Abstract
Angiogenesis plays an important role in neovascularization in tumors. Glycodelin, a hormone-responsive protein, has been detected in tumors of reproductive organs and is found in high levels in the plasma of subjects with gynecological malignancies. Glycodelin is also found in the endothelial cells of the umbilical cord and in the blood vessels of tumors. In this study, we tested whether glycodelin-rich amniotic fluid and a synthetic peptide derived from the sequence of glycodelin peptide (Gp) might promote angiogenic response by examining the migration and tube formation in human umbilical cord vein endothelial cells (HUVECs). Increased migration and tube formation of HUVECs were found in the presence of amniotic fluid and Gp, and this increase was blocked by antibody to Gp and by an anti-vascular endothelial growth factor (VEGF) antibody, suggesting that the angiogenic effects of glycodelin might be mediated by VEGF. The results also showed that Gp significantly increased the release of VEGF protein and mRNA expression in HUVECs, RL-95 (human endometrial carcinoma cells), OVCAR-3 (human ovarian adenocarcinoma cells), EM42 (human endometrial epithelial cells), THP-1 (human monocyte), and MCF-7 and MDA-MB-231 (human breast adenocarcinoma cells) cell lines. VEGF receptor Fit-1 mRNA expression in HUVECs was also increased in the presence of Gp. These findings, together with the suggestion from the literature that glycodelin may have immunosuppressive properties, suggest that glycodelin might play an important role in neovascularization during embryogenesis and tumor development.
Angiogenesis is the process of new blood vessel formation from preexisting vessels and is a fundamental requirement for embryogenesis, wound healing, and reproductive functions in the adult; it is also involved in the pathogenesis of tumor progression (1). Studies have shown that without angiogenesis, solid tumor would grow slowly, and the degree of neovascularization within the primary tumor is related to the prognostic significance of some malignant tumors (2–4).
Vascular endothelial growth factor (VEGF) is a potent angiogenic factor that might play a major role in the proliferation and migration of endothelial cells and neovascularization (4). VEGF can be secreted by cells of tumors of lung, thyroid, breast, gastrointestinal tract, ovary, and others (5–9), and might induce neovascularization through at least two tyrosine kinase receptors, Flt-1 and Flk-1, on endothelial cells (10–12). Granulocyte-macrophage colony-stimulating factor, interleukin-5, and other cytokines have been reported to induce the expression of VEGF (13, 14).
Glycodelin is a 28-kDa glycoprotein that has been considered to be specific for the reproductive tract and can be synthesized by the endometrial tissue and the decidua during pregnancy (14, 15). Serum levels of glycodelin are elevated in the late secretory phase and in the first trimester of pregnancy (16). The physiological role of glycodelin is not well known, but in vitro studies have shown that it can inhibit natural killer cell activity (17–19). Elevated levels of glycodelin also are seen in the plasma of patients with ovarian and uterine malignancies (20). Thus, there is abundant evidence to support the view that glycodelin is associated with rapidly growing tissue.
We recently demonstrated the presence of glycodelin in the endothelial cells of both the umbilical cord and the artery, and also observed that human umbilical cord vein endothelial cells (HUVECs) could accumulate glycodelin when incubated with glycodelin in vitro (21, 22). Our immunohistochemical studies on gynecological tumor tissues also showed increased glycodelin expression and vascularization in tumor cells compared with normal tissue. More importantly, the endothelium of the tumor blood vessels was predominantly stained with an antibody that was raised against peptide that derived from the sequence of glycodelin peptide (Gp) (20). Based on these results, we considered that the presence of glycodelin in endothelial cells of blood vessels might suggest an angiogenic role for glycodelin.
We studied angiogenesis activity of glycodelin in HUVECs by using Gp and glycodelin-rich amniotic fluid (AF) of the second trimester. We also used ELISA, Western blot, and reverse transcription (RT)-PCR analysis methods to examine whether Gp can induce VEGF and its receptor Flt-1 expression in several cell lines.
Materials and Methods
Materials.
Gp (H2N-YKKVLGEKTENPKKFK-COOH) was synthesized by the Microchemical Facility of Emory University, and an antibody to Gp was generated in chicken as described (23). Anti-von Willebrand factor antibody was purchased from Dako. Monoclonal anti-human VEGF antibody, chicken IgG, mouse anti-human gastrin I antibody, secondary antibodies, and substrates were purchased from Sigma.
Cell Lines.
Cell lines used in this study were obtained from American Type Culture Collection. Primary HUVECs were isolated and cultured (21) for studying angiogenic activity of Gp. HUVECs, RL-95 (human endometrial carcinoma cells), OVCAR-3 (human ovarian adenocarcinoma cells), EM42 (human endometrial epithelial cells), MCF-7 and MDA-MB-231 (human breast adenocarcinoma cells), and THP-1 (human monocyte) cells were used to study the induction of VEGF and its receptor expression.
Tissue Preparation and Immunohistochemistry.
Human umbilical cords and tumor samples were collected after normal delivery or after surgical procedures. Patient consent was obtained, and the protocol for the collection of samples was approved by the Emory University Human Investigation Committee. The samples were transferred to the laboratory on ice, washed with PBS, fixed with formal sucrose [4% (wt/vol) paraformaldehyde/7.5% (wt/vol) sucrose/20 μM butylated hydroxytoluene/2 mM EDTA, pH 7.4], and embedded in paraffin. Tissue sections were incubated for 2 hr with a 1:400 dilution of chicken anti-Gp antibody and a 1:200 dilution of an antibody to von Willebrand factor in PBS containing 3% (wt/vol) BSA. For negative control, the primary antibody was omitted. After washing, tissue sections were incubated for 2 hr with secondary antibody conjugated with alkaline phosphatase. Rabbit anti-chicken IgG (1:200 dilution) for anti-Gp antibody and goat anti-rabbit IgG (1:200 dilution) for anti-von Willebrand factor antibody were used. Tissue sections were washed with PBS three times, and fast red was added as chromogen in conjunction with alkaline phosphatase-substrate naphthol phosphate. Positive immunostaining that appeared as a pink color was visualized under a microscope, and the results were analyzed.
Migration Assay.
Migration assay was done by using 12-well Transwell culture plate inserts with polycarbonate filters (Corning; ref. 24). In this setup, cultured HUVECs were starved 2 hr in M199 medium (Mediatech, Herndon, VA) containing 0.1% fatty-acid-free BSA, then trypsinized and suspended at a concentration of 5 × 104 cells per well in the same medium. Cells were placed in the upper chambers with 12-μm pore polycarbonate filters (Corning), and the same medium containing the reagents (Gp or AF in the presence or absence of specific antibodies) was added to the lower chamber. The cells incubated in medium alone were used as control. The chambers were incubated at 37°C under 5% CO2 in air for 2 hr. After incubation, cells on the upper side of the filter were washed off. The cells that had migrated to the lower side of the filter were fixed in methanol, stained with Diff-Quick staining solution (Behring), and counted under a microscope for quantification of cell migration. Migration was calculated as the average number of cells observed in five random high-power (×400) fields per well in triplicate wells.
Collagen Tube Formation.
Tube formation assay was done according to a modified Wang's method (24). Three-dimensional collagen gel plates (12 well) were prepared by adding 0.8 ml of chilled rat tail collagen into each well and adjusted to neutral pH with NaHCO3. Collagen was allowed to solidify at 37°C for 30 min; 1 × 105 cells per well HUVECs were plated on collagen gel in M199 medium containing 15% (vol/vol) FBS and endothelial cell growth factor (EGF). When the cells were confluent, the medium was replaced by M199 medium [15% (vol/vol) FBS but without EGF] containing the various compounds of our interest (Gp or AF in the presence or absence of specific antibodies). Wells were monitored and pictures were taken at 48 hr after the addition of reagents.
ELISA of VEGF Expression.
RL-95, OVCAR-3, EM42, MCF-7, MDA-MB-231, and THP-1 cells were cultured in T-75 flasks by using 10 ml of medium containing penicillin and streptomycin and were incubated at 37°C until 80% confluence. The culture medium was removed, and fresh medium with 0.1% FBS was added for 24 hr; then the cells were incubated with 50 ng/ml Gp in medium containing 0.1% FBS for 24 hr. The cells cultured in medium only were used as control. The medium was taken out for ELISA, and the cells were used for Western blot analysis.
Wells in 96-well ELISA plates were filled with 100 μl of culture media. Medium incubated in the absence of cells served as negative control. All of the sets were done in triplicate, and the plates were incubated overnight at 37°C. The wells then were washed three times with PBS and blocked with 1% BSA for 2 hr at 37°C. Mouse monoclonal anti-human VEGF antibody at a dilution of 1 μg/ml in PBS containing 1% BSA was added and incubated for 2 hr at 37°C. Wells were again washed three times with PBS. The secondary antibody (goat anti-mouse IgG conjugated with alkaline phosphatase) was added at a dilution of 1:30,000 in PBS containing 1% BSA and incubated for 2 hr at 37°C. The wells were washed again with PBS, and p-nitrophenyl phosphate was added as the substrate. Color development was read in an ELISA plate reader (Lab-Tek). The optical density of each well was read at 405 nm, and the mean of the triplicates was determined.
Western Blot Analysis of VEGF Expression.
VEGF expression in the cells induced by Gp was determined by Western blot analysis. Proteins were separated in a 12% polyacrylamide gel and transferred onto nitrocellulose membranes. Western blot analyses were performed with the use of mouse monoclonal anti-human VEGF antibody at a dilution of 2 μg/ml. The membrane was then incubated with the secondary antibody goat anti-mouse IgG conjugated to horseradish peroxidase (dilution of 1:3,000; Sigma). The protein was detected with the ECL (enhanced chemiluminescence) detection system (RPN 2106, Amersham Pharmacia). The chemiluminescence signal was detected by a short exposure of the membrane to autoradiography film (RPN 2114H, Hyperfilm ECL, Amersham Pharmacia).
RT-PCR Analysis for VEGF.
Total RNA was isolated from the cells by using Tri reagent (Sigma). RNA concentrations were determined spectrophotometrically by absorption at 260 nm. The RT-PCR was done by using the method of Shen et al. (25). Briefly, 4 μg of RNA was reverse transcribed, and nested PCR was carried out by using 2 μl of cDNA and the following primers: (i) 5′-GCTACTGCCATCCAATCGAGACC-3′ (exon 3, forward); (ii) 5′-GTTTCTGGATTAAGGACTGTTCTGTCG-3′ (exon 8, reverse); and (iii) 5′-AATCCAATTCCAAGAGGGACCGTGC-3′ (exon 8, reverse). First-round amplification was carried out by using primers i and iii for 15 cycles. Then 2 μl of the first-round PCR products was used for second-round amplification with the nested primers i and ii. Amplification was for 30 cycles under the same conditions as for the first-round amplification. The PCR condition consisted of denaturation for 1 min at 94°C, annealing for 2 min at 62°C, and extension for 3 min at 72°C. RT-PCR product was analyzed on 2% agarose gel stained by ethidium bromide. The gene expression levels were standardized by using glyceraldehyde-3-phosphate dehydrogenase gene expression. By using the primers described, the expected PCR products for each VEGF variant were calculated as 440 bp, 572 bp, 644 bp, and 695 bp for isoforms of 121, 165, 189, and 206, respectively. The primers used for Flt-1 were 5′-GATGTTGAGGAAGAGGAGGATT-3′ (forward) and 5′-AAGCTAGTTTCCTGGGGGTATA-3′ (reverse) (26). The PCR conditions consisted of 30 cycles of denaturation for 1 min at 94°C, annealing for 2 min at 64°C, and extension for 3 min at 72°C; PCR product was analyzed on a 2% agarose gel stained by ethidium bromide.
Statistical Analysis.
Results are expressed as mean ± SD. The difference between two groups was tested for significance by using the Student two-tailed t test.
Results
Immunohistochemical Detection of Glycodelin in Tissues.
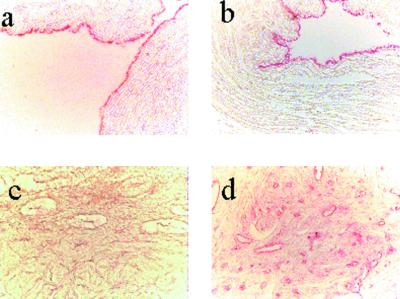
Human umbilical cords collected after full-term delivery and the gynecological tumor tissues were obtained and immunostained with an anti-Gp antibody. Human umbilical cord vein and artery endothelial cells were positively immunostained with anti-Gp antibody (Fig. 1a). These cells were confirmed as endothelial cells by positive immunostaining with anti-von Willebrand factor (Fig. 1b).
Figure 1.
(a) Immunohistochemistry with anti-Gp antibody in human umbilical cord vein, and (b) with anti-von Willebrand factor in human umbilical cord vein. (c and d) Immunohistochemistry with anti-Gp antibody in normal and adenocarcinoma endometrium tissue. In c, no obvious staining in endothelial cells of the blood vessels was seen, but in d, intense pink staining in endothelial cells of the blood vessels could be seen in endometrium adenocarcinoma tissue.
Next, we compared the expression of glycodelin in endometrial samples from normal and endometrial cancer subjects. There was a very low level of immunoreactivity in normal endometrial tissues (Fig. 1c), but the endometrial cancer tissues were strongly immunostained with anti-Gp antibody (Fig. 1d). Intense immunostaining could be seen in the blood vessels of the tumor tissues. This immunoreactivity in blood vessels was colocalized with immunostaining with anti-von Willebrand factor antibody, confirming the presence of glycodelin in endothelial cells of blood vessels (data not shown). A similar expression of glycodelin also was observed in samples of ovarian, uterine, cervical, and prostate cancers, and in benign leiomyoma (20).
Effect of Gp on Migration of HUVECs.
The migration of endothelial cells is an important process during angiogenesis. A significant increase in the migration of HUVECs was seen in the presence of 50 ng/ml Gp (Fig. 2A, bar 2, 31.6 ± 5.5, P < 0.01; Fig. 2Bii) and 10 μl/ml AF (Fig. 2A, bar 7, 22.0 ± 5.3, P < 0.05; Fig. 2Biii) as compared with the control cells (Fig. 2A, bar 1, 14.0 ± 2.4; Fig. 2Bi). When anti-Gp antibody (10 μg/ml) was added along with the Gp (Fig. 2A, bar 3, 12.8 ± 3.6, P < 0.01; Fig. 2Biv), the migration of HUVECs was inhibited as compared with the cells that migrated in the presence Gp alone (Fig. 2A, bar 2; Fig. 2Bii). Similarly, the cell number decreased when HUVECs were exposed to AF plus anti-Gp antibody (Fig. 2A, bar 8, 10.4 ± 3.8, P < 0.01) compared with cells exposed to AF only (Fig. 2A, bar 7; Fig. 2Biii). The presence of nonspecific IgG, anti-gastrin antibody, and chicken IgG together with Gp had no significant effect on cell migration induced by Gp (Fig. 2A, bars 5 and 6, respectively; 41.6 ± 11.3 and 30.8 ± 7.6, respectively; P > 0.05). Interestingly, when cells were exposed to Gp plus anti-VEGF antibody (1 μg/ml) (Fig. 2A, bar 4, 13.2 ± 2.3; P < 0.01), a significant decrease in the migration of HUVECs was observed compared with the cells exposed to Gp alone (Fig. 2A, bar 2; Fig. 2Bii), suggesting that the effect of Gp on the migration of HUVECs could be mediated by VEGF.
Figure 2.
(A) Effect of Gp on migration of HUVECs. HUVECs were incubated with medium only (bar 1), or with Gp (bar 2), Gp plus anti-Gp antibody (bar 3), Gp plus anti-VEGF antibody (bar 4), Gp plus anti-gastrin I antibody (bar 5), Gp plus chicken IgG (bar 6), AF (bar 7), and AF plus anti-Gp antibody (bar 8). The number of migrating cells was counted under a phase-contrast light microscope at magnification = ×400. Results are presented as mean ± SD of each group. **, P < 0.01 vs. control; $, P < 0.01 vs. Gp; @, P > 0.05 vs. Gp; *, P < 0.05 vs. control; #, P < 0.01 vs. AF. (B) Staining of migrating cells with Diff-Quick staining solution. (i) HUVECs (control). (ii) HUVECs exposed to Gp. (iii) HUVECs exposed to AF. (iv) HUVECs exposed to Gp plus anti-Gp antibody. More cell migration was observed in cells treated with Gp and AF. (Magnification = ×200.)
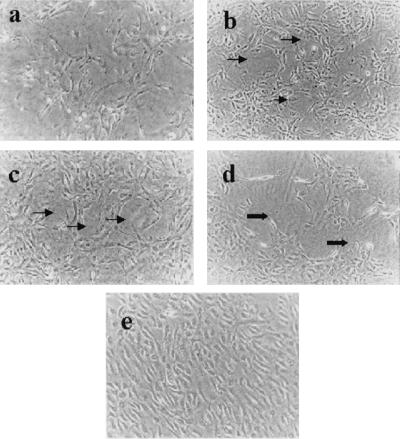
Effect of Gp on HUVECs Tube Formation in Collagen Gels.
An in vitro tube formation assay was performed to assess whether Gp could provide an angiogenic stimulus. HUVECs formed elongated tube-like structures within 24–48 hr when grown on a collagen matrix, and the lumen was composed of several endothelial cells linked to each other. Tube formation was increased in response to Gp (Fig. 3b) and AF (Fig. 3c) compared with the control cells (Fig. 3a). While in the presence of Gp plus anti-Gp antibody, tube formation was less, and some tubes were broken (Fig. 3d). In addition, when cells were exposed to Gp plus anti-VEGF antibody, the tube formation of HUVECs was decreased compared with the cells exposed to Gp alone (Fig. 3e). These findings demonstrate that Gp can promote an endothelial angiogenic response in vitro.
Figure 3.
Tube formation of HUVECs in collagen gels. (a) Control. (b) HUVECs exposed to Gp. (c) HUVECs exposed to AF. (d) HUVECs exposed to Gp plus anti-Gp antibody. (e) HUVECs exposed to Gp plus anti-VEGF antibody. Increased tube formation (thin arrows, b and c) was observed in HUVECs exposed to Gp and AF when compared with untreated cells. Compared with Gp alone, HUVECs exposed to Gp plus the antibodies to Gp or VEGF showed broken tube formation (thick arrows, d) or less tube formation (e), respectively. (Magnification = ×100.)
ELISA for VEGF Expression.
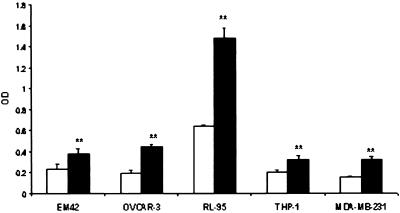
Our results indicate that an antibody to VEGF could block migration and tube formation of HUVECs induced by Gp, suggesting that the action of Gp might be mediated by VEGF. To test whether Gp could induce VEGF synthesis, we determined the levels of VEGF in the media of cells cultured with or without Gp (50 ng/ml). When compared with untreated cells, supernatant from several cell lines cultured with Gp showed increased VEGF levels (Fig. 4; P < 0.01).
Figure 4.
Effect of Gp on VEGF level in different cell culture media by ELISA analysis. C, control (white bars); GP, cultured with 50 ng/ml Gp (black bars). **, P < 0.01 vs. control.
Western Blot Analysis for VEGF Expression.
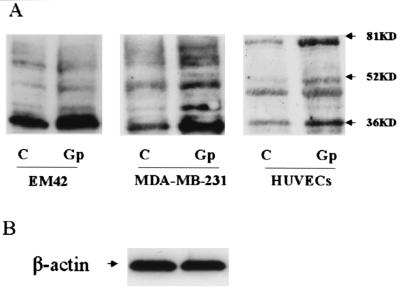
Similarly, we also did a Western blot analysis to check whether Gp could induce VEGF protein expression in the cell lines. By using an antibody against VEGF, four bands between 36 and 80 kDa were detected. The predominant band was ≈36 kDa. The intensity of each band in the cells incubated with Gp was increased compared with untreated cells (Fig. 5A). An equal amount of protein loading was justified by analyzing actin expression (Fig. 5B).
Figure 5.
(A) Western blot analysis of VEGF expression in EM42, MDA-MB-231, and HUVECs cells. C, control; Gp, cells cultured in presence of 50 ng/ml Gp. (B) β-Actin was used as internal control.
RT-PCR Analysis.
RT-PCR was used to assess the differential expression of VEGF mRNA-splice isoforms in cells cultured with or without Gp. Four bands of 440, 572, 644, and 695 bp corresponding to VEGF isoforms of 121, 165, 189, and 206 amino acids, respectively, were expected. In addition to these four bands, another band of about 500 bp was observed. In untreated RL-95, OVCAR-3, THP-1, MCF-7, and MDA-MB-231 cells, there were predominantly three bands of 440, 500, and 572 bp, whereas 644- and 695-bp isoforms were lacking or faint. After the cells were cultured with Gp, the bands of 644 and 695 bp could be seen clearly, and the intensity of each band was increased (Fig. 6A).
Figure 6.
(A) RT-PCR analysis of VEGF expression in the cells cultured with or without Gp. The 440-, 572-, 644-, and 695-bp products correspond to VEGF121, VEGF165, VEGF189, and VEGF206 isoforms, respectively; the 500-bp product may correspond to the VEGF145 isoform. (B) Flt-1 expression in HUVECs cultured with or without Gp. M, 100-bp DNA marker; C, control; Gp, cells cultured in presence of 50 ng/ml Gp. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as internal control in A and B.
As VEGF induces neovascularization through receptors Flt-1 and Flk-1 on endothelial cells, it is of interest to know whether Gp can induce Flt-1 expression in HUVECs. Results also showed Flt-1 mRNA in HUVECs was increased in the cells cultured with Gp, as compared with controls (Fig. 6B). Glyceraldehyde-3-phosphate dehydrogenase mRNA was used as the internal control to indicate that an equal amount of RNA was amplified (Fig. 6).
Discussion
Glycodelin, also known as placental protein (PP14), was identified immunochemically in extracts of early human placenta (27, 28). A potential role for glycodelin in embryonic implantation has been confirmed as facilitating evasion of the maternal immune response (17–19). The biological function of glycodelin in tumor has not been clarified yet. There is speculation that glycodelin may modulate the reaction of the immune system of the patient toward the malignant tumor (28). Both our findings (20) and those of others (14, 29) have demonstrated that glycodelin expression is increased in tumor tissues. Glycodelin is expressed mainly in tumor cells and endothelial cells of small blood vessels nearby, which could suggest that the presence of glycodelin might be related to neovascularization in tumor tissues.
Migration of endothelial cells and formation of new capillary tubes are two of the required events in the angiogenic response. Our results showed that both AF and Gp increased the migration and tube formation of HUVECs. The antibodies against Gp and VEGF decreased the effects of both AF and Gp, which suggests that glycodelin could induce angiogenesis in vitro and might be mediated through VEGF.
We considered the possibility that the angiogenic effect of the AF could be caused by the presence of VEGF. VEGF is expressed in the placenta and in several fetal tissues. Levels of glycodelin in early second trimester AF used in the current study are much higher than those in a full-term pregnancy (20). However, levels of VEGF both in the early and term AF are less than the detection limit of assays, which is 16 ng/liter (30). VEGF plays a key role in angiogenesis. Several studies have demonstrated that VEGF is up-regulated in various human malignancies. A variety of solid tumors also are known to secrete VEGF as an important mediator of angiogenesis to support their growth in vivo (5–10). Because glycodelin expression is also up-regulated in tumor cells and some infiltrating cells, the question of whether glycodelin can induce VEGF expression is an interesting one.
Results from the present studies demonstrate that glycodelin could induce VEGF expression at both protein and mRNA levels in HUVECs, RL-95, OVCAR-3, EM42, MCF-7, MDA-MB-231, and THP-1 cells. VEGF protein and mRNA were constitutively expressed in these cell lines. By ELISA, we observed also that Gp increased the VEGF protein level in cell culture medium significantly. VEGF was reported to be 34- to 50-kDa proteins (31, 32), although additional anti-VEGF reactive proteins of ≈60 and 90 kDa or 90 and 110 kDa also were reported (33, 34). We found that VEGF proteins in the cell lines were detected as four obvious bands between 36 and 80 kDa, which probably represent different isoforms of VEGF. The predominant band was ≈36 kDa. The intensity of each band in the cells incubated with Gp was increased compared with untreated cells.
Four isoforms of VEGF usually reported are VEGF121, VEGF165, VEGF189, and VEGF206. VEGF145 has been detected as a rare VEGF mRNA species in placenta (35, 36) and in several tumorigenic cell types that originated from the female reproductive system (37). VEGF121, VEGF145, and VEGF165 are secreted in the medium by cells, whereas VEGF189 and VEGF206 are sequestered by cell-surface heparin sulfates (38). By RT-PCR technique, we also found another band of ≈500-bp length, bigger than VEGF121 mRNA and smaller than VEGF165 mRNA. Because those cells are all from the female reproductive system or related to reproduction, we predict that the band represents the mRNA of VEGF145 (37). The results showed that in untreated RL-95, OVCAR-3, THP-1, MCF-7, and MDA-MB-231 cells there were predominantly three bands of 440, 500, and 572 bp. Isoforms of 644 and 695 bp were lacking or faint. After the cells were cultured with Gp, the bands of 644 and 695 bp could be seen clearly, and the intensity of each band was increased, which indicates that VEGF121, VEGF145, and VEGF165 are constitutively expressed in these cell lines. Gp increased the expression of the three isoforms, but the most important finding is the ability to induce VEGF189 and VEGF206 expression in the cell lines.
Not all VEGF isoforms are present in all cells; some cell types could produce several VEGF forms simultaneously (39, 40). It has been reported that each form of VEGF offers advantages in different situations and, therefore, the simultaneous production of several different forms may ensure a balanced angiogenic response under diverse circumstances (37). The result of Gp inducing the expression of all isoforms in the cancer cell lines can make tumor cells have strong angiogenic activity.
VEGF is thought to play an angiogenic role by binding to Flt-1 and Flk/KDR, two plasma membrane tyrosine kinase VEGF receptors, predominantly expressed on endothelial cells (11). Our results also showed Flt-1 mRNA expression increased significantly in HUVECs after incubation with Gp. Angiogenesis is a fundamental action in many physiological and pathological processes. Gp can induce angiogenesis not only by enhancing migration and tube formation of endothelial cells directly, but also by increasing Flt-1 in endothelial cells and increasing VEGF production in VEGF-producing cells as a paracrine factor to induce angiogenesis. This activity may be another important function of glycodelin in tumor development and embryogenesis.
In conclusion, this report shows that Gp can induce angiogenesis in vitro. Our results indicate that Gp can induce VEGF expression in some cell lines and VEGF receptor Flt-1 in HUVECs. Glycodelin usually has been considered to play an immunosuppressive function in embryo implantation and tumor development (18, 19). Our results suggest that glycodelin also might play an important role in embryogenesis and tumor development by inducing angiogenesis. These results also raise the possibility of manipulating either desirable or undesirable angiogenesis by using Gp or controlling glycodelin expression.
Acknowledgments
We thank Dr. Denise Raynor for providing the umbilical cords used in this study. This study was supported by Grant 5POIHD35276-02 from the National Institute of Child and Human Development, and by the Vesa W. & William J. Hardman, Jr., Charitable Foundation, Inc.
Abbreviations
- VEGF
vascular endothelium-derived growth factor
- HUVECs
human umbilical cord vein endothelial cells
- Gp
glycodelin-derived peptide
- AF
amniotic fluid
- EGF
endothelial cell growth factor
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ferrara N, Davis-Smyth T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Cancer Res. 1986;46:467–473. [PubMed] [Google Scholar]
- 3.Weidner N, Semple J P, Welch W R, Folkman J. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 5.Mattern J, Koomagi R, Volm M. Br J Cancer. 1996;73:931–934. doi: 10.1038/bjc.1996.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viglietto G, Maglione D, Rambaldi M, Cerutti J, Romano A, Trapasso F, Fedele M, Ippolito P, Chiappetta G, Botti G, et al. Oncogene. 1995;11:1569–1579. [PubMed] [Google Scholar]
- 7.Yoshiji H, Gomez D E, Shibuya M, Thorgeirsson U P. Cancer Res. 1996;6:2013–2016. [PubMed] [Google Scholar]
- 8.Brown L F, Berse B, Jackman R W, Tognazzi K, Manseau E J, Senger D R, Dvorak H F. Cancer Res. 1993;53:4727–4753. [PubMed] [Google Scholar]
- 9.Olson T A, Mohanraj D, Carson L F, Ramakrishnan S. Cancer Res. 1994;54:276–280. [PubMed] [Google Scholar]
- 10.Ferrara N, Houck K, Jakeman L, Leung D W. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 11.de Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 12.Terman B I, Dougher-Vermazen M, Carrion M E, Dimitrov D, Armellino D C, Gospodarowicz D, Bohlen P. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi T, Weller P F. Am J Respir Cell Mol Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- 14.Kamarainen M, Miettinen M, Seppala M, Von Boguslawsky K, Benassi M S, Bohling T, Andersson L C. Int J Cancer. 1998;76:487–490. doi: 10.1002/(sici)1097-0215(19980518)76:4<487::aid-ijc7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Fazleabas A T, Donnelly K M, Hild-Petito S, Hausermann H M, Verhage H G. Hum Reprod Update. 1997;3:553–559. doi: 10.1093/humupd/3.6.553. [DOI] [PubMed] [Google Scholar]
- 16.Lalitkumar P G, Sengupta J, Karande A A, Ghosh D. Hum Reprod. 1998;13:3478–3486. doi: 10.1093/humrep/13.12.3478. [DOI] [PubMed] [Google Scholar]
- 17.Morrow D M, Xiong N, Getty R R, Ratajczak M Z, Morgan D, Seppala M. Am J Pathol. 1994;145:1485–1495. [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto N, Uchida A, Takakura K, Kariya Y, Kanzaki H, Rittinen L, Koistinen R, Seppala M, Mori T. Am J Reprod Immunol. 1991;26:137–142. doi: 10.1111/j.1600-0897.1991.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 19.Rachmilewitz J, Riely G J, Tykocinski M L. Cell Immunol. 1999;191:126–113. doi: 10.1006/cimm.1998.1408. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz, I. R., Cho, C. H., Song, M. Q., Flowers, L. C., Santanam, N., Parthasarathy, S. & Ramachandran, S. (2001) Int. J. Gynecol. Cancer, in press. [DOI] [PubMed]
- 21.Zhou H M, Ramachandran S, Kim J G, Raynor D B, Rock J A, Parthasarathy S. Fertil Steril. 2000;73:843–847. doi: 10.1016/s0015-0282(99)00600-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim J G, Ramachandran S, Zhou H M, Rayner D B, Parthasarathy S. Fertil Steril. 2000;73:839–842. doi: 10.1016/s0015-0282(99)00599-3. [DOI] [PubMed] [Google Scholar]
- 23.Poddar A S, Kim J G, Gill K P, Bates B N, Santanam N, Rock J A, Murphy A A, Parthasarathy S. Fertil Steril. 1998;69:543–548. doi: 10.1016/s0015-0282(97)00558-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Van Brocklyn J R, Hobson J P, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 25.Shen G H, Ghazizadeh M, Kawanami O, Shimizu H, Jin E, Araki T, Sugisaki Y. Br J Cancer. 2000;83:196–203. doi: 10.1054/bjoc.2000.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E. J Pathol. 2000;191:426–433. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Seppala M, Koistinen H, Koistinen R, Dell A, Morris H R, Oehninger S, Clark G F. Clin Endocrinol (Oxford) 1997;46:381–386. doi: 10.1046/j.1365-2265.1997.1510943.x. [DOI] [PubMed] [Google Scholar]
- 28.Seppala M, Bohn H, Tatarinov Y. Tumour Biol. 1998;19:213–220. doi: 10.1159/000030009. [DOI] [PubMed] [Google Scholar]
- 29.Chatzaki E, Gallagher C J, Iles R K, Ind T E, Nouri A M, Bax C M, Grudzinskas J G. Br J Cancer. 1994;69:1010–1014. doi: 10.1038/bjc.1994.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuorela-Vepsalainen P, Alfthan H, Orpana A, Alitalo K, Stenman U H, Halmesmaki E. Hum Reprod. 1999;14:1346–1351. doi: 10.1093/humrep/14.5.1346. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima T, Hudson M, Claymon G L. Head Neck. 2000;22:483–488. doi: 10.1002/1097-0347(200008)22:5<483::aid-hed7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Henzel W J. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 33.Pammer J, Weninger W, Mildner M, Burian M, Wojta J, Tschachler E. J Pathol. 1998;186:186–191. doi: 10.1002/(SICI)1096-9896(1998100)186:2<186::AID-PATH148>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 34.Jackson M W, Bentel J M, Tilley W D. J Urol. 1997;157:2323–2328. [PubMed] [Google Scholar]
- 35.Charnock-Jones S D, Sharkey A M, Rajput-Williams J, Burch D, Schofield J P, Fountain S A, Boocock C, Smith S K. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- 36.Cheung C Y, Singh M, Ebaugh M J, Brace R A. Am J Obstet Gynecol. 1995;173:753–759. doi: 10.1016/0002-9378(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak X, Cohen T, Sivan R, Kandies T, Ohnishi Y, Tokunaga T, Tomii Y, Kijima H, Yamazaki G. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- 38.Houck K A, Leung D W, Rowland A M, Winer J, Ferrara N. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 39.Neufeld G, Cohen T, Gitay-Goren H, Poltorak Z, Tessler S, Gengrinovitch S, Levi B. Cancer Metastasis Rev. 1996;15:153–158. doi: 10.1007/BF00437467. [DOI] [PubMed] [Google Scholar]
- 40.Bacic M, Edwards N A, Merrill M J. Growth Factors. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]