Abstract
Purpose
The current live attenuated rabies vaccine must be replaced with a safer vaccine based on the ERAGS strain to prevent rabies in South Korea. We evaluated the safety and immunogenicity of a new strain in dogs and cattle.
Materials and Methods
The ERAGS strain, featuring two mutations altering two amino acids in a glycoprotein of rabies virus, was propagated in NG108-15 cells. We lyophilized the virus in the presence of two different stabilizers to evaluate the utilities of such preparations as novel rabies vaccines for animals. To explore safety and immunogenicity, dogs and cattle were inoculated with the vaccine at various doses via different routes and observed daily for 8 weeks post-inoculation (WPI). Immunogenicity was evaluated using a fluorescent antibody virus neutralization test or enzyme-linked immunosorbent assay.
Results
The two different stabilizers did not differ greatly in terms of maintenance of virus viability in accelerated stability testing. No clinical signs of rabies developed in dogs or cattle inoculated with the vaccines (107.0 FAID50/mL). Dogs and cattle inoculated intramuscularly with 105.0 FAID50/mL exhibited virus neutralization assay titers of 4.6 IU/mL and 1.5 to 0.87 IU/mL at 4 WPI, respectively. All control animals remained rabies virus–seronegative throughout, confirming that no contact transmission occurred between vaccinated and control animals.
Conclusion
Our findings indicate that the new rabies vaccine is safe and immunogenic in dogs and cattle.
Keywords: Rabies, Vaccines, Dogs, Cattle
Introduction
Rabies is a fatal disease caused by rabies virus (RABV) of the family Rhabdoviridae and the genus Lyssavirus. RABV kills warm-blooded animals including dogs and cattle. Pets and domestic animals, particularly dogs, are at increased risk if they live in regions populated by raccoon dogs (Nyctereutes procyonoises koreensis), which is a known reservoir of RABV and has played a key role in rabies transmission in South Korea [1,2]. A total of 486 animals infected by rabid raccoons have been identified since 1993; these include 221 cattle, 184 dogs, 76 raccoon dogs, four feral cats, and one deer (http://www.kahis.go.kr). Prevention of rabies in animals is essential to reduce human rabies; more than 95% of all human cases are caused by dog bites [3,4]. Both vaccination and the use of a bait vaccine (vaccinia-rabies glycoprotein) have been used successfully in South Korea; no case of animal rabies was recorded during 2014-2016.
Reverse genetics rescuing new rabies vaccine strains has rendered it possible to produce safe and efficient RABV vaccines for animals [5,6]. Using reverse genetics, we constructed a recombinant Evelyn-Rokitnicki-Abelseth (ERA) G3G viral strain with an amino acid substitution (Arg → Glu) at position 333 of the glycoprotein gene of ERA and evaluated vaccine safety, efficacy, and immunogenicity. Mice (4–6 weeks of age) inoculated with the ERAG3G strain via the intracranial route did not show any clinical sign of rabies. Furthermore, single immunization via the intramuscular (IM) or oral route completely protected mice and induced a protective immune response in dogs, cats, and wild raccoon dogs [7]. However, the single mutation was associated with the risk of reversion to virulence after three continuous viral passages in suckling mice [8]. Therefore, newer RABVs with multiple mutations were constructed using reverse genetics; these safely induced virus-neutralizing antibodies (VNAs) in immunized mice and protected the animals from lethal challenge [5,8,9]. In addition, other recombinant RABVs carrying immunostimulatory genes such as those encoding granulocyte/macrophage colony-stimulating factor or interferon α1 have been rescued and exhibited enhanced immunostimulatory capacities in mice [10,11].
We previously constructed the recombinant rabies vaccine strain ERAGS with two mutations in the glycoprotein gene using reverse genetics. The strain was immunogenic in both mice and raccoon dogs when given either IM or orally [12]. In the present study, we evaluated the safety and immunogenicity of the vaccine in dogs and cattle.
Materials and Methods
Cells and viruses
Murine neuroblastoma (NG108-15, ATCC HB-12317) cells grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with antibiotics (100 IU/mL penicillin, 10 µg/mL streptomycin, and 0.25 µg/mL amphotericin B) and 5% (v/v) fetal bovine serum (FBS) were used for propagation of the virus. BHK-21 cells (ATCC CCL-10) were grown in DMEM supplemented with 10% (v/v) heat-inactivated FBS and the same antibiotic/antimycotic solution. These BHK-21 cells were used in the fluorescent assay virus neutralization (FAVN) test. The construction of ERAGS has been described previously [12]. CVS11, a standard rabies strain, was used in the FAVN test.
Propagation of the ERAGS strain and lyophilization
NG108-15 cells plated in 150 cm2 tissue culture flasks were infected with the ERAGS strain at a multiplicity of infection of 0.1. After incubation at 37℃ for 72 hours, the cells were frozen and thawed three times. The virus was harvested and titered using the same cells growing in 96-well microplates; titers were determined by the method of Reed and Muench [13]. Two formulations containing different stabilizers were prepared; these were slight modifications of the standard vaccine stabilizer [14]. The first stabilizing formulation was lactose/phosphate/glutamate/gelatin (LPGG) and the second trehalose/phosphate/glutamate/gelatin (TPGG) (Table 1). Equal volumes of the viral preparation and 2× stabilizer solutions were mixed, dispensed into vaccine bottles, and lyophilized using a VirTis instrument according to the manufacturer's instructions (VirTis, Gardiner, NY, USA). The lyophilized vaccines were initially stored at 4℃ and subjected to accelerated stability testing after storage at 4℃, 24℃, and 37℃ for 7 days.
Table 1. The composition of stabilizers used during lyophilization of the ERAGS strain.
| Component | LPGG, g (mM) | TPGG, g (mM) |
|---|---|---|
| Lactose (C12H22O11) | 100 (292.2)a) | - |
| Trehalose dehydrate (C12H22O11) | - | 100 (264.3) |
| Monopotassium phosphate (KH2PO4) | 0.52 (3.8) | 0.52 (3.8) |
| Potassium phosphate dibasic (K2HPO4) | 0.854 (4.85) | 0.854 (4.85) |
| Monosodium L-glutamate (C5H8NNaO4) | 0.829 (4.9) | 10 (59.1) |
| Sorbitol (C6H14O6) | - | 20 |
| Gelatin | 5 | 5 |
LPGG, lactose/phosphate/glutamate/gelatin; TPGG, trehalose/phosphate/glutamate/gelatin.
a)The amounts are those required for lyophilization of 1 L of the vaccine.
Safety and immunogenicity of the ERAGS strain in animals
Animal ethics committee of Animal and Plane Quarantine Agency (APQA, Gimcheon, Korea) approved the experimental design (approval No. 2016-453). Four-month-old dogs seronegative for RABV were divided into 10 groups (1–10) of four dogs each. Group 1 was inoculated with 107.0 FAID50 (50% fluorescent antibody infectious dose per milliliter) of the vaccine via IM injection into a hind leg for a safety test. Groups 2-5 were inoculated with 105.0 to 102.0 FAID50/mL of the vaccine via IM injection. Groups 6-9 were inoculated with 105.0 to 102.0 FAID50/mL of the vaccine via the subcutaneous route (SC route). Group 10 served as a control group. Two-year-old cattle were divided into six groups (11-16) of five animals each. Group 11 was inoculated with 107.0 FAID50/mL of the vaccine via IM injection for a safety test. Groups 12–15 were inoculated with 105.0 to 102.0 FAID50/mL of the vaccine via IM injection. Group 16 served as an untreated control group. At 0, 4, and 8 weeks post-inoculation (WPI), blood was collected from all dogs and cattle, and antibody titers against RABV were measured. Safety and immunogenicity were monitored daily. We sought to record all adverse events such as hyper-salivation, anorexia, prostration, anxiety, agitation, aggression, paralysis, and sudden death.
Serological assays
VNA titers were measured using the FAVN test. A positive reference serum sample was obtained from the World Health Organization and used at 0.5 IU/mL [15]. Briefly, all sera were heated at 56℃ for 30 minutes. Fifty-microliter volumes of the sera, and positive and negative controls, were placed in the first four wells of a microplate and then serially diluted threefold. The CVS11 RABV strain (100 FAID50/50 µL) was added to each well followed by incubation for 1 hour at 37℃. Next, 50 µL of a BHK-21 cell suspension (4×105 cells/mL) were added to each well and the microplates incubated for 72 hours under 5% (v/v) CO2 at 37℃. The cells were fixed in 80% of cold acetone (-20℃) and stained with a monoclonal antibody against the RABV nucleoprotein and fluorescent isothiocyanate–conjugated goat/anti-mouse IgG+IgM (KPL, Gaithersburg, MD, USA). After washing and drying, the microplates were examined under a fluorescence microscope (Nikon, Tokyo, Japan) at 200×. Serum VNA titers were expressed in IU/mL by reference to those of the positive standard.
Commercial Platelia Rabies enzyme-linked immunosorbent assay (ELISA) kits (Bio-Rad, Hercules, CA, USA) were used, according to the manufacturer's instructions, to detect rabies-specific anti-glycoprotein antibodies in dog sera. The ELISA kit employed protein-A–conjugated anti-glycoprotein antibodies to this end. As the kit can detect RABV-specific anti-glycoprotein antibodies in dogs and cats only, the kit was used to evaluate dog sera only.
Statistical analysis
Serological data were expressed as mean±standard deviation. SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA) was used to compare FAVN and ELISA titers between groups. The differences were subjected to one-way ANOVA followed by Tukey's post hoc test and the paired Student's t test. A p-value <0.05 was considered to indicate statistical significance.
Results
Accelerated stability testing of the lyophilized rabies vaccine
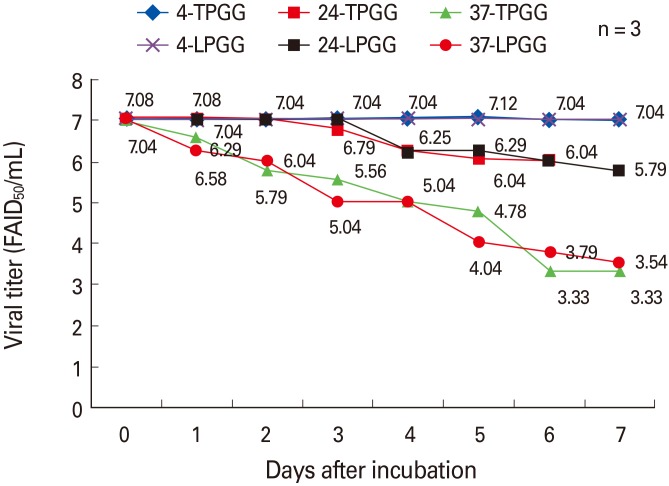
The ERAGS strain was inoculated into NG108-15 cells and harvested 60 hours later. After freezing and thawing three times, the titer was 107.8 FAID50/mL (data not shown). Equal volumes of the ERAGS suspension and 2× stabilizer solutions were combined, lyophilized, and three lyophilized vaccines were subjected to accelerated stability testing to determine changes in titer over time after exposure to temperatures higher (4℃, 24℃, and 37℃ for 7 days) than normal storage temperatures (Fig. 1). When held at 4℃, neither preparation exhibited loss of viability (the mean titers were 107.04 FAID50/mL at 7 days); storage at 24℃ was associated with lower loss of viability than that induced by storage at 37℃. The mean titers decreased from 105.79 to 103.33 FAID50/mL over 7 days. Thus, viral viability was similar when either stabilizer (LPGG or TPGG) was employed.
Fig. 1. Accelerated stability testing of the freeze-dried rabies vaccine at various temperatures (4℃, 24℃, and 37℃). Virus was prepared with two types of stabilizer (LPGG or TPGG), and viral titers in NG108-15 cells were measured at the times shown. LPGG, lactose/phosphate/glutamate/gelatin; TPGG, trehalose/phosphate/glutamate/gelatin.
Vaccine safety and immunogenicity
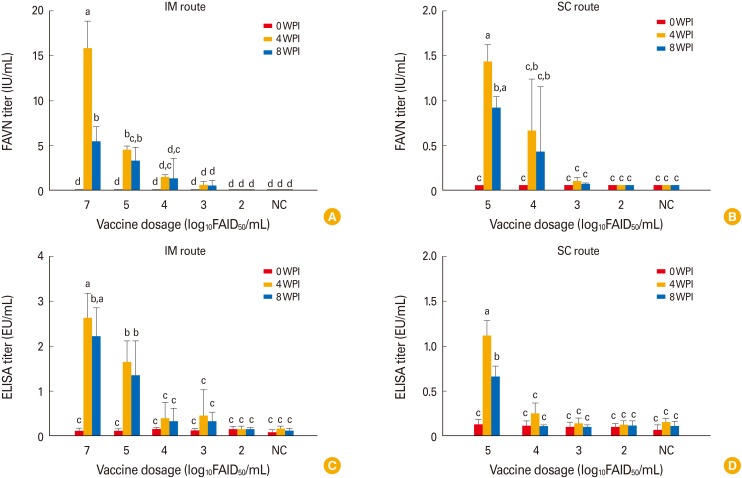
Vaccine lyophilized with TPGG (a universal protein stabilizer [16]) was used to evaluate safety and immunogenicity in dogs and cattle. No clinical sign of rabies was noted after inoculation via the IM or SC route (Table 2); unvaccinated controls also remained normal through 8 WPI. All dogs in group 1 inoculated with 107.0 FAID50/mL vaccine via the IM route developed high VNA titers (7.9-23.9 IU/mL [mean, 15.9 IU/mL]) by 4 WPI (Fig. 2A). Dogs in groups 2-4 inoculated with 105.0 to 103.0 FAID50/mL vaccine via the IM route developed mean VNA titers of 4.56 to 0.61 IU/mL by 4 WPI. However, moderate decreases in mean VNA titers were evident in all groups by 8 WPI (Fig. 2B). Dogs in groups 6-8 inoculated with 105.0 to 103.0 FAID50/mL vaccine via the SC route exhibited a slightly lower mean VNA titer (1.44-0.1 IU/mL) at 4 WPI. Dogs in groups 5 and 9 inoculated with 102.0 FAID50/mL vaccine via the IM and SC routes, respectively did not develop VNAs by 4 or 8 WPI. All dog sera were subjected to the Platelia Rabies ELISA to detect anti-RABV antibodies. As shown in Fig. 2C and D, dogs in groups 1-4 inoculated with >103.0 FAID50/mL via the IM route and groups 7 and 8 inoculated with >104.0 FAID50/mL via the SC route developed antibodies at titers >0.24 equivalent units/mL by 4 WPI. Although the average antibody levels measured by the ELISA kit were lower than those measured by the FAVN test, the trends in antibody levels were similar between the two tests at 0, 4, and 8 WPI.
Table 2. Clinical signs in dogs and cattle inoculated with the new rabies vaccine.
| Animal | Group | Dosea) | Inoculation route | Age of animals | No. of animals | Clinical signsb) | Observational periodc) |
|---|---|---|---|---|---|---|---|
| Dog | 1 | 7.0 | IM | 4 mo | 4 | Normal | 8 |
| 2 | 5.0 | IM | 4 mo | 4 | Normal | 8 | |
| 3 | 4.0 | IM | 4 mo | 4 | Normal | 8 | |
| 4 | 3.0 | IM | 4 mo | 4 | Normal | 8 | |
| 5 | 2.0 | IM | 4 mo | 4 | Normal | 8 | |
| 6 | 5.0 | SC | 4 mo | 4 | Normal | 8 | |
| 7 | 4.0 | SC | 4 mo | 4 | Normal | 8 | |
| 8 | 3.0 | SC | 4 mo | 4 | Normal | 8 | |
| 9 | 2.0 | SC | 4 mo | 4 | Normal | 8 | |
| 10 | Control | - | 4 mo | 4 | Normal | 8 | |
| Cattle | 11 | 7.0 | IM | 2 yr | 5 | Normal | 8 |
| 12 | 5.0 | IM | 2 yr | 5 | Normal | 8 | |
| 13 | 4.0 | IM | 2 yr | 5 | Normal | 8 | |
| 14 | 3.0 | IM | 2 yr | 5 | Normal | 8 | |
| 15 | 2.0 | IM | 2 yr | 5 | Normal | 8 | |
| 16 | Control | - | 2 yr | 5 | Normal | 8 |
IM, intramuscular; SC, subcutaneous.
a)Log10FAID50/mL.
b)Hyper-salivation, anorexia, paralysis, sudden death, etc.
c)Weeks post-inoculation.
Fig. 2. The immune response of dogs inoculated dose-dependently with the ERAGS strain via the intramuscular (IM) (A and C) and subcutaneous (SC) (B and D) routes. The fluorescent assay virus neutralization and enzyme-linked immunosorbent assay titers of the sera were measured. Dogs inoculated with >103.0 FAID50/mL vaccine via the IM route exhibited protective immune responses by 4 weeks post-inoculation (WPI). Each bar represents the mean±standard deviation from four independent samples. NC, negative control. Different lower-case letters above the bars indicate significant differences among groups (p<0.05, Tukey's post-hoc test).
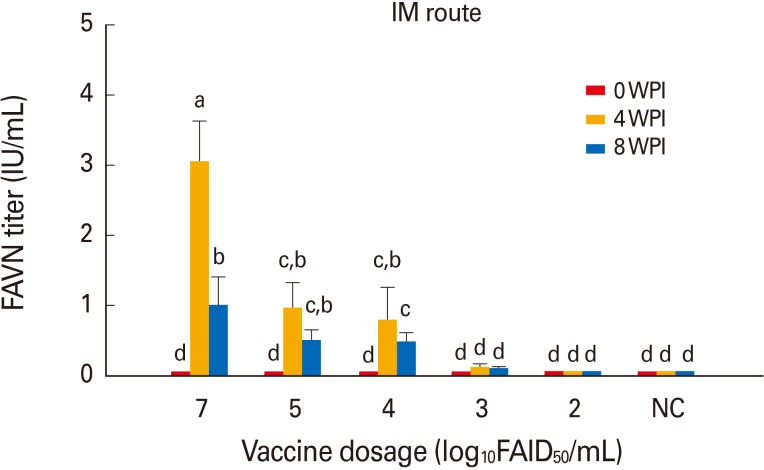
Cattle in group 11 inoculated with 107.0 FAID50/mL vaccine via the IM route developed a moderate mean VNA titer of 3.05 IU/mL by 4 WPI (Fig. 3). Cattle in groups 12-14 inoculated with diluted vaccine at 105.0 to 103.0 FAID50/mL via the IM route developed mean VNA titers of 0.97-0.13 IU/mL by 4 WPI. However, moderate decreases in the mean VNA titers were apparent by 8 WPI. Cattle in group 15 inoculated with 102.0 FAID50/mL vaccine via the IM route did not develop VNA. All non-vaccinated dogs and cattle remained RABV-seronegative throughout the test, indicating that no contact transmission had occurred between vaccinated and control animals. Thus, the new rabies vaccine prepared at 107.0 FAID50/mL IM was safe in dogs and cattle and, when given at ≥103.0 or 104.0 FAID50/mL via the IM route, induced protective immune responses by 4 WPI.
Fig. 3. Immune responses in cattle given vaccine via the intramuscular (IM) route. Antibody titers were measured using the fluorescent assay virus neutralization (FAVN) test. Cattle inoculated with >104.0 FAID50/mL vaccine developed protective levels (0.81 IU/mL) of anti-rabies antibodies. NC, negative control; WPI, weeks post-inoculation. Different lower-case letters above the bars indicate significant differences among groups (p<0.05, Tukey's post hoc test).
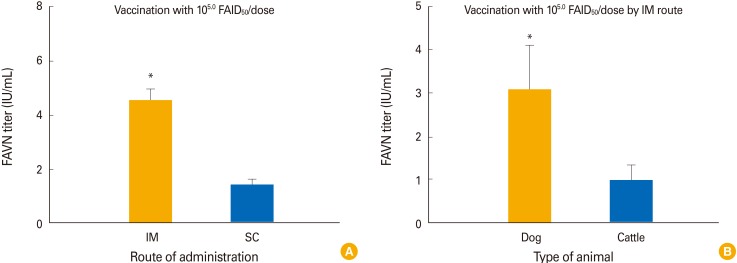
In terms of the route of administration, dogs in group 2 inoculated with 105.0 FAID50/mL via the IM route developed a higher mean VNA titer (4.56 IU/mL) than did dogs in group 6 inoculated with the same amount of vaccine via the SC route by 4 WPI (1.44 IU/mL; p<0.05) (Fig. 4A). Dogs inoculated with 105.0 FAID50/mL of the vaccine developed a higher mean VNA titer (4.56 IU/mL) than did cattle (0.97 IU/mL) inoculated with the same dose by 4 WPI (p<0.05) (Fig. 4B).
Fig. 4. Comparison of virus-neutralizing antibody titers in animals given 105.0 FAID50/mL vaccine via different inoculation routes (A). Comparison of data between the two animal species (B). FAVN, fluorescent assay virus neutralization. *Significant difference between the intramuscular (IM) and subcutaneous (SC) routes or between dogs and cattle (p<0.05, paired Student's t test).
Discussion
In South Korea, three rabies vaccines (inactivated, live, and oral vaccines) have played key roles in eliminating animal rabies via a vigorous nationwide eradication program that commenced in 2000 [2,17]. Several inactivated rabies vaccines, either imported or manufactured in South Korea, continue to be given to dogs, cats, and cattle annually. In addition, live attenuated vaccines based on the ERA strain, produced by Korean companies, have been given to both dogs and cattle in some regions. Finally, an oral vaccine (the VRG bait) has been distributed in high-risk rabies regions to block viral expansion in raccoon dogs. The live attenuated vaccine is preferred to the inactivated vaccine, as it is more cost-effective and easier to manufacture. However, safety concerns remain. There are indirect indications that live attenuated vaccines such as the ERA strain should be replaced; the ERA strain is pathogenic in adult mice [18]. Therefore, we constructed the ERAGS strain with multiple mutations in the glycoprotein gene and evaluated safety and immunogenicity in mice, raccoon dogs, and pigs [12].
Most live vaccines propagated in cell culture are freeze-dried. Viral titers must be maintained during storage to minimize costs. The Korean Standard Assay of Veterinary Biological Products requires that a live rabies vaccine should maintain a minimum titer of 103.5 FAID50/mL over a defined period. We subjected the new rabies vaccines, admixed with different stabilizers, to accelerated stability testing at three temperatures. Such testing yields useful preliminary information on the stability of the live vaccine. TPGG and LPGG are commonly used to stabilize live vaccines in South Korea [14]. We found that both stabilizers were equally effective. However, viral titers steadily decreased during storage at 24℃ or 37℃ for 7 days, suggesting that hydrogen bonds between viral proteins and the stabilizers were disrupted over this time [19]. Therefore, long-term stability testing should be performed at 4℃. In addition, other stabilizers, such as sorbitol, should be evaluated.
We evaluated vaccine safety and immunogenicity in dogs and cattle inoculated via the IM or SC route. Since 1993, dogs and cattle have been the animals principally infected by rabid raccoon dogs in Korea; dogs and cattle were thus appropriate experimental animals. Previous studies found that various recombinant RABVs bearing multiple mutations and sometimes immunostimulatory genes were safe; no replication was evident in organs including the brain and salivary glands of inoculated animals [6,8,20]. We found that no dog or cattle given vaccine IM at 107.0 FAID50/mL exhibited any clinical sign of disease during the observational period, and all developed high VNA titers (15.9 to 3.05 IU/mL) by 4 WPI. The FAVN and ELISA immunogenicity tests showed that the mean VNA titers in dogs and cattle depended on the vaccine dose; vaccination via the IM route induced higher VNA titers than did vaccination via the SC route. Earlier studies showed that both the vaccination route and the dose significantly influenced the immune response and extent of protection from RABV challenge, regardless of the vaccine strain employed. Moreover, the response after IM vaccination in mice given a DNA vaccine was superior to that after SC vaccination [21,22]. This may be because in dogs and mice, connective tissue and subcutaneous fat prevent vaccine administered SC from reaching antigen-presenting cells. IM administration allows the virus to contact immune cells of the muscle directly. In terms of immunogenicity in dogs and cattle vaccinated IM with 105.0 FAID50/mL, dogs developed higher VNA titers than did cattle, indicating that larger animal such as cattle require higher amounts of the new vaccine.
In summary, the two new freeze-dried rabies vaccines containing different stabilizers (LPGG or TPGG) were similar in terms of viability after storage. No dog or cattle inoculated at 107.0 FAID50/mL via the IM route exhibited any adverse effects; animals given >103.0 or 104.0 FAID50/mL developed VNA titers ≥0.5 IU/mL by 4 WPI. Therefore, the new rabies vaccine may be safe and immunogenic in dogs and cattle. Future studies must explore safety and immunogenicity in cats which are sensitive to RABV.
Footnotes
No potential conflict of interest relevant to this article was reported.
This work was supported financially by a grant (B1543083-2016-18-01) from Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.
References
- 1.Kim CH, Lee CG, Yoon HC, et al. Rabies, an emerging disease in Korea. J Vet Med B Infect Dis Vet Public Health. 2006;53:111–115. doi: 10.1111/j.1439-0450.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 2.Cheong Y, Kim B, Lee KJ, et al. Strategic model of national rabies control in Korea. Clin Exp Vaccine Res. 2014;3:78–90. doi: 10.7774/cevr.2014.3.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenzin, Dhand NK, Gyeltshen T, et al. Dog bites in humans and estimating human rabies mortality in rabies endemic areas of Bhutan. PLoS Negl Trop Dis. 2011;5:e1391. doi: 10.1371/journal.pntd.0001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudarshan MK, Madhusudana SN, Mahendra BJ, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis. 2007;11:29–35. doi: 10.1016/j.ijid.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa K, Ito N, Masatani T, et al. Generation of a live rabies vaccine strain attenuated by multiple mutations and evaluation of its safety and efficacy. Vaccine. 2012;30:3610–3617. doi: 10.1016/j.vaccine.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Guo L, Feng N, Yang S, et al. Reverse genetic system for rabies virus vaccine Evelyn-Rokitnicki-Abelseth strain. Wei Sheng Wu Xue Bao. 2009;49:949–954. [PubMed] [Google Scholar]
- 7.Yang DK, Kim HH, Jo HY, Kim HW, Choi SS, Cho IS. Safety and immunogenicity of a recombinant rabies virus strain (ERAG3G) in Korean raccoon dogs. J Bacteriol Virol. 2015;45:250–255. [Google Scholar]
- 8.Faber M, Faber ML, Papaneri A, et al. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005;79:14141–14148. doi: 10.1128/JVI.79.22.14141-14148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J Virol. 2001;75:11496–11502. doi: 10.1128/JVI.75.23.11496-11502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Tian Q, Xu X, et al. Recombinant rabies virus expressing IFNalpha1 enhanced immune responses resulting in its attenuation and stronger immunogenicity. Virology. 2014;468-470:621–630. doi: 10.1016/j.virol.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Wen Y, Wang H, Wu H, et al. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol. 2011;85:1634–1644. doi: 10.1128/JVI.01552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang DK, Kim HH, Choi SS, et al. Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs. Clin Exp Vaccine Res. 2016;5:159–168. doi: 10.7774/cevr.2016.5.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed LJ, Muench H. Simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 14.Kang MS, Jang H, Kim MC, et al. Development of a stabilizer for lyophilization of an attenuated duck viral hepatitis vaccine. Poult Sci. 2010;89:1167–1170. doi: 10.3382/ps.2009-00620. [DOI] [PubMed] [Google Scholar]
- 15.Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998;212:79–87. doi: 10.1016/s0022-1759(97)00212-3. [DOI] [PubMed] [Google Scholar]
- 16.Habib S, Khan MA, Younus H. Thermal destabilization of stem bromelain by trehalose. Protein J. 2007;26:117–124. doi: 10.1007/s10930-006-9052-1. [DOI] [PubMed] [Google Scholar]
- 17.Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011;64:513–515. [PubMed] [Google Scholar]
- 18.Shuai L, Feng N, Wang X, et al. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antiviral Res. 2015;121:9–15. doi: 10.1016/j.antiviral.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Kissmann J, Ausar SF, Rudolph A, et al. Stabilization of measles virus for vaccine formulation. Hum Vaccin. 2008;4:350–359. doi: 10.4161/hv.4.5.5863. [DOI] [PubMed] [Google Scholar]
- 20.Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007;25:3409–3418. doi: 10.1016/j.vaccine.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Wunderli PS, Dreesen DW, Miller TJ, Baer GM. Effects of vaccine route and dosage on protection from rabies after intracerebral challenge in mice. Am J Vet Res. 2003;64:491–498. doi: 10.2460/ajvr.2003.64.491. [DOI] [PubMed] [Google Scholar]
- 22.Osinubi MO, Wu X, Franka R, et al. Enhancing comparative rabies DNA vaccine effectiveness through glycoprotein gene modifications. Vaccine. 2009;27:7214–7218. doi: 10.1016/j.vaccine.2009.09.031. [DOI] [PubMed] [Google Scholar]