Abstract
Objective
To study prognostic characteristics of cardiac troponin I (cTnI) elevation in acute ischemic stroke.
Methods
We retrospectively studied patients (n = 248) with acute ischemic stroke, acute ST-segment elevation myocardial infarction, and acute non-ST-elevation myocardial infarction who were treated between January 2013 and October 2015. Baseline demographic data and changes in cTnI levels among these three groups were compared. Patients with acute ischemic stroke were assigned to either the cTnI elevation group (cTnI > 0.034 ng/mL) or the no cTnI elevation group (cTnI ≤ 0.034 ng/mL). Logistic regression analysis was used to identify risk factors associated with elevated serum cTnI in patients with acute ischemic stroke. Moreover, the duration of hospital stay and incidence of major cardiovascular outcomes were compared in patients with acute ischemic stroke, with or without elevated cTnI.
Results
In this study population of patients with acute ischemic stroke (n = 178), acute ST-segment elevation myocardial infarction (n = 35), and acute non-ST-elevation myocardial infarction (n = 35), patients with acute ischemic stroke with elevated cTnI comprised 18.5% of subjects. Patients with elevated cTnI were older and more likely to have a history of hypertension. In addition, these patients had higher levels of inflammatory markers, reduced renal functions, increased D-dimer levels, higher NIH stroke scores, and lower left ventricular ejection fractions. Logistic regression analysis showed that both percentage of neutrophil and NIH stroke scores were elevated; estimated glomerular filtration rate and left ventricular ejection fraction were decreased in patients with acute ischemic stroke who had elevated cTnI, and they had more frequent major cardiovascular events during hospital stay.
Conclusion
Elevated cTnI detected in patients with acute ischemic stroke, indicated a greater likelihood of poor short-term prognosis during hospital stay.
Keywords: Acute ischemic stroke, Acute myocardial infarction, Cardiac troponin I
1. Introduction
Elevation of cardiac troponin I (cTnI) usually indicates disruption of myocardial cell membrane integrity and injury to myocardial cells. It is the gold standard for the diagnosis of acute myocardial infarction. However, elevated cTnI has been found to occur in some non-coronary artery diseases. A large number of studies have shown that some patients with acute stroke had troponin elevations, without obvious clinical cardiac symptoms in most cases.[1] In addition, cTnI level was closely associated with prognosis in these patients. In the current study, we aim to explore characteristics of elevated cTnI in patients with acute ischemic stroke and relevant risk factors to establish an evidence base to understand cTnI elevations and its clinical implications.
2. Methods
2.1. Study design and participants
This retrospective study was undertaken in the Neurology and Cardiology Departments of Peking University People's Hospital. Patient medical records from the records database of the hospital were reviewed, and 513 consecutive patients treated between January 2013 and October 2015 were screened initially. Of these, 335 patients were excluded, and 248 STEMI and NSTEMI age- and sex-matched patients were included in the study. The study protocol was approved by the institutional ethics committee.
Inclusion criteria were acute ischemic stroke confirmed by head CT and diagnosed based on the 2014 diagnostic criteria, specified in the guidelines issued by the Chinese Medical Association, Neurology Branch for the diagnosis and treatment criteria of acute ischemic stroke in China.[2] Exclusion criteria were (1) patients with disease onset time > 1 week; (2) patients who did not undergo cTnI testing or those with incomplete clinical data; and (3) where elevated cTnI was caused by some other diseases, including chronic heart failure, severe liver and kidney dysfunction, muscle diseases, tumors, infections, and immune diseases.
Criteria for the diagnosis of acute ST-segment elevation myocardial infarction (STEMI) and acute non-ST-segment elevation myocardial infarction (NSTEMI) were based on the 3rd Global Myocardial Infarction Diagnostic Criteria.[3]
2.2. Study outcome measurements
After study enrolment, patient demographic data, including age and gender, were recorded. Laboratory tests were conducted for routine blood cell counts (cat#XN9000; Automatic Blood Cell Analyzer, Sysmex, Japan), biochemical tests (cat#5832; Biochemical Analyzer, Beckman Coulter, United States), and D-dimer level (Instrumentation Laboratory, United States). Levels of myocardial injury markers including cTnI, creatine kinase MB (CK-MB), and myoglobin, were also conducted from hospitalization days 1 to 6 (Dxi800 Chemiluminescence Immunoassay Analyzer, Beckman Coulter, United States). A cTnI > 0.034 ng/mL, CK-MB > 5.0 ng/mL, and myoglobin > 91.2 ng/mL were defined as cut-off levels indicating marker elevation. The NIH Stroke Score (NIHSS) was used to evaluate the severity of stroke.[4] Duration of hospital stay and the incidence of major cardiovascular events in patients with acute ischemic stroke were also recorded. Major cardiovascular events included death, bleeding, recurrent stroke, myocardial infarction, acute heart failure, and malignant arrhythmia.
2.3. Statistical analysis
Continuous data were presented as mean ± SD after evaluating for normal distribution, and were tested by independent sample t tests. Categorical data were presented as percentage and examined by chi-square analysis. Logistic analysis was applied to test risk factors associated with elevated cTnI. P < 0.05 was considered indicative of statistical significance. All statistical analyses were conducted using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
The study enrolled 248 patients, including 178 patients with acute ischemic stroke and 70 with acute myocardial infarction (n = 35 STEMI, n = 35 NSTEMI). Of the 178 patients with acute ischemic stroke, cTnI elevations were detected in 33 patients (18.5%), including two patients with acute ischemic stroke after diagnosis of acute myocardial infarction. Both patients with acute ischemic stroke after acute myocardial infarction had acute NSTEMI, with acute ischemic stroke onset on day 4 and day 1 after acute myocardial infarction, and the trends in cTnI changes were consistent with those of acute myocardial infarction.
Changes in markers of myocardial injury in patients with acute ischemic stroke and acute myocardial infarction from day 1 to day 6 after disease onset are shown in Table 1. Abnormal electrocardiograms occurred in 42.9% of the patients with acute ischemic stroke, mainly T-wave changes, whereas abnormal ECG changes occurred in all of the patients with acute myocardial infarction, with ECG in STEMI patients showing ST-segment elevation and in NSTEMI patients showing ST-segment depression or T-wave changes. There was no statistically significant difference in age, gender, and body mass index between patients with elevated cTnI and those with NSTEMI and STEMI (P > 0.05, Table 2), whereas D-dimer in patients with acute stroke with elevated cTnI was significantly increased, compared with that in patients with NSTEMI and STEMI (P < 0.05, Table 2).
Table 1. Changes of myocardial injury markers in patients with acute ischemic stroke, NSTEMI, and STEMI.
| cTnI (ng/mL) | CK-MB (ng/mL) | Myoglobin (ng/mL) | ||
| Acute ischemic stroke | Day 1 | 0.094 (0.065, 0.185) | 3.350 (2.300, 6.550) | 102.100 (35.950, 280.400) |
| Day 2 | 0.201 (0.085, 0.340) | 2.650 (1.550, 4.250) | 72.400 (58.725, 87.975) | |
| Day 3 | 0.118 (0.041, 0.207) | 2.700 (1.200, 4.650) | 65.000 (27.450, 95.100) | |
| Day 4 | 0.086 (0.046, 0.143) | 1.800 (1.500, 3.900) | 43.100 (39.300, 94.000) | |
| Day 5 | 0.090 (0.043, 0.330) | 1.300 (0.700, 2.900) | 48.500 (17.600, 81.400) | |
| Day 6 | 0.067 (0.019, 0.206) | 1.700 (0.750, 2.900) | 50.900 (17.250, 98.600) | |
| NSTEMI | Day 1 | 2.711 (0.504, 7.809) | 12.850 (4.725, 43.800) | 42.000 (26.825, 83.750) |
| Day 2 | 1.566 (0.407, 3.800) | 4.000 (1.950, 7.750) | 27.200 (21.850, 48.900) | |
| Day 3 | 1.092 (0.343, 2.210) | 2.000 (1.300, 3.200) | 25.000 (18.800, 36.200) | |
| Day 4 | 0.638 (0.168, 1.569) | 1.500 (1.100, 2.300) | 23.600 (19.600, 31.900) | |
| Day 5 | 0.492 (0.176, 1.200) | 1.300 (1.000, 2.125) | 20.300 (16.775, 32.850) | |
| Day 6 | 0.426 (0.112, 0.725) | 1.200 (0.900, 1.800) | 19.600 (16.600, 29.850) | |
| STEMI | Day 1 | 25.725 (10.947, 65.638) | 69.350 (27.200, 123.875) | 78.600 (44.325, 152.650) |
| Day 2 | 13.652 (4.353, 28.689) | 10.800 (6.500, 22.800) | 40.200 (20.400, 67.400) | |
| Day 3 | 9.955 (3.771, 17.519) | 3.400 (2.200, 7.200) | 29.350 (17.450, 38.725) | |
| Day 4 | 6.947 (3.285, 13.249) | 2.150 (1.450, 5.100) | 28.050 (21.450, 38.725) | |
| Day 5 | 4.440 (1.657, 10.598) | 1.700 (1.100, 2.755) | 21.700 (16.075, 28.600) | |
| Day 6 | 1.937 (0.563, 5.975) | 1.300 (1.000, 1.950) | 20.800 (15.750, 30.700) |
Data were presented as median (interquartile range). CK-MB: creatine kinase isoenzyme; cTnI: cardiac troponin I; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST segment elevation myocardial infarction.
Table 2. Demographic characteristics and laboratory examinations.
| Acute ischemic stroke with cTnI elevation (n = 31) | NSTEMI (n = 35) | STEMI (n = 35) | |
| Age, yrs | 75.45 ± 8.51 | 73.23 ± 8.29 | 71.69 ± 9.23 |
| Male gender | 54.8% | 58.4% | 60.0% |
| BMI, kg/m2 | 24.67 ± 3.96 | 24.81 ± 2.72 | 23.82 ± 2.29 |
| WBC, ×109/L | 8.46 ± 2.20 | 8.20 ± 1.92 | 10.13 ± 2.96* |
| NE, % | 77.81% ± 11.02% | 69.01% ± 10.29%* | 80.24% ± 10.08% |
| AST, U/L | 20 (18, 30) | 16 (14, 23) | 29 (19, 58) |
| ALT, U/L | 15 (10, 23) | 28 (22, 46) | 46 (24, 107) |
| LDH, U/L | 240 (201, 328) | 247 (179, 270) | 263 (209, 498) |
| TC, mmol/L | 3.95 ± 1.30 | 4.40 ± 0.92 | 4.02 ± 1.16 |
| TG, mmol/L | 1.85 ± 1.21 | 1.81 ± 0.97 | 1.43 ± 0.88 |
| HDL-C, mmol/L | 0.84 ± 0.24 | 0.92 ± 0.21 | 0.92 ± 0.28 |
| LDL-C, mmol/L | 2.28 ± 1.00 | 2.74 ± 0.84* | 2.45 ± 0.97 |
| CRE, µmol/L | 79.81 ± 13.02 | 76.20 ± 14.75 | 77.49 ± 11.70 |
| UA, µmol/L) | 332.13 ± 137.22 | 359.94 ± 92.34 | 331.49 ± 95.32 |
| Urea, mmol/L | 7.72 (3.84, 10.70) | 6.06 (4.91, 7.14)* | 6.25 (4.73, 8.40) |
| eGFR, mL/min per 1.73 m2 | 78.88 ± 12.28 | 80.33 ± 13.77 | 79.55 ± 11.79 |
| D-D, ng/mL | 322 (215, 545) | 98 (78, 241) | 124 (69, 288) |
Data were presented as mean ± SD or median (interquartile range). *P < 0.05; P values were obtained through comparison between patients with NSTEMI or STEMI and patients with acute ischemic stroke. ALT: alanine aminotransferase; AST: aspartate transaminase; CRE: creatinine; D-D: D-dimer; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDH: lactate dehydrogenase; LDL-C: low-density lipoprotein cholesterol; NE: neutrophil; NSTEMI: non-ST segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; TC: total cholesterol; TG: triglyceride; UA: uric acid; WBC: white blood cells.
Compared to patients with acute ischemic stroke without cTnI elevation, patients who had acute ischemic stroke with associated cTnI elevation were significantly older, more likely to be hypertensive, had higher inflammatory indicators, including white blood cell count and neutrophil percentage and lower renal function [increased creatinine and declined estimated glomerular filtration rate (eGFR), increased D-dimer, higher NIHSS scores, and decreased left ventricular ejection fraction (LVEF; P < 0.05, Tables 3 and 4). Infarctions in patients with cTnI elevation were more likely to be located in the posterior vasculature of the brain, whereas those in patients without cTnI elevation were more likely located in the anterior vasculature of the brain. Logistic regression analysis showed that increase in neutrophil percentage and NHISS scores, as well as decrease in eGFR and LVEF, were predictive for cTnI elevation in patients with acute ischemic stroke (Table 5).
Table 3. Comparison of demographic characteristics and medical history in patients with acute ischemic stroke with or without elevated cTnI.
| cTnI elevation (n = 31) | No cTnI elevation (n = 145) | |
| Male gender | 54.80% | 53.10% |
| Age, yrs | 75.45 ± 8.51 | 69.83 ± 13.43* |
| BMI, kg/m2 | 24.67 ± 3.96 | 24.65 ± 3.15 |
| DM | 12 (38.7%) | 63 (43.4%) |
| Hypertension | 27 (87.1%) | 108 (72.5%)* |
| Hyperlipidemia | 13 (41.9%) | 90 (62.1%)* |
| Smoking | 11 (35.5%) | 46 (31.7%) |
| Alcohol consumption | 5 (16.1%) | 26 (17.9%) |
| Aspirin | 21 (67.7%) | 93 (64.1%) |
| Statin | 10 (33.3%) | 57 (39.3%) |
| CCB | 14 (45.2%) | 72 (49.7%) |
| β-blocker | 19 (61.3%) | 71 (49.0%)* |
| ACEI | 16 (51.6%) | 80 (55.2%) |
| ARB | 9 (29.0%) | 35 (24.1%) |
Data are presented as mean ± SD or n (%) unless other indicated. *P < 0.05 indicated statistically significant difference. ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; BMI: body mass index; CCB: calcium channel blocker; cTnI: cardiac troponin I; DM: diabetes mellitus.
Table 4. Comparison of laboratory and imaging examination results in patients of acute ischemic stroke with or without elevated cTnI.
| cTnI elevation (n = 31) | No cTnI elevation (n = 145) | |
| WBC, ×109/L | 8.46 ± 2.20 | 6.81 ± 2.07* |
| NE | 77.81% ± 11.02% | 66.68% ± 10.38%* |
| AST | 20 (18, 30) | 20 (16, 25) |
| ALT | 15 (10, 23) | 17 (11, 22) |
| LDH | 240 (201, 328) | 178 (151, 227) |
| TC, mmol/L | 3.95 ± 1.30 | 4.38 ± 1.20 |
| TG, mmol/L | 1.85 ± 1.21 | 1.66 ± 1.06 |
| HDL-C, mmol/L | 0.84 ± 0.24 | 1.00 ± 0.27* |
| LDL-C, mmol/L | 2.28 ± 1.00 | 2.66 ± 0.92 |
| CRE, µmol/L | 79.81 ± 13.02 | 68.34 ± 13.79* |
| UA, µmol/L | 332.13 ± 137.22 | 306.39 ± 95.83 |
| UREA, mmol/L | 7.72 (3.84, 10.70) | 4.76 (3.92, 5.82) |
| eGFR, mL/min per 1.73 m2 | 78.88 ± 12.28 | 90.88 ± 19.12* |
| D-D, ng/mL | 322 (215, 545) | 198 (90, 289)* |
| LVEF | 58.32% ± 10.75% | 66.02% ± 8.63%* |
| Infarction site | ||
| Anterior cerebral circulation | 70.9% | 84.8%* |
| Posterior cerebral circulation | 29.1% | 15.2% |
| NIHSS score | 10 (6, 17) | 4 (2, 8)* |
Data were presented as mean ± SD or median (interquartile range). *P < 0.05 indicated statistically significant differences. ALT: alanine aminotransferase; AST: aspartate transaminase; CRE: creatinine; cTnI: cardiac troponin I; D-D: D-dimer; eGFR: estimated glomerular filtration rate; HDL-C: high density lipoprotein cholesterol; LDH: lactate dehydrogenase; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; NE: Neutrophil; NIHSS: NIH Stroke Score; TC: total cholesterol; TG: triglyceride; UA: uric acid; UREA: urea; WBC: White blood cells.
Table 5. Logistic regression analysis of cTnI elevation.
| Risk factors | B | OR | 95% CI | P value |
| NE% | 0.076 | 1.079 | 1.013–1.150 | 0.018 |
| NIHSS score | 0.085 | 1.089 | 1.008–1.176 | 0.030 |
| eGFR | −0.065 | 0.937 | 0.897–0.979 | 0.004 |
| LVEF | −0.106 | 0.900 | 0.852–0.949 | < 0.001 |
cTnI: cardiac troponin I; eGFR, estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; NE: Neutrophil; NIHSS: NIH Stroke Score.
There was no significant difference in duration of hospital stay between patients with acute ischemic stroke, with or without elevated cTnI (P > 0.05, Table 6); the duration of hospitalization in the former group tended to increase, and the incidence of major cardiovascular events during hospitalization was significantly higher as compared to the latter group (P < 0.05, Table 6). To further investigate whether cTnI elevation was associated with short-term prognosis in patients with acute ischemic stroke, we further subdivided all patients with acute ischemic stroke (n = 176) into the in-hospital adverse event group (n = 13) and the non-in-hospital adverse event group (n = 163); logistic regression analysis on these two groups showed cTnI elevation was an independent risk factor for in-hospital major cardiovascular events in patients with acute ischemic stroke (B = 1.946, OR: 7.002; 95% CI: 1.372–35.728, P = 0.019).
Table 6. Comparison of duration of hospitalization and in-hospital adverse events.
| cTnI elevation (n = 31) | No cTnI elevation (n = 145) | |
| Duration of hospitalization, days | 22 (14, 31) | 18 (10, 25) |
| Adverse events duringhospitalization | 8 (25.8%) | 5 (3.4%)* |
Data were presented as mean (interquartile range) or n (%). *P < 0.05 indicated statistically significant differences. cTnI: cardiac troponin I.
4. Discussion
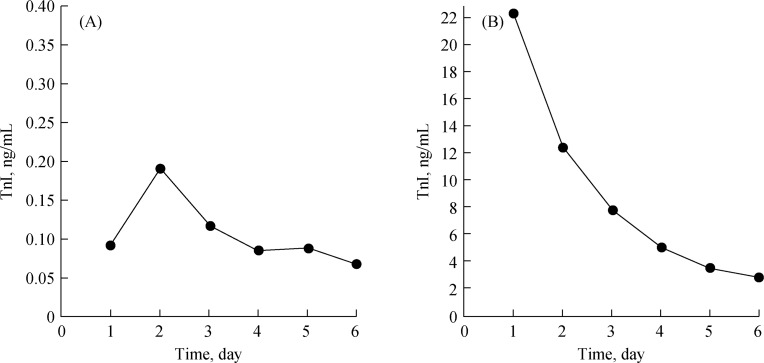
Previous studies have shown that cTnI is not only elevated in patients with myocardial injury but also is increased in patients with other diseases.[9]–[11] In the current study, our results demonstrated that cTnI could increase in patients with acute ischemic stroke (18.5% patients), but the level usually did not exceed 6-folds of the 99th percentile value of the normal reference range, whereas cTnI could be significantly increased in patients with myocardial infarction, exceeding 10- to 100-fold of the 99th percentile value of the normal reference range. Furthermore, we showed that, in patients with acute ischemic stroke, the level of cTnI reached its peak two days after disease onset and, thereafter, exhibited a slowly fluctuated decline; however, in patients with acute myocardial infarction, cTnI reached its peak 24 h after infarction onset, which was followed by a continuous decline (Figure 1). Patients with elevated cTnI were more likely to be older and to have greater severity of stroke, coagulation disorders, stronger inflammatory responses, and impaired renal or cardiac function.
Figure 1. Trend of cTnI changes in patients with (A) acute ischemic stroke and (B) acute myocardial infarction.
cTnI: cardiac troponin I.
In addition to cTnI, we studied the other markers of myocardial injury, CK-MB and myoglobin, in this research. We found that levels of CK-MB and myoglobin increased rapidly after the onset of NSTEMI and STEMI, and then declined gradually. However, the levels of CK-MB and myoglobin in patients with acute ischemic stroke fluctuated three to five days after disease onset and, sometimes, were higher than those patients with NSTEMI or STEMI. Some previous studies have found that levels of myocardial injury markers of patients with acute ischemic stroke manifested the most significant changes within five days,[5] which were consistent with our finding. Increased sympathetic activity secondary to insular cortical damage may be one of the factors leading to changes in cardiac enzyme levels.[6]–[7] Other studies have shown that patients with a normal cTn can have a high CK-MB level, indicating that CK-MB might not be a specific biochemical marker for cardiac myocytolysis.[6]–[8] Butcher, et al.,[6] reported that increased CK-MB levels in some patients with stroke may not be of cardiac origin.
Currently, the mechanism underlying cTnI elevation in patients with acute stroke has not been fully understood. The present study showed that cTnI elevation might be associated with increase in the percentage of neutrophils, indicating that patients with stroke who have relatively strong inflammatory responses are at an increased risk for myocardial injury.[14] This further suggested that non-ischemic factors, such as cytokine-mediated myocardial injury, catecholamine-mediated myocardial toxicity, microvascular spasm, and endothelial dysfunction may be associated with myocardial injury.[12] Some recent studies have demonstrated that the mechanism of pathogenesis underlying cTnI elevation might be similar to that in Takotsubo cardiomyopathy, caused by stress-mediated myocardial “supply and demand imbalance”.[13]
NIHSS scores are mainly used to assess neurological dysfunction in patients with stroke, and patients with higher scores, most often, experience a greater severity stroke. Some studies have shown that the NIHSS score is an independent risk factor for troponin elevation in patients with stroke.[15]–[17] In the present study, the average NIHSS scores of patients in the cTnI elevation group were 10 points (mostly moderate to severe stroke), which was significantly higher than in the normal group (4 points), and this was consistent with previous studies.
The decline in renal and cardiac function can, furthermore, contribute to the elevation of cTnI, which is mainly eliminated by the kidneys. Decreased eGFR can not only result in reduced cTnI clearance but also increase cardiac load and damage myocardial cells. Both of these changes would elevate cTnI level. Christopher et al.[18] found that cTnI elevation was closely associated with decline in eGFR. The same group also detected 1864 patients and also found that the level of cTnI was correlated with eGFR.[19] Manea reported cTnI elevations in patients with ischemic stroke and a history of ischemic heart disease. This high level was attributable to renal or cardiac insufficiency, rather than to myocardial infarction.[20] Our study showed that patients with acute ischemic stroke who had elevated cTnI were more likely to experience major cardiovascular events during hospital stay, which was consistent with previous reports.[21] Su, et al.,[17] conducted a retrospective study on 871 patients with acute ischemic stroke, and showed that elevated cTnI was an important predictive factor for in-hospital death and adverse outcomes in patients. Thålin, et al.[22] carried out a 5-year follow-up observation on 247 hospitalized patients with acute stroke, and found the 5-year risk of death in patients with elevated cTnI at admission to be increased by 1.9-fold. Although most patients with acute stroke and acute cTnI elevation have no clinical symptoms of acute coronary ischemia, mortality and morbidity rates in these patients are relatively high. Therefore, the 2013 American Heart Association and American Stroke Association Guideline recommend that all patients with acute ischemic stroke should undergo routine cTnI detection.[23]
Limitations of the current study include its small sample size, its design as a single-center study, and the lack of long-term follow-up.
In summary, cTnI elevation could occur in patients with acute ischemic stroke and is associated with poor short-term prognosis. Patients with elevated cTnI levels should be closely monitored and receive appropriate care to improve their prognosis.
Acknowledgments
This study was supported by the Beijing Science and Technology Major Project (No. D141100003014002), and the Capital Health Research and Development of Special (No.2016-2-4083). There are no conflicts of interest, including related consultancies, shareholdings, and grant funding, with regard to this study. We thank Hong-Xiao LI, Si-Qi WANG, and colleagues in Department of Cardiology and Neurology for their technical assistance during the study.
References
- 1.Mochmann HC, Jan F, Scheitz, Gabor C, et al. Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the troponin elevation in acute ischemic stroke (TRELAS) Study. Circulation. 2016;133:1264–1271. doi: 10.1161/CIRCULATIONAHA.115.018547. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Medical Association Neurogical Subsection Diagnostic key points of different cerebrovascular diseases. Chin Neuropsychiatric J. 1996;29:379. [Google Scholar]
- 3.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Muir KW, Weir CJ, Murray GD, et al. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27:1817–1820. doi: 10.1161/01.str.27.10.1817. [DOI] [PubMed] [Google Scholar]
- 5.Arboix Adrià, Alió Josefina. Acute Cardioembolic Cerebral Infarction: Answers to Clinical Questions. Curr Cardiol Rev. 2012;8:54–67. doi: 10.2174/157340312801215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher KS, Parsons MW. Cardiac enzyme elevations after stroke: the importance of specificity. Stroke. 2002;33:1944–1945. doi: 10.1161/01.str.0000023346.80463.a4. [DOI] [PubMed] [Google Scholar]
- 7.Manea MM, Comsa M, Minca A, et al. Brain-heart axis––review article. J Med Life. 2015;8:266–271. [PMC free article] [PubMed] [Google Scholar]
- 8.Ay H, Arsava EM, Saribaş O. Creatine kinase-MB elevation after stroke is not cardiac in origin: comparison with troponin T levels. Stroke. 2002;33:286–289. doi: 10.1161/hs0102.101544. [DOI] [PubMed] [Google Scholar]
- 9.Di Angelantonio E, Fiorelli M, Toni D, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:76–81. doi: 10.1136/jnnp.2004.041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghali J, Allison D, Kleinig T, et al. Elevated serum concentrations of troponin T in acute stroke: what do they mean? J Clin Neurosci. 2010;17:69–73. doi: 10.1016/j.jocn.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Lasek-Bal A, Kowalewska-Twardela T, Gasior Z, et al. The significance of troponin elevation for the clinical course and outcome of first-ever ischaemic stroke. Cerebrovasc Dis. 2014;38:212–218. doi: 10.1159/000365839. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JK, Atar D, Mickley H. Mechanism of troponin elevations in patients with acute ischemic stroke. Am J Cardiol. 2007;99:867–870. doi: 10.1016/j.amjcard.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Batal O, Jentzer J, Balaney B, et al. The prognostic significance of troponin I elevation in acute ischemic stroke. J Crit Care. 2016;31:41–47. doi: 10.1016/j.jcrc.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Haumer M, Amighi J, Exner M, et al. Association of neutrophils and future cardiovascular events in patients with peripheral artery disease. J Vasc Surg. 2005;41:610–617. doi: 10.1016/j.jvs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Chalela JA, Ezzeddine MA, Davis L, et al. Myocardial injury in acute stroke: a troponin I study. Neurocrit Care. 2004;1:343–346. doi: 10.1385/NCC:1:3:343. [DOI] [PubMed] [Google Scholar]
- 16.Abdi S, Oveis-Gharan S, Sinaei F, et al. Elevated troponin T after acute ischemic stroke: Association with severity and location of infarction. Iran J Neurol. 2015;14:35–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Su YC, Huang KF, Yang FY, et al. Elevation of troponin I in acute ischemic stroke. Peer J. 2016;4:e1866. doi: 10.7717/peerj.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.deFilippi C, Seliger SL, Kelley W, et al. Interpreting Cardiac Troponin Results from High-Sensitivity Assays in Chronic Kidney Disease without Acute Coronary Syndrome. Clin Chem. 2012;58: 9:1342–1351. doi: 10.1373/clinchem.2012.185322. [DOI] [PubMed] [Google Scholar]
- 19.Cardinaels EP, Altintas S, Versteylen Mo, et al. High-sensitivity cardiac tropon in concentrations in patients with chest discomfort: is it the heart or the kidneys as well? PLoS One. 2016;11:e0153300. doi: 10.1371/journal.pone.0153300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32:404–411. doi: 10.1093/eurheartj/ehq456. [DOI] [PubMed] [Google Scholar]
- 21.Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009;28:220–226. doi: 10.1159/000226773. [DOI] [PubMed] [Google Scholar]
- 22.Thålin C, Rudberg AS, Johansson F, et al. J Stroke Cerebrovasc Dis. 2015;24:2390–2396. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 23.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]