available, but where biological market models have provided guidance and predictions; especially for studies of humans [23–27]. However, whereas the general origin models are useful as a scaffold for identifying general principles in the evolution of sex roles, an empirical ‘bottom-up approach’ used in various taxa has also generated a number of important insights.
First, wide variation in ASR exists across taxa as well as across space and time within species [22]. For example, of 183 species of bird examined in one of the first studies of ASR variation, only 35% had an ASR that did not deviate significantly from a balanced sex ratio [21], and in a marsupial population, the ASR changed more than twofold over just 5 years [28]. Second, biases in ASR can arise as a result of deviations from an even primary sex ratio at conception, sex-biased mortality rates at all ages between conception and sexual maturity, a sex difference in rates of sexual maturation as well as from mortality stemming from sex-biased natal dispersal and migration [29–31], and are therefore not always easy to estimate [32]. In addition, ASR is the result of sex-specific reproductive strategies, which, for example, influence the number of males per group ([33], see below), and therefore has cumulative effects at the population level. Third, eminent evolutionary biologists had long noticed variation in ASR [34,35], but neither sufficiently detailed data nor sophisticated theoretical concepts were available to them at the time to link this variation explicitly to sex roles. Today, experimental [36,37] and correlational [5,16,22,38] evidence indicates that ASR influences, or is at least correlated with, processes of mate acquisition, breeding system, patterns of biparental care and sexual conflict. Furthermore, some adaptive behavioural responses to a biased ASR, like increased mate harassment in male-biased populations [39], feed back on the ASR via sex-specific mortality rates.
The focus of this paper is on the relationships between the ASR and sex roles in mammals. After discussing three general points about mammalian sex roles, I will review behavioural correlates and consequences of ASR variation in the mammalian order for which most behavioural data exist—non-human primates [40]—to examine whether and how behavioural differences between species and within species over time relate to different ASRs. Some of these insights might be useful for future attempts at modelling sex role variation across and within mammalian societies.
2. Sex role variation and dynamics in mammals
In this section, I will discuss three general points about mammalian sex roles and sex ratios. First, variation in sex roles among and within species can be affected by the ASR at the population level, but perhaps also by the ASR of taxon-specific social units because mating in mammals is socially structured below the population level. However, the distinction between ASRs at the species, population and group level (figure 1) has not been systematically considered in previous studies and models. Second, most previous studies have examined the effects of ASR on sex roles. This relationship is not unidirectional, however, because the competitive component of sex roles can themselves contribute to biases in ASRs, for example, exacerbating sex differences in mortality. Finally, lineage-specific life-history traits constrain sex role flexibility generally, for example, by committing one sex to parental care, but they also impact sex roles more specifically by affecting the operational sex ratio (OSR), defined as the ratio of fertilizable females to sexually active males [4,41], by determining the sex-specific duration of time outs from reproduction [42]. I will discuss these aspects in more detail below because they have not yet received much explicit acknowledgement in the literature.
(a). Adult sex ratio and social organization
The social organization of a species is defined by the distribution and association pattern of adult males and females in space and time [43]. Among mammals, three types of outcomes are most common: adult individuals of a species can either be solitary, associated with a member of the opposite sex in pairs, or they live in groups of three or more adults, with some group-living species exhibiting interesting multi-level structuring [44,45] or internal association dynamics [46–49]. Level 1 models investigating the evolution of sex roles use population-wide numbers of adult males and females and implicitly assume that all individuals can potentially mate with each other or that the population ASR corresponds closely to the local ASR. When access to mates is limited to a subset of the population, explicit acknowledgement of intra-specific social stratification may be required, however, if the objective is to explain and predict sex role variation at level 2 or 3.
Classes of vertebrates differ widely in their modal type of social organization [50,51], but it has not been considered whether these differences should be taken into consideration in studies examining the relationships between sex roles and ASR in particular taxa. Inter- and intra-specific variation in ASR was first reported for birds [35], where data have been recorded and summarized at the level of species or individual populations. Because of the practical problems associated with various methods used to collect these data [32], and because the vast majority of birds are organized into pairs [11], considering social organization in comparative studies of birds may be of little practical importance. In other species of vertebrates, which are either solitary or organized into large schools or flocks, population ASR is presumably also an adequate measure of the number of competitors and potential mates because mating opportunities are less socially constrained. Many mammals, however, live in groups of multiple adults, often, but not always, including members of both sexes, typically in uneven numbers [52]. The question therefore arises whether ASR at the species, population and group level represent similar selective agents for mammals, and whether variation at one level is biologically more meaningful for explaining variation in sex roles.
With rare exceptions [53,54], demographic variables of a species can typically only be sampled rather than directly measured. As a result, ASR can either be estimated at the population level or it can be expressed as a group average (also referred to as the socionomic sex ratio, SSR [55]). In solitary species, ASR can only be estimated from a population sample. However, additional behavioural data may be useful for more realistic assessments of the actual local intensity of mating competition and opportunities for mate choice, for example, in species where males roam widely instead of defending territories [56,57] or where mating access is dependent on dominance. ASR estimates in group- or pair-living mammals should be less biased at the population level, because they may also include potential floater and bachelor males. However, such non-resident males may be much more difficult to observe or capture, especially in nocturnal or arboreal species. In species that live in pairs or groups, average SSR is more easily determined in practice, but it will misestimate sex ratio biases because it does not include non-group members [58]. As in solitary species, only behavioural data can help identifying biologically meaningful units for assessing the effects of ASR on sex roles. For example, it may be important to know whether individuals interact exclusively with own group members or whether they perceive and respond to the ASR beyond their own group based on direct contact, range overlap, scent marks, long-range vocalizations or temporary contact at clumped resources. Long-term data on known individuals typically provide information about group histories and dispersal status. In mammals, this information about the effective number of potential rivals and mates should influence male reproductive strategies, in particular, but only if non-resident males pose a significant challenge for the reproductive success of residents [59].
Given common practical differences associated with behavioural observations of matings with non-residents, genetic paternity analyses could provide estimates of the strength of breeding competition coming from outside a social unit. In reptiles, multiple paternity of clutches is widespread, both among species and in over 50% of individual clutches [60]. Most reptiles are solitary, but in the few taxa with strong pair-bonding, multiple paternity occurs in 10–30% of clutches. In birds, extra-pair paternity occurs in about 90% of species, and over 11% of offspring are on average not sired by the resident male [61]. In mammals, the proportion of offspring fathered by non-resident males varies between 0 and 80% [62]. In groups with multiple males and females, and in species where single males are only intermittently associated with one or several females, about 35% of offspring are sired by non-resident males [62]; among socially monogamous mammals, extra-pair paternity rates are around 20% [63]. Across social and mating systems, extra-group paternity in mammals is negatively correlated with the length of the mating season and positively correlated with the number of resident females ([64], see also [65]). These very crude estimates indicate that opportunities for mating with non-resident males are ubiquitous across terrestrial vertebrates, but future studies should aim at better resolution by determining the identity of extra-group males to distinguish between offspring sired by true floaters or males from neighbouring groups. Thus, it appears that population-level measures of ASR should provide more meaningful estimates of the respective potential for male reproductive competition and female choice. Including information about the species-specific social organization and sex-specific behavioural strategies may refine these ASR estimates, however.
(b). Dynamic interactions between sex roles and adult sex ratio
Previous models and empirical studies have explicitly or implicitly examined the consequences of ASR variation for sex roles and the effects of (mortality costs of) parental care for ASR and OSR, respectively. However, sex-specific competitive strategies may also affect ASR through their effect on group composition and biased mortality. These effects are evident at the level of social units, where they can be most easily measured (see above), but they might also be reflected at the population level if more intense competition leads to higher mortality rates or if particular forms of parental care are very costly. Thus, the relationships between ASR and the components of sex roles are more dynamic than previously thought because of multiple feedback loops.
How sex role components impact the SSR is explained by socio-ecological theory. Accordingly, interspecific variation in mammalian social organization can been explained by sex-specific adaptations to fitness-limiting resources [41,66,67]. Thus, females integrate information about the distribution of food, predators, parasites and shelters in their environment and either space out or associate with other females in response. While these ecological factors also affect male fitness, their distribution is primarily determined by the distribution of females [68,69] because male fitness is typically constrained by access to receptive females. The spatial and temporal distribution of receptive females determine male monopolization potential [70], leading to four principle outcomes. Males can either associate with a single female, they can defend access to multiple solitary females more or less exclusively, they can establish exclusive access to a group of females or groups of females are joined by multiple males [43]. Males unable to secure access to females in all these types of social organization may either float solitarily among social units or form all-male groups [58,71,72]. Thus, male competition shapes the ASR of basic social units, but how this variation corresponds to the population-level ASR is an open empirical question (see above). In any event, future models and empirical studies of sex role variation between and within species should be aware of the dynamic feedback loop between sex roles and ASR.
(c). Sex roles and life history
Sex roles are also fundamentally related to the biological realities of different taxa because animals differ widely in their life histories [73]. The number, size and developmental state of offspring at birth, in particular, have direct consequences for the necessity and opportunities for parental care across taxa [14]. Some of these life-history traits also lead to sex differences in, or sexual conflict over, parental care. Such taxon-specific life-history constraints should be considered in models of sex role evolution in specific lineages.
Mammals provide a particularly compelling example of the effects of a particular life-history trait on sex roles because few other taxa exhibit more asymmetrical, consistent sex differences in parental investment. Owing to the physiological constraints of internal gestation and lactation, which are a defining feature of the mammals, females provide the bulk of post-zygotic care, most notably in the form of lactation [74]. Because of these constraints, male mammals are exceptional (together with reptiles) among vertebrates in that uniparental male care for offspring is absent [15]. In addition, following fertilization, females enter an anovulatory period extending into lactational amenorrhoea. As a result, female mammals have longer mandatory ‘time outs’ from reproduction than males [42,75], affording successful males with faster potential reproductive rates and making access to receptive females the limiting factor for male reproductive success [4,41]. Male mammals were therefore traditionally seen as competing with rivals for multiple mating opportunities, whereas females were considered to be the coy, choosy sex [76].
The universality of this classical notion of sex-specific mammalian reproductive strategies was subsequently modified by recognizing that it can also pay for male mammals to be choosy under certain circumstances [13,77–80], if only by strategically allocating sperm [81], and that females can also compete with each other over mates and reproductive opportunities [1,82–86]. Hence, the fundamental sex roles of mammals are obviously constrained by internal, biological traits, but there are also interesting cases of intra- and interspecific variation in sex roles, including paternal care [87,88]. Thus, mammalian sex roles are generally, but not invariably constrained by a fundamental life-history trait, and models attempting to predict this variation among mammalian species need to take this fact into account.
Moreover, species-specific life-history traits, such as the length of gestation and lactation, can also affect sex roles through their impact on OSR. Specifically, a bias in the OSR can create frequency-dependent selection favouring increased mate guarding and reduced parental care by the sex facing more intense completion for mates [4,89,90]. In species where one sex is committed to parental care, the effects of ASR variation might be magnified by the OSR and more probably affect mate acquisition or mate guarding strategies than parental care [38]. I will illustrate some practical problems associated with spatial and temporal variation in ASR and OSR using some primate examples below.
3. Adult sex ratio and primate behaviour
Variation in ASR is both a cause and a consequence of sex-specific reproductive strategies, and these inter-relationships can be studied across and within species. To disentangle these different levels and mutual inter-dependencies, I will first illustrate why and how sex-specific reproductive strategies contribute to ASR variation across primate species by shaping species-typical types of social organization. Next, I will examine the consequences of ASR variation for opportunities for different forms of paternal care. The subsequent discussions of male and female reproductive strategies under variable ASRs will also emphasize intra-specific variation in ASR, and especially in OSR. The final section will be devoted to the effects of ASR on inter-sexual relationships. Throughout this section, I will review reports from the primate literature and formulate predictions for future quantitative tests.
(a). Adult sex ratio and primate social organization
Quantifying ASR within primate groups is relatively straightforward where species form groups and records of the number of adult males and females can be averaged across multiple study groups, at least in the larger and diurnal species [91]. These species-specific values have been referred to as the SSR and were used in many comparative studies [55,92]. Because only a few primate species form all-male groups and because immigrants will be recognized in long-term studies, ASR estimates for group-living species should be fairly robust. Estimates of the number of adult floater males are more difficult because it is not always possible to distinguish between death and emigration when males disappear and because the time transferring males spend in transit between groups varies between and within species [93,94]. The same applies to pair-living species, which tend to have relatively even SSRs by definition, but including floaters, also through genetic paternity analyses, may lead to minor deviations from the expected even sex ratio [58,95–97]. In addition, there is often variation in the SSR of pair-living species at the population level because some social units include more than one adult member of each sex [98–101]. Only long-term observations of known individuals can reveal whether these cases really represent deviations from the modal pattern or cases of mature offspring awaiting dispersal opportunities while remaining on the parental territory.
The situation is more complicated for solitary species, which lack obvious social boundaries, even though they sometimes cluster into matrilineal kin clusters in neighbourhoods [102–104]. Here, it might be most practical to work with counts or long-term averages obtained from (repeated) sampling of a defined study area. Genetic typing based on non-invasively collected samples (e.g. faeces or hair) may also provide information about elusive individuals, and cohort-based survival models may be useful in certain cases to estimate ASR from the stable age distribution. The accuracy of ASR estimates in all primates, irrespective of their social organization, is further complicated by the fact that only long-term studies of known individuals have sufficient information about age and maturation schedules to classify individuals as adults. This problem is particularly acute in species with sexual bimaturism, where males may be spermatogenic for years before they achieve the adult phenotype [105], but subtler developmental sex differences are widespread across primates [106]. Empirical studies of ASR variation should therefore be very explicit about how the number of adult males and females was determined and how adulthood was defined [107].
Factors explaining species differences in social organization should also explain a large proportion of interspecific variation in SSR. Birth sex ratios typically exhibit only a small male bias in most primates [108], so they cannot explain the common female-biased ASR. Comparative analyses revealed that this pre-natal bias in primate birth sex ratios in favour of the dispersing sex across species is explained by local resource competition [109]; the opposite bias in cooperatively breeding species is predicted by the local resource enhancement hypothesis. Important post-natal factors responsible for sex ratio variation include sex differences in maturation schedules and other sources of sex-differential juvenile mortality [110–112]. As in other mammals, all else being equal, larger and stronger males should enjoy above-average reproductive success, and males may pay the costs of the required somatic investment through compromised health and condition, greater susceptibility to parasites and a higher risk of wounding [113].
In species where birth ratios are unequal, they are typically biased in favour of sons [114], presumably because mothers in good condition benefit from overproducing male offspring [115]. However, empirical [116] and theoretical [117] studies have questioned the strength of this relationship, and the sex-specific reproductive value of offspring should also be considered in interpreting biases in maternal sex allocation [118]. Such biases in birth sex ratios tend to be found most consistently in polgynous species with intense male competition, which also tend to have the most female-biased ASRs [114,119,120]. Thus, changes in sex ratios between birth and adulthood should be most pronounced in species living in one-male groups, followed by species living in multi-male groups and pairs.
At the population level, sex differences in dispersal and the attendant sex differences in mortality are a major source of ASR variation [30–32]. Immigration and emigration may not have major effects on SSRs, however, because they should cancel each other out in species with socially structured populations in cases of successful dispersal. In other words, one group's emigrant is another group's immigrant, so that taking averages from several groups should account for successful dispersal, given a long enough time scale and low rates of dispersal-associated mortality. In addition, female interests also influence immigration decisions [121–123], making sexual conflict over group composition an interesting demographic aspect of the more general conflict between the sexes. Several studies indicate that the group sex ratio is indeed a good predictor of dispersal decisions in primates. Based on the expected level of male reproductive skew, males in high-skew populations tend to target groups based on qualities of the dominant male(s), whereas in species with lower male reproductive skew males tended to immigrate into groups with either fewer rivals or more sexually active females [124,125]. Species with female or bisexual dispersal may be responding primarily to other factors, such as the risk of infanticide [126], also suggesting that the local SSR is an important proximate determinant of behavioural strategies.
(b). Adult sex ratio and paternal care
Primate males can exhibit two major types of paternal care [127,128]. Direct beneficial interactions with dependent infants in the form of carrying or provisioning are found in less than 10% of all primate species, reflecting the general physiological constraints obligating maternal care in mammals. More indirect beneficial effects for offspring accrue in the form of paternal protection from major risks, such as predation and infanticide. The fitness consequences of other types of male offspring interactions, such as grooming or playing, remain to be systematically explored. As I will argue below, paternal care in primates has been primarily examined as a species-level trait, where it is poorly related to ASR, but some intra-specific variation may be sensitive to ASR variation.
Most female primates regularly mate with multiple males during one receptive cycle [129]. In addition, compared with other mammals, primates have relatively slow life histories [130]. This combination of conditions makes it both risky and costly for male primate to exhibit direct paternal care. Paternal care is risky because female polyandrous mating reduces paternity certainty, so that males may end up investing in others' offspring, unless they have sophisticated kin recognition mechanisms [131] or rely on reliable proxies for paternity, such as mate guarding during the fertile period of their ovulatory cycle. Paternal care is costly not only in terms of the direct physical costs, but also in terms of foregoing additional mating opportunities because direct care among primates is limited to monogamous species. It is therefore not surprising that direct paternal care is generally rare.
Interspecific variation in the occurrence of paternal care is not clearly predicted by ASR, however. As discussed above, different types of social organization are not only characterized by a particular ASR but also by different mating systems. Males are more likely to engage in paternal care if their probability of paternity is high. Paternity certainty is highest in monogamous species and in species with high male reproductive skew, e.g. one-male groups [132]. However, whereas paternal care is indeed found in some monogamous species (e.g. callitrichids, owl monkeys, titi monkeys), it is absent in others (gibbons, indri) and non-existent in polygynous species, such as gorillas (where immatures associate with high-ranking males that are not necessarily their fathers [133]), colobines or some sifakas [127,128]. Because paternal care also occurs in polyandrous callitrichids, paternity certainty is neither sufficient nor necessary for the occurrence of paternal care [134]. Thus, although female-biased ASRs in birds are associated with little or no paternal care [21,31], and a male-biased OSR in humans has been linked to the evolution of male parenting [135], the occurrence of direct paternal care across primates is not obviously related to ASR variation; a prediction that can be tested with phylogenetically controlled comparative analyses.
Indirect forms of paternal care may be more widespread because they are less costly and because predation and infanticide jeopardize infant survival more than other sources of early infant death in many species [136,137]. The permanent association between males and infant-carrying females, which distinguishes primates from other mammalian orders, has been proposed to reflect an anti-infanticide strategy [138]. Infant protection is not only in the father's direct interest, but it can also reflect mating effort. It is therefore not surprising that in some species, males and females form temporary ‘friendships’ that extend from mating to weaning [139], with male care for infants being conditional upon prior mating, at least in baboons and chimpanzees [140,141], so that these friendships seem to reflect parental rather than mating effort. In this particular context, ASR variation can apparently lead to behavioural consequences. For example, female baboons compete over such male friends when ASR becomes more female-biased [142,143], indicating that ASR models should not only consider male competition for mates. In particular, pregnant and lactating females exhibit higher rates of aggression when they share a male friend, providing evidence for ASR-dependent female competition over paternal care in a promiscuous species [144]. From a theoretical perspective, it would be interesting to examine the relationship between paternal care and mating effort, which is assumed to be structured by a trade-off in most sex role models, in more detail, and to ask whether and how males adjust their parental effort to variable group ASRs. Although this example indicates that primates can respond flexibly to variation in ASR in the larger context of indirect paternal care, more work is clearly required to understand the behavioural consequences of ASR variation, especially within species.
(c). Adult sex ratio and male reproductive strategies
Male reproductive performance typically exhibits greater variance than that of females (but see, e.g. [145,146]), and the number of males in a social unit appears to explain most of this variation [62,147]. Because a given social organization reflects the outcome of male and female interests, as well as of the resulting conflict between the sexes, studying variation in ASRs may further illuminate the sources of this variation in reproductive success. In several primate species from all radiations, including lemurs, howler monkeys, langurs or mountain gorillas, the modal group composition includes only a single male, but groups with a second male are regularly found in these species [33]. This variation in the number of males appears to be related to local variation in fecundity and mortality, i.e. demographic traits that modulate intruder pressure [148], providing another example of how components of sex-specific reproductive strategies affect ASR.
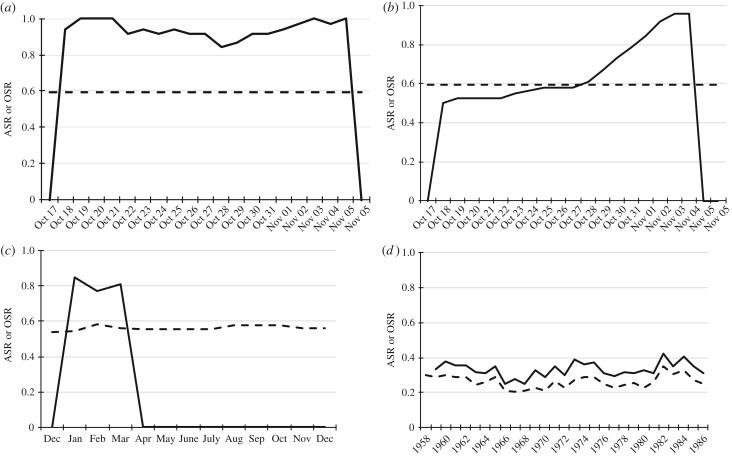
Independent of the actual number of males per group, another fundamental life-history variable has important effects on OSR. As pointed out by Mitani et al. [149], the long time outs of female primates from reproduction may actually shift a typically female-biased ASR towards a male-biased OSR, especially when female receptive periods are not synchronized [41,70]. Seasonal reproduction in annual breeders will trigger a similar shift towards successively more male-biased OSR as fertilized females drop out of the mating pool in the course of the mating season [57,150]. In some species, males may also drop out of the mating pool as the mating season progresses [119,151]. However, it is not straightforward to decide at which temporal scale, variation in the OSR is relevant because females of different species differ in the duration and degree of synchrony of their receptive periods (figure 2). This problem highlights the more general question of how animals proximately perceive variation in the number of adult males and females at a biologically meaningful scale. The fact that ASR and OSR are only poorly correlated in some (bird) species [155] underscores the importance of choosing the right measure.
Figure 2.
Relationship between ASR and OSR in three primate species. (a) Gray mouse lemurs (Microcebus murinus) are a solitary species, where females are receptive on a single night once per year. Depicted is the daily OSR (=adult males/(adult males + receptive females)) during the three-week annual mating season of 22 females (individual receptive period known) and 27 males (solid) and the ASR (dashed) for one study population. Details in [152]. (b) Dynamic cumulative depiction of the same data, assuming that all females are potentially ready to mate at the beginning of the mating season and gradually drop out of the mating pool as they get impregnated. (c) ASR (dashed) and OSR (solid) for a population of Verreaux's sifaka (Propithecus verreauxi), a group-living species with seasonal annual reproduction. Depicted are monthly means averaged across eight groups over 1 year [153]. (d) ASR (dashed) and OSR (solid) for a group of Japanese macaques (Macaca fuscata), who breed seasonally and females have an average inter-birth interval of 1.56 years. Depicted are annual means for ASR and annual estimates of OSR (number of females present, corrected for average inter-birth interval of females reproducing in the previous year resulting in time outs) over 27 years. Reported numbers are based on one annual census [154].
Hence, exclusive reliance on the more readily available data on SSR to analyse male reproductive strategies and their consequences may fall short for two reasons. First, the constraints imposed by a species' social organization may result in identical numbers of adult males per social unit, which can vary greatly in the number of adult females, however. Every comparison between any gibbon species and a sympatric langur species with one-male groups will illustrate the point that they differ greatly in ASR but not in OSR if receptive langur females are not synchronized in their reproductive activity. Second, as with other relatively large and long-lived mammals, primate females have long inter-birth intervals [130]. Thus, a substantial proportion of adult females may not be ready to mate at any given point in time, creating a strongly male-biased OSR, despite a female-biased ASR in multi-male, multi-female species, or an even OSR in one-male, multi-female groups if females are receptive asynchronously.
Several previous studies have examined consequences or correlates of interspecific variation in ASR and OSR across primates. Most research in this context has focused on morphological correlates of male–male competition, assuming that body size and weapons mediate components of sex roles related to competition. For example, controlling for body size and phylogeny, Mitani et al. [149] found that OSR (taking female time outs into consideration) is a strong predictor of sexual size dimorphism across 18 polygynous primate species, with the degree of sexual size dimorphism reflecting evolutionary advantages for larger males in competing with rivals or excluding them from a group altogether. However, similar analyses revealed no correlation between OSR and canine dimorphism [156], even though sexual selection on canine size appears to be stronger than on body size [157]. Because estimates of OSR are not perfect [156] and only available for relatively few haplorrhine primates, more detailed comparative analyses are indicated to resolve this apparent discrepancy between interspecific variation in canine and body size dimorphism.
Male reproductive skew provides another example of a variable that is influenced by sex ratios. The priority-of-access model predicts that the dominant male in a multi-male group monopolizes reproduction [158], but it does not take the number of males in a group into account. An extended version of this model explicitly included the number of rivals, but not the number of females, and found this to be the only predictor of reproductive skew in phylogenetically controlled species comparisons [159]. Thus, mating skew decreases as the number of males increases, making it more difficult for the dominant male to monopolize females. Studies of individual species also demonstrated this effect in mandrills [160] and chimpanzees [161]. Single-species studies indicated that the number of females can also be a powerful predictor of male reproductive skew [162], whereas the degree of reproductive synchrony had variable effects on measures of skew in comparative studies [159,163,164]. Additional males can also contribute to an increase in the dominant's tenure length [165] or reduce the risk that a group will be taken over by outside males and the dominant expelled [166,167]. In these situations, the presence of additional males also reduces mating skew, presumably through a concession mechanism [168], but such an effect is not obligate, for example, in Verreaux's sifakas [169].
When the number of co-resident male rivals is large and the expected mating rates of subordinates are low, alternative reproductive tactics may evolve. Theoretical models predict that with increasingly male-biased ASR, mate guarding, rather than roaming or competing, will be favoured [170]. In several invertebrates, the fitness benefits of mate guarding indeed increase with increasing ASR, even favouring the evolution of monogyny [171,172]. Such flexible responses within species (level 3) have been observed in Soay sheep [52,173], and recent models suggest the importance of a similar mechanism in humans [25]. In group-living primates, where females are clumped and receptive asynchronously, the dominant male may use mate guarding as a reproductive tactic to exclude rivals [160,174]. In an exceptionally large chimpanzee community with unusually many males, mate guarding was indeed more common than in other communities, but also performed by coalitions of males [153]. The proportion of males in the adult population in this community was also about twice as high as in other communities. Male–female friendships in several Old World monkeys (see above) may functionally correspond to extended mate guarding. Increased mate selectivity may represent another variation on this theme [175], but there is as of yet little evidence from primate studies supporting this notion [129]. Other alternative male reproductive tactics, including dispersal decisions, temporary influxes of males from all-male groups, coercive or surreptitious mating and coalition formation, have been described [176], but they have not yet been systematically linked to variation in ASR.
(d). Adult sex ratio and female reproductive strategies
The fact that female primates and other mammals not only compete with each other for food, but also in the context of reproduction has only recently been fully appreciated [1,82,85,86,177]. Sex ratios may play an important role in this context as well because female-biased ASRs may exacerbate this competition by increasing the number of competitors relative to males when female mammals compete directly for access to mates and paternal investment [82,83,178]. In a solitary ground squirrel, female mating failure varied indeed predictably with changes in the colony's ASR: a female's probability of breeding increased under more male-biased OSR and it was independent of local female density [150]. Among primates, increases in female aggression were observed among sexually receptive females in baboons [142], and in one population increases in female aggression were prompted by more female-biased ASR [143], indicating intrasexual competition for indirect paternal investment. In some group-living lemurs, female reproduction is compromised in larger groups, and some females are forcefully evicted by their relatives above a critical group size [179], but it is not yet known whether variation in ASR predicts any of these events. Similarly, we do not know whether primate females exercise more mate choice, e.g. by sampling more potential mates, when the ASR is more male-biased [129]. The level of female polyandry may also increase with increases in male bias in the ASR because this will reduce the risk of infanticide and provide other benefits of polyandry [180], but also increase the prevalence of sexually transmitted disease [181]. Despite numerous possible predictions, only one study has compared the effects of different sources of variation on rates of female agonism across primates, revealing that female group size is the best and only significant predictor thereof, but ASR was not considered in these analyses [182].
(e). Adult sex ratio and inter-sexual relationships
Finally, variation in ASR is also expected to have consequences for how males and females adapt their interactions to variable proportions of potential mates and rivals. These links between ASR variation and social interactions have been systematically explored in agent-based models, and results of these models have been compared with behavioural variation across several macaque species. Specifically, an increase in the proportion of males in the models resulted in higher rates of more intense aggression, which in turn prompted a greater differentiation of dominance values in both sexes, resulting in an increase in the proportion of females that dominate males [183]. Subsequent analysis of inter-sexual dominance relations in macaques confirmed that the degree of female dominance in despotic species, but not in more egalitarian species, increases with the percentage of males in the group, either because they have to deal with aggressive males or because of an increase in female aggression. More male-biased ASR can also lead to more male harassment of females, which can contribute to increased female mortality and, hence, a stronger male bias in ASR [184,185], but these effects have not yet been demonstrated in primates. Because male sexual coercion can provide males with increased probability of paternity, for example, in chimpanzees [186], its potential variation with ASR is to be expected, however. In mountain gorillas, male–female associations were weaker in groups with more males [187], suggesting that association patterns are also sensitive to variation in ASR. A sudden change in ASR in a baboon group due to the death of half of the adult males from tuberculosis also led to a more relaxed dominance hierarchy and an increase in male–female affiliation [188]. Because this pacific culture persisted after the ASR returned to values before the outbreak, a causal role of the change in ASR is not established, however. Thus, patterns of inter-sexual association, affiliation and aggression appear to be sensitive to ASR variation, but more research is clearly indicated to substantiate these relationships.
A final point relates to the fact both sexes might derive benefits from a biased ASR. Males not only benefit from an increase in the number of potential mates, but females may also derive direct fitness benefits from an increase in the number of males in their group. These benefits are primarily related to the risks of infanticide and predation and therefore also affect male–female relations in the context of sexual selection. First, additional males, especially in modally single-male groups, can reduce the risk of takeover and subsequent infanticide by outside males [137], thereby also providing a direct benefit to females with dependent infants. Second, additional males afford better protection from predators through a combination of mechanisms, and with increasing predation risk, the ASR becomes indeed less female-biased across species [189,190]. Single-species studies of populations with temporal variation in ASR demonstrate these effects more directly: for example, with a shift towards more female-biased ASR, female mortality due to predation increased in chacma baboons [143]. Furthermore, males may also participate in other potentially costly group activities, such as inter-group encounters, to improve their status as cooperation partners for females, who may return the service by offering other services like sex [191]. If this interpretation is correct, the frequency or costs of such male services should also vary with ASR.
4. Conclusion
This review indicated that existing theoretical models examining the evolution of sex roles can only offer broad guidance for studies examining sex role variation across and within species of primates and other mammals. To make progress with such taxon-oriented studies, three points should be acknowledged. First, even though the number of adult males and females is typically estimated by averaging SSRs because of the difficulties of sampling outside males, ASR estimates should be preferred. Additional behavioural data, preferably from long-term studies, may provide the required details, and the utility of demographic modelling for estimating mammalian ASRs could be explored. Second, ASR not only influences sex roles, but especially the competitive component of sex roles may affect ASR, creating a dynamic feedback. Third, lineage-specific life-history traits, like mammalian gestation and lactation, may constrain flexibility in parental care, and species-specific variation in such life-history traits may impact the OSR through its effects on time out from reproduction. Whether ASR or OSR is a better and more meaningful predictor of sex role variation, and how individuals perceive either one, remains to be determined empirically. Thus, next-generation modelling should appreciate key aspects of taxon-relevant natural history. The scant available evidence from primate field studies indicates that they are indeed sensitive to variation in ASR, inviting additional behavioural and comparative studies focusing on the causes and consequences of sex ratio variation.
Acknowledgements
I am grateful to Joan Silk, Tim Clutton-Brock, Tamas Székely, Steve Beissinger, the participants of the Wiko ASR workshop and, in particular, to Michael Jennions for helpful discussions and constructive feedback on this manuscript.
Data accessibility
This article has no additional data.
Funding
This paper was written while the author was a Fellow at the Wissenschaftskolleg zu Berlin.
Competing interests
I declare I have no competing interests.
References
- 1.Clutton-Brock TH. 2007. Sexual selection in males and females. Science 318, 1882–1885. ( 10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 2.Schärer L, Rowe L, Arnqvist G. 2012. Anisogamy, chance and the evolution of sex roles. Trends Ecol. Evol. 27, 260–264. ( 10.1016/j.tree.2011.12.006) [DOI] [PubMed] [Google Scholar]
- 3.Parker GA, Baker RR, Smith VCF. 1972. The origin and evolution of gamete dimorphism and the male–female phenomenon. J. Theor. Biol. 36, 529–553. ( 10.1016/0022-5193(72)90007-0) [DOI] [PubMed] [Google Scholar]
- 4.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 5.Kokko H, Jennions MD. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919–948. ( 10.1111/j.1420-9101.2008.01540.x) [DOI] [PubMed] [Google Scholar]
- 6.Gowaty PA. 2004. Sex roles, contests for the control of reproduction, and sexual selection. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP), pp. 37–54. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Gowaty PA, Hubbell SP. 2009. Reproductive decisions under ecological constraints: it's about time. Proc. Natl Acad. Sci. USA 106, 10 017–10 024. ( 10.1073/pnas.0901130106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roughgarden J, Oishi M, Akcay E. 2006. Reproductive social behavior: cooperative games to replace sexual selection. Science 311, 965–969. ( 10.1126/science.1110105) [DOI] [PubMed] [Google Scholar]
- 9.Ah-King M, Nylin S. 2010. Sex in an evolutionary perspective: just another reaction norm. Evol. Biol. 37, 234–246. ( 10.1007/s11692-010-9101-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ah-King M. 2012. On anisogamy and the evolution of ‘sex roles’. Trends Ecol. Evol. 28, 1–2. ( 10.1016/j.tree.2012.04.004) [DOI] [PubMed] [Google Scholar]
- 11.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. ( 10.1016/S0003-3472(80)80103-5) [DOI] [Google Scholar]
- 12.Andersson M, Iwasa Y. 1996. Sexual selection. Trends Ecol. Evol. 11, 53–58. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 13.Reynolds JD. 1996. Animal breeding systems. Trends Ecol. Evol. 11, 68–72. ( 10.1016/0169-5347(96)81045-7) [DOI] [PubMed] [Google Scholar]
- 14.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 15.Dulac C, O'Connell LA, Wu Z. 2014. Neural control of maternal and paternal behaviors. Science 345, 765–770. ( 10.1126/science.1253291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokko H, Jennions MD. 2012. Sex differences in parental care. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 101–116. Oxford, UK: University of Oxford Press. [Google Scholar]
- 17.Queller DC. 1997. Why do females care more than males? Proc. R. Soc. Lond. B 264, 1555–1557. ( 10.1098/rspb.1997.0216) [DOI] [Google Scholar]
- 18.Fromhage L, Jennions MD. 2016. Coevolution of parental investment and sexually selected traits drives sex-role divergence. Nat. Commun. 7, 12517 ( 10.1038/ncomms12517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennions MD, Fromhage L. 2017. Not all sex ratios are equal: the Fisher condition, parental care and sexual selection. Phil. Trans. R. Soc. B 372, 20160312 ( 10.1098/rstb.2016.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noë R, Hammerstein P. 1994. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11. ( 10.1007/BF00167053) [DOI] [Google Scholar]
- 21.Donald PF. 2007. Adult sex ratios in wild bird populations. Ibis 149, 671–692. ( 10.1111/j.1474-919X.2007.00724.x) [DOI] [Google Scholar]
- 22.Székely T, Weissing FJ, Komdeur J. 2014. Adult sex ratio variation: implications for breeding system evolution. J. Evol. Biol. 27, 1500–1512. ( 10.1111/jeb.12415) [DOI] [PubMed] [Google Scholar]
- 23.Schacht R, Rauch KL, Borgerhoff Mulder M. 2014. Too many men: the violence problem? Trends Ecol. Evol. 29, 214–222. ( 10.1016/j.tree.2014.02.001) [DOI] [PubMed] [Google Scholar]
- 24.Schacht R, Borgerhoff Mulder M. 2015. Sex ratio effects on reproductive strategies in humans. R. Soc. open. sci. 2, 140402 ( 10.1098/rsos.140402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schacht R, Bell AV. 2016. The evolution of monogamy in response to partner scarcity. Sci. Rep. 6, 32472 (doi:10.10.1038/srep32472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schacht R, Kramer KL. 2016. Patterns of family formation in response to sex ratio variation. PLoS ONE 11, e0160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schacht R, Tharp D, Smith KR. 2016. Marriage markets and male mating effort: violence and crime are elevated where men are rare. Hum. Nat. 27, 489 (doi10.1007/s12110-016-9271-x) [DOI] [PubMed] [Google Scholar]
- 28.Wayne AF, Maxwell MA, Ward CG, Vellios CV, Wilson I, Wayne JC, Williams MR. 2015. Sudden and rapid decline of the abundant marsupial Bettongia penicillata in Australia. Oryx, 49, 175–185. ( 10.1017/S0030605313000677) [DOI] [Google Scholar]
- 29.Parkes AS. 1926. The mammalian sex ratio. Biol. Rev. 2, 1–51. ( 10.1111/j.1469-185X.1926.tb00600.x) [DOI] [Google Scholar]
- 30.Veran S, Beissinger SR. 2009. Demographic origins of skewed operational and adult sex ratios: perturbation analyses of two-sex models. Ecol. Lett. 12, 129–143. ( 10.1111/j.14610248.2008.01268.x) [DOI] [PubMed] [Google Scholar]
- 31.Liker A, Freckleton RP, Szekely T. 2013. The evolution of sex roles in birds is related to adult sex ratio. Nat. Commun. 4, 1587 ( 10.1038/ncomms2600) [DOI] [PubMed] [Google Scholar]
- 32.Ancona S, Dénes FV, Krüger O, Székely T, Beissinger SR. 2017. Estimating adult sex ratios in nature. Phil. Trans. R. Soc. B 372, 20160313 ( 10.1098/rstb.2016.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kappeler PM. 2000. Primate males: causes and consequences of variation in group composition. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: Murray. [Google Scholar]
- 35.Mayr E. 1939. The sex ratio in wild birds. Am. Nat. 73, 156–179. ( 10.1086/280824) [DOI] [Google Scholar]
- 36.Clutton-Brock TH, Rose KE, Guiness FE. 1997. Density-related changes in sexual selection in red deer. Proc. R. Soc. Lond. B 264, 1509–1516. ( 10.1098/rspb.1997.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritzsche K, Booksmythe I, Arnqvist G. 2016. Sex ratio bias leads to the evolution of sex role reversal in honey locust beetles. Curr. Biol. 26, 2522–2526. ( 10.1016/j.cub.2016.07.018) [DOI] [PubMed] [Google Scholar]
- 38.Kokko H, Klug H, Jennions MD. 2012. Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient and the scope for competitive investment. Ecol. Lett. 15, 1340–1351. ( 10.1111/j.1461-0248.2012.01859.x) [DOI] [PubMed] [Google Scholar]
- 39.Le Galliard J-F, Fitze PS, Ferrière R, Clobert J. 2005. Sex ratio bias, male aggression, and population collapse in lizards. Proc. Natl Acad. Sci. USA 102, 18 231–18 236. ( 10.1073/pnas.0505172102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB.. 2012. The evolution of primate societies. Chicago, IL: University of Chicago Press. [Google Scholar]
- 41.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.32754) [DOI] [PubMed] [Google Scholar]
- 42.Clutton-Brock TH, Parker GA. 1992. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 67, 437–456. ( 10.1086/417793) [DOI] [Google Scholar]
- 43.Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707–740. ( 10.1023/A:1015520830318) [DOI] [Google Scholar]
- 44.Rubenstein DI, Hack M. 2004. Natural and sexual selection and the evolution of multi-level societies: insights from zebras with comparisons to primates. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP), pp. 266–278. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Grueter CC, Chapais B, Zinner D. 2012. Evolution of multilevel social systems in nonhuman primates and humans. Int. J. Primatol. 33, 1002–1037. ( 10.1007/s10764-012-9618-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead H. 1997. Analysing animal social structure. Anim. Behav. 53, 1053–1067. ( 10.1006/anbe.1996.0358) [DOI] [Google Scholar]
- 47.Ruckstuhl KE, Neuhaus P. 2002. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 77, 77–96. ( 10.1017/S1464793101005814) [DOI] [PubMed] [Google Scholar]
- 48.Aureli F, et al. 2008. Fission-fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. ( 10.1086/586708) [DOI] [Google Scholar]
- 49.Schneider TC, Kappeler PM. 2016. Gregarious sexual segregation: the unusual social organization of the Malagasy narrow-striped mongoose (Mungotictis decemlineata). Behav. Ecol. Sociobiol. 70, 913 ( 10.1007/s00265-016-2113-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrish JK, Edelstein-Keshet L. 1999. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101. ( 10.1126/science.284.5411.99) [DOI] [PubMed] [Google Scholar]
- 51.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 52.Clutton-Brock TH. 2016. Mammal societies. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 53.Komdeur J. 1992. Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358, 493–495. ( 10.1038/358493a0) [DOI] [Google Scholar]
- 54.Clutton-Brock TH, Guinness FE, Albon SD. 1982. Red deer: behavior and ecology of two sexes. Chicago, IL: University of Chicago Press. [Google Scholar]
- 55.Clutton-Brock TH, Harvey PH, Rudder B. 1977. Sexual dimorphism, socionomic sex ratio and body weight in primates. Nature 269, 797–800. ( 10.1038/269797a0). [DOI] [PubMed] [Google Scholar]
- 56.Schwagmeyer PL, Woontner SJ. 1986. Scramble competition polygyny in thirteen-lined ground squirrels: the relative contributions of overt conflict and competitive mate searching. Behav. Ecol. Sociobiol. 19, 359–364. ( 10.1007/BF00295709) [DOI] [Google Scholar]
- 57.Kappeler PM. 1997. Intrasexual selection in Mirza coquereli: evidence for scramble competition polygyny in a solitary primate. Behav. Ecol. Sociobiol. 41, 115–127. ( 10.1007/s002650050371). [DOI] [Google Scholar]
- 58.Fernandez-Duque E, Huck M.. 2013. Till death (or an intruder) do us part: intrasexual competition in a monogamous primate. PLoS ONE 8, e53724 ( 10.1371/journal.pone.0053724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Port M, Kappeler PM, Johnstone RA. 2011. Communal defense of territories and the evolution of sociality. Am. Nat. 178, 787–800. ( 10.1086/662672) [DOI] [PubMed] [Google Scholar]
- 60.Uller T, Olsson M. 2008. Multiple paternity in reptiles: patterns and processes. Mol. Ecol. 17, 2566–2580. ( 10.1111/j.1365-294X.2008.03772.x) [DOI] [PubMed] [Google Scholar]
- 61.Griffith SC, Owens IPF, Thuman KA. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 62.Clutton-Brock T, Isvaran K. 2006. Paternity loss in contrasting mammalian societies. Biol. Lett. 2, 513–516. ( 10.1098/rsbl.2006.0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohas A, Allainé D. 2009. Social structure influences extra-pair paternity in socially monogamous mammals. Biol. Lett. 5, 313–316. ( 10.1098/rsbl.2008.0760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isvaran K, Clutton-Brock TH. 2007. Ecological correlates of extra-group paternity in mammals. Proc. R. Soc. B 274, 219–224. ( 10.1098/rspb.2006.3723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soulsbury CD. 2010. Genetic patterns of paternity and testes size in mammals. PLoS ONE 5, e9581 ( 10.1371/journal.pone.0009581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crook JH, Gartlan JS. 1966. Evolution of primate societies. Nature 210, 1200–1203. ( 10.1038/2101200a0) [DOI] [PubMed] [Google Scholar]
- 67.Clutton-Brock TH, Janson CH. 2012. Primate socioecology at the crossroads: past, present, and future. Evol. Anthropol. 21, 136–150. ( 10.1002/evan.21316). [DOI] [PubMed] [Google Scholar]
- 68.Ims RA. 1988. Spatial clumping of sexually receptive females induces space sharing among male voles. Nature 335, 541–543. ( 10.1038/335541a0) [DOI] [PubMed] [Google Scholar]
- 69.Altmann J. 1990. Primate males go where the females are. Anim. Behav. 39, 193–195. ( 10.1016/S0003-3472(05)80740-7) [DOI] [Google Scholar]
- 70.Nunn CL. 1999. The number of males in primate social groups: a comparative test of the socioecological model. Behav. Ecol. Sociobiol. 46, 1–13. ( 10.1007/s002650050586) [DOI] [Google Scholar]
- 71.Utami AS, van Hooff JARAM. 2004. Alternative male reproductive strategies: male bimaturism in orangutans. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP), pp. 196–207. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 72.Grueter CC, van Schaik CP. 2009. Evolutionary determinants of modular societies in colobines. Behav. Ecol. 21, 63–71. ( 10.1093/beheco/arp149) [DOI] [Google Scholar]
- 73.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 74.Hinde K, Milligan LA. 2011. Primate milk: proximate mechanisms and ultimate perspectives. Evol. Anthropol. 20, 9–23. ( 10.1002/evan.20289) [DOI] [PubMed] [Google Scholar]
- 75.Williams GC. 1966. Adaptation and natural selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 76.Clutton-Brock T. 2017. Reproductive competition and sexual selection. Phil. Trans. R. Soc. B 372, 20160310 ( 10.1098/rstb.2016.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kokko H, Johnstone RA. 2002. Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Phil. Trans. R. Soc. B 357, 319–330. ( 10.1098/rstb.2001.0926). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gowaty PA, Drickamer LC, Schmid-Holmes S. 2003. Male house mice produce fewer offspring with lower viability and poorer performance when mated with females they do not prefer. Anim. Behav. 65, 95–103. ( 10.1006/anbe.2002.2026) [DOI] [Google Scholar]
- 79.Preston BT, Stevenson IR, Pemberton JM, Coltman DW, Wilson K. 2005. Male mate choice influences female promiscuity in Soay sheep. Proc. R. Soc. B 272, 365–373. ( 10.1098/rspb.2004.2977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramm SA, Stockley P. 2014. Sequential male mate choice under sperm competition risk. Behav. Ecol. 25, 660–667. ( 10.1093/beheco/aru037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly CD, Jennions MD. 2011. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 86, 863–884. ( 10.1111/j.1469-185X.2011.00175.x) [DOI] [PubMed] [Google Scholar]
- 82.Stockley P, Bro-Jørgensen J. 2011. Female competition and its evolutionary consequences in mammals. Biol. Rev. 86, 341–366. ( 10.1111/j.1469-185X.2010.00149.x) [DOI] [PubMed] [Google Scholar]
- 83.Stockley P, Campbell A. 2013. Female competition and aggression: interdisciplinary perspectives. Phil. Trans. R. Soc. B 368, 20130073 ( 10.1098/rstb.2013.0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bro-Jørgensen J. 2007. Reversed sexual conflict in a promiscuous antelope. Curr. Biol. 17, 2157–2161. ( 10.1016/j.cub.2007.11.026) [DOI] [PubMed] [Google Scholar]
- 85.Clutton-Brock TH. 2009. Sexual selection in females. Anim. Behav. 77, 3–11. ( 10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 86.Clutton-Brock C TH, Huchard E. 2013. Social competition and selection in males and females. Phil. Trans. R. Soc. B 368, 20130074 ( 10.1098/rstb.2013.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.French JA, Mustoe AC, Cavanaugh J, Birnie AK. 2013. The influence of androgenic steroid hormones on female aggression in atypical mammals. Phil. Trans. R. Soc. B 368, 20130084 ( 10.1098/rstb.2013.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kappeler PM, Fichtel C. 2015. Eco-evo-devo of the lemur syndrome: did adaptive behavioral plasticity get canalized in a large primate radiation? Front. Zool. 12, S15 ( 10.1186/1742-9994-12-S1-S15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kvarnemo C, Ahnesjö I. 1996. The dynamics of operation sex ratios and competition for mates. Trends Ecol. Evol. 11, 404–408. ( 10.1016/0169-5347(96)10056-2) [DOI] [PubMed] [Google Scholar]
- 90.Weir LK, Grant JW, Hutchings JA. 2011. The influence of operational sex ratio on the intensity of competition for mates. Am. Nat. 177, 167–176. ( 10.1086/657918) [DOI] [PubMed] [Google Scholar]
- 91.Patterson S, Sandel A, Miller J, Mitani J. 2014. Data quality and the comparative method: the case of primate group size. Int. J. Primatol. 35, 990–1003. ( 10.1007/s10764-014-9777-1) [DOI] [Google Scholar]
- 92.Clutton-Brock TH, Harvey PH. 1977. Primate ecology and social organization. J. Zool. Lond 183, 1–39. ( 10.1111/j.1469-7998.1977.tb04171.x). [DOI] [Google Scholar]
- 93.Pusey AE, Packer C. 1987. Dispersal and philopatry. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT), pp. 250–266. Chicago, IL: University of Chicago Press. [Google Scholar]
- 94.Alberts S, Altmann J. 1995. Balancing costs and opportunities: dispersal in male baboons. Am. Nat. 145, 279–306. ( 10.1086/285740) [DOI] [Google Scholar]
- 95.Huck M, Fernandez-Duque E, Babb P, Schurr T. 2014. Correlates of genetic monogamy in socially monogamous mammals: insights from Azara's owl monkeys. Proc. R. Soc. B 281, 20140195 ( 10.1098/rspb.2014.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernandez-Duque E. 2015. Social monogamy in wild owl monkeys (Aotus azarae) of Argentina: the potential influences of resource distribution and ranging patterns. Am. J. Primatol. 78, 355–371. ( 10.1002/ajp.22397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Belle S, Fernandez-Duque E, Di Fiore A. 2016. Demography and life history of wild red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador: a 12-year study. Am. J. Primatol. 78, 204–215. ( 10.1002/ajp.22493) [DOI] [PubMed] [Google Scholar]
- 98.Hilgartner RD, Fichtel C, Kappeler PM, Zinner DP. 2012. Determinants of pair-living in red-tailed sportive lemurs (Lepilemur ruficaudatus). Ethology 118, 466–479. ( 10.1111/j.1439-0310.2012.02033.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dröscher I, Kappeler PM. 2013. Defining the low end of primate social complexity: the social organization of the nocturnal white-footed sportive lemur (Lepilemur leucopus). Int. J. Primatol. 34, 1225–1243. ( 10.1007/s10764-013-9735-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kappeler PM. 2014. Lemur behaviour informs the evolution of social monogamy. Trends Ecol. Evol. 29, 591–593. ( 10.1016/j.tree.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 101.Garber PA, Porter LM, Spross J, Fiore AD. 2015. Tamarins: insights into monogamous and non-monogamous single female social and breeding systems. Am. J. Primatol. 78, 298–314. ( 10.1002/ajp.22370) [DOI] [PubMed] [Google Scholar]
- 102.Radespiel U, Sarikaya Z, Zimmermann E, Bruford MW. 2001. Sociogenetic structure in a free-living nocturnal primate population: sex specific differences in the grey mouse lemur (Microcebus murinus). Behav. Ecol. Sociobiol. 50, 493–502. ( 10.1007/s002650100402) [DOI] [Google Scholar]
- 103.Kappeler PM, Wimmer B, Zinner D, Tautz D. 2002. The hidden matrilineal structure of a solitary lemur: implications for primate social evolution. Proc. R. Soc. Lond. B 269, 1755–1763. ( 10.1098/rspb.2002.2066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wimmer B, Tautz D, Kappeler PM. 2002. The genetic population structure of the gray mouse lemur (Microcebus murinus), a basal primate from Madagascar. Behav. Ecol. Sociobiol. 52, 166–175. ( 10.1007/s00265-002-0497-8) [DOI] [Google Scholar]
- 105.Utami SS, Goossens B, Bruford MW, de Ruiter JR, van Hooff JARAM. 2002. Male bimaturism and reproductive success in Sumatran orang-utans. Behav. Ecol. 13, 643–652. ( 10.1093/beheco/13.5.643) [DOI] [Google Scholar]
- 106.Setchell JM, Lee PC. 2004. Development and sexual selection in primates. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP), pp. 175–195. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 107.Alberts SC, Altmann J. 1995. Preparation and activation: determinants of age at reproductive maturity in male baboons. Behav. Ecol. Sociobiol. 36, 397–406. ( 10.1007/BF00177335) [DOI] [Google Scholar]
- 108.Brown GR, Silk JB. 2002. Reconsidering the null hypothesis: is maternal rank associated with birth sex ratios in primate groups? Proc. Natl Acad. Sci. USA 99, 11 252–11 255. ( 10.1073/pnas.162360599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silk JB, Brown GR. 2008. Local resource competition and local resource enhancement shape primate birth sex ratios. Proc. R. Soc. B 275, 1761–1765. ( 10.1098/rspb.2008.0340). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Schaik CP, de Visser JAGM. 1990. Fragile sons or harassed daughters? Sex differences in mortality among juvenile primates. Folia Primatol. 55, 10–23. ( 10.1159/000156493). [DOI] [PubMed] [Google Scholar]
- 111.Rajpurohit LS, Sommer V. 1991. Sex differences in mortality among langurs (Presbytis entellus) of Jodhpur, Rajasthan. Folia Primatol. 56, 17–27. ( 10.1159/000156523). [DOI] [Google Scholar]
- 112.Tecot SR, Gerber BD, King SJ, Verdolin JL, Wright PC. 2013. Risky business: sex differences in mortality and dispersal in a polygynous, monomorphic lemur. Behav. Ecol. 24, 987–996. ( 10.1093/beheco/art008). [DOI] [Google Scholar]
- 113.Promislow DEL. 1992. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. Lond. B 247, 203–210. ( 10.1098/rspb.1992.0030) [DOI] [Google Scholar]
- 114.Cameron EZ. 2004. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. Lond. B 271, 1723–1728. ( 10.1098/rspb.2004.2773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. ( 10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 116.Sheldon Ben C, West Stuart A. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54. ( 10.1086/381003). [DOI] [PubMed] [Google Scholar]
- 117.Fawcett TW, Kuijper B, Weissing FJ, Pen I. 2011. Sex-ratio control erodes sexual selection, revealing evolutionary feedback from adaptive plasticity. Proc. Natl Acad. Sci. USA 108, 15 925–15 930. ( 10.1073/pnas.1105721108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schindler S, Gaillard J-M, Gruning A, Neuhaus P, Traill LW, Tuljapurkar S, Coulson T. 2015. Sex-specific demography and generalization of the Trivers–Willard theory. Nature 526, 249–252. ( 10.1038/nature14968) [DOI] [PubMed] [Google Scholar]
- 119.Lukas D, Clutton-Brock TH. 2014. Costs of mating competition limit male lifetime breeding success in polygynous mammals. Proc. R. Soc. B 281, 20140418 ( 10.1098/rspb.2014.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Booksmythe I, Mautz B, Davis J, Nakagawa S, Jennions MD. 2017. Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta-analysis. Biol. Rev. 92, 108–134. ( 10.1111/brv.12220) [DOI] [PubMed] [Google Scholar]
- 121.Kappeler PM. 2000. Causes and consequences of unusual sex ratios among lemurs. In Primate males: causes and consequences of variation in group composition (ed. Kappeler PM.), pp. 55–63. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 122.Lawson Handley LJ, Perrin N. 2007. Advances in our understanding of mammalian sex-biased dispersal. Mol. Ecol. 16, 1559–1578. ( 10.1111/j.1365-294X.2006.03152.x) [DOI] [PubMed] [Google Scholar]
- 123.Kappeler PM, Mass V, Port M. 2009. Even adult sex ratios in lemurs: potential costs and benefits of subordinate males in Verreaux's sifaka (Propithecus verreauxi) in the Kirindy Forest CFPF, Madagascar. Am. J. Phys. Anthropol. 140, 487–497. ( 10.1002/ajpa.21091) [DOI] [PubMed] [Google Scholar]
- 124.van Noordwijk MA, van Schaik CP. 2004. Sexual selection and the careers of primate males: paternity concentration, dominance acquisition tactics and transfer decisions. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP), pp. 208–229. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 125.Alberts SC. 2012. Magnitude and sources of variation in male reproductive performance. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 412–431. Chicago, IL: University of Chicago Press. [Google Scholar]
- 126.Sterck EHM, Korstjens AH. 2000. Female dispersal and infanticide avoidance in primates. In Infanticide by males and its implications (eds van Schaik CP, Janson CH), pp. 293–321. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 127.Muller MN, Emery Thompson M. 2012. Mating, parenting and male reproductive strategies. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 387–411. Chicago, IL: University of Chicago Press. [Google Scholar]
- 128.Huck M, Fernandez-Duque E. 2012. Building babies when dads help: infant development of owl monkeys and other primates with allo-maternal care. In Building babies: primate development in proximate and ultimate perspective (eds Clancy KBH, Hinde K, Rutherford JN), pp. 361–385. New York, NY: Springer US. [Google Scholar]
- 129.Kappeler PM. 2012. Mate choice. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 343–366. Chicago, IL: University of Chicago Press. [Google Scholar]
- 130.van Schaik CP, Isler K. 2012. Life-history evolution. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 220–244. Chicago, IL: University of Chicago Press. [Google Scholar]
- 131.Levrero F, Carrete-Vega G, Herbert A, Lawabi I, Courtiol A, Willaume E, Kappeler PM, Charpentier MJ. 2015. Social shaping of voices does not impair phenotype matching of kinship in mandrills. Nat. Commun. 6, 7609 ( 10.1038/ncomms8609) [DOI] [PubMed] [Google Scholar]
- 132.Port M, Kappeler PM. 2010. The utility of reproductive skew models in the study of male primates, a critical evaluation. Evol. Anthropol. 19, 46–56. ( 10.1002/evan.20243) [DOI] [Google Scholar]
- 133.Rosenbaum S, Hirwa JP, Silk JB, Vigilant L, Stoinski TS. 2015. Male rank, not paternity, predicts male–immature relationships in mountain gorillas, Gorilla beringei beringei. Anim. Behav. 104, 13–24. (doi:0.1016/j.anbehav.2015.02.025). [Google Scholar]
- 134.Smuts BB, Gubernick DJ. 1992. Male–infant relationships in nonhuman primates: paternal investment or mating effort? In Father–child relations: cultural and bioscoial contexts (ed. Hewlett BS.), pp. 1–30. New York, NY: Aldine de Gruyter. [Google Scholar]
- 135.Coxworth JE, Kim PS, McQueen JS, Hawkes K. 2015. Grandmothering life histories and human pair bonding. Proc. Natl Acad. Sci. USA 112, 11 806–11 811. ( 10.1073/pnas.1599993112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Noordwijk MA. 2012. From maternal investment to to lifetime maternal care. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 321–342. Chicago, IL: University of Chicago Press. [Google Scholar]
- 137.Palombit RA. 2012. Infanticide: male strategies and female counterstrategies. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 432–468. Chicago, IL: University of Chicago Press. [Google Scholar]
- 138.van Schaik CP, Kappeler PM. 1997. Infanticide risk and the evolution of male–female association in primates. Proc. R. Soc. Lond. B 264, 1687–1694. ( 10.1098/rspb.1997.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ostner J, Vigilant L, Bhagavatula J, Franz M, Schülke O. 2013. Stable heterosexual associations in a promiscuous primate. Anim. Behav. 86, 623–631. ( 10.1016/j.anbehav.2013.07.004) [DOI] [Google Scholar]
- 140.Baniel A, Cowlishaw G, Huchard E. 2016. Stability and strength of male-female associations in a promiscuous primate society. Behav. Ecol. Sociobiol. 70, 761–775. ( 10.1007/s00265-016-2100-8) [DOI] [Google Scholar]
- 141.Murray CM, Stanton MA, Lonsdorf EV, Wroblewski EE, Pusey AE. 2016. Chimpanzee fathers bias their behaviour towards their offspring. R. Soc. open sci. 3, 160441 ( 10.1098/rsos.160441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huchard E, Cowlishaw G. 2011. Female–female aggression around mating: an extra cost of sociality in a multimale primate society. Behav. Ecol. 22, 1003–1011. ( 10.1093/beheco/arr083) [DOI] [Google Scholar]
- 143.Cheney DL, Silk JB, Seyfarth RM. 2012. Evidence for intrasexual selection in wild female baboons. Anim. Behav. 84, 21–27. ( 10.1016/j.anbehav.2012.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baniel A. 2016. Conflits reproductifs chez un primate social vivant en milieu naturel, le babouin chacma (Papio ursinus), PhD thesis University of Montpellier, Montpellier, France. [Google Scholar]
- 145.Klug H, Heuschele J, Jennions MD, Kokko H. 2010. The mismeasurement of sexual selection. J. Evol. Biol. 23, 447–462. ( 10.1111/j.1420-9101.2009.01921.x). [DOI] [PubMed] [Google Scholar]
- 146.Young AJ, Bennett NC. 2013. Intra-sexual selection in cooperative mammals and birds: why are females not bigger and better armed? Phil. Trans. R. Soc. B 368, 20130075 ( 10.1098/rstb.2013.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alberts SC, Watts HE, Altmann J. 2003. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim. Behav. 65, 821–840. ( 10.1006/anbe.2003.2106) [DOI] [Google Scholar]
- 148.Port M, Johnstone RA. 2013. Facing the crowd: intruder pressure, within-group competition, and the resolution of conflicts over group-membership. Ecol. Evol. 3, 1209–1218. ( 10.1002/ece3.533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mitani JC, Gros-Louis J, Richards AF. 1996. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am. Nat. 147, 966–980. ( 10.1086/285888) [DOI] [Google Scholar]
- 150.Vasilieva N, Tchabovsky A. 2015. A shortage of males causes female reproductive failure in yellow ground squirrels. Sci. Adv. 1, e1500401 ( 10.1126/sciadv.1500401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clutton-Brock TH, Albon SD, Gibson RM, Guinness FE. 1979. The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim. Behav. 27, 211–225. ( 10.1016/0003-3472(79)90141-6) [DOI] [Google Scholar]
- 152.Eberle M, Kappeler PM. 2004. Sex in the dark: determinants and consequences of mixed male mating tactics in microcebus murinus, a small solitary nocturnal primate. Behav. Ecol. Sociobiol. 57, 77–90. ( 10.1007/s00265-004-0826-1). [DOI] [Google Scholar]
- 153.Watts DP. 1998. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 44, 43–56. ( 10.1007/s002650050513) [DOI] [Google Scholar]
- 154.Itoigawa N, Tanaka T, Ukai N, Fujii H, Kurokawa T, Koyama T, Ando A, Watanable Y, Imakawa S. et al. 1992. Demography and reproductive parameters of a free-ranging group of Japanese macaques (Macaca fuscata) at Katsuyama. Primates 33, 49–68. ( 10.1007/BF02382762) [DOI] [Google Scholar]
- 155.Carmona-Isunza MC, Ancona S, Székely T, Ramallo-González AP, Cruz-López M, Serrano-Meneses MA, Küpper C. 2017. Adult sex ratio and operational sex ratio exhibit different temporal dynamics in the wild. Behav. Ecol. 28, 523–532. ( 10.1093/beheco/arw183) [DOI] [Google Scholar]
- 156.Plavcan JM. 2004. Sexual selection, measures of sexual selection, and sexual dimorphism in primates. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP), pp. 230–252. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 157.Thorén S, Lindenfors P, Kappeler PM. 2006. Phylogenetic analyses of dimorphism in primates: evidence for stronger selection on canine size than on body size. Am. J. Phys. Anthropol. 130, 50–59. ( 10.1002/ajpa.20321) [DOI] [PubMed] [Google Scholar]
- 158.Altmann SA. 1962. A field study of the sociobiology of the rhesus monkey, Macaca mulatta. Ann. NY Acad. Sci. 102, 338–435. ( 10.1111/j.1749-6632.1962.tb13650.x) [DOI] [PubMed] [Google Scholar]
- 159.Kutsukake N, Nunn CL. 2006. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav. Ecol. Sociobiol. 60, 695–706. ( 10.1007/s00265-006-0213-1) [DOI] [Google Scholar]
- 160.Setchell JM, Charpentier M, Wickings EJ. 2005. Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Anim. Behav. 70, 1105–1120. ( 10.1016/j.anbehav.2005.02.021) [DOI] [Google Scholar]
- 161.Boesch C, Kohou G, Néné H, Vigilant L. 2006. Male competition and paternity in wild chimpanzees of the Taï forest. Am. J. Phys. Anthropol. 130, 103–115. ( 10.1002/ajpa.20341) [DOI] [PubMed] [Google Scholar]
- 162.Kappeler PM, Port M. 2008. Mutual tolerance or reproductive competition? Patterns of reproductive skew among male redfronted lemurs (Eulemur fulvus rufus). Behav. Ecol. Sociobiol. 62, 1477–1488. ( 10.1007/s00265-008-0577-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ostner J, Nunn CL, Schülke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150–1158. ( 10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gogarten JF, Koenig A. 2013. Reproductive seasonality is a poor predictor of receptive synchrony and male reproductive skew among nonhuman primates. Behav. Ecol. Sociobiol. 67, 123–134. ( 10.1007/s00265-012-1432-2) [DOI] [Google Scholar]
- 165.Snyder-Mackler N, Alberts SC, Bergman TJ. 2012. Concessions of an alpha male? Cooperative defence and shared reproduction in multi-male primate groups. Proc. R. Soc. B 279, 3788–3795. ( 10.1098/rspb.2012.0842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ostner J, Kappeler PM. 2004. Male life history and the unusual adult sex ratios of redfronted lemur, Eulemur fulvus rufus, groups. Anim. Behav. 67, 249–259. ( 10.1016/j.anbehav.2003.05.012) [DOI] [Google Scholar]
- 167.Port M, Johnstone RA, Kappeler PM. 2012. The evolution of multimale groups in Verreaux's sifaka, or how to test an evolutionary demographic model. Behav. Ecol. 23, 889–897. ( 10.1093/beheco/ars053) [DOI] [Google Scholar]
- 168.Vigilant L, Roy J, Bradley BJ, Stoneking CJ, Robbins MM, Stoinski TS. 2015. Reproductive competition and inbreeding avoidance in a primate species with habitual female dispersal. Behav. Ecol. Sociobiol. 69, 1163–1172. ( 10.1007/s00265-015-1930-0). [DOI] [Google Scholar]
- 169.Kappeler PM, Schäffler L. 2008. The lemur syndrome unresolved: extreme male reproductive skew in sifakas (Propithecus verreauxi), a sexually monomorphic primate with female dominance. Behav. Ecol. Sociobiol. 62, 1007–1015. ( 10.1007/s00265-007-0528-6) [DOI] [Google Scholar]
- 170.Harts AMF, Kokko H. 2013. Understanding promiscuity: when is seeking additional mates better than guarding an already found one? Evolution 67, 2838–2848. ( 10.1111/evo.12163) [DOI] [PubMed] [Google Scholar]
- 171.Parker GA. 1974. Courtship persistence and female-guarding as male time investment strategies. Behaviour 48, 157–184. ( 10.1163/156853974%D700327) [DOI] [Google Scholar]
- 172.Fromhage L, Elgar MA, Schneider JM. 2005. Faithful without care: the evolution of monogyny. Evolution 59, 1400–1405. ( 10.1111/j.0014-3820.2005.tb01790.x) [DOI] [PubMed] [Google Scholar]
- 173.Johnston SE, Gratten J, Berenos C, Pilkington JG, Clutton-Brock TH, Pemberton JM, Slate J. 2013. Life history trade-offs at a single locus maintain sexually selected genetic variation. Nature 502, 93–95. ( 10.1038/nature12489) [DOI] [PubMed] [Google Scholar]
- 174.Mass V, Heistermann M, Kappeler PM. 2009. Mate-guarding as a male reproductive tactic in Propithecus verreauxi. Int. J. Primatol. 30, 389–409. ( 10.1007/s10764-009-9345-2) [DOI] [Google Scholar]
- 175.Edward DA, Chapman T. 2011. The evolution and significance of male mate choice. Trends Ecol. Evol. 26, 647–654. ( 10.1016/j.tree.2011.07.012) [DOI] [PubMed] [Google Scholar]
- 176.Setchell JM. 2008. Alternative reproductive tactics in primates. In Alternative reproductive tactics: an integrative approach (eds Oliveira RF, Taborsky M, Brockman HJ), pp. 373–398. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 177.Bro-Jørgensen J. 2002. Overt female mate competition and preference for central males in a lekking antelope. Proc. Natl Acad. Sci. USA 99, 9290–9293. ( 10.1073/pnas.142125899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Clutton-Brock TH, Huchard E. 2013. Social competition and its consequences in female mammals. J. Zool. Lond. 289, 151–171. ( 10.1111/jzo.12023) [DOI] [Google Scholar]
- 179.Kappeler PM, Fichtel C. 2012. Female reproductive competition in Eulemur rufifrons: eviction and reproductive restraint in a plurally breeding Malagasy primate. Mol. Ecol. 21, 685–698. ( 10.1111/j.1365-294X.2011.05255.x) [DOI] [PubMed] [Google Scholar]
- 180.Parker GA, Birkhead TR. 2013. Polyandry: the history of a revolution. Phil. Trans. R. Soc. B 368, 20120335 ( 10.1098/rstb.2012.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nunn CL, Scully E, Kutsukake N, Ostner J, Schülke O, Thrall P. 2014. Mating competition, promiscuity, and life history traits as predictors of sexually transmitted disease risk in primates. Int. J. Primatol. 35, 764–786. ( 10.1007/s10764-014-9781-5) [DOI] [Google Scholar]
- 182.Wheeler BC, Scarry CJ, Koenig A. 2013. Rates of agonism among female primates: a cross-taxon perspective. Behav. Ecol. 24, 1369–1380. ( 10.1093/beheco/art076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hemelrijk CK, Wantia J, Isler K. 2008. Female dominance over males in primates: self-organisation and sexual dimorphism. PLoS ONE 3, e2678 ( 10.1371/journal.pone.0002678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Johanos TC, Becker BL, Baker JD, Ragen TJ, Gilmartin WG, Gerrodette T. 2010. Impacts of sex ratio reduction on male aggression in the critically endangered Hawaiian monk seal Monachus schauinslandi. Endanger. Species Res. 11, 123–132. ( 10.3354/esr00259) [DOI] [Google Scholar]
- 185.Barrientos R. 2015. Adult sex-ratio distortion in the native European polecat is related to the expansion of the invasive American mink. Biol. Conserv. 186, 28–34. ( 10.1016/j.biocon.2015.02.030) [DOI] [Google Scholar]
- 186.Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby IC. 2014. Sexually coercive male chimpanzees sire more offspring. Curr. Biol. 24, 2855–2860. ( 10.1016/j.cub.2014.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rosenbaum S, Maldonado-Chaparro AA, Stoinski TS. 2016. Group structure predicts variation in proximity relationships between male–female and male–infant pairs of mountain gorillas (Gorilla beringei beringei). Primates 57, 17–28. ( 10.1007/s10329-015-0490-2) [DOI] [PubMed] [Google Scholar]
- 188.Sapolsky RM, Share LJ. 2004. A pacific culture among wild baboons: its emergence and transmission. PLoS Biol. 2, 534–541. ( 10.1371/journal.pbio.0020106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hill RA, Lee PC. 1998. Predation risk as an influence on group size in cercopithecoid primates: implications for social structure. J. Zool. Lond. 245, 447–456. ( 10.1111/j.1469-7998.1998.tb00119.x) [DOI] [Google Scholar]
- 190.van Schaik CP, Hörstermann M. 1994. Predation risk and the number of adult males in a primate group: a comparative test. Behav. Ecol. Sociobiol. 35, 261–272. ( 10.1007/BF00170707) [DOI] [Google Scholar]
- 191.Arseneau-Robar TJM, Müller E, Taucher AL, van Schaik CP, Willems EP. 2016. Male food defence as a by-product of intersexual cooperation in a non-human primate. Sci. Rep. 6, 35800 ( 10.1038/srep35800) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.