Abstract
During sex determination, genetic and/or environmental factors determine the cascade of processes of gonad development. Many organisms, therefore, have a developmental window in which their sex determination can be sensitive to, for example, unusual temperatures or chemical pollutants. Disturbed environments can distort population sex ratios and may even cause sex reversal in species with genetic sex determination. The resulting genotype–phenotype mismatches can have long-lasting effects on population demography and genetics. I review the theoretical and empirical work in this context and explore in a simple population model the role of the fitness vyy of chromosomally aberrant YY genotypes that are a consequence of environmentally induced feminization. Low vyy is mostly beneficial for population growth. During feminization, low vyy reduces the proportion of genetic males and hence accelerates population growth, especially at low rates of feminization and at high fitness costs of the feminization itself (i.e. when feminization would otherwise not affect population dynamics much). When sex reversal ceases, low vyy mitigates the negative effects of feminization and can even prevent population extinction. Little is known about vyy in natural populations. The available models now need to be parametrized in order to better predict the long-term consequences of disturbed sex determination.
This article is part of the themed issue ‘Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies’.
Keywords: sex determination, environmental sex reversal, climate change, endocrine-disrupting chemicals, population growth, extinction
1. Introduction
Sex determination is strictly genetic in nearly all mammals and birds, mostly with male (XY) or female (ZW) heterogamety, and purely environmental in, for example, many reptiles. However, in various taxa sex determination is neither purely genetic nor purely environmental [1,2]. It is therefore often useful to see the phenotypic sex as the result of the three major drivers of phenotypic variation, namely genes, the environment and developmental noise (stochasticity due to random factors) [3]. It is then easy to see why disturbed environments can affect sex determination and hence population sex ratios. Such disturbances have genetic and demographic consequences that can sometimes threaten the viability of populations.
Authors often make a distinction between sex determination, i.e. the developmental step that decides whether an individual becomes female or male, and sex differentiation, i.e. the subsequent steps in developmental pathways during which the female or male phenotype is built up after the initial step of sex determination has occurred. However, abandoning a fundamental distinction between sex determination and gonad differentiation may help to better understand the evolution of sex-determining systems [1,4]. Sex is then still a threshold trait, with processes early in development regulating later processes, and with some of these processes occurring directly in the gonads, while others occur elsewhere in the organism. While sex is often a trait that has a single main trigger (e.g. DMRT1 expression above-critical level in chicken [5]), there are many species with several master triggers, for example, in plants [6], fishes [7] or gastropods [8]. It is therefore more useful to understand sex determination as a developmental switch that is composed of various regulatory elements. These elements can be both genetic and non-genetic and may even include maternal strategies [1,4].
Thinking of sex determination as a developmental process with one or several initial triggers raises interesting questions, including (i) what prevents in some taxa the emergence of a single master trigger of sex, i.e. why do so many species have several types of factors that determine sex [9], (ii) how do novel sex-determining systems arise from existing single or multi-factorial systems, and (iii) what are the demographic and genetic consequences of different sex-determining systems in changing environments? This article focuses on the latter question.
2. Sex determination in disturbed and undisturbed environments
Many environmental factors can affect sex determination in species with primarily environmental or genetic sex determination. Temperature is certainly the most important environmental factor that can potentially influence sex determination and hence adult sex ratios (ASR) in undisturbed environments [2,10,11]. In pure temperature-dependent sex determination (TSD), temperature during a thermosensitive period triggers male or female gonad development. TSD occurs in crocodiles, most turtles and some fish [12,13]. Other factors that drive environmental sex determination in undisturbed environments are photoperiod in some amphipods and barnacles [14], social influences in some fish and aquatic snails [15,16], pathogens [17], and pH or oxygen levels [13]. Temperature often acts in combination with other environmental effects on sex determination [1,18]. These other factors include maternal environmental effects like egg size [19] and yolk steroid hormones [20], which appear to reflect differential maternal investment [21,22]. It may therefore not be surprising that several endocrine-disrupting chemicals have also been found to interfere with sex determination in species with TSD [23]. For instance, embryos of the turtle Trachemys scripta that are incubated at male-producing temperatures often turn into females when exposed to oestradiol [24], different types of polychlorinated biphenyls (PCBs) [25], the herbicide atrazine [26] or other compounds of which the insecticide chlordane is synergistic with oestradiol when applied in combination [24].
Sex determination can also be altered in species with genetic sex determination. In this context, the most important anthropogenic changes to the environment are temperature (due to climate change or, for example, power plants that increase river water temperatures) and micropollutants [27]. Various endocrine-disrupting chemicals have been shown to interfere with the endocrine system and affect sex determination. Exogenous chemicals are therefore often used in aquaculture and research to override genetic sex determination [28]. Piferrer [28] lists over 50 fish species and hybrids whose sex determination has been successfully manipulated. The oestrogens used most often in such treatments are natural oestrone (E1), 17β-oestradiol (E2), the synthetic 17α-ethinylestradiol (EE2). However, fishes vary in their susceptibility to exogenous chemicals, i.e. the potential of a given oestrogen to feminize needs to be separately evaluated for each species [13,28].
While many of these oestrogens usually play a minor role in aquatic systems because of their low prevalence and relatively short half-life, EE2 is a prevalent pollutant that is globally relevant. It is used in most formulations of oral contraceptives, and its half-life in aquatic environments is around 14 days [29]. EE2 is now commonly found in surface and groundwater at concentrations around 1 ng l−1 [30], but concentrations of up to 273 ng l−1 have been reported [31]. Concentrations as low as 1 ng l−1 are known to affect embryo growth and to induce vitellogenin production, i.e. the precursor protein of egg yolk, in fish [32–34]. EE2 is also a potential endocrine-disrupting chemical in amphibians [35].
Other micropollutants that can affect sex determination are pesticides, including atrazine that has been shown to interfere with sex determination ([36,37], see also [38] and subsequent discussion in the same journal), PCBs [23], and some of the most widely used plasticizers (additives that increase the viscosity or plasticity of certain industrial products), including phthalates and bisphenol A (BPA) that can interfere with hormone systems and may hence affect sex determination [39]. Within aquatic systems, molluscs, crustacean and amphibians generally seem to be more susceptible to these plasticizers than fish, but disturbance of fish spermatogenesis has also been found even at low concentrations of BPA [39].
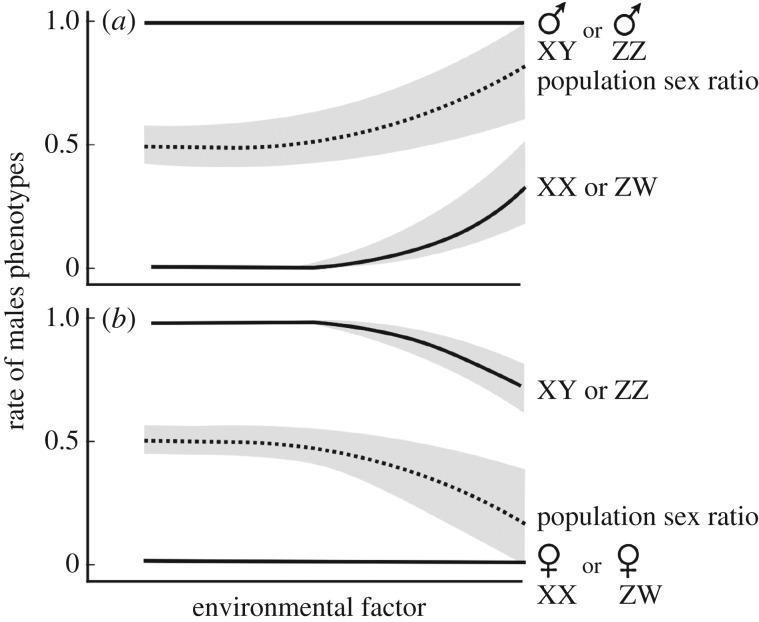
There are many cases of unusual temperatures or micropollutants overriding genetic factors of sex determination and causing environmental sex reversal (ESR), resulting in a mismatch between an organism's phenotype and genotype. Figure 1 illustrates possible patterns of genetic versus environmental contributions to sex determination. The figure only illustrates the principles. The link between sex determination and environment need not be linear or even continuous, the variance need not be constant in different environments, and environmental inputs may completely override genetic sex-determining factors. The resulting phenotype–genotype mismatches can then affect sex ratios in subsequent generations, as explained below. The potential significance of the interaction between genetic and environmental factors is further explored by Bokony et al. [11], who argue that male heterogametic and female heterogametic amphibians are likely to respond differently to temperature-induced sex reversal.
Figure 1.
Illustrating the continuum of genetic and environmental sex determination. Examples of possible effects of environmental factors (e.g. temperature or concentration of endocrine-disrupting micropollutants) on sex determination in (a) a hypothetical population with genetic sex determination and the female genotype being susceptible to environmental factors that masculinize (i.e. turning some XX or ZW individuals into males) and (b) a population with genetic sex-determining factors and the male genotype being susceptible to environmental factors that feminize (i.e. turning some XY or ZZ individuals into females). The shaded area indicates the within-population variance that could be due to additive genetic variance in the reaction norms or due to random effects at the start of the sex determination cascade. The hatched line gives the population sex ratio (proportion of males) if all clutches experience the same environmental conditions. This population sex ratio will equal adult sex ratio (ASR) if there is no sex-specific mortality.
There are other types of anthropogenic changes of the environment that can influence individual sex determination and hence population sex ratios, e.g. non-random exploitation of sequential hermaphrodites that can affect their life history and their timing of sex change [40]. These other anthropogenic changes will not be further discussed here. In the following section, I concentrate on environmental changes that override genetic sex determination.
3. Relevance of different mating types after disturbed sex determination
While ESR can immediately affect the phenotypic sex ratio of a population, it also creates phenotype–genotype mismatches that can have potentially counterintuitive consequences for future generations (e.g. environmental feminization may sometimes explain male-biased ASR). Such long-term consequences depend on the various possible mating types. Some of these mating types can therefore be relevant for the management of wild and captive populations.
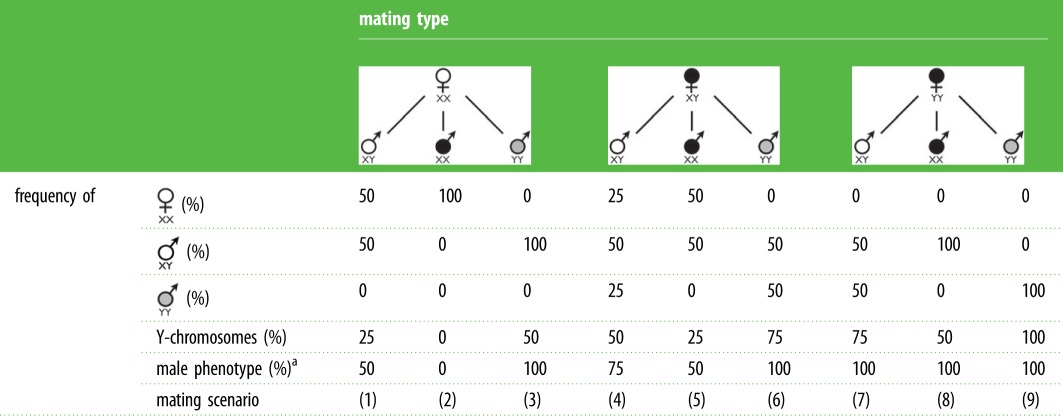
Table 1 shows the effect of ESR on all possible mating types in an XY sex determination system with ESR (both masculinization and feminization), the frequency of the sex chromosomes in the resulting offspring and the family sex ratios (here defined as frequency of the male phenotype before possible further sex reversal; assuming that the YY genotype naturally leads to the male phenotype). These family sex ratios will equal the ASR in the F1 if there is no further sex reversal and no sex-specific mortality.
Table 1.
Mating types with XY sex determination and ESR. The expected consequences of all possible mating types in a XY sex determination system, i.e. of males or females with no phenotype–genotype mismatch (open symbols), sex-reversed individuals (black symbols) or with karyotypes that can results from sex reversal in the parental generation (grey symbols), assuming that all mating types are possible and have the same effect on the viability of all types of offspring, and that the YY genotype naturally leads to the male phenotype, i.e. sex reversal is necessary to produce YY females. The figure gives the expected frequencies of XX-female, XY-male and YY-male offspring, the expected frequencies of Y-chromosomes, and the expected frequencies of male phenotypes in the F1. See text for a discussion of the various mating scenarios.
 |
aBefore possible further sex reversal.
Apart from the FXX × MXY mating (scenario 1 in table 1), there are eight further possible mating types that can result from ESR. Some scenarios are only possible after sex reversal occurs in a previous generation (e.g. MYY and FYY must be offspring of sex-reversed FYY or FXY). The nine scenarios vary in their genetic and demographic effects on future generations [41,42]. They also vary in their potential relevance for population management, including the management of threatened wild populations that may [43] or may not suffer from distorted sex ratio [44], the management of undesired populations (e.g. invasive species) [45] and the management of captive populations (e.g. in aquaculture) [28].
In aquaculture, one-generation mono-sex cultures are often economically advantageous because, for example, they avoid the problems of early maturation and uncontrolled reproduction [28]. Masculinization of XX individuals (via hormone treatment) and mating scenario 2 could be relevant for the production of female mono-sex cultures in fish farming [46]. They may also be relevant in managing wild populations, for example, for boosting population growth to above-critical levels in order to reduce the risk of extinction [44]. Scenarios 3, 4, 6, 7 and 9 (all based on feminization of XY or YY individuals, e.g. via hormones) pertain to population management that is based on ‘Trojan Y-chromosomes’ [45,47]. The idea here is to produce YY individuals and release them into natural populations in order to distort population sex ratios towards the male sex in order to control growth of undesired populations (e.g. of invasive fish or amphibians). This type of population management would ideally be based on broodstocks of YY males and YY females (if males are the heterogametic sex) or of ZZ males and ZZ females (if females are the normally heterogametic sex, see below).
YY-broodstocks would ideally aim for mating scenario 3 in table 1 if the release of hormone-treated individuals into a natural population is to be avoided, e.g. to avoid anglers catching and consuming hormone-treated fish [48]. Consumption of non-treated offspring of hormone-treated fish seems accepted from a food-safety standpoint, as made evident by the large amounts of commercially grown offspring of sex-reversed fish that have been consumed over the last decades [49]. Scenarios 7 and 9 could become relevant if progeny of a YY-broodstock can be released after hormone treatment [45,47], with scenario 9 as a possibility when wild-born offspring of FYY mate with introduced FYY. Scenarios 4 and 9 also describe stages in a YY broodstock production [48]. Scenarios 5 and 8 seem to have no or limited relevance in aquaculture or for the control of undesired natural populations but could be used in experimental research to study, for example, viability effects of sex reversal in the different karyotypes. These are crucial parameters in various types of population models [41,42,45,50]. Scenario 6 could become relevant if the second phase in a YY-broodstock production needs to be repeated, e.g. in order to increase the genetic diversity of the broodstock.
Table 2 shows the analogous demographic and genetic effects of all other possible mating types in a ZW sex determination system with wild-type and artificially constructed genotype–phenotype combinations on the subsequent generation, assuming that the WW genotype naturally leads to the female phenotype (analogous to the assumption above that the YY genotype naturally leads to the male genotype). Scenario 10 describes the natural FZW × MZZ mating. The release of sex-reversed ZW and WW individuals into a natural population with ZW females (scenarios 11 and 12) would be expected to bias the population sex ratio towards the female sex and hence boost population growth. This could potentially be an option for boosting population growth to above-critical levels in order to reduce the risk of extinction [44], analogously to scenario 2 in table 1. Scenario 13 offers such a potential boost in population growth while avoiding the release of hormone-treated individuals. Such non-hormone treated FWW would ideally be produced in scenario 15. Scenarios 12–15 and 18 would be possible broodstocks for mono-sex cultures in fish farming if females are the preferred sex. Scenarios 17 seems of no or limited relevance in aquaculture or for the management of natural populations but could potentially be used in experimental research to study viability effects of sex reversal in the different karyotypes, analogously to scenarios 5 and 8 in table 1. Scenario 16 is an interesting one: it may not only be the ideal broodstock for mono-sex cultures if males are the preferred sex in fish farming, but it could also describe the type of mating that a release of sex-reversed ZZ individuals into a natural population with ZZ males would lead to if the Z-chromosome is used as Trojan element to control the growth of an undesired population.
Table 2.
Mating types with ZW sex determination and ESR. The expected consequences of all possible mating types in a ZW sex determination system, analogous to table 1 (assuming that the WW genotype naturally leads to the female phenotype, i.e. sex reversal is necessary to produce WW males). See text for a discussion of the mating scenarios.
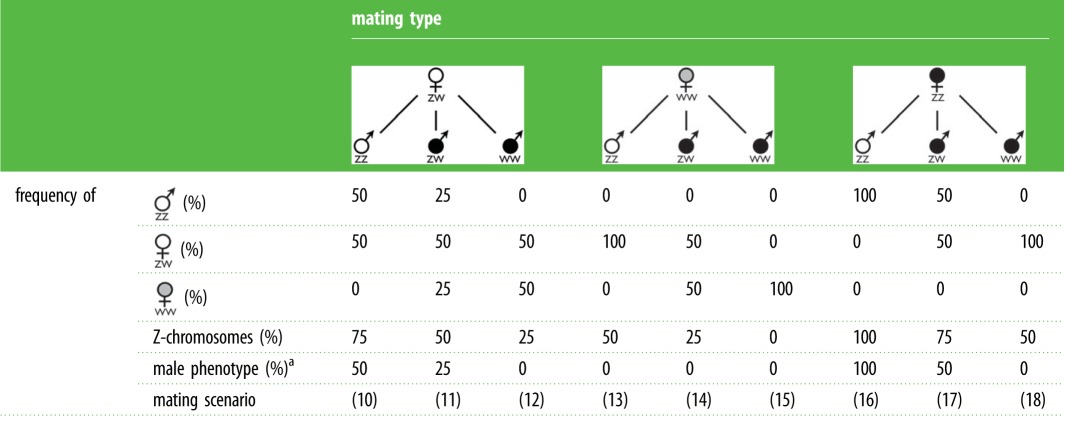 |
aBefore possible further sex reversal.
4. Demographic and genetic consequences of phenotype–genotype mismatches
If sex determination is predominantly genetic but can be reversed by environmental factors, immediate shifts in population sex ratios and in the frequencies of the sex chromosomes are likely and can extend over several generations [51,52]. The demographic and genetic consequences then need to be modelled. They depend on the frequencies of the all possible mating types that were discussed in §3 and that are likely to change over time, depending on the fitness (viability and reproductive success) of the various possible combinations of phenotypes and genotypes. The present section summarizes the available models and later meta-analyses and case studies that help to better define the relevant parameter space of such models. Recent empirical work suggests that the fitness of sex-reversed individuals is probably not as decisive as previously assumed in some models. However, the fitness of aberrant karyotypes (YY and WW) may be more important than sometimes assumed. Section 5 will therefore focus on the fitness of aberrant karyotypes and demonstrate its relevance for demographic and population-genetic models.
Environmental masculinization (figure 1a) reduces the proportion of genetic males and can eventually lead to the extinction of Y-chromosomes, while environmental feminization (figure 1b) can elevate the proportion of genetic males and can theoretically drive X-chromosomes to extinction [41,42,53] (but extinction of X-chromosomes requires far stronger rates of ESR than extinction of Y-chromosomes [42]). Ceasing sex reversal (e.g. by stopping pollution) could then lead to extreme population sex ratios and quickly drive populations to extinction [42]. Another important consequence of environmentally induced sex reversal can be a switching between sex determination systems, for example, switching from XY/XX to ZW/ZZ or from genetic to environmental sex determination [11,54–56].
Apart from these extreme scenarios, ESR can have marked effects on population growth, depending on the kind of sex reversal and on the fitness costs of the sex reversal [42]. If these fitness costs are small and males are not needed for parental care, population census sizes (Nc) tend to react positively to environmental feminization. Genetically effective population sizes (Ne, i.e. the size of a model population that loses genetic variation at the same rate as the study population [57]) suffer from distorted sex ratios. However, this effect is likely to be compensated in subsequent generations by increased census sizes [58,59]. On the other hand, masculinization is generally expected to reduce population growth [42]. Moreover, Ne is negatively affected if masculinization increases the variance in reproductive success among phenotypic males, for example, because sexual selection may act differently on XX- and XY-males or because of possible effects of distorted sex ratios on male and female life history [60,61]. This is because Ne also decreases with increasing variation in family size among males [57].
The viability of sex-reversed individuals has been assumed to be a key variable determining the dynamics of populations that are exposed to ESR [42,62]. However, a first meta-analysis of the available data concluded that ESR by itself does generally not seem to significantly reduce individual health and vigor [62]. Exposure to endocrine-disrupting chemicals often reduces individual growth during some developmental stages, but individuals seem often able to recover from such temporary effects [62]. In a more recent review, Senior et al. [63] found little evidence for significant effects of ESR on sperm characteristics. They concluded that ‘…masculinized genotypic females may enjoy reproductive success comparable to genotypic males’ [63], and hence that ESR is more likely to influence the genetics and demography of wild populations than has previously been assumed. On the same line, Holleley et al. [64] argue in their review that ESR is unlikely to reduce viability and fertility in reptiles.
While the effects of masculinization or feminization on individual viability and fertility may typically be smaller than previously assumed [42], the effects of aberrant karyotypes (YY or WW) on viability and fertility can still be significant. Sex chromosomes evolve from autosomes and are likely to become heteromorphic because of repressed recombination on Y- and W-chromosomes [65]. Repressed recombination reduces the efficiency of natural selection and is expected to cause the kind of degeneration of Y- and W-chromosomes that is observed in many taxa, including humans [66].
Taxa in which ESR occasionally occurs under natural conditions (e.g. many fish and amphibians) typically show lower levels of degeneration of Y- and W-chromosomes than taxa that are less susceptible to ESR (e.g. birds and mammals). This may be because such taxa benefit from phenotype-specific recombination of sex chromosomes (e.g. X–Y recombination in FXY). Perrin [9] suggested that this phenotype-specific recombination in sex-reversed individuals (e.g. recombination between X and Y in phenotypic females), followed by selection, is a ‘fountain of youth’ for sex chromosomes and may explain the high rate of homomorphic sex chromosomes in fish and amphibians. Indeed, viable and fertile YY and WW genotypes could repeatedly be produced in some fish and amphibians [13,67]. Such aberrant genotypes could even be sex-reversed for subsequent breeding programmes (recent examples include Liu et al. [68] and Schill et al. [48]). However, because of their reduced recombination rate and their relatively small effective size compared with X- and Z-chromosomes (Y- and W-chromosomes are rarer in natural populations than X and Z), Y- and W-chromosomes will generally show higher levels of degeneration than X- and Z-chromosomes. Therefore, the aberrant YY and WW karyotypes usually suffer from reduced individual fitness when compared with the XX, XY, ZZ and ZW genotypes.
Not much is known about the relative viability and reproductive success of karyotypes within fishes and amphibians. When Schill et al. [48] produced a YY-broodstock of brook trout (Salvelinus fontinalis) for potential use in eradication programmes, they found the expected number of YY offspring in FXY × MXY matings, i.e. YY individuals did not seem to suffer from higher embryo or juvenile mortality under the protected hatchery conditions. However, feminization of YY individuals was more difficult than feminization of XY individuals, and E2-treatment led to higher rates of individuals with intersex characteristics among the YY than the XY individuals. Theoretical treatments of the long-term demographic and genetic effects of environmentally induced sex reversal should therefore distinguish between (the possibly minor) fitness effects on sex-reversed normal genotypes (e.g. FXY or MXX) and (the possibly higher) fitness effects of chromosomally aberrant individuals (e.g. MYY or the sex-reversed FYY). Fitness reduction in aberrant karyotypes are predicted to affect an evolutionary transition from one sex-determining system to another [55,69]. They have also been predicted to affect population sex ratios [41].
5. Modelling effects of environmental sex reversal and YY karyotypes on population dynamics
To study the demographic and genetic effects of reduced fitness in chromosomally aberrant individuals, I adopt Cotton & Wedekind's [42] deterministic model and largely followed their settings (box 1). Cotton & Wedekind's [42] analyses were based on the assumption that ESR-linked individual fitness was identical for YY and XY genotypes. In order to relax this assumption, YY genotypes now have a fitness of vYY ≤ 1. I analysed 20 generations, with a constant feminization rate during the first 10 generations and no feminization in the remaining 10 generations (i.e. a cease of ESR at generation 10).
Box 1. Settings of the model.
The present analysis of potential effects of ESR and YY karyotypes on population dynamics is based on Cotton & Wedekind's [42] deterministic model (i.e. excluding mutation-based evolution and random sex determination). Their settings were as follows: discrete generations, male heterogamety, population size at generation 0 = 1000, initial 1 : 1 sex ratio, random mating, females mate only once and contribute r offspring to the next generation, environmental feminization p ≤ 1 (identical for YY and XY genotypes), environmental masculinization q ≤ 1, and ESR-linked individual fitness vESR ≤ 1 (with fitness including survival and reproduction). In the new model, YY genotypes have a fitness of vYY ≤ 1, and the following simplifications are implemented: (i) no limitations on male mating ability (including the extreme case when one male is sufficient to fertilize all available eggs), (ii) carrying capacity K = 2000 and (iii) number of offspring per female r = 2 when NF ≤ K/2, otherwise r = K/NF (ceiling model of density-dependent reproduction).
The effects of environmental feminization and a ceasing of sex reversal are then analysed with regard to the population census sizes (Nc) and the genetically effective population sizes (Ne). Ne corrects for the effects of unequal sex ratios by Ne = 4NMNF/(NM + NF) and for the effects of variation of population size over time, e.g. of population bottlenecks, by using the harmonic mean each over all Ne from generation 0 on [57].
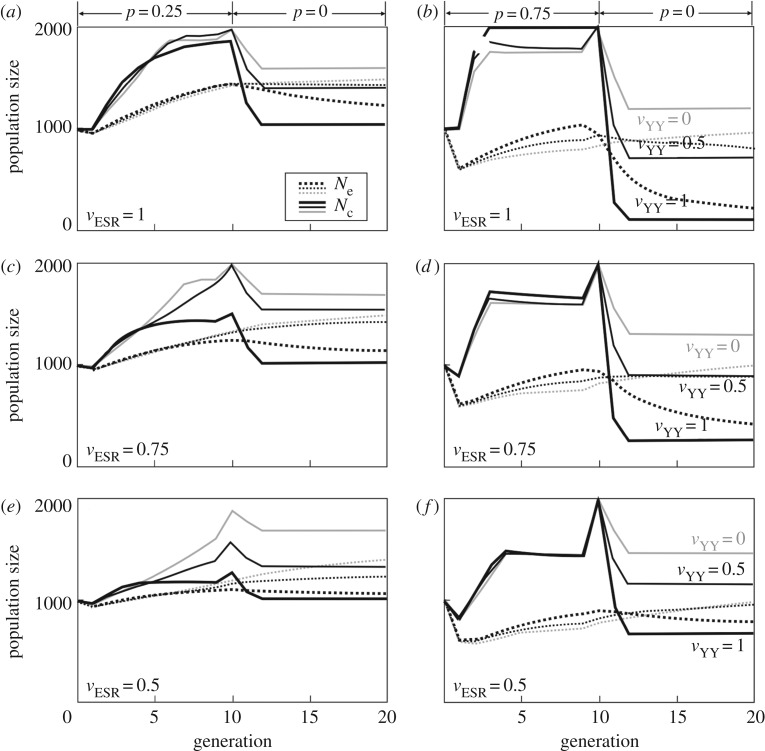
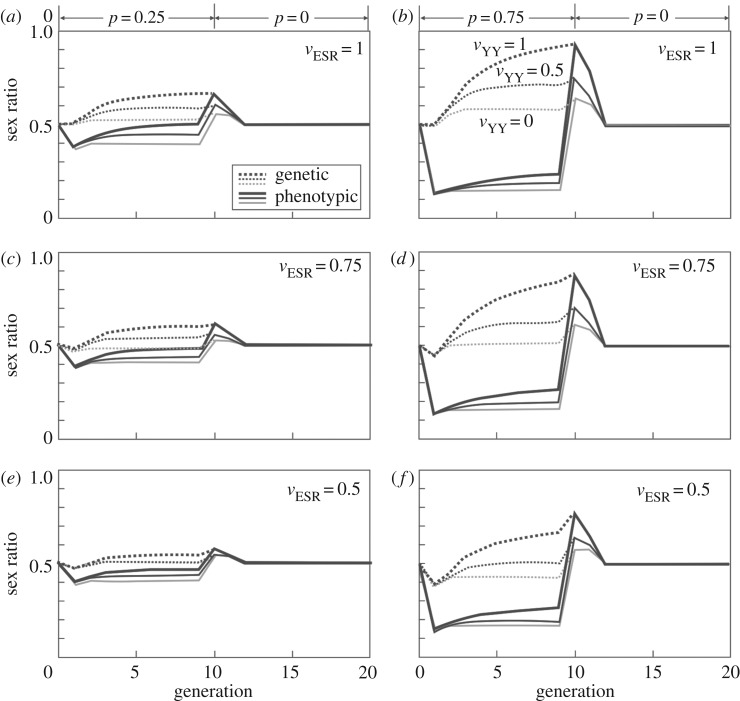
Environmental feminization has first a positive effect on the population census sizes Nc (figure 2). However, ESR changes the population sex ratio and hence reduces the genetically effective population size Ne, at least in the first generation after ESR has started (figure 2). Environmental masculinization generally reduces population sizes (both Nc and Ne) because of the high rate of males in the population [42], and, at the present parameter setting, quickly leads to population extinction at high rates of masculinization (electronic supplementary material, figure S1). In environmental feminization, the negative effects on Ne can be compensated later by the increased Nc, depending on the strength of the feminization and the population's carrying capacity (figure 2). However, ceasing sex reversal after generation 10 reduces population sizes (both Nc and Ne). The higher the feminization rate in the first 10 generations, the more pronounced is this drop in population sizes (approaching N = 0 with high p, vESR and vYY; figure 2b). This effect is mitigated with increased reduction of vESR, and especially so with increased reduction of vYY (figure 2), because low vYY cause low ratios of genetic males (Y-carriers) in the population during feminization (figure 3).
Figure 2.
The effects of environmental feminization and various types of fitness reduction on population size and genetics. Low fitness of YY genotypes (vYY) can significantly mitigate the negative long-term effects of feminization when sex reversal ceases. Low vYY can also produce positive effects on population growth during feminization, especially at low rates and high costs of feminization. The figure shows the population census sizes Nc (non-hatched lines) and the genetically effective population sizes Ne (hatched lines) when sex reversal (here only feminization, i.e. q = 0) causes no fitness reduction (vESR = 1; panels a,b) or fitness reductions of vESR = 0.75 (panels c and d) or vESR = 0.5 (panels e,f). Feminization is either weak (p = 0.25; panels a,c,e) or strong (p = 0.75; panels b,d,f) during the first 10 generations (q always = 0). Feminization ceases from generation 10 on (p = 0). The aberrant YY karyotype either causes no additional fitness reduction (vYY = 1; thick black lines) or a fitness of vYY = 0.5 (thin black lines) or vYY = 0 (thin grey lines). See box 1 for the settings of the model.
Figure 3.
The effects of environmental feminization and various types of fitness reduction on phenotypic and genetic sex ratio. Feminization reduces the proportion of phenotypic males while it increases the proportion of genetic males. Both effects are dependent on the fitness of YY genotypes (vYY). Low vYY can significantly reduce the proportion of genetic males, especially so at high rates of feminization. The figure shows the phenotypic population sex ratio (proportion of males; non-hatched lines) and the genetic sex ratio, i.e. the rate of individuals with Y-chromosomes (hatched lines). The parameter setting are as in figure 2, i.e. the fitness effect of sex reversal is either vESR = 1 (panels a,b), vESR = 0.75 (panels c,d), or vESR = 0.5 (panels e,f), feminization is either weak (p = 0.25; panels a,c,e) or strong (p = 0.75; panels b,d,f) during the first 10 generations, feminization ceases from generation 10 on (p = 0), and the aberrant YY karyotype has a fitness of either vYY = 1 (thick black lines), vYY = 0.5 (thin black lines), or vYY = 0 (thin grey lines).
The role of vYY on population growth during feminization depends both on p and vESR. At high p, variation in vYY has little effects on population growth during feminization. At low p and low vESR, overall population grow is nearly unaffected by the feminization when vYY is high. However, population growth then increases with declining vYY (figure 2e) because declining vYY reduce the rate of male genotypes in the population (figure 3).
6. Rapid evolutionary responses to environmentally disturbed sex determination?
The mechanisms of sex determination are rapidly evolving in many animal and plant clades [2]. The diversity of sex determination systems within fish, for example, extends deep into families [13], and there are several cases of within-species population differences in fish and other taxa [70]. Pen et al. [71] found, for example, sex determination to be mostly temperature-dependent in snow skink (Niveoscincus ocellatus) living in the lowlands of Tasmania, while it was predominantly genetic in adjacent highland populations. The authors argued that warm incubation temperatures lead to earlier births in the year and hence an improved opportunity for growing to large body until maturation. In lowland populations, females seem to profit more from large body sizes than males, and this might have selected for TSD. In their simulation models, they assumed sex to be determined by a combination of incubation temperature and of the alleles at four diploid loci. Under lowland conditions, genetic sex determination is then likely to turn into TSD within few thousands generations [71].
Such a transition from genetic to temperature-dependent sex determination can be dramatically faster if temperature induces sex reversal. The Australian bearded dragon (Pogona vitticeps), for example, has a ZW sex determination system that can be overridden by warm temperatures such that ZZ individuals turn into females who seem to be at least as viable and fertile as the wild-type ZW females [56]. By mating sex-reversed individuals, Holleley et al. [56] could experimentally induce a transition from genetic to solely temperature-dependent sex determination within only one generation (because sex-reversed ZZ females mated to wild-type ZZ males can only produce ZZ offspring). The environmental temperatures that allow for such transitions are within the range the species is currently exposed to, i.e. sex-reversed ZZ female bearded dragons can be found in the wild, and probably in increasing frequencies as observations between 2003 and 2011 suggest [56]. This species is hence susceptible to local extinction of W-chromosomes due to extreme environmental conditions, especially if combined with small population sizes (drift effects). Analogous rapid transitions are possible in a XY sex determination system when XX individuals are masculinized and mate with wild-type XX females to produce only XX offspring [41,42,69].
Further examples of diversity in sex determination system within species include the recent work of Rodrigues et al. [72,73], who found significant difference in sex determination among populations of the common frog (Rana temporaria), Ribas et al. [74], who found the masculinizing effects of elevated environmental temperatures to be family-specific in zebra fish (Danio rerio) and Shen et al. [75], who found strain-specific reaction norms in TSD in four strains of bluegill sunfish (Lepomis macrochirus). In the latter example, the authors suggested that the genotype–temperature interactions they found could be exploited to more efficiently manipulate sex determination in aquaculture, because males grow faster and larger than females in this species.
Given that even populations of the same species can differ in sex determination, it seems unsurprising that closely related species often differ in their reaction norms in feminization rate after exposure to micropollutants. A recent example includes Tamschick et al. [35], who found species-specific reaction norms in the response of three amphibians to exposure to EE2. Mizoguchi & Valenzuela [23] discuss possible species-specific reaction norms to various micropollutants in reptiles.
The evolutionary potential of natural populations to adapt to anthropogenic changes in the environment critically depends on the existence of additive genetic variation in the response to the change [76,77]. Such heritabilities are typically difficult to estimate, especially in the presence of non-genetic parental effects [78]. However, recent analyses of genome sequences and transcriptomes of Atlantic killifish (Fundulus heteroclitus) and of blue mussel (Mytilus edulis) populations sampled from polluted sites and from geographically paired non-polluted sites suggest pollution-induced genetic differentiation [79,80]. Brazzola et al. [32] used full-factorial in vitro breeding experiments (i.e. several males crossed with several females in all possible combination to control for maternal environmental effects and for any form of differential parental investments) and found significant additive genetic variance in the tolerance to EE2 pollution within two whitefish species (Coregonus sp.). In addition, Hamilton et al. [81] found roach (Rutilus rutilus) populations to be self-sustaining in heavily polluted habitats of Southern England despite widespread feminization (see also discussion in [82,83]).
These examples suggest that rapid genetic adaptation to some forms of pollution could be possible in some taxa. The basis of such tolerances needs to be further studied in order to better understand the potential for rapid adaptive evolution in response to environmentally disrupted sex determination. Data about the liability of sex determination and about the critical heritabilities are often lacking, and it is possible that many taxa might not be capable of rapid adaptation to environments that disturb sex determination [84].
7. Conclusion and implications for conservation and pest management
Fishes, amphibians and reptiles are often susceptible to anthropogenic disturbance of sex determination caused either by extreme temperatures or various types of micropollutants. This may occur either because their sex determination is environmental, or because their sex determination has a genetic basis that can be overruled. Such ESR creates phenotype–genotype mismatches that are often exploited in aquaculture to produce more profitable mono-sex cultures. In natural populations, phenotype–genotype mismatches can sometimes boost population growth if they reduce the ratio of males in the population and if females are limiting population growth. However, in most cases, disturbed sex determination and ESR is a threat to natural populations because it distorts the rates of sex chromosomes. Distorted rates of sex chromosomes can severely affect population growth and even cause extinction, e.g. during masculinization or when an environmental force that induces feminization ceases after sex reversal over several generations.
Recent meta-analyses suggest that ESR has little effect on individual survival and reproduction, and that the significance of vESR for population dynamics was sometimes overrated. However, the extended model presented here reveals that the fitness (survival and reproduction) of individuals with the aberrant YY genotype (vyy) plays an important role especially when feminization ceases and populations experience a sudden consequent drop in Nc and Ne. Low vyy significantly mitigates population decline. During feminization, vyy has little effect on population growth except when the rate of feminization is small and feminization affects individual fitness. Low vyy then boosts population growth because it reduces the rate of individuals carrying Y-chromosomes.
While ESR commonly threatens natural populations, it also creates interesting management options for problem populations, such as invasive fish or amphibians. This is true for both species with a ZW/ZZ and species with a XY/XX sex determination system. In ZW/ZZ species, the release of sex-reversed ZZ females into natural populations (and the subsequent mating of ZZ females with wild-type ZZ males) is expected to increase the rate of males in future generations and hence to reduce population growth. Analogously, in XY/XX species, the release of sex-reversed XY females and especially of YY males or even of sex-reversed YY females into a natural population is also expected to increase the ratio of males to females in future generations and to reduce population growth. This idea is based on the assumption that vESR and vyy are high, which is often the case for vESR, but needs to be further examined for vyy. The potential of this ‘Trojan Y-chromosome hypothesis’ then needs to be evaluated in field trials.
Supplementary Material
Acknowledgements
I thank Michael Jennions, Karen Kramer, Ryan Schacht, Tamás Székely and an anonymous reviewer for helpful comments, and the participants of a workshop on sex ratios at the Wissenschaftskolleg Berlin for discussion.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
Swiss National Science Foundation (31003A_125396 and 31003A_159579).
References
- 1.Beukeboom LW, Perrin N. 2014. The evolution of sex determination, 1–222 p Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Bachtrog D. et al 2014. Sex determination: Why so many ways of doing it? PLoS Biol. 12, e1001899 ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrin N. 2016. Random sex determination: when developmental noise tips the sex balance. Bioessays 38, 1218–1226. ( 10.1002/bies.201600093) [DOI] [PubMed] [Google Scholar]
- 4.Uller T, Helantera H. 2011. From the origin of sex-determining factors to the evolution of sex-determining systems. Q. Rev. Biol. 86, 163–180. ( 10.1086/661118) [DOI] [PubMed] [Google Scholar]
- 5.Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271. ( 10.1038/nature08298) [DOI] [PubMed] [Google Scholar]
- 6.Shannon RK, Holsinger KE. 2007. The genetics of sex determination in stinging nettle (Urtica dioica). Sexual Plant Reprod. 20, 35–43. ( 10.1007/s00497-006-0041-5) [DOI] [Google Scholar]
- 7.Liew WC, Bartfai R, Lim ZJ, Sreenivasan R, Siegfried KR, Orban L. 2012. Polygenic sex determination system in zebrafish. PLoS ONE 7, e34397 ( 10.1371/journal.pone.0034397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusa Y. 2007. Nuclear sex-determining genes cause large sex-ratio variation in the apple snail Pomacea canaliculata. Genetics 175, 179–184. ( 10.1534/genetics.106.060400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrin N. 2009. Sex reversal: a fountain of youth for sex chromosomes? Evolution 63, 3043–3049. ( 10.1111/j.1558-5646.2009.00837.x) [DOI] [PubMed] [Google Scholar]
- 10.Valenzuela N, Lance VA. 2004. Temperature-dependent sex determination in vertebrates. Washington, DC: Smithsonian Books. [Google Scholar]
- 11.Bókony V, Kövér S, Nemesházi E, Liker A, Székely T. 2017. Climate-driven shifts in adult sex ratios via sex reversals. Phil. Trans. R. Soc. B 372, 20160325 ( 10.1098/rstb.2016.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchant-Larios H, Diaz-Hernandez V. 2013. Environmental sex determination mechanisms in reptiles. Sex. Dev. 7, 95–103. ( 10.1159/000341936) [DOI] [PubMed] [Google Scholar]
- 13.Devlin RH, Nagahama Y. 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208, 191–364. ( 10.1016/s0044-8486(02)00057-1) [DOI] [Google Scholar]
- 14.Guler Y, Short S, Kile P, Ford AT. 2012. Integrating field and laboratory evidence for environmental sex determination in the amphipod, Echinogammarus marinus. Mar. Biol. 159, 2885–2890. ( 10.1007/s00227-012-2042-2) [DOI] [Google Scholar]
- 15.Walker G. 2005. Sex determination in the larvae of the parasitic barnacle Heterosaccus lunatus: an experimental approach. J. Exp. Mar. Biol. Ecol. 318, 31–38. ( 10.1016/j.jembe.2004.12.008) [DOI] [PubMed] [Google Scholar]
- 16.Geffroy B, Bardonnet A. 2016. Sex differentiation and sex determination in eels: consequences for management. Fish Fish. 17, 375–398. ( 10.1111/faf.12113) [DOI] [Google Scholar]
- 17.Bouchon D, Rigaud T, Juchault P. 1998. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc. R. Soc. Lond. B 265, 1081–1090. ( 10.1098/rspb.1998.0402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarre SD, Georges A, Quinn A. 2004. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays 26, 639–645. ( 10.1002/bies.20050) [DOI] [PubMed] [Google Scholar]
- 19.Warner DA, Lovern MB, Shine R. 2007. Maternal nutrition affects reproductive output and sex allocation in a lizard with environmental sex determination. Proc. R. Soc. B 274, 883–890. ( 10.1098/rspb.2006.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding GH, Yang J, Wang J, Ji X. 2012. Offspring sex in a TSD gecko correlates with an interaction between incubation temperature and yolk steroid hormones. Naturwissenschaften 99, 999–1006. ( 10.1007/s00114-012-0981-6) [DOI] [PubMed] [Google Scholar]
- 21.Horvathova T, Nakagawa S, Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. B 279, 163–170. ( 10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil D, Graves J, Hazon N, Wells A. 1999. Male at attractiveness and differential testosterone investment in zebra finch eggs. Science 286, 126–128. ( 10.1126/science.286.5437.126) [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi BA, Valenzuela N. 2016. Ecotoxicological perspectives of sex determination. Sex. Dev. 10, 45–57. ( 10.1159/000444770) [DOI] [PubMed] [Google Scholar]
- 24.Willingham E, Crews D. 1999. Sex reversal effects of environmentally relevant xenobiotic concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination. Gen. Comp. Endocrinol. 113, 429–435. ( 10.1006/gcen.1998.7221) [DOI] [PubMed] [Google Scholar]
- 25.Bergeron JM, Crews D, McLachlan JA. 1994. PCBs as environmental estrogens – turtle sex determination as a biomarker of environmental contamination. Environ. Health Persp. 102, 780–781. ( 10.1289/ehp.94102780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willingham EJ. 2005. The effects of atrazine and temperature on turtle hatchling size and sex ratios. Front. Ecol. Environ. 3, 309–313. ( 10.1890/1540-9295(2005)003%5B0309:TEOAAT%5D2.0.CO;2) [DOI] [Google Scholar]
- 27.Johnson AC, Sumpter JP. 2014. Putting pharmaceuticals into the wider context of challenges to fish populations in rivers. Phil. Trans. R. Soc. B 369, 20130581 ( 10.1098/rstb.2013.0581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piferrer F. 2001. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197, 229–281. ( 10.1016/s0044-8486(01)00589-0) [DOI] [Google Scholar]
- 29.Shore LS, Gurevitz M, Shemesh M. 1993. Estrogen as an environmental pollutant. Bull. Environ. Contam. Toxicol. 51, 361–366. ( 10.1002/jssc.200800673) [DOI] [PubMed] [Google Scholar]
- 30.Vulliet E, Cren-Olive C. 2011. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environm. Pollut. 159, 2929–2934. ( 10.1016/j.envpol.2011.04.033) [DOI] [PubMed] [Google Scholar]
- 31.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. 2002. Response to comment on, ‘Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance’. Environ. Sci. Technol. 36, 4007–4008. ( 10.1021/es020136s) [DOI] [PubMed] [Google Scholar]
- 32.Brazzola G, Chèvre N, Wedekind C. 2014. Additive genetic variation for tolerance to estrogen pollution in natural populations of Alpine whitefish (Coregonus sp., Salmonidae). Evol. Appl. 7, 1084–1093. ( 10.1111/eva.12216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose J, Holbech H, Lindholst C, Norum U, Povlsen A, Korsgaard B, Bjerregaard P. 2002. Vitellogenin induction by 17 beta-estradiol and 17 alpha-ethinylestradiol in male zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 131, 531–539. ( 10.1016/S1532-0456(02)00035-2) [DOI] [PubMed] [Google Scholar]
- 34.Kaptaner B, Kankaya E, Unal G. 2009. Effects of 17 alpha-ethynylestradiol on hepatosomatic index, plasma vitellogenin levels and liver glutathione-S-transferade activity in lake Van fish (Chalcalburnus tarichi Pallas, 1811). Fresen. Environ. Bull. 18, 2366–2372. [Google Scholar]
- 35.Tamschick S, Rozenblut-Kościsty B, Ogielska M, Lehmann A, Lymberakis P, Hoffmann F, Lutz I, Kloas W, Stöck M. 2016. Sex reversal assessments reveal different vulnerability to endocrine disruption between deeply diverged anuran lineages. Sci. Rep. 6(23825), 1–8. ( 10.1038/srep23825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes TB, et al. 2010. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl Acad. Sci. USA 107, 4612–4617. ( 10.1073/pnas.0909519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. 2002. Herbicides: feminization of male frogs in the wild. Nature 419, 895–896. ( 10.1038/419895a) [DOI] [PubMed] [Google Scholar]
- 38.Jooste AM, Du Preez LH, Carr JA, Giesy JP, Gross TS, Kendall RJ, Smith EE, Van der Kraak GL, Solomon KR. 2005. Gonadal development of larval male Xenopus laevis exposed to atrazine in outdoor microcosms. Environ. Sci. Technol. 39, 5255–5261. ( 10.1021/es048134q) [DOI] [PubMed] [Google Scholar]
- 39.Oehlmann J, et al. 2009. A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062. ( 10.1098/rstb.2008.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provost MM, Jensen OP. 2015. The impacts of fishing on hermaphroditic species and treatment of sex change in stock assessments. Fisheries 40, 536–545. ( 10.1080/03632415.2015.1093471) [DOI] [Google Scholar]
- 41.Hurley MA, Matthiessen P, Pickering AD. 2004. A model for environmental sex reversal in fish. J. theor. Biol. 227, 159–165. ( 10.1016/j.jtbi.2003.10.010) [DOI] [PubMed] [Google Scholar]
- 42.Cotton S, Wedekind C. 2009. Population consequences of environmental sex reversal. Conserv. Biol. 23, 196–206. ( 10.1111/j.1523-1739.2008.01053.x) [DOI] [PubMed] [Google Scholar]
- 43.Wedekind C, Evanno G, Székely T, Pompini M, Darbellay O, Guthruf J. 2013. Persistent unequal sex ratio in a population of grayling (Salmonidae) and possible role of temperature increase. Conserv. Biol. 27, 229–234. ( 10.1111/j.1523-1739.2012.01909.x) [DOI] [PubMed] [Google Scholar]
- 44.Cotton S, Wedekind C. 2007. Introduction of Trojan sex chromosomes to boost population growth. J. Theor. Biol. 249, 153–161. ( 10.1016/j.jtbi.2007.07.016) [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez JB, Teem JL. 2006. A model describing the effect of sex-reversed YY fish in an established wild population: the use of a Trojan Y chromosome to cause extinction of an introduced exotic species. J. Theor. Biol. 241, 333–341. ( 10.1016/j.jtbi.2005.11.032) [DOI] [PubMed] [Google Scholar]
- 46.Razmi K, Naji T, Alizadeh M, Sahafi HH. 2011. Hormonal sex reversal of rainbow trout (Oncorhynchus mykiss) by ethynylestradiol-17 alpha (EE2). Iran. J. Fish. Sci. 10, 304–315. [Google Scholar]
- 47.Cotton S, Wedekind C. 2007. Control of introduced species using Trojan sex chromosomes. Trends Ecol. Evol. 22, 441–443. ( 10.1016/j.tree.2007.06.010) [DOI] [PubMed] [Google Scholar]
- 48.Schill DJ, Heindel JA, Campbell MR, Meyer KA, Mamer E. 2016. Production of a YY male brook trout broodstock for potential eradication of undesired brook trout populations. N. Am. J. Aquac. 78, 72–83. ( 10.1080/15222055.2015.1100149) [DOI] [Google Scholar]
- 49.Bye VJ, Lincoln RF. 1986. Commercial methods for the control of sexual maturation in rainbow trout (Salmo gairdneri). Aquaculture 57, 299–309. ( 10.1016/0044-8486(86)90208-5) [DOI] [Google Scholar]
- 50.Cotton S, Wedekind C. 2010. Male mutation bias and possible long-term effects of human activities. Conserv. Biol. 24, 1190–1197. ( 10.1111/j.1523-1739.2010.01524.x) [DOI] [PubMed] [Google Scholar]
- 51.Stelkens RB, Wedekind C. 2010. Environmental sex reversal, Trojan sex genes, and sex ratio adjustment: conditions and population consequences. Mol. Ecol. 19, 627–646. ( 10.1111/j.1365-294X.2010.04526.x) [DOI] [PubMed] [Google Scholar]
- 52.Senior AM, Lokman PM, Closs GP, Nakagawa S. 2015. Ecological and evolutionary applications for environmental sex reversal of fish. Q. Rev. Biol. 90, 23–44. ( 10.1086/679762) [DOI] [PubMed] [Google Scholar]
- 53.Kanaiwa M, Harada Y. 2002. Genetic risk involved in stock enhancement of fish having environmental sex determination. Popul. Ecol. 44, 7–15. ( 10.1007/s101440200001) [DOI] [Google Scholar]
- 54.Quinn AE, Sarre SD, Ezaz T, Marshall Graves JA, Georges A. 2011. Evolutionary transitions between mechanisms of sex determination in vertebrates. Biol. Lett. 7, 443–448. ( 10.1098/rsbl.2010.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossen C, Neuenschwander S, Perrin N. 2011. Temperature-dependent turnovers in sex-determination mechanisms: a quantitative model. Evolution 65, 64–78. ( 10.1111/j.1558-5646.2010.01098.x) [DOI] [PubMed] [Google Scholar]
- 56.Holleley CE, O'Meally D, Sarre SD, Graves JAM, Ezaz T, Matsubara K, Azad B, Zhang XW, Georges A. 2015. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523, 79–82. ( 10.1038/nature14574) [DOI] [PubMed] [Google Scholar]
- 57.Allendorf FW, Luikard G. 2007. Conservation and the genetics of populations. Malden, MA: Oxford University Press. [Google Scholar]
- 58.Wedekind C. 2002. Manipulating sex ratios for conservation: short-term risks and long-term benefits. Anim. Conserv. 5, 13–20. ( 10.1017/S1367943002001026) [DOI] [Google Scholar]
- 59.Lenz TL, Jacob A, Wedekind C. 2007. Manipulating sex ratio to increase population growth: the example of the Lesser Kestrel. Anim. Conserv. 10, 236–244. ( 10.1111/j.1469-1795.2007.00099.x) [DOI] [Google Scholar]
- 60.Clutton-Brock T. 2017. Reproductive competition and sexual selection. Phil. Trans. R. Soc. B 372, 20160310 ( 10.1098/rstb.2016.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jennions MD, Fromhage L. 2017. Not all sex ratios are equal: the Fisher condition, parental care and sexual selection. Phil. Trans. R. Soc. B 372, 20160312 ( 10.1098/rstb.2016.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senior AM, Lim JN, Nakagawa S. 2012. The fitness consequences of environmental sex reversal in fish: a quantitative review. Biol. Rev. 87, 900–911. ( 10.1111/j.1469-185X.2012.00230.x) [DOI] [PubMed] [Google Scholar]
- 63.Senior AM, Johnson SL, Nakagawa S. 2016. Sperm traits of masculinized fish relative to wild-type males: a systematic review and meta-analyses. Fish Fish. 17, 143–164. ( 10.1111/faf.12096) [DOI] [Google Scholar]
- 64.Holleley CE, Sarre SD, O'Meally D, Georges A. 2016. Sex reversal in reptiles: reproductive oddity or powerful driver of evolutionary change? Sex. Dev. 10, 279–287. ( 10.1159/000450972) [DOI] [PubMed] [Google Scholar]
- 65.Bachtrog D. 2013. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 14, 113–124. ( 10.1038/nrg3366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes JF, et al. 2012. Strict evolutionary conservation followed rapid gene loss on human and rhesus Y chromosomes. Nature 483, U82–U124. ( 10.1038/nature10843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallace H, Badawy GMI, Wallace BMN. 1999. Amphibian sex determination and sex reversal. CMLS Cell. Mol. Life Sci. 55, 901–909. ( 10.1007/s000180050343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu HQ, Guan B, Xu J, Hou CC, Tian H, Chen HX. 2013. Genetic manipulation of sex ratio for the large-scale breeding of YY super-male and XY all-male yellow catfish (Pelteobagrus fulvidraco (Richardson)). Mar. Biotechnol. 15, 321–328. ( 10.1007/s10126-012-9487-7) [DOI] [PubMed] [Google Scholar]
- 69.Schwanz LE, Ezaz T, Gruber B, Georges A. 2013. Novel evolutionary pathways of sex-determining mechanisms. J. Evol. Biol. 26, 2544–2557. ( 10.1111/jeb.12258) [DOI] [PubMed] [Google Scholar]
- 70.Sarre SD, Ezaz T, Georges A. 2011. Transitions between sex-determining systems in reptiles and amphibians. Annu. Rev. Genomics Hum. Genet. 12, 391–406. ( 10.1146/annurev-genom-082410-101518) [DOI] [PubMed] [Google Scholar]
- 71.Pen I, Uller T, Feldmeyer B, Harts A, While GM, Wapstra E. 2010. Climate-driven population divergence in sex-determining systems. Nature 468, U436–U262. ( 10.1038/nature09512) [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues N, Vuille Y, Loman J, Perrin N. 2015. Sex-chromosome differentiation and ‘sex races’ in the common frog (Rana temporaria). Proc. R. Soc. B 282, 20142726 ( 10.1098/rspb.2014.2726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodrigues N, Vuille Y, Brelsford A, Merilä J, Perrin N. 2016. The genetic contribution to sex determination and number of sex chromosomes vary among populations of common frogs (Rana temporaria). Heredity 117, 25–32. ( 10.1038/hdy.2016.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ribas L, Liew WC, Diaz N, Sreenivasan R, Orban L, Piferrer F. 2017. Heat-induced masculinization in domesticated zebrafish is family-specific and yields a set of different gonadal transcriptomes. Proc. Natl Acad. Sci. USA 114, E941–E950. ( 10.1073/pnas.1609411114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen ZG, Wang HP, Yao H, O'Bryant P, Rapp D, Zhu KQ. 2016. Sex determination in bluegill sunfish Lepomis macrochirus: effect of temperature on sex ratio of four geographic strains. Biol. Bull. 230, 197–208. ( 10.1086/BBLv230n3p197) [DOI] [PubMed] [Google Scholar]
- 76.Eizaguirre C, Baltazar-Soares M. 2014. Evolutionary conservation-evaluating the adaptive potential of species. Evol. Appl. 7, 963–967. ( 10.1111/eva.12227) [DOI] [Google Scholar]
- 77.Hamilton JA, Miller JM. 2016. Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conserv. Biol. 30, 33–41. ( 10.1111/cobi.12574) [DOI] [PubMed] [Google Scholar]
- 78.Warner DA, Uller T, Shine R. 2013. Transgenerational sex determination: the embryonic environment experienced by a male affects offspring sex ratio. Sci. Rep. 3, 293 ( 10.1038/srep02709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larsson J, Lönn M, Lind EE, Swiezak J, Smolarz K, Grahn M. 2016. Sewage treatment plant associated genetic differentiation in the blue mussel from the Baltic Sea and Swedish west coast. PeerJ 4, e2628 ( 10.7717/peerj.2628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reid NM, et al. 2016. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science 354, 1305–1308. ( 10.1126/science.aah4993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamilton PB, Nicol E, De-Bastos ESR, Williams RJ, Sumpter JP, Jobling S, Stevens JR, Tyler CR. 2014. Populations of a cyprinid fish are self-sustaining despite widespread feminization of. BMC Biol. 12, 1 ( 10.1186/1741-7007-12-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson AC, Sumpter JP. 2016. Are we going about chemical risk assessment for the aquatic environment the wrong way? Environ. Toxicol. Chem. 35, 1609–1616. ( 10.1002/etc.3441) [DOI] [PubMed] [Google Scholar]
- 83.Wedekind C. 2014. Fish populations surviving estrogen pollution. BMC Biol. 12, 10 ( 10.1186/1741-7007-12-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitchell NJ, Janzen FJ. 2010. Temperature-dependent sex determination and contemporary climate change. Sex. Dev. 4, 129–140. ( 10.1159/000282494) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.