Abstract
Sex reversals whereby individuals of one genetic sex develop the phenotype of the opposite sex occur in ectothermic vertebrates with genetic sex-determination systems that are sensitive to extreme temperatures during sexual differentiation. Recent rises in global temperatures have led researchers to predict that sex reversals will become more common, resulting in the distortion of many populations' sex ratios. However, it is unclear whether susceptibility to climate-driven sex-ratio shifts depends on the type of sex determination that varies across species. First, we show here using individual-based theoretical models that XX/XY (male-heterogametic) and ZZ/ZW (female-heterogametic) sex-determination systems can respond differentially to temperature-induced sex reversals. Interestingly, the impacts of climate warming on adult sex ratio (ASR) depend on the effects of both genotypic and phenotypic sex on survival and reproduction. Second, we analyse the temporal changes of ASR in natural amphibian populations using data from the literature, and find that ASR shifted towards males in ZZ/ZW species over the past 60 years, but did not change significantly in XX/XY species. Our results highlight the fact that we need a better understanding of the interactions between genetic and environmental sex-determining mechanisms to predict the responses of ectotherms to climate change and the associated extinction risks.
This article is part of the themed issue ‘Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies’.
Keywords: adult sex ratio, amphibians, climate change, masculinization, sex-determination systems, temperature-induced sex reversal
1. Introduction
Global temperatures have been rising in recent decades, resulting in manifold effects on the Earth's ecosystems from shifts in phenology to altered distribution ranges to disease dynamics [1,2]. One particular biological process that is influenced by temperature is the development of the sexual phenotype in many ectothermic vertebrates. Temperature-dependent sex determination (TSD), whereby offspring sex is determined by post-fertilization environmental temperatures during a susceptible period of development, has been demonstrated in various species of fish and reptiles [3–5]. The concern that climate change may distort the sex ratio of TSD species has been raised repeatedly [5–7], as mounting evidence shows that unusually warm years yield hatchling sex ratios that are skewed towards the sex produced near the upper limit of tolerated incubation temperatures, which can result in biased sex ratio of the adult population [8]. It has even been proposed that past climate-change effects on sex ratios might have played a role in dinosaur extinctions [9].
Climate change, however, may also influence the sex ratios of populations with genetic sex-determination (GSD) systems. In these species, sex is determined at fertilization by the sex chromosomes, such that heterogametic individuals (i.e. those with two different sex chromosomes, XY or ZW) develop into males in XX/XY systems and into females in ZZ/ZW systems [3]. Both systems are widespread in vertebrates, and in contrast with mammals (all male-heterogametic) and birds (all female-heterogametic), in ectotherms, the type of sex determination is evolutionarily labile, differing even among closely related species of amphibians, reptiles and fish [3,4]. In these ectotherms, GSD can be overridden by unusually high or low temperatures during the sensitive period of ontogeny, resulting in sex-reversed individuals whose phenotypic sex differs from their genetic sex [10–14]. Theoretical models show that such sex reversals can lead to biased sex ratios [15–18], with far-reaching consequences for population viability [16,17,19] and the evolution of sex-determining mechanisms [14,20–23]. For example, sex reversals can either extirpate the population or boost its size [16,17], and they can propel evolutionary transitions from GSD to TSD [14,21,22] and between XX/XY and ZZ/ZW systems [20,23].
Intuitively, the impact of temperature-induced sex reversals on the population sex ratio may differ between the two major types of GSD: for example, if high temperatures masculinize genetic females, which is characteristic of amphibians and fish [5,10,11], in species with XX/XY systems, the resulting XX males will mate with XX females, producing 100% XX offspring which may counter the sex-ratio distorting effect of masculinization. By contrast, in species with ZZ/ZW systems, masculinized ZW individuals will mate with ZW females, producing 25% male offspring (or 33% males if the WW genotype is not viable); thus, this system may have lower capacity to compensate for masculinization by female-biased offspring sex ratios. Although a few previous theoretical models indicated that the two types of GSD might differ in their susceptibility to the effects of sex reversals on population demography [16,19], no study has yet formally compared the performance of the XX/XY and ZZ/ZW systems to test whether, and under what circumstances, they are differently affected by temperature-induced sex reversals.
In this study, we employ a two-pronged approach to investigate climate-driven sex-ratio shifts in ectotherms with GSD. First, we develop an individual-based theoretical framework to investigate the effects of temperature-induced sex reversals on population sex ratios under various conditions and contrast these effects between the two types of GSD. Second, we compile empirical data from the literature to analyse the temporal changes of sex ratios in natural populations, and contrast these findings with the predictions of our model. We focus on amphibians, although our model is applicable to any taxon with GSD and naturally occurring masculinization. Out of the amphibian species studied so far, all have GSD [4,24] (but see [25]), and almost all can be experimentally masculinized with high temperatures (approx. above 30°C) during larval life, probably due to the temperature-dependence of aromatase (or its inhibitors), which converts androgens into oestrogens [10,11]. It has long been suspected that reproductively functional sex-reversed individuals occur in natural amphibian populations [12,26]. A recent study found that 9% of genetically female adults were phenotypic males in a population of common frogs (Rana temporaria) and 17% of the genotyped clutches were all-female, probably resulting from the matings between two XX genotypes, i.e. a normal female and a masculinized (genotypically female but phenotypically male) individual [18]. While sex reversals are difficult to study in the field due to a general lack of genetic sexing methods in amphibians [18,27], phenotypic sex ratios of adults have been studied extensively in some species over the past decades. Therefore, we tested whether adult sex ratios (ASRs) changed over the last 60 years in parallel with climate warming [28], whether this change differed between species with different GSD systems, and whether the empirical results can be explained by increasing masculinization rates according to our model.
2. Material and methods
(a). Theoretical modelling
We modelled the effects of increasing masculinization rate using an individual-based model in a diploid population with overlapping generations and either XX/XY or ZZ/ZW sex determination. Our specific parameter values are explained in electronic supplementary material, table S1. Each year, a maximum number of Nmax offspring were allowed to complete metamorphosis; thereby, we assumed that survival from eggs to metamorphosis is density-dependent due to limited carrying capacity of the environment. The actual number of newly metamorphosed offspring in each year was calculated as N = min(Nmax; fert × Nfemale), where Nfemale is the number of adult females and fert is the average number of offspring each female can recruit in the absence of density-dependence (i.e. when larval density is low due to scarcity of breeding females). Metamorphs' survival during the first winter was independent of phenotypic sex, but we allowed for an effect of genotype according to the ‘unguarded sex chromosome hypothesis' such that heterogametic individuals (XY or ZW) could have reduced survival, due to rare mutations on the X or Z chromosome that we assumed to exert deleterious effects primarily during early ontogeny (electronic supplementary material, table S1).
After surviving the first winter, the individuals turned into juveniles with an annual survival rate independent of both genotypic and phenotypic sex (electronic supplementary material, table S1). When reaching the age of maturity, which was allowed to differ between phenotypic males and females, juveniles turned into adults with an annual survival rate independent of age and genotype but dependent on phenotypic sex. For simplicity, we assumed a fixed lifespan; all adults died upon reaching this age (electronic supplementary material, table S1). Each year, adults participated in a single breeding event, during which the mother of each of the N offspring was chosen randomly out of all phenotypically female adults, while the fathers were chosen from among the phenotypically male adults according to their mating success (α), which was allowed to vary with genotype (electronic supplementary material, table S1).
Each model was run with these baseline settings and without masculinization for 50 years to allow the population structure (age and sex ratios) to stabilize. Then, each model was run for a further 350 years, during which the probability of masculinization increased linearly with some stochasticity as pmasc = bmasc × t ± sdmasc where bmasc is the year-to-year increase in the average probability of masculinization while sdmasc is its standard deviation. Each year, the sex of newly produced, genotypically female (XX, ZW and WW) offspring was reverted to the male phenotype by the probability pmasc. Thus, we assumed that with rising average temperatures, the chances of stochastic variation resulting in temperatures high enough to trigger sex reversal will increase as well. We assumed no individual variability in the likelihood of masculinization and we did not allow resistance to sex reversal to evolve because we were primarily interested in the effects over an evolutionarily short time span to facilitate comparison with available empirical data (see below). However, in the ZZ/ZW system, neofemales with the WW genotype (resulting from the matings between normal ZW females and sex-reversed ZW males) were either allowed to get masculinized with the same probability as ZW females, or not at all (electronic supplementary material, table S1). Masculinized individuals were then allowed to reproduce according to their phenotype and produce gametes according to their genotype, with no epigenetic inheritance of sex reversal [14,29].
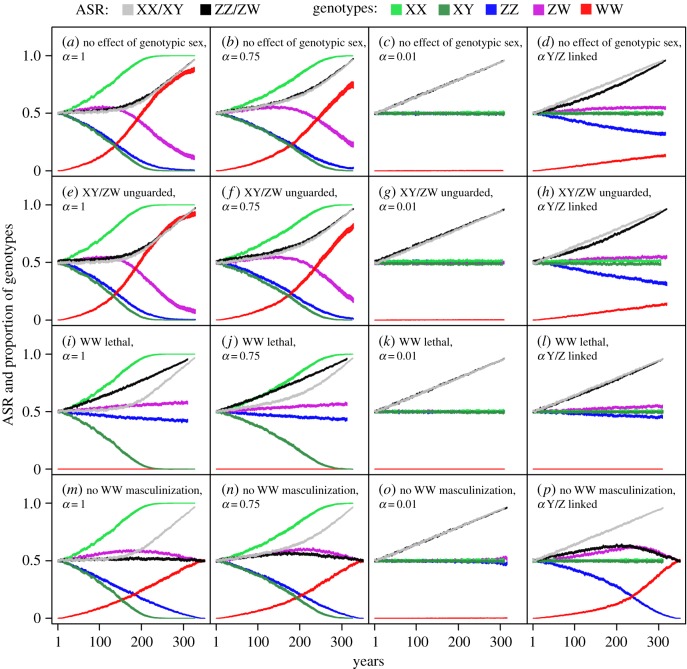
We ran the model with 32 different combinations of parameter settings (hereafter scenarios, see below), each repeated in 100 runs. We examined two alternative sex-specific life histories: one characterizing urodelans (i.e. the age of first reproduction and adult survival being similar in both sexes), whereas the other corresponding to anurans (i.e. males maturing earlier but experiencing higher mortality than females; see the electronic supplementary material, table S1). With each of these two life histories, the models were run with 16 scenarios (electronic supplementary material, table S1), representing four different effects of genotypic sex (see panel rows in figure 1) combined with four different effects of phenotypic sex (see panel columns in figure 1). Specifically, the four different scenarios of genotypic sex effects assumed, respectively, that (i) genotypic sex has no effect on survival or masculinization, (ii) the heterogametic sex suffers extra mortality early in life due to the ‘unguarded sex chromosome’ effect, (iii) the WW genotype is lethal, and (iv) WW females cannot be masculinized. Each of these four scenarios was run with four different phenotypic sex effects, assuming that the mating success of masculinized individuals (i.e. phenotypic males with the XX, ZW or WW genotype) is 100, 75 or 1% compared with normal males, or linked to the Y or Z chromosomes (i.e. 50% in ZW males and 1% in WW and XX males).
Figure 1.
Model-predicted changes over 350 years in ASR (proportion of phenotypic males) and relative frequencies of genotypes, in scenarios with no sex difference in maturation age and adult survival. The width of each curve shows the 95% confidence band from 100 runs. The average rate of masculinization increases from zero by 0.003 each year; α denotes the mating success of masculinized individuals (i.e. phenotypic males with the XX, ZW or WW genotype) relative to normal males. Note that the parameter settings for the XX/XY system are the same in the scenarios ‘α = 0.01’ and ‘α Y-linked’ (see the electronic supplementary material, table S1).
For each scenario, we report the results by plotting the qualitative effects of increasing masculinization rate on the phenotypic ASR and the relative frequency of each genotype until pmasc reaches 1. From the same models, we statistically analysed the phenotypic ASR over the first 60 years for comparison with the empirical data (see below). For each scenario, we extracted the regression slope of ASR change over 60 years from each run and calculated its 95% confidence interval for the two GSD systems. We compared the slope of ASR change between the XX/XY and ZZ/ZW system within each scenario by Welch tests and corrected the p-values for false discovery rate. All models were run in R v. 3.1.0 [30]; the R code of our model is available in electronic supplementary material, S2.
(b). Empirical study
We compiled data on the ASR (proportion of males in the adult population) from the literature as described earlier [31]. We searched in Google Scholar for the terms ‘species name’ and ‘sex ratio’ for all amphibian species for which either male or female heterogamety has been demonstrated according to the Tree of Sex database [24]. We excluded studies where the authors stated or speculated that their data may not represent the population sex ratio, or when the methods were not described in enough detail to assess the adequacy of the study (for more details on collecting, filtering and validating the data, see [31]). We found data on natural populations for 39 species; the repeatability of ASR among populations within species was 0.56 [31]. However, long-term data were not available; for the vast majority of the studied populations, ASR has been reported for 1 or 2 years (maximum: 6 years). From each study, we extracted ASR records for each year per population, keeping only the records with N > 20 individuals. We restricted our analyses to species for which there were at least 10 records and at least 10 years between the earliest and latest study year. If a study did not report annual ASR but provided average ASR over several (2–4) years, we assigned the midpoint of the study period as study year of that record.
The data that fit these criteria totalled 125 records of six species (two anurans and four urodelans) from 51 studies conducted between 1953 and 2011. As the ASR data for each species came from several populations, we collected data for three potentially confounding variables that may influence ASR: latitude, altitude and morph. As amphibian demography and life history can vary along geographical gradients [32], we extracted the latitude and altitude of the study location for each ASR record. As urodelans can be either metamorphic or paedomorphic, and the two morphs can differ in sex ratio [33], we categorized morph for each record as the presence or absence of paedomorphic adults (i.e. sexually mature individuals that retain larval somatic traits) in the population. Our dataset is available in the electronic supplementary material, S3.
To analyse the relationship between ASR and time expressed as the number of years since 1950, we used a mixed-effects modelling framework. By estimating random intercepts and random slopes, we allowed the six species to differ in average ASR and in the slope of the change of ASR with time, respectively. As fixed effects, we included time, GSD and the time × GSD interaction to test whether species with XX/XY and ZZ/ZW systems differed in the slope of ASR change over the years. We also included the potentially confounding effects of latitude and altitude as covariates, and morph as a fixed factor. This approach assumes that each ASR record is a random sample taken from the pool of each species' all populations at various points in time and space, testing whether time (a proxy for climate warming) or geographical gradients have a systematic effect on amphibian sex ratios (see [34] for a similar approach applied to turtle sex ratios).
We implemented the mixed-effects model by Bayesian approach, using the R package ‘MCMCglmm’ [35]. We ran four MCMC chains with inverse Wishart priors (V = 1, ν = 0.002; [35]), each with 7 000 000 iterations, a thinning interval of 500 and a burn-in of 2 000 000 iterations, yielding 10 000 samples per chain, from which we calculated the posterior distribution of all parameter estimates (fixed and random effects). We tested the convergence of model parameters among the chains using the Gelman–Rubin statistic; the potential scale reduction factor was 1 in all cases, showing that model convergence was appropriate. We report the parameter estimates (posterior means) and corresponding 95% credibility intervals (CI) from the first chain. Autocorrelation was less than 0.02 for all estimated parameters, and there was no heteroscedasticity by sample size (electronic supplementary material, figure S1).
3. Results
(a). Theoretical modelling
As the rate of masculinization increased over several hundred years, the model predicted increasingly male-biased ASR in most scenarios (figure 1). ASR started to shift later if the sex-reversed individuals had relatively high mating success (figure 1). When the masculinized individuals reproduced just as well as normal males, the normal male genotype went extinct in most scenarios by the time masculinization rate reached 100% and the initial GSD transitioned into a TSD system in which all individuals have female genotypes (XX, or WW and ZW) and phenotypic males are produced solely by temperature-induced masculinization (figure 1a,e,i,m). WW lethality sped up the ASR shift while slowing down the change of genotype frequencies (figure 1i–l). When WW females could not be masculinized, ZW females and ZZ males were replaced by WW females and ZW males, respectively, thus leading to a switch from ZZ/ZW to XX/XY system (figure 1m,n,p). Similar changes occurred, albeit more slowly, when the mating success of masculinized individuals was 75% compared with normal males (figure 1b,f,j,n). By contrast, when their mating success was close to zero, both systems shifted rapidly to male-biased ASR with little change in the genotype frequencies (figure 1c,g,k,o). For the XX/XY system, this latter scenario is equivalent to the cases when male mating success is Y-linked, whereas for the ZZ/ZW system, Z-linked mating success predicted the same, albeit slower, trends as did the scenarios with 75% mating success of masculinized individuals (figure 1d,h,l,p). The corresponding ‘urodelan’ and ‘anuran’ scenarios yielded similar results (electronic supplementary material, figure S2).
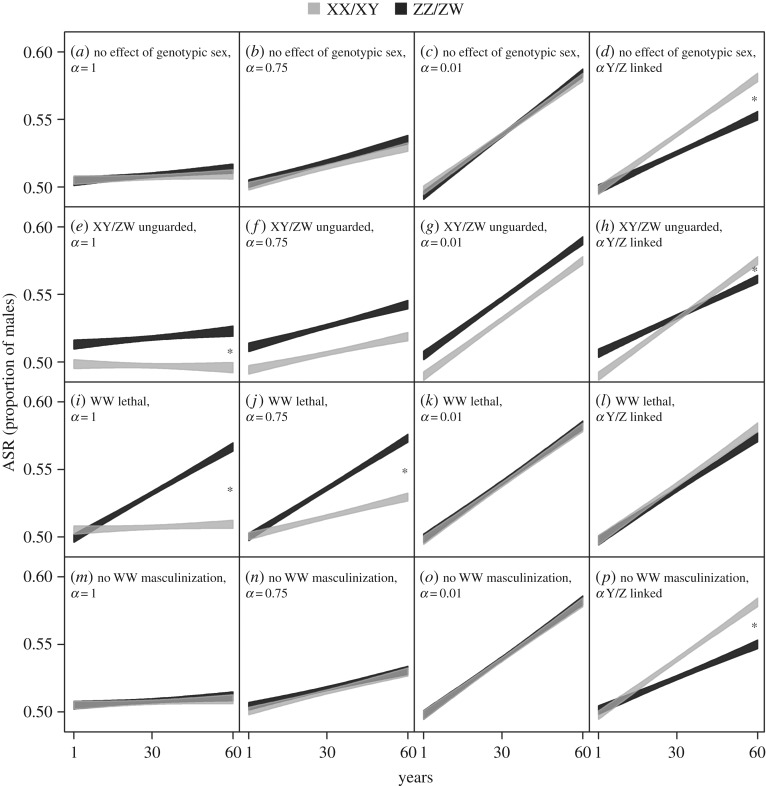
Over the first 60 years after masculinization rate had started to increase, both GSD systems shifted towards significantly more male-biased ASR in most scenarios (figure 2; electronic supplementary material, table S2 and figure S3). This shift was significantly stronger in the XX/XY than in the ZZ/ZW system in seven of the eight scenarios when male mating success was Y/Z linked (electronic supplementary material, table S2; figure 2). By contrast, the shift was significantly stronger in the ZZ/ZW than in the XX/XY system in three scenarios (electronic supplementary material, table S2): when masculinized individuals had high mating success (100 or 75% of normal males) and the WW genotype was lethal (figure 2i,j), or when masculinized individuals had 100% mating success and heterogametic offspring suffered extra mortality early in life (figure 2e). The slope of ASR change never differed between the two GSD systems when the masculinized individuals had very low mating success (electronic supplementary material, table S2; figure 2).
Figure 2.
Model-predicted changes of ASR over the first 60 years in the scenarios with no sex difference in maturation age and adult survival. Black and grey polygons show the 95% confidence bands of the slopes in ZZ/ZW and XX/XY systems, respectively. Asterisks mark the scenarios in which the slopes differ significantly between the two systems (p < 0.05 after correction for false discovery rate). The average rate of masculinization increases from zero by 0.003 each year; α denotes the mating success of masculinized individuals relative to normal males. Note that the parameter settings for the XX/XY system are the same in the scenarios ‘α = 0.01’ and ‘α Y-linked’ (see the electronic supplementary material, table S1).
(b). Empirical study
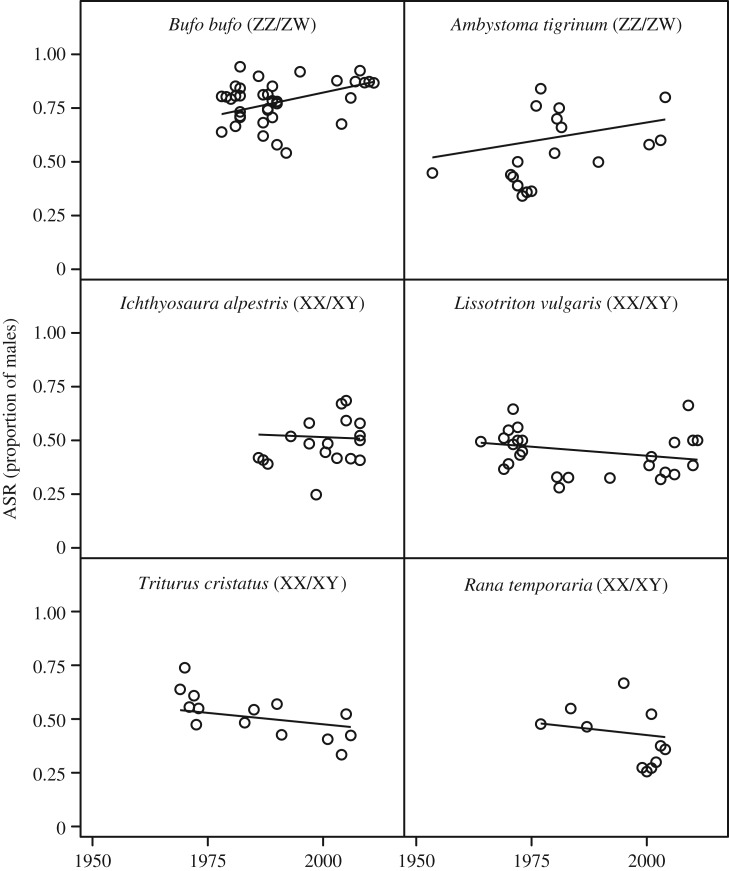
The change of ASR over the years differed significantly between species with different GSD (table 1). The two species with female heterogamety (ZZ/ZW) shifted significantly towards more male-biased ASR over time (table 2 and figure 3), with an average increase of 0.4% per year (CI: 0.03, 0.77). By contrast, the four species with male heterogamety (XX/XY) showed no significant change in ASR over the years (table 2 and figure 3), with an average decrease of 0.17% per year (CI: −0.43, 0.08). This observed pattern, i.e. increasing male bias in ZZ/ZW and no change in XX/XY, is qualitatively consistent with the theoretical scenarios where α = 1 and the WW genotype is lethal or the ‘unguarded’ heterogametic offspring suffer extra mortality (electronic supplementary material, table S2 and figure S3e,i; figure 2e,i).
Table 1.
Linear mixed-effects model for empirical ASR in relation to time (years since 1950) and GSD (genetic sex-determination system) in six amphibian species. ASR is expressed as percentage of males in the adult population to avoid very small model parameter values.
| 95% CI |
||||
|---|---|---|---|---|
| model parameters | posterior mean | lower bound | upper bound | p-value |
| intercept (ZZ/ZW, metamorphic) | 55.140 | 39.030 | 70.760 | 0.001 |
| latitude | −0.205 | −0.621 | 0.240 | 0.348 |
| altitude | −0.003 | −0.007 | 0.001 | 0.123 |
| morph (difference of paedomorphic) | −17.860 | −26.310 | −9.185 | <0.001 |
| GSD (difference of XX/XY from ZZ/ZW) | −0.140 | −19.310 | 21.210 | 0.964 |
| time (slope in ZZ/ZW species) | 0.403 | 0.018 | 0.766 | 0.036 |
| time × GSD (difference of slope) | −0.581 | −1.010 | −0.103 | 0.016 |
Table 2.
Annual change of empirical ASR in six amphibian species with different GSD system, estimated from the model in table 1. ASR is expressed as percentage of males in the adult population to avoid very small model parameter values.
| 95% CI |
||||
|---|---|---|---|---|
| species | GSD | slope | lower bound | upper bound |
| Ambystoma tigrinum | ZZ/ZW | 0.350 | 0.030 | 0.671 |
| Bufo bufo | ZZ/ZW | 0.455 | 0.134 | 0.777 |
| Ichthyosaura alpestris | XX/XY | −0.085 | −0.356 | 0.186 |
| Lissotriton vulgaris | XX/XY | −0.168 | −0.376 | 0.041 |
| Triturus cristatus | XX/XY | −0.212 | −0.498 | 0.074 |
| Rana temporaria | XX/XY | −0.234 | −0.499 | 0.031 |
Figure 3.
Empirical ASRs over time in amphibians with either XX/XY or ZZ/ZW sex-determination systems. Slopes are fitted from the model in table 1; species are ordered by slope from most positive to most negative.
ASR did not vary systematically with latitude and altitude, while it was significantly lower in populations containing paedomorphic adults (table 1). The difference among species or between the two GSD systems was not attributable to spatial differences in the rate of climate warming (electronic supplementary material, figure S4).
4. Discussion
Our most important novel finding, as demonstrated by the theoretical models and indirectly supported by the empirical results, is that the XX/XY and ZZ/ZW systems can differ remarkably in their susceptibility to climate change. Our model showed that the direction and extent of this difference varies with the mating success of sex-reversed individuals, the nature of genotype-dependent differences in offspring viability and the ability of WW individuals to sex-reverse. The empirical data showed that the ASR shifted towards males in ZZ/ZW but not in XX/XY species of temperate-zone amphibians over the past decades while climate has been warming in their habitats. Comparing these changes of ASR (figure 3) to the patterns predicted by our model (figure 2), the theoretical scenario that qualitatively best matches the empirical data is the case where masculinized individuals can reproduce as well as normal males but offspring survival is genotype-dependent, due to either WW lethality or extra mortality of the ‘unguarded’ heterogametic offspring. In both of these scenarios, the potential to dampen the effects of increasing masculinization rate by the production of female-biased offspring is constrained in the ZZ/ZW system compared with the XX/XY system, because early-life mortality of the genotypes is female-biased in the former but not in the latter. To explore whether these conditions are met in natural populations, and to be able to predict the impacts of climate change, detailed knowledge has to be accumulated on each species regarding the type of sex-determination system, the reproductive success of sex-reversed individuals and the effects of genotypic sex on offspring mortality. As most of this information is lacking for the majority of amphibian species and ectotherms in general, this challenging task will hinge on the development of genetic sexing methods [27].
Our model corroborates several findings from earlier theoretical studies that showed that sex ratios can vary with climate warming in species with GSD via temperature-induced sex reversals. Firstly, if masculinized individuals are not handicapped in reproduction, higher than 50% masculinization rates lead to the extinction of the male genotype, and phenotypic males are produced solely by temperature-induced masculinization [15]. Thus, the system switches from GSD to TSD even in the absence of selection for TSD, i.e. without any sex-specific fitness benefit favouring sex-specific developmental temperatures [23]. Secondly, if sex-reversed individuals have reduced reproductive success, masculinization shifts ASR towards males more rapidly, while genotypic sex ratios change more slowly [17]. Thirdly, WW lethality can affect the outcome [22] by saving the Z chromosome from extinction but rapidly shifting the ASR towards males [15]. Finally, if WW females are resistant to masculinization, for example, due to accumulation of male-antagonistic alleles on the W chromosome [22], increasing masculinization rates trigger a switch between GSD systems from ZZ/ZW to XX/XY [20].
Notably, these changes occurred in our models over several hundreds of years; and similarly, theoretical studies that allowed the sex-determination system to evolve via mutations (which was not permitted in our models) found such switches after hundreds or thousands of years [21–23]. Given the rapidity of contemporary environmental change [28], the question remains whether species can adapt fast enough [7,14]. The latest evolutionary transition between XX/XY and ZZ/ZW systems in amphibians is dated ca 1 Mya [36], and the youngest neo-sex chromosome, presumably representing a switch from a mixed GSD–TSD system to a pure XX/XY system, has been documented from a lineage that diverged ca 10 kya [25]. Nevertheless, several empirical findings suggest ample potential for adaptation. Even individuals with the same sex chromosomes under identical environmental conditions can vary in their propensity to develop into male or female, as pointed out by a recent review [37] and by studies reporting that extreme temperatures do not always induce sex reversal in all individuals [11]. It is not yet known whether the individual variation in sex-reversal propensity is heritable, although TSD species exhibit considerable heritability in the sex-determination threshold [38], and in a GSD lizard, the offspring of sex-reversed (ZZ) mothers were found to have greater temperature sensitivity, suggesting heritable variation in susceptibility to feminization [14]. Heritable variation in sex-reversal propensity would allow sex-determination thresholds to evolve, and this may fundamentally change the effects of climate change as well [20–22]. Thus, future models should allow for adaptation when comparing the responses of XX/XY and ZZ/ZW systems.
A further limitation of our theoretical study is that it did not allow for phenotypically plastic adjustments of parental or offspring behaviour in choice of thermal environment. For example, many species have been responding to climate change by breeding earlier or changing nest depth [6]; it is unclear whether or not such behavioural adjustments can counter-balance the effects of climate change on sex ratios [6–8]. Because the thermal environment has critical effects on growth and developmental rates as well as on the exposure to pathogens, predators and competitors, the consequences of changing the time or place of offspring development are non-trivial and can be counterintuitive. For example, a shift of spawning to earlier dates in a fish species has led to increasingly colder environment for the offspring at the time of sex determination, because water temperatures rose more slowly in early spring than later in the season [39]. Future theoretical studies of sex reversals should account for such effects of phenotypic plasticity.
Over 60 years of climate warming, our model predicted small, although ecologically still relevant, increases in ASR averaging an additional 0.08% of males per year (electronic supplementary material, table S2), which adds up to an extra approximately 5% of males in total over 6 decades. The data observed in nature showed relatively rapid changes in ASR, adding up to an extra 21–27% of males in ZZ/ZW species over 60 years (i.e. the slope values in table 2 multiplied by 60), although these estimates inevitably had relatively high uncertainty. Changes of such magnitude are significant from evolutionary and conservationist viewpoints alike, as sex ratios have far-reaching consequences for sexual and parental behaviours, demography and population viability [40–43]. For example, male-biased ASR may reduce effective population size and the population's intrinsic rate of increase [7,8], intensify sexual competition including sexual coercion and male–male contest that can be harmful or even fatal to females [44–46], or induce homosexual behaviours [46].
It is unlikely that the difference we found between the two GSD systems was a by-product of spatially heterogeneous climate change, because the increase in maximum temperatures at the study locations was similar in XX/XY and ZZ/ZW species. Also, because we controlled for geographical variability and morph differences in our analyses, the contrasting temporal trends we found are unlikely to be artefacts of different populations being studied in different years, although among-population heterogeneity is a likely cause for the high uncertainty of our slope estimates. Owing to the lack of long-term datasets, the number of species in our analysis is very small, especially for the ZZ/ZW species, so further evidence will be needed to corroborate the pattern we found. Nevertheless, the two ZZ/ZW species have little in common in terms of life history or phylogeny that would make them stand apart from the four XX/XY species, suggesting that the difference we found is related to the sex-determination system. It is also notable that in two additional ZZ/ZW species for which we found data with at least 10 years between the earliest and latest study year (two records for each species), ASR increased from 41% in 1958–1959 to 64% in 2002–2003 in Duttaphrynus melanostictus and from 34% in 1981–1984 to 42% in 1997–2002 in Crinia signifera [47–50].
We caution that the observed changes of ASR do not necessarily result from global warming; direct evidence for the role of climate change and sex reversals in these empirical trends should come from long-term studies of sex ratios in various age groups within natural populations coupled with extensive genetic analyses. Until such data become available, it has to be born in mind that the temporal changes of ASR may be influenced by factors unrelated to temperature. For example, pesticides widely used in agricultural practice [51,52] and pharmaceuticals and other xenoestrogens associated with urbanization [53,54] have the potential to interfere with sexual development and induce sex reversal, and species can differ substantially in their susceptibility to sex reversal by the same chemicals [27]. Furthermore, because both temperature and chemicals can disrupt the same endocrine pathway that is responsible for sexual differentiation [11,51], and high temperatures can exacerbate pesticide toxicity [51,52], chemical pollution and climate change may interact to affect sex ratios in complex ways. Such synergistic effects may be difficult to predict unless both chemical and temperature effects are considered simultaneously in theoretical and empirical studies.
Sex-determining mechanisms exhibit astonishing diversity among taxa [3], and evidence is accumulating that this diversity contributes profoundly to variation in demographic traits such as ASR [31] and evolutionary processes such as adaptive radiation [55] and genome evolution [56]. Our results highlight the fact that the type of GSD can also influence the species' susceptibility to sex-ratio shifts driven by environmentally induced sex reversals. Thus, understanding the interactions between genetic and environmental effects on sex determination will be essential for predicting the among-species variation in vulnerability and adaptability to environmental changes such as climate warming. Well-informed predictions about these issues, in turn, may prove crucial in the future for the conservation of ectothermic species.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Piotr Tryjanowski and Michael Jennions for comments on an earlier draft.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
V.B. conceived of the study, collected and analysed the data and wrote the manuscript. V.B., S.K. and E.N. designed the modelling study; S.K. and E.N. programmed the models. S.K., E.N., A.L. and T.S. critically reviewed the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The study was financed by the National Research, Development and Innovation Office (NKFIH) of Hungary (grant no. 115402). V.B. was supported by the János Bolyai Scholarship of the Hungarian Academy of Sciences. T.S. was supported by the NKFIH (grant no. 116310) and was a Fellow at the Wissenschaftskolleg zu Berlin—Institute for Advanced Study.
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.2307/annurev.ecolsys.37.091305.30000024) [DOI] [Google Scholar]
- 2.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 3.Bachtrog D, et al. 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarre SD, Ezaz T, Georges A. 2011. Transitions between sex-determining systems in reptiles and amphibians. Annu. Rev. Genomics Hum. Genet. 12, 391–406. ( 10.1146/annurev-genom-082410-101518) [DOI] [PubMed] [Google Scholar]
- 5.Ospina-Álvarez N, Piferrer F. 2008. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 3, 2–4. ( 10.1371/journal.pone.0002837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban MC, Richardson JL, Freidenfelds NA. 2014. Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evol. Appl. 7, 88–103. ( 10.1111/eva.12114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pezaro N, Doody JS, Thompson MB. 2016. The ecology and evolution of temperature-dependent reaction norms for sex determination in reptiles: a mechanistic conceptual model. Biol. Rev. in press ( 10.1111/brv.12285) [DOI] [PubMed] [Google Scholar]
- 8.Mitchell NJ, Janzen FJ. 2010. Temperature-dependent sex determination and contemporary climate change. Sex. Dev. 4, 129–140. ( 10.1159/000282494) [DOI] [PubMed] [Google Scholar]
- 9.Miller D, Summers J, Silber S. 2004. Environmental versus genetic sex determination: a possible factor in dinosaur extinction? Fertil. Steril. 81, 954–964. ( 10.1016/j.fertnstert.2003.09.051) [DOI] [PubMed] [Google Scholar]
- 10.Wallace H, Badawy GMI, Wallace BMN. 1999. Amphibian sex determination and sex reversal. Cell. Mol. Life Sci. 55, 901–909. ( 10.1007/s000180050343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chardard D, Penrad-Mobayed M, Chesnel A, Pieau C, Dournon C. 2004. Thermal sex reversals in amphibians. In Temperature-dependent sex determination in vertebrates (eds Valenzuela N, Lance V), pp. 59–67. Washington, DC: Smithsonian Books. [Google Scholar]
- 12.Perrin N. 2009. Sex reversal: a fountain of youth for sex chromosomes? Evolution 63, 3043–3049. ( 10.1111/j.l558-5646.2009.00837.x) [DOI] [PubMed] [Google Scholar]
- 13.Radder RS, Quinn AE, Georges A, Sarre SD, Shine R. 2008. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol. Lett 4, 176–178. ( 10.1098/rsbl.2007.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holleley CE, O'Meally D, Sarre SD, Marshall-Graves JA, Ezaz T, Matsubara K, Azad B, Zhang X, Georges A. 2015. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523, 79–82. ( 10.1038/nature14574) [DOI] [PubMed] [Google Scholar]
- 15.Hurley MA, Matthiessen P, Pickering AD. 2004. A model for environmental sex reversal in fish. J. Theor. Biol. 227, 159–165. ( 10.1016/j.jtbi.2003.10.010) [DOI] [PubMed] [Google Scholar]
- 16.Cotton S, Wedekind C. 2007. Introduction of Trojan sex chromosomes to boost population growth. J. Theor. Biol. 249, 153–161. ( 10.1016/j.jtbi.2007.07.016) [DOI] [PubMed] [Google Scholar]
- 17.Cotton S, Wedekind C. 2009. Population consequences of environmental sex reversal. Conserv. Biol. 23, 196–206. ( 10.1111/j.1523-1739.2008.01053.x) [DOI] [PubMed] [Google Scholar]
- 18.Alho JS, Matsuba C, Merilä J. 2010. Sex reversal and primary sex ratios in the common frog (Rana temporaria). Mol. Ecol. 19, 1763–1773. ( 10.1111/j.1365-294X.2010.04607.x) [DOI] [PubMed] [Google Scholar]
- 19.Senior AM, Krkosek M, Nakagawa S. 2013. The practicality of Trojan sex chromosomes as a biological control: an agent based model of two highly invasive Gambusia species. Biol. Invasions 15, 1765–1782. ( 10.1007/s10530-013-0407-1) [DOI] [Google Scholar]
- 20.Quinn AE, Sarre SD, Ezaz T, Marshall Graves JA, Georges A. 2011. Evolutionary transitions between mechanisms of sex determination in vertebrates. Biol. Lett. 7, 443–448. ( 10.1098/rsbl.2010.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pen I, Uller T, Feldmeyer B, Harts A, While GM, Wapstra E. 2010. Climate-driven population divergence in sex-determining systems. Nature 468, 436–438. ( 10.1038/nature09512) [DOI] [PubMed] [Google Scholar]
- 22.Schwanz LE, Ezaz T, Gruber B, Georges A. 2013. Novel evolutionary pathways of sex-determining mechanisms. J. Evol. Biol. 26, 2544–2557. ( 10.1111/jeb.12258) [DOI] [PubMed] [Google Scholar]
- 23.Grossen C, Neuenschwander S, Perrin N. 2011. Temperature-dependent turnovers in sex-determination mechanisms: a quantitative model. Evolution 65, 64–78. ( 10.1111/j.1558-5646.2010.01098.x) [DOI] [PubMed] [Google Scholar]
- 24.Ashman T-L, et al. 2014. Tree of sex: a database of sexual systems. Sci. Data 1, 561–572. ( 10.1038/sdata.2014.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues N, Vuille Y, Brelsford A, Merilä J, Perrin N. 2016. The genetic contribution to sex determination and number of sex chromosomes vary among populations of common frogs (Rana temporaria). Heredity 117, 25–32. ( 10.1038/hdy.2016.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crew FAE. 1921. Sex-reversal in frogs and toads. A review of the recorded cases of abnormality of the reproductive system and an account of a breeding experiment. J. Genet. 11, 141–181. ( 10.1007/BF02983047) [DOI] [Google Scholar]
- 27.Tamschick S, Rozenblut-Kościsty B, Ogielska M, Lehmann A, Lymberakis P, Hoffmann F, Lutz I, Kloas W, Stöck M. 2016. Sex reversal assessments reveal different vulnerability to endocrine disruption between deeply diverged anuran lineages. Sci. Rep. 6, 1–8. ( 10.1038/srep23825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seneviratne SI, Donat MG, Mueller B, Alexander L V. 2014. No pause in the increase of hot temperature extremes. Nat. Clim. Chang. 4, 161–163. ( 10.1038/nclimate2145) [DOI] [Google Scholar]
- 29.Shao C, et al. 2014. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 24, 604–615. ( 10.1101/gr.162172.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.r-projectorg/). [Google Scholar]
- 31.Pipoly I, Bókony V, Kirkpatrick M, Donald PF, Székely T, Liker A. 2015. The genetic sex-determination system predicts adult sex ratios in tetrapods. Nature 527, 91–94. ( 10.1038/nature15380) [DOI] [PubMed] [Google Scholar]
- 32.Morrison C, Hero J. 2003. Geographic variation in life history characteristics of amphibians: a review. J. Anim. Ecol. 72, 270–279. ( 10.1046/j.1365-2656.2003.00696.x) [DOI] [Google Scholar]
- 33.Denoël M, Joly P, Whiteman HH. 2005. Evolutionary ecology of facultative paedomorphosis in newts and salamanders. Biol. Rev. 80, 663–671. ( 10.1017/S1464793105006858) [DOI] [PubMed] [Google Scholar]
- 34.Gibbs JP, Steen DA. 2005. Trends in sex ratios of turtles in the United States: implications of road mortality. Conserv. Biol. 19, 552–556. ( 10.1111/j.1523-1739.2005.000155.x) [DOI] [Google Scholar]
- 35.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 36.Miura I. 2008. An evolutionary witness: the frog Rana rugosa underwent change of heterogametic sex from XY male to ZW female. Sex. Dev. 1, 323–331. ( 10.1159/000111764) [DOI] [PubMed] [Google Scholar]
- 37.Perrin N. 2016. Random sex determination: when developmental noise tips the sex balance. Bioessays 38, 1218–1226. ( 10.1002/bies.201600093) [DOI] [PubMed] [Google Scholar]
- 38.McGaugh SE, Janzen FJ. 2011. Effective heritability of targets of sex-ratio selection under environmental sex determination. J. Evol. Biol. 24, 784–794. ( 10.1111/j.1420-9101.2010.02211.x) [DOI] [PubMed] [Google Scholar]
- 39.Wedekind C, Küng C. 2010. Shift of spawning season and effects of climate warming on developmental stages of a grayling (Salmonidae). Conserv. Biol. 24, 1418–1423. ( 10.1111/j.1523-1739.2010.01534.x) [DOI] [PubMed] [Google Scholar]
- 40.Donald PF. 2007. Adult sex ratios in wild bird populations. Ibis 149, 671–692. ( 10.1111/j.1474-919x.2007.00724.x) [DOI] [Google Scholar]
- 41.Liker A, Freckleton RP, Székely T. 2013. The evolution of sex roles in birds is related to adult sex ratio. Nat. Commun. 4, 1587 ( 10.1038/ncomms2600) [DOI] [PubMed] [Google Scholar]
- 42.Liker A, Freckleton RP, Székely T. 2014. Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr. Biol. 24, 880–884. ( 10.1016/j.cub.2014.02.059) [DOI] [PubMed] [Google Scholar]
- 43.Székely T, Weissing FJ, Komdeur J. 2014. Adult sex ratio variation: implications for breeding system evolution. J. Evol. Biol. 27, 1500–1512. ( 10.1111/jeb.12415) [DOI] [PubMed] [Google Scholar]
- 44.Wells KD. 2007. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- 45.Hailey A, Willemsen RE. 2000. Population density and adult sex ratio of the tortoise Testudo hermanni in Greece: evidence for intrinsic population regulation. J. Zool. 251, 325–338. ( 10.1017/S0952836900007068) [DOI] [Google Scholar]
- 46.Bonnet X, Golubović A, Arsovski D, Dević S, Ballouard JM, Sterijovski B, Ajtić R, Barbraud C, Tomović L. 2016. A prison effect in a wild population: a scarcity of females induces homosexual behaviors in males. Behav. Ecol. 27, 1206–1215. ( 10.1093/beheco/arw023) [DOI] [Google Scholar]
- 47.Berry PY. 1964. The breeding patterns of seven species of Singapore Anura. J. Anim. Ecol. 33, 227–243. ( 10.2307/2629) [DOI] [Google Scholar]
- 48.Gramapurohit NP, Radder RS. 2012. Mating pattern, spawning behavior, and sexual size dimorphism in the tropical toad Bufo melanostictus (Schn.). J. Herpetol. 46, 412–416. ( 10.1670/11-096) [DOI] [Google Scholar]
- 49.Williamson I, Bull CM. 1996. Population ecology of the Australian frog Crinia signifera: adults and juveniles. Wildl. Res. 23, 249 ( 10.1071/WR9960249) [DOI] [Google Scholar]
- 50.Lauck B. 2005. Life history of the frog Crinia signifera in Tasmania, Australia. Aust. J. Zool. 53, 21–27. ( 10.1071/ZO04028) [DOI] [Google Scholar]
- 51.Mann RM, Hyne RV, Choung CB, Wilson SP. 2009. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ. Pollut. 157, 2903–2927. ( 10.1016/j.envpol.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 52.Köhler H-R, Triebskorn R. 2013. Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341, 759–765. ( 10.1126/science.1237591) [DOI] [PubMed] [Google Scholar]
- 53.Skelly DK, Bolden SR, Dion KB. 2010. Intersex frogs concentrated in suburban and urban landscapes. Ecohealth 7, 374–379. ( 10.1007/s10393-010-0348-4) [DOI] [PubMed] [Google Scholar]
- 54.Smits AP, Skelly DK, Bolden SR. 2014. Amphibian intersex in suburban landscapes. Ecosphere 5, 11 ( 10.1890/ES13-00353.1) [DOI] [Google Scholar]
- 55.Organ CL, Janes DE, Meade A, Pagel M. 2009. Genotypic sex determination enabled adaptive radiations of extinct marine reptiles. Nature 461, 389–392. ( 10.1038/nature08523) [DOI] [PubMed] [Google Scholar]
- 56.Valenzuela N, Adams DC. 2011. Chromosome number and sex determination coevolve in turtles. Evolution 65, 1808–1813. ( 10.1111/j.1558-5646.2011.01258.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.