Abstract
Outbreaks of H5 highly pathogenic avian influenza viruses (HPAIVs) have caused economic loss for the poultry industry and posed a threat to public health. In South Korea, novel reassortants of HPAIVs such as H5N6 and H5N8 had been circulating in poultry. Here, we will discuss the identity of recent novel reassortants of Korean H5 HPAIVs and the recent advances in vaccine development, which will be useful for controlling HPAIV transmission in poultry and for effectively preventing future epidemics and pandemics.
Keywords: Avian influenza, H5N1 subtype, H5N6 subtype, H5N8 subtype, Epidemiology, Vaccines
Introduction
Influenza A virus is a well-known zoonotic pathogen that can infect a broad range of hosts such as birds, swine, companion animals, marine animals, and humans, causing annual epidemics and pandemics. Currently, influenza A viruses are categorized into 18 hemagglutinin (HA) subtypes (H1 to H16 from wild waterfowl, and H17 and H18 from bats) and 11 neuraminidase (NA) subtypes (N1 to N9 from wild waterfowl, and N10 and N11 from bats) [1,2].
In 1996, the highly pathogenic avian influenza (HPAI) H5N1 A/Goose/Guangdong/1/1996 virus was first detected on a goose farm in China [3]. Since then, H5 viruses have developed novel characteristics by genetic reassortment with other avian influenza (AI) viruses that infect wild bird and poultry. For example, various subtypes of H5 highly pathogenic avian influenza viruses (HPAIVs) have been detected worldwide, including H5N2, H5N5, H5N6, and H5N8 viruses that disseminate via wild birds [4,5,6,7]. Recently, two novel HPAIs, H5N8 [4], and H5N6 [8], from wild migratory birds in South Korea caused outbreaks in domestic poultry. An H5N8 influenza virus was first reported in South Korea in 2014, which belongs to clade 2.3.4.4 and spread to a large number of countries, including East Asia, Europe, and further to North America, and subsequently created novel H5Nx subtypes [9,10,11,12]. In November 2016, a novel genotype of the H5N6 HPAIV first isolated from migratory birds in South Korea, caused outbreaks in domestic poultry [8,13,14]. According to genetic analysis, this was a novel Korean isolate of the H5N6 HPAIV belonging to clade 2.3.4.4, which was newly reassorted by three different subtypes of AI viruses, namely, H5N6, H4N2, and H1N1.
In this review, we have discussed the zoonotic characteristics of Korean H5 HPAIVs and presented an overview of vaccine strategies for AI vaccine, including development of immunogenicity, vaccine safety, and cross-protective vaccines for controlling HPAIs in poultry.
Current Epidemiology of HPAI in South Korea
H5N1 HPAI in Korea
Since the first identification of the H5 subtype HPAI virus A/goose/Guangdong/1/1996 (H5N1) in 1996 in China, these viruses have evolved into diverse lineages as well as reassortants, generating H5N2, H5N3, H5N5, H5N6, and H5N8 [14,15,16] strains. Although the HA cleavage site in the HA protein is known to be critical for viral pathogenicity in avian species [17], only the H5 and H7 subtypes of AI viruses are highly pathogenic for poultry.
In Korea, the first outbreak of H5N1 HPAI caused the 2003-2004 epidemic in poultry farms with high mortality [18,19]. Since then, second and third outbreaks of H5N1 HPAI occurred in 2006 and 2008, respectively. The HPAI outbreak in 2008 spread to 11 provinces and affected not only the poultry farms but also diverse bird species in live bird markets [20]. Based on the sequence of the gene encoding HA, the H5N1 HPAIVs of the 2008 Korean epidemic belong to clade 2.3.2, unlike those of the 2003 (clade 2.5) and 2006 (clade 2.2) outbreaks [20,21].
Following the detection of the H5N1 HPAIV in migratory birds in 2010, a fourth outbreak of the virus was inevitable, and around fourteen bird species, including poultry and wild bird were affected [22]. Although the H5N1 HPAIVs of the 2010 outbreak were clustered into clade 2.3.2, they were not closely related to the H5N1 HPAIV of 2008.
The causative AI viruses of the four HPAIV outbreaks between 2003 and 2011 belonged to the H5N1 subtypes; however, phylogenetic relationship indicated that the viruses belonged to different lineages. Therefore, from the epidemiological point of view, these strains were introduced from abroad, rather than evolving by persistent circulation inside the country.
H5N8 HPAI in Korea
An outbreak of a novel subtype of the HPAIV progressed between January 16 and May 8, 2014, as phases I, II, and III [23]. The causative AI viruses were reported to be novel reassorted influenza A (H5N8) viruses of clade 2.3.4.6, which was later more frequently called clade 2.3.4.4 [24,25]. Following an outbreak in poultry in South Korea in January 2014, the H5N8 viruses rapidly spread worldwide in 2014-2015, and long-distance migratory birds played a major role in the global dissemination of these viruses [26]. In addition, since the first outbreak of the H5N8 viruses in early 2014, these viruses were reintroduced into Korea by the migratory waterfowl in 2014-2015 [27], and have caused sporadic outbreaks of HPAI in Korea ever since 2014.
In the experimental infection study, the viral replication and shedding were greater in H5N8-infected ducks than in H5N1-infected ducks [28], and it had lower pathogenicity and transmissibility in poultry species compared to the previously reported H5N1 HPAIVs [29]. These characteristics of H5N8 viruses might lead to late recognition of the symptoms associated with HPAI and increase the chances of viral transmission among poultry farms. However, the transmission mechanism of the HPAIVs is still not fully understood. Further studies on risk factors in terms of biosecurity of poultry farms and transmission ecology of the virus in migratory and indigenous animals are required [30].
H5N6 HPAI in Korea
A reassortant clade 2.3.4.4 of the H5N6 HPAIVs was isolated from migratory birds in South Korea during October and November 2016 [8,27]. Subsequently, HPAI outbreaks in approximately 380 poultry farms were reported by April 4th, 2017, in South Korea (http://www.qia.go.kr). The H5N6 HPAIVs were first reported in China in 2013, which disseminated not only within China but also in Vietnam and Laos [31,32]. While the first Chinese H5N6 viruses were novel reassortants of NA from the H6N6 viruses and seven other genes from H5N1 viruses, the Korean H5N6 HPAIVs were novel reassortants of Chinese H5N6 viruses and Eurasian low pathogenicity AI viruses of wild birds [14].
The recently emerging H5N6 HPAIVs in Korea are continuously evolving and are found in wild migratory birds [33]. Since the H5N6 HPAIVs are also related to human infections [34], additional studies on their pathogenicity, interspecies transmission, and potential risk for public health and poultry industries are required.
Perspective on HPAI in Korea
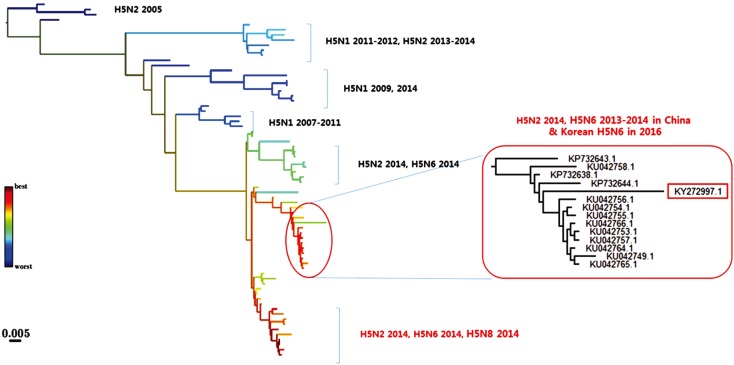
The HPAIVs identified since 2003 are all H5-subtype viruses that are genetically distant from each other, and most of them were introduced from outside Korea (Table 1). The continuous HPAI outbreaks by novel H5Nx viruses in Korea is closely related to migratory birds, and most HPAIV strains are genetically similar to those from China and Southeast Asian countries [18,26,27,28]. As continuous evolution and reassortment of AI viruses occur in live poultry markets of China, which might result in the emergence of diverse AI viruses in China [31], future outbreaks of HPAI by other novel HPAIVs may be inevitable in Korea. Therefore, monitoring Chinese HPAIVs and predicting the HPAIV lineages that can be introduced to Korea is one of the strategies for controlling future HPAI epidemics. This can be accomplished with the recent advance in bioinformatics, which can predict evolution of strains from the shape of genealogical trees [35]. Using this approach, we collected HA sequences of Chinese H5-subtype influenza viruses and predicted lineages with high fitness in a preliminary study (Fig. 1). Two lineages, including the H5N2, H5N6, and H5N8 viruses of the 2014 outbreaks, were inferred to have high fitness, and the Korean H5N6 HPAIV (2016) belonged to one of those lineages, showing 97.5%-98.4% amino acid similarities. This preliminary result indicates that the fitness inference tool developed by Neher et al. [35] can be applied to study the molecular epidemiology of HPAIVs.
Table 1. Subtypes and clades of HPAIVs in Korea.
| Year of HPAI outbreaks | ||||||
|---|---|---|---|---|---|---|
| 2003-2004 | 2006-2007 | 2008-2009 | 2010-2011 | 2014-2015 | 2016-2017 | |
| Subtype | H5N1 | H5N1 | H5N1 | H5N1 | H5N8 | H5N6 |
| Clade | 2.5 | 2.2 | 2.3.2 | 2.3.2 | 2.3.4.4 (2.3.4.6)a) | 2.3.4.4 |
Fig. 1. Predicting the evolution of Chinese H5Nx viruses. A genealogical tree of hemagglutinin sequences of H5Nx viruses by 2014 in China and H5N6 highly pathogenic avian influenza virus (HPAIV), 2016 in Korea. Nodes are colored according to the fitness ranking. Overall amino acids similarities of the input sequences were 86.2%-98.4%, while those of the lineage to which Korean H5N6 virus belonged were 97.5%-98.4%. A box indicates a H5N6 HPAIV in Korea, 2016.
In addition, continuous monitoring of migratory birds and indigenous animals should be conducted to understand the interspecies transmission ecology of HPAIVs. Although the global transmission of these viruses is closely associated with migratory birds, their mode of transmission from migratory birds to poultry farms is not known. While poultry farm-based risk factors of HPAI outbreaks might be important as management factors, ecological risk factors can be also investigated considering interspecies transmission of influenza viruses [29]. In our monitoring system, several indigenous avian and mammalian species showed serological evidence of H5-subtype influenza virus infections (unpublished data). Therefore, consideration of both biosecurity of poultry farms and the transmission ecology of HPAIVs in different hosts can provide additional information for the development of control policies.
Current HPAI H5 Vaccine
HPAIVs are economically important diseases of poultry. In particular, highly pathogenic subtypes of the H5 and H7 AI viruses cause devastating mortality in poultry industries, reduce egg production, and decrease bird weight gain. Unfortunately, the AI viruses are able to infect humans, which cause severe diseases with high mortality rates and are a cause of serious public health concern [36,37]. Therefore, many scientists suggest vaccination as one of the effective methods for prevention of AI virus infection.
Conventional AI vaccines, which are based on inactivated whole viruses [38] from an HPAI outbreak or a low pathogenicity isolate with a well-matched HA, have been shown to be effective at preventing clinical disease and decreasing virus shed. The first HPAI vaccine manufactured in China used the inactivated low pathogenic AI H5 virus A/Turkey/England/N-28/1973 (H5N2) and rapidly controlled the H5N1 outbreaks in China in 2004 [39]. However, the seed virus of the inactivated H5N2 vaccine was limited to the control of antigenic diversity within the H5N1 strains of China. To circumvent this problem, an advanced H5 vaccine using HA and NA from the Re-1 strain of H5N1 (A/goose/Guangdong/1/1996) and 6 internal genes from A/Puerto Rico/8/1934 (PR8) was produced using reverse genetics, which was antigenically well-matched with the epidemic strains of that time [40,41]. The inactivated vaccines used in China were also exported to several other countries, including Egypt, Indonesia, Vietnam, Bangladesh, Burma, and Mongolia for effective control of H5N1 HPAIVs [42].
To control H5 HPAIV epidemics, vaccine candidates are continuously improved and developed using a recombinant virus based on modified HA and NA, which is designated as H5N1/PR8 (2+6) (Table 2) [39,43,44]. However, since 2010, H5 AI virus with other NA subtypes including N2, N3, N6, and N8 have been detected in poultry and wild birds in China, which may have contributed to the spread of H5N8 viruses to North America, Europe, and neighboring Asian countries [4,5,12,26]. Therefore, to improve vaccine efficacy against the newly emerging or re-emerging H5 HPAIVs in poultry, it is necessary to develop or induce a bigger spectrum of protective immunity.
Table 2. Vaccine strains for immunization of chickens against H5N1 HPAIVs.
| Candidate vaccine virus | Subtype | Clade | Seed name |
|---|---|---|---|
| A/turky/England/N-28/73 | H5N2 | Classical | - |
| A/chicken/Legok(Indonesia)/2003 | H5N1 | Clade 2.1.1 | - |
| A/duck/Novosibirsk/02/2005 | H5N1 | Clade 2.2 | - |
| A/goose/Guandong/1996 | H5N1 | Clade 0 | Re-1 |
| A/chicken/Shanxi/2/2006 | H5N1 | Clade 7 | Re-4 |
| A/duck/Anhui/1/2006 | H5N1 | Clade 2.3.4 | Re-5 |
| A/duck/Guangdong/S1322/2010 | H5N1 | Clade 2.3.2 | Re-6 |
| A/chicken/Egypt/18-H/2008 | H5N1 | Clade 2.2.1 | - |
| A/chicken/Liaoning/S4092/2011 | H5N1 | Clade 7.2 | Re-7 |
| A/pollo/Guizhou/4/13 | H5N1 | Clade 2.3.4.4 | Re-8 |
Generation of Novel AI Vaccines
Recombinant virus vector–based vaccines
Recombinant virus vector–based vaccines can express any antigen with or without modification in vivo [45]. Previous reports suggest that recombinant virus vector vaccines can stimulate a wide range of immune responses compared to conventional inactivated vaccines [46,47,48]. So far, a variety of recombinant H5N1 influenza vaccines have been developed using fowl pox virus, Newcastle disease virus (NDV), Turkey herpes virus, duck enteritis virus, and infectious laryngotracheitis virus. In particular, a recombinant NDV vector–based H5N1 AI vaccine was approved for use in chickens in China and the bivalent recombinant NDV vector–based H5N1 AI vaccine was manufactured and used in chickens in China between 2006 and 2012 [49,50,51]. To improve the NDV vaccine efficacy, researchers are not only inducing AI-specific immune response to protect against viral infection, but are also considering the limitations of anti-NDV antibodies derived from routine NDV vaccination [52,53].
Nucleic acid–based vaccines
DNA vaccines offer a number of advantages over conventional vaccines [54]. For example, an administered DNA vaccine can elicit both humoral and cellular immunity and can be administered multiple times to enhance immune efficacy. Several studies showed that protection of highly pathogenic H5 virus–infected chickens or mammals was improved by codon optimization of DNA vaccines based on H5N1 HAs [55,56,57] DNA vaccines are usually constructed based on the HA of the matching subtype; however, recent studies demonstrated that vaccines can be developed as a mixture expressing HA from different clades of H5N1 viruses, and this new DNA vaccine candidate could protect chickens challenged with heterologous H5N1 viruses [57]. However, despite positive results regarding vaccine efficacy, the use of DNA vaccines for vaccination of poultry is still limited by the expenses of mass vaccination and the requirement of devices for efficient on-site vaccination such as jet injection [58] and electroporation [59]. Although licensed DNA vaccines against AI for poultry are currently unavailable, several DNA vaccine candidates are being studied for providing protection against HPAIVs in poultry.
Recombinant protein vaccines
The use of current conventional egg-based H5 AI vaccines are limited by subtype-specific vaccine efficacy and long period of vaccine production (approximately 6 months) [38]. Therefore, previous studies have suggested the use of recombinant protein vaccines based on bacterial [60], mammalian [61], and recombinant baculovirus/insect cell expression system [62] for controlling HPAIVs. Among the viral proteins of H5 AI viruses, the HA and matrix protein 2 ectodomains (M2e) have been developed as candidate antigens for recombinant protein vaccines [60,62,63].
HA is an envelope glycoprotein and it is best-suited for inducing the production of neutralizing antibodies. Thus, to control H5 HPAIVs, HA is being developed predominantly using several recombinant expression systems [64,65,66]. Saczynska [66] and Liu et al. [67] used bacterial and baculoviral systems, respectively, for expressing HA of H5N1. These studies demonstrated that HA was able to induce antigen-specific neutralizing antibody response and vaccinated chickens could be protected against the highly pathogenic H5 influenza virus.
Matrix 2 (M2) is transmembrane protein and it plays key roles in viral replication and structural integrity [68,69]. The extracellular domain of M2 (M2e) consists of 24 amino acids and it is one of the highly conserved domains across different subtypes of influenza viruses. Thus, M2e is one of the candidates for developing a universal influenza vaccine [70,71]. The M2e vaccine candidates have induced immune responses and protective efficacy against highly pathogenic H5N1 viruses in chickens [72,73]. However, several studies showed that M2e has lower immunogenicity, and therefore, requires carrier proteins [74,75] and co-administration with vaccine adjuvants such as water-in-oil based adjuvants [76] for inducing antigen-specific immunogenicity.
Universal vaccines
Universal vaccines provide broad cross-protective immunity than the currently licensed vaccines and should provide longer protection against several relevant influenza viruses. The globular head domain of HA plays a critical role in producing potent neutralizing antibodies, which indicates that HA can induce the production of neutralizing antibodies against only homologous viruses [1,77]. Therefore, many investigators demonstrated that highly conserved sequences such as the stem region of HA (called HA2) and M2e are more desirable targets for generating a universal vaccine [78,79,80]. Consist with the above results, our group also confirmed that the HA2 domain induced the production of a broad spectrum of neutralizing activities against different avian strains containing H5 subtypes (unpublished). In addition, recent studies demonstrated that different combinations of vaccines increased the efficacy of universal vaccines. The prime-boost strategy with plasmid DNA and other vaccine candidates such as virus-like particle [81] and inactivated viruses [82] increased neutralizing activities with homologous and heterologous viruses. Thus, strategies involving recombinant proteins and combinatorial vaccines should be developed for generating effective and safe universal influenza vaccines.
Conclusion
Nowadays, sub-lineages of influenza H5 virus are rapidly evolving and they are considered to be the most likely cause of infectious outbreaks in poultry. Recently, the outbreak of the novel and highly pathogenic H5N6 and H5N8 AI virus was reported in poultry in South Korea. These viruses belong to clade 2.3.4.4 and showed a close relationship with HPAIs identified from Eastern China and South Korea. However, the route of virus dissemination is still unclear. Therefore, these concerns collectively call for continued virus monitoring, surveillance of poultry and wild birds, and characterization and pathogenicity assessment of these viruses, the information pertaining to which should be made available before any outbreak in poultry.
To protect against novel H5 HPAIs in poultry and to overcome the limitations of conventional vaccines, we attempted to develop new vaccine candidates with broad cross-protectivity against influenza viruses. However, H5 HPAI vaccination is still a complex issue considering scientific regulations and policies of vaccine application in the field.
Footnotes
No potential conflict of interest relevant to this article was reported.
This work was supported grants of the KRIBB Initiative program, supported by the BioNano Health-Guard Research Center funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea as Global Frontier Project (Grant No. H-GUARD 2013M3A6B2078954) and supported by Animal Disease Management Technology Development, Ministry of Agriculture, Food and Rural Affairs (Grant No. 116101-03), and supported by Korea Ministry of Environment (MOE) as “Public Technology Program based on Environmental Policy (No. 2016000210002)”.
References
- 1.Medina RA, Garcia-Sastre A. Influenza A viruses: new research developments. Nat Rev Microbiol. 2011;9:590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong S, Zhu X, Li Y, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza A viruses. Curr Top Microbiol Immunol. 2014;385:359–375. doi: 10.1007/82_2014_396. [DOI] [PubMed] [Google Scholar]
- 4.Kim YI, Pascua PN, Kwon HI, et al. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerg Microbes Infect. 2014;3:e75. doi: 10.1038/emi.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu M, Zhao G, Zhao K, et al. Novel variants of clade 2.3.4 highly pathogenic avian influenza A(H5N1) viruses, China. Emerg Infect Dis. 2013;19:2021–2024. doi: 10.3201/eid1912.130340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasick J, Berhane Y, Joseph T, et al. Reassortant highly pathogenic influenza A H5N2 virus containing gene segments related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci Rep. 2015;5:9484. doi: 10.1038/srep09484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeJesus E, Costa-Hurtado M, Smith D, et al. Changes in adaptation of H5N2 highly pathogenic avian influenza H5 clade 2.3.4.4 viruses in chickens and mallards. Virology. 2016;499:52–64. doi: 10.1016/j.virol.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Si YJ, Lee IW, Kim EH, et al. Genetic characterisation of novel, highly pathogenic avian influenza (HPAI) H5N6 viruses isolated in birds, South Korea, November 2016. Euro Surveill. 2017;22:30434. doi: 10.2807/1560-7917.ES.2017.22.1.30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torremorell M, Alonso C, Davies PR, et al. Investigation into the airborne dissemination of H5N2 highly pathogenic avian influenza virus during the 2015 spring outbreaks in the midwestern United States. Avian Dis. 2016;60:637–643. doi: 10.1637/11395-021816-Reg.1. [DOI] [PubMed] [Google Scholar]
- 10.Stoute S, Chin R, Crossley B, et al. Highly pathogenic Eurasian H5N8 avian influenza outbreaks in two commercial poultry flocks in California. Avian Dis. 2016;60:688–693. doi: 10.1637/11314-110615-Case.1. [DOI] [PubMed] [Google Scholar]
- 11.Adlhoch C, Brown IH, Angelova SG, et al. Highly pathogenic avian influenza A(H5N8) outbreaks: protection and management of exposed people in Europe, 2014/15 and 2016. Euro Surveill. 2016;21:30419. doi: 10.2807/1560-7917.ES.2016.21.49.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan BS, Russier M, Jeevan T, et al. Novel highly pathogenic avian A(H5N2) and A(H5N8) influenza viruses of clade 2.3.4.4 from north America have limited capacity for replication and transmission in mammals. mSphere. 2016;1:e00003–e00016. doi: 10.1128/mSphere.00003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon JH, Lee DH, Swayne DE, et al. Reassortant clade 2.3.4.4 avian influenza A(H5N6) virus in a wild mandarin duck, South Korea, 2016. Emerg Infect Dis. 2017;23:822–826. doi: 10.3201/eid2305.161905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EK, Song BM, Lee YN, et al. Multiple novel H5N6 highly pathogenic avian influenza viruses, South Korea, 2016. Infect Genet Evol. 2017;51:21–23. doi: 10.1016/j.meegid.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Sonnberg S, Webby RJ, Webster RG. Natural history of highly pathogenic avian influenza H5N1. Virus Res. 2013;178:63–77. doi: 10.1016/j.virusres.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries E, Guo H, Dai M, Rottier PJ, van Kuppeveld FJ, de Haan CA. Rapid emergence of highly pathogenic avian influenza subtypes from asubtype H5N1 hemagglutinin variant. Emerg Infect Dis. 2015;21:842–846. doi: 10.3201/eid2105.141927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abolnik C. Evolution of H5 highly pathogenic avian influenza: sequence data indicate stepwise changes in the cleavage site. Arch Virol. 2017 Mar 30; doi: 10.1007/s00705-017-3337-x. [Epub] [DOI] [PubMed] [Google Scholar]
- 18.Lee CW, Suarez DL, Tumpey TM, et al. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol. 2005;79:3692–3702. doi: 10.1128/JVI.79.6.3692-3702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon H, Park CK, Nam HM, Wee SH. Virus spread pattern within infected chicken farms using regression model: the 2003-2004 HPAI epidemic in the Republic of Korea. J Vet Med B Infect Dis Vet Public Health. 2005;52:428–431. doi: 10.1111/j.1439-0450.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim HR, Park CK, Lee YJ, et al. An outbreak of highly pathogenic H5N1 avian influenza in Korea, 2008. Vet Microbiol. 2010;141:362–366. doi: 10.1016/j.vetmic.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Choi YK, Kim YJ, et al. Highly pathogenic avian influenza virus (H5N1) in domestic poultry and relationship with migratory birds, South Korea. Emerg Infect Dis. 2008;14:487–490. doi: 10.3201/eid1403.070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HR, Lee YJ, Park CK, et al. Highly pathogenic avian influenza (H5N1) outbreaks in wild birds and poultry, South Korea. Emerg Infect Dis. 2012;18:480–483. doi: 10.3201/eid1803.111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong J, Kang HM, Lee EK, et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet Microbiol. 2014;173:249–257. doi: 10.1016/j.vetmic.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee YJ, Kang HM, Lee EK, et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis. 2014;20:1087–1089. doi: 10.3201/eid2006.140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song BM, Lee EK, Lee YN, Heo GB, Lee HS, Lee YJ. Phylogeographical characterization of H5N8 viruses isolated from poultry and wild birds during 2014-2016 in South Korea. J Vet Sci. 2017;18:89–94. doi: 10.4142/jvs.2017.18.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Consortium for H5N8 and Related Influenza Viruses. Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 2016;354:213–217. doi: 10.1126/science.aaf8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon JH, Lee DH, Swayne DE, et al. Highly pathogenic avian influenza A(H5N8) viruses reintroduced into South Korea by migratory waterfowl, 2014-2015. Emerg Infect Dis. 2016;22:507–510. doi: 10.3201/eid2203.151006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang HM, Lee EK, Song BM, et al. Experimental infection of mandarin duck with highly pathogenic avian influenza A (H5N8 and H5N1) viruses. Vet Microbiol. 2017;198:59–63. doi: 10.1016/j.vetmic.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Chen LH, Chen YP, et al. Highly pathogenic avian influenza viruses H5N2, H5N3, and H5N8 in Taiwan in 2015. Vet Microbiol. 2016;187:50–57. doi: 10.1016/j.vetmic.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Short KR, Richard M, Verhagen JH, et al. One health, multiple challenges: the inter-species transmission of influenza A virus. One Health. 2015;1:1–13. doi: 10.1016/j.onehlt.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi X, Cui L, Yu H, Ge Y, Tang F. Whole-genome sequence of a reassortant H5N6 avian influenza virus isolated from a live poultry market in China, 2013. Genome Announc. 2014;2:e00706–e00714. doi: 10.1128/genomeA.00706-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su S, Bi Y, Wong G, Gray GC, Gao GF, Li S. Epidemiology, evolution, and recent outbreaks of avian influenza virus in China. J Virol. 2015;89:8671–8676. doi: 10.1128/JVI.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong J, Woo C, Ip HS, et al. Identification of two novel reassortant avian influenza a (H5N6) viruses in whooper swans in Korea, 2016. Virol J. 2017;14:60. doi: 10.1186/s12985-017-0731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Wu P, Uyeki TM, et al. Preliminary epidemiologic assessment of human infections with highly pathogenic avian influenza A(H5N6) virus, China. Clin Infect Dis. 2017 Apr 12; doi: 10.1093/cid/cix334. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neher RA, Russell CA, Shraiman BI. Predicting evolution from the shape of genealogical trees. Elife. 2014;3 doi: 10.7554/eLife.03568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 37.Uyeki TM. Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin Infect Dis. 2009;49:279–290. doi: 10.1086/600035. [DOI] [PubMed] [Google Scholar]
- 38.Check E. Avian flu special: is this our best shot? Nature. 2005;435:404–406. doi: 10.1038/435404a. [DOI] [PubMed] [Google Scholar]
- 39.Chen H. H5N1 avian influenza in China. Sci China C Life Sci. 2009;52:419–427. doi: 10.1007/s11427-009-0068-6. [DOI] [PubMed] [Google Scholar]
- 40.Webby RJ, Perez DR, Coleman JS, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian G, Zhang S, Li Y, et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology. 2005;341:153–162. doi: 10.1016/j.virol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Capua I, Cattoli G. Prevention and control of highly pathogenic avian influenza with particular reference to H5N1. Virus Res. 2013;178:114–120. doi: 10.1016/j.virusres.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 43.WHO/OIE/FAO H5N1 Evolution Working Group. Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:e1. doi: 10.3201/eid1407.071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng X, Chen P, Liu L, et al. Protective Efficacy of an H5N1 inactivated vaccine against challenge with lethal H5N1, H5N2, H5N6, and H5N8 influenza viruses in chickens. Avian Dis. 2016;60(1 Suppl):253–255. doi: 10.1637/11179-052015-ResNoteR. [DOI] [PubMed] [Google Scholar]
- 45.Choi Y, Chang J. Viral vectors for vaccine applications. Clin Exp Vaccine Res. 2013;2:97–105. doi: 10.7774/cevr.2013.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niqueux E, Guionie O, Amelot M, Jestin V. Prime-boost vaccination with recombinant H5-fowlpox and Newcastle disease virus vectors affords lasting protection in SPF Muscovy ducks against highly pathogenic H5N1 influenza virus. Vaccine. 2013;31:4121–4128. doi: 10.1016/j.vaccine.2013.06.074. [DOI] [PubMed] [Google Scholar]
- 47.Pantin-Jackwood MJ, Kapczynski DR, DeJesus E, et al. Efficacy of a recombinant Turkey herpesvirus H5 vaccine against challenge with H5N1 clades 1.1.2 and 2.3.2.1 highly pathogenic avian influenza viruses in domestic ducks (Anas platyrhynchos domesticus) Avian Dis. 2016;60:22–32. doi: 10.1637/11282-091615-Reg.1. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Ge A, Xu M, et al. Construction of a recombinant duck enteritis virus (DEV) expressing hemagglutinin of H5N1 avian influenza virus based on an infectious clone of DEV vaccine strain and evaluation of its efficacy in ducks and chickens. Virol J. 2015;12:126. doi: 10.1186/s12985-015-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H. Avian influenza vaccination: the experience in China. Rev Sci Tech. 2009;28:267–274. doi: 10.20506/rst.28.1.1860. [DOI] [PubMed] [Google Scholar]
- 50.van den Berg T, Lambrecht B, Marche S, Steensels M, Van Borm S, Bublot M. Influenza vaccines and vaccination strategies in birds. Comp Immunol Microbiol Infect Dis. 2008;31:121–165. doi: 10.1016/j.cimid.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Kim SH, Samal SK. Newcastle disease virus as a vaccine vector for development of human and veterinary vaccines. Viruses. 2016;8:E183. doi: 10.3390/v8070183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge J, Deng G, Wen Z, et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81:150–158. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JK, Lee DH, Yuk SS, et al. Virus-like particle vaccine confers protection against a lethal newcastle disease virus challenge in chickens and allows a strategy of differentiating infected from vaccinated animals. Clin Vaccine Immunol. 2014;21:360–365. doi: 10.1128/CVI.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stachyra A, Gora-Sochacka A, Sirko A. DNA vaccines against influenza. Acta Biochim Pol. 2014;61:515–522. [PubMed] [Google Scholar]
- 55.Jiang Y, Yu K, Zhang H, et al. Enhanced protective efficacy of H5 subtype avian influenza DNA vaccine with codon optimized HA gene in a pCAGGS plasmid vector. Antiviral Res. 2007;75:234–241. doi: 10.1016/j.antiviral.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stachyra A, Redkiewicz P, Kosson P, et al. Codon optimization of antigen coding sequences improves the immune potential of DNA vaccines against avian influenza virus H5N1 in mice and chickens. Virol J. 2016;13:143. doi: 10.1186/s12985-016-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith LR, Wloch MK, Ye M, et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine. 2010;28:2565–2572. doi: 10.1016/j.vaccine.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Shen X, Soderholm J, Lin F, et al. Influenza A vaccines using linear expression cassettes delivered via electroporation afford full protection against challenge in a mouse model. Vaccine. 2012;30:6946–6954. doi: 10.1016/j.vaccine.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 60.Saczynska V, Romanik A, Florys K, et al. A novel hemagglutinin protein produced in bacteria protects chickens against H5N1 highly pathogenic avian influenza viruses by inducing H5 subtype-specific neutralizing antibodies. PLoS One. 2017;12:e0172008. doi: 10.1371/journal.pone.0172008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu CY, Yeh YC, Yang YC, et al. Mammalian expression of virus-like particles for advanced mimicry of authentic influenza virus. PLoS One. 2010;5:e9784. doi: 10.1371/journal.pone.0009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nwe N, He Q, Damrongwatanapokin S, et al. Expression of hemagglutinin protein from the avian influenza virus H5N1 in a baculovirus/insect cell system significantly enhanced by suspension culture. BMC Microbiol. 2006;6:16. doi: 10.1186/1471-2180-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ernst WA, Kim HJ, Tumpey TM, et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Crawford J, Wilkinson B, Vosnesensky A, et al. Baculovirus-derived hemagglutinin vaccines protect against lethal influenza infections by avian H5 and H7 subtypes. Vaccine. 1999;17:2265–2274. doi: 10.1016/s0264-410x(98)00494-0. [DOI] [PubMed] [Google Scholar]
- 65.Cornelissen LA, de Vries RP, de Boer-Luijtze EA, Rigter A, Rottier PJ, de Haan CA. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PLoS One. 2010;5:e10645. doi: 10.1371/journal.pone.0010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saczynska V. Influenza virus hemagglutinin as a vaccine antigen produced in bacteria. Acta Biochim Pol. 2014;61:561–572. [PubMed] [Google Scholar]
- 67.Liu G, Zhang F, Shi J, et al. A subunit vaccine candidate derived from a classic H5N1 avian influenza virus in China protects fowls and BALB/c mice from lethal challenge. Vaccine. 2013;31:5398–5404. doi: 10.1016/j.vaccine.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 69.Pinto LH, Lamb RA. The M2 proton channels of influenza A and B viruses. J Biol Chem. 2006;281:8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 70.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 71.Hughey PG, Roberts PC, Holsinger LJ, Zebedee SL, Lamb RA, Compans RW. Effects of antibody to the influenza A virus M2 protein on M2 surface expression and virus assembly. Virology. 1995;212:411–421. doi: 10.1006/viro.1995.1498. [DOI] [PubMed] [Google Scholar]
- 72.Zhao G, Lin Y, Du L, et al. An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol J. 2010;7:9. doi: 10.1186/1743-422X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elaish M, Ngunjiri JM, Ali A, et al. Supplementation of inactivated influenza vaccine with norovirus P particle-M2e chimeric vaccine enhances protection against heterologous virus challenge in chickens. PLoS One. 2017;12:e0171174. doi: 10.1371/journal.pone.0171174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dabaghian M, Latify AM, Tebianian M, et al. Vaccination with recombinant 4 x M2e.HSP70c fusion protein as a universal vaccine candidate enhances both humoral and cell-mediated immune responses and decreases viral shedding against experimental challenge of H9N2 influenza in chickens. Vet Microbiol. 2014;174:116–126. doi: 10.1016/j.vetmic.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 75.De Filette M, Martens W, Smet A, et al. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine. 2008;26:6503–6507. doi: 10.1016/j.vaccine.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 76.Wu F, Yuan XY, Huang WS, Chen YH. Heterosubtypic protection conferred by combined vaccination with M2e peptide and split influenza vaccine. Vaccine. 2009;27:6095–6101. doi: 10.1016/j.vaccine.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 77.Ndifon W, Wingreen NS, Levin SA. Differential neutralization efficiency of hemagglutinin epitopes, antibody interference, and the design of influenza vaccines. Proc Natl Acad Sci U S A. 2009;106:8701–8706. doi: 10.1073/pnas.0903427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uranowska K, Tyborowska J, Jurek A, Szewczyk B, Gromadzka B. Hemagglutinin stalk domain from H5N1 strain as a potentially universal antigen. Acta Biochim Pol. 2014;61:541–550. [PubMed] [Google Scholar]
- 79.Ekiert DC, Wilson IA. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr Opin Virol. 2012;2:134–141. doi: 10.1016/j.coviro.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nayak B, Kumar S, DiNapoli JM, et al. Contributions of the avian influenza virus HA, NA, and M2 surface proteins to the induction of neutralizing antibodies and protective immunity. J Virol. 2010;84:2408–2420. doi: 10.1128/JVI.02135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steel J, Lowen AC, Wang TT, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1:e00018–e00010. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suguitan AL, Jr, Cheng X, Wang W, Wang S, Jin H, Lu S. Influenza H5 hemagglutinin DNA primes the antibody response elicited by the live attenuated influenza A/Vietnam/1203/2004 vaccine in ferrets. PLoS One. 2011;6:e21942. doi: 10.1371/journal.pone.0021942. [DOI] [PMC free article] [PubMed] [Google Scholar]