Abstract
The aging brain undergoes several anatomical changes that can be measured with Magnetic Resonance Imaging (MRI). Early studies using lower field strengths have assessed changes in tissue properties mainly qualitatively, using - or - weighted images to provide image contrast. With the development of higher field strengths (7 T and above) and more advanced MRI contrasts, quantitative measures can be acquired even of small subcortical structures. This study investigates volumetric, spatial, and quantitative MRI parameter changes associated with healthy aging in a range of subcortical nuclei, including the basal ganglia, red nucleus, and the periaqueductal grey. The results show that aging has a heterogenous effects across regions. Across the subcortical areas an increase of values is observed, most likely indicating a loss of myelin. Only for a number of areas, a decrease of and increase of QSM is found, indicating an increase of iron. Aging also results in a location shift for a number of structures indicating the need for visualization of the anatomy of individual brains.
Keywords: Ultra-high field 7 T MRI, , , QSM, Subcortex, Basal ganglia, Aging
Introduction
Healthy aging across the adult lifespan is known to have a diverse effect on the anatomy of the brain. Historically, anatomical studies were performed on post-mortem specimens but with the development of magnetic resonance imaging (MRI) in vivo individual anatomy can now be visualized non-invasively. Several age-related findings which are frequently reported are the ventricular enlargement (e.g., Good et al. 2001; Raz and Rodrigue 2006; Greenberg et al. 2008; Keuken et al. 2013), changes in white matter microstructure (e.g., Walhovd et al. 2005; Benedetti et al. 2006; Raz and Rodrigue 2006), reduced grey matter volume (e.g., Courchesne et al. 2000; Good et al. 2001; Cherubini et al. 2009; Lemaitre et al. 2012), shifted location of gray matter nuclei (e.g., Dunnen and Staal 2005; Kitajima et al. 2008; Keuken et al. 2013; Mavridis et al. 2014), and an increase in iron deposition (e.g., Hallgren and Sourander 1958; Schenker et al. 1993; Zecca et al. 2004; Raz and Rodrigue 2006; Aquino et al. 2009; Pfefferbaum et al. 2009). These aging effects can vary locally across the brain (e.g., Greenberg et al. 2008; Cherubini et al. 2009; Draganski et al. 2011; Lemaitre et al. 2012). In addition to these anatomical changes, previous work shows changes in MRI parameters with age (e.g., Schenker et al. 1993; Steen et al. 1995; Courchesne et al. 2000; Good et al. 2001; Benedetti et al. 2006; Bastin et al. 2009; Saito et al. 2009; Cherubini et al. 2009; Draganski et al. 2011; Bilgic et al. 2012; Lebel et al. 2012; Lemaitre et al. 2012; Li et al. 2013; Yeatman et al. 2014; Lorio et al. 2014; Persson et al. 2015; Acosta-Cabronero et al. 2016; Betts et al. 2016). Changes in values are frequently reported and have been interpreted as changes in myelin structure (e.g., Silver et al. 1997; Maniega et al. 2015). Another common finding is the shortening of values, which have been interpreted as an indication of iron accumulation (Daugherty and Raz 2013).
The value indicates the recovery time of the longitudinal component of the magnetization following the application of a radio frequency excitation pulse. The quantitative values correlate highly with myelin content (Koenig 1991; Stüber et al. 2014) and can be used as an in vivo proxy for the underlying myeloarchitecture (Lutti et al. 2014; Dinse et al. 2015). The value indicates the decay of the transverse magnetization component as a result of proton interactions and magnetic field inhomogeneity. The values have been used as a proxy for iron, but recently several studies have demonstrated that the values are also strongly influenced by the presence and orientation of myelin (Fukunaga et al. 2010; Lee et al. 2010; Cohen-Adad et al. 2012; Stüber et al. 2014). Iron content in gray matter can be estimated more precisely using a post-processing technique called Quantitative Susceptibility Mapping (QSM) on the phase signal present in the -weighted volumes (Schweser et al. 2011, 2016; Bilgic et al. 2012; Langkammer et al. 2012; Stüber et al. 2014; Ropele and Langkammer 2016). QSM quantifies the susceptibility distribution by estimating the magnetic field distribution, removing the background field contribution, and solves the inverse problem from field perturbation to magnetic susceptibility (Schweser et al. 2016).
Many age-related MRI studies have been limited by the use of low field strength or qualitative MRI sequences. Using low field strength such as 1.5 or 3T makes it difficult to visualize smaller nuclei such as the subthalamic nucleus or to discriminate the internal and external segment of the globus pallidus (e.g., Cho et al. 2008, 2010; Abosch et al. 2010; Beisteiner et al. 2011; Lenglet et al. 2012; Keuken et al. 2014).
The current study set out to describe the effects of aging on the volumetric and spatial properties, and to provide quantitative , , and QSM values at 7T static magnetic field for the striatum (STR), external segment of the globus pallidus (GPe), the internal segment of the globus pallidus (GPi), the red nucleus (RN), the subthalamic nucleus (STN), the substantia nigra (SN), and the periaqueductal grey (PAG). This was done by manually parcellating the different regions in structural ultra-high resolution 7T MRI data in three different age groups.
Methods
Participants
Thirty young participants with an average age of 23.8 years (age range 19–29; SD = 2.3, 14 females) which have been previously reported (Keuken et al. 2014) were included. In addition to the young participants, a second group of 14 middle-aged participants were scanned, with an average age of 52.5 years (age range 40–60, SD = 6.6, 7 females). Finally, a third group of 10 elderly participants were included, with an average age of 69.6 years (age range 60–75, SD = 4.6, 3 females). One elderly subject (male, age 73) did not complete all scans due to time constraints and was excluded from all further analyses. All subjects were right-handed, as confirmed by the Edinburgh Inventory (Oldfield 1971). None of the participants had a history of neurological disorders or currently suffered from psychiatric disorders as indicated by self-report and structured clinical interview. The study was approved by the local ethical committee of the Max Planck Institute for Human Brian and Cognitive Sciences in Leipzig, Germany.
MRI acquisition
The structural data were acquired using a 7T Siemens Magnetom MRI scanner using a 24-channel head array Nova coil (NOVA Medical Inc., Wilmington MA) and consisted of three sequences: a whole brain MP2RAGE (Marques et al. 2010), a MP2RAGE covering a smaller slab, and a multi-echo 3D FLASH (Haase et al. 1986). The whole brain MP2RAGE had 240 sagittal slices with an acquisition time of 10:57 min (repetition time (TR) = 5000 ms; echo time (TE) = 2.45 ms; inversion times TI1/TI2 = 900/2750 ms; flip angle = 5°/3°; bandwidth = 250 Hz/Px; voxel size = 0.7 mm isotropic). The MP2RAGE slab consisted of 128 slices with an acquisition time of 9:07 min (TR = 5,000 ms; TE = 3.71 ms; TI1/TI2 = 900/2,750 ms; flip angle = 5°/3°; bandwidth = 240 Hz/Px; voxel size = 0.6 mm isotropic). The MP2RAGE sequence is a -weighted structural scan but with the additional feature that it also provides a so-called map (Marques et al. 2010). The sequence is based on two volumes with different inversion times (the INV1 and INV2 volumes), which can be combined into a single -weighted volume (UNI) or used to estimate the values. The resulting map gives reasonable estimates of the underlying values and has been shown to be highly reliable within subjects across scan sessions and scanners (Okubo et al. 2015; Voelker et al. 2016). It should, however, be noted that these maps may still contain residual transmit field bias (Marques and Gruetter 2013; Lutti et al. 2014).
The FLASH slab consisted of 128 slices with an acquisition time of 17:18 min (TR = 41 ms and three different TE: 11.22/20.39/29.57 ms; flip angle = 14°; bandwidth = 160 Hz/Px; voxel size = 0.5 mm isotropic). Both slab sequences consisted of axial slices tilted −23° to the true axial plane in scanner coordinates. See (Forstmann et al. 2014) for more information regarding the exact MRI parameters and data quality. All structural data have been made freely available and can be found on http://www.nitrc.org/projects/atag_mri_scans/ and http://datadryad.org/resource/doi:10.5061/dryad.fb41s.
Manual parcellation of subcortical structures
The STR, GPe, GPi, RN, STN, and SN masks for the young group have been previously presented in (Keuken et al. 2014). For the young group, the PAG, lateral ventricle, third ventricle, cerebral aqueduct, and fourth ventricle were additionally parcellated and have not been published previously in the young group for this study. For the middle-aged and elderly group the STR, GPe, GPi, RN, STN, SN, PAG, lateral ventricle, third ventricle, cerebral aqueduct, and fourth ventricle were parcellated using the same parcellation protocol (Keuken et al. 2014). In short, the manual parcellation was performed using the FSL 4.1.4 viewer (http://fsl.fmrib.ox.ac.uk/fsl/fslview/) by two independent raters. Based on previous results (Keuken et al. 2014) the STR was segmented on the UNI MP2RAGE slab volumes, the STN, SN, and RN on the FLASH volumes, and finally the GPe and GPi on the QSM volumes. The PAG and ventricle system were parcellated on the UNI MP2RAGE whole brain volumes. The resolution and lack of contrast of the in vivo MP2RAGE scans made it very difficult to distinguish the different sub compartments of the STR (Voorn et al. 2004; Haber and Gdowski 2004; Neto et al. 2008; Haber and Knutson 2009; Keuken et al. 2014). Therefore, the STR was segmented as a whole without attempting to make distinctions between the different subparts which would have to be based on surrounding landmarks. The GPe and GPi were segmented on the QSM images as the image quality was not sufficient to clearly separate the interlamina between the GPe and GPi, whereas this was the case for the QSM (Keuken et al. 2014).
After parcellation of the structure, the inter-rater agreement was assessed using the Dice coefficient (Dice 1945). The resulting conjunction masks were used for further analysis unless stated otherwise. All volume estimates are calculated in the native space of the MRI sequence in which the structure was parcellated. The intracranial volume estimate was calculated using the skull stripped whole brain MP2RAGE unified volume, using BET (Smith 2002). Because the whole brain MP2RAGE scan was skull stripped automatically, no inter-rater agreement was assessed.
Due to the time-consuming nature of manual parcellation, it was not possible to have the same raters for the middle-aged and elderly group as in the young group that was published previously (Keuken et al. 2014). Therefore, it might be the case that some of the effects of aging are actually due to different raters for the young age group versus the middle-aged and elderly group. To test whether this could influence the general conclusion, the same raters that segmented the RN and STN for the middle-aged and elderly group, redid the parcellation for the young RN and STN masks, which allowed testing the influence on the results of different raters for different age groups.
Registration to standard stereotactic MNI-space
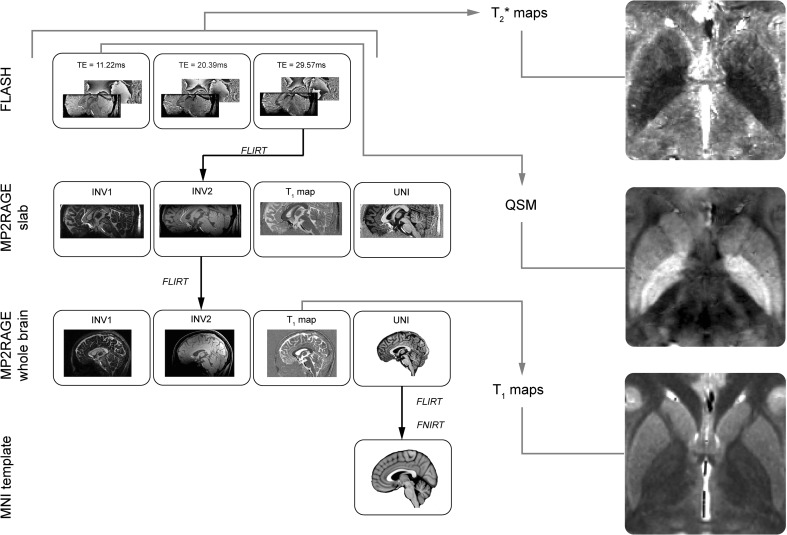
The third echo time of the FLASH sequence was linearly registered to the MP2RAGE slab second inversion volume using Mutual information and 6 Degrees of Freedom (DoF). The MP2RAGE slab second inversion volume was registered to the MP2RAGE whole brain second inversion volume using correlation ratio information and 6 DoF. The MP2RAGE whole brain unified volumes were registered to the MNI152 0.5 mm template using correlation ratio information and 12 DoF. All linear registrations used tri-linear interpolation and were done using FLIRT (Jenkinson and Smith 2001; Jenkinson et al. 2002) as implemented in FSL 5.0.2. Prior to the registration to MNI standard space, both the MP2RAGE whole brain and MNI152 template were skull stripped using BET (Smith 2002). Finally, the MP2RAGE whole brain unified volumes were non-linearly registered to the MNI152 0.5 mm template using the linearly registered volumes as input for FNIRT in combination with the default settings of FNIRT (Andersson et al. 2007; Jenkinson et al. 2012). The FLASH and MP2RAGE slab images were transformed to the MNI152 template by combining the resulting transformation matrices from the FLASH to MP2RAGE slab, MP2RAGE slab to MP2RAGE whole brain, and MP2RAGE whole brain to MNI152 registration. All individual registration steps were visually checked for misalignment. All parcellated structures were transformed to the MNI152 template using the corresponding transformation matrices and added together to create a probabilistic atlas of each structure separately. This was done both for the linear and non-linearly registered structures. All the probabilistic atlases are made freely available and can be found on https://www.nitric.org/projects/atag/. See Fig. 1 for a flowchart of the registration pipeline.
Fig. 1.
The registration pipeline. The RN, STN, and SN were parcellated on the FLASH volumes. The GPe and GPi were parcellated on the QSM volumes which were based on the first echo time of the FLASH volumes. The STR was parcellated on the MP2RAGE slab UNI volume whereas the ventricle compartments and the PAG were parcellated on the MP2RAGE whole brain UNI volume. Using the resulting transformation matrices, the conjunction masks were transformed to the , QSM, or map space so that the quantitative MRI values could be extracted. Examples of the , QSM, and maps of a young participant are shown on the right
Statistical analyses were done in R 3.2.4 (http://www.r-project.org; R Development Core Team 2013). All correlation and partial correlation tests were done using a two-sided Pearson’s r test with a critical α of 0.05 using the individual age as a continuous variable. The three age groups were only used as a discrete variable to provide the summary statistics. A Bonferroni correction was used to correct for multiple comparisons per family of tests. For instance, the Bonferroni correction for the correlations between age and the Dice coefficient was based on 11 tests which correspond to the number of structures for which the Dice coefficient was calculated. All reported p values are after Bonferroni correction. For all significant correlations, 95% confidence intervals were estimated using the function predict as implemented in R and plotted in Figs. 2 and 3
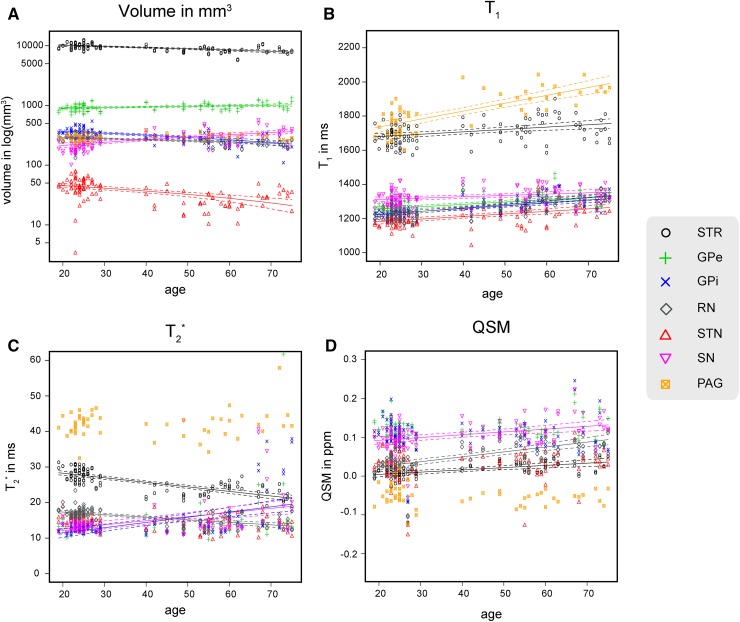
Fig. 2.
The volume, , , and QSM values per structure for each individual participant. The regression lines indicate a significant correlation with age. The dotted lines indicate the 95% confidence interval. STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey
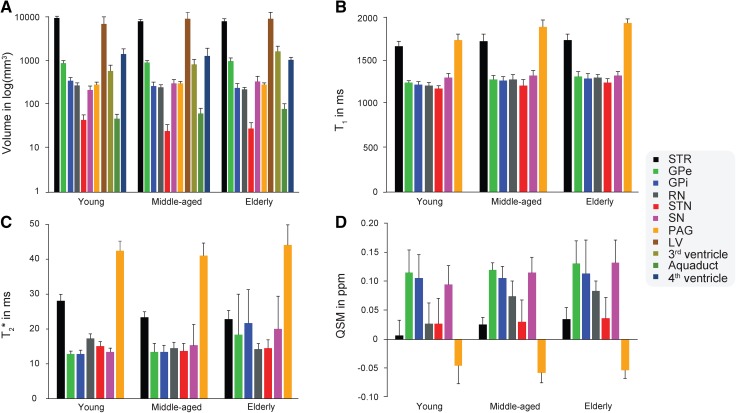
Fig. 3.
The average volume, , , and QSM values across hemispheres per structure for each age group. The error bars indicate 1 sd. STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey, LV lateral ventricle
Effect of age on location
To test whether age had an effect on the location of the gray matter nuclei in standard MNI space, the following analysis was done. First, the Center of Mass (CoM) was calculated for each linear registered structure using FSLUTILS (FSL 5.0.2). To reduce the number of tests, a Principal Component Analysis (PCA) was computed on the X, Y, and Z CoM coordinates of each individual structure using princomp in R Team (2013). Since we had no a-priori hypothesis regarding lateralization and age, the negative X-coordinate values, corresponding to the left hemisphere, were converted to positive values and combined in a single PCA analysis. The resulting first principal component corresponds to a new latent variable which captures the maximal amount of variance. Finally, the eigenvector scores of the first principal component of each structure and participant were correlated with age. This analysis allows us to test whether there is a relationship between the individual spatial location, as indicated by the individual eigenvector scores, and age.
Computation of the , , and QSM values
The values for the STR, GPe, GPi, RN, STN, SN, and PAG were extracted from the MP2RAGE whole brain map. The values for the STR, GPe, GPi, RN, STN, SN, and PAG were calculated using the fitting module as implemented in the CBS High-Res Brain Processing Tools for MIPAV (http://www.nitrc.org/projects/cbs-tools/) (Bazin et al. 2013). This module uses a nonlinear least squares function to estimate a single-component map (Whittall et al. 1997):
Possibly due to the interpolation between different MRI scans or intrinsic partial voluming, the STR and PAG masks contained voxels which, based on the , values, and the spatial location, belonged to the lateral ventricle or cerebral aqueduct. Extracting the mean and values from these voxels would result in a skewed estimate given that the and values of the CSF are considerably distinct from those in the gray matter ROIs (McRobbie et al. 2006). We addressed this by calculating the mean and values of the ventricle compartment or the cerebral aqueduct and used that value as an upper threshold when extracting the mean and values for the STR and PAG. For completeness we also tested the effects of aging on the non-thresholded and values for the STR and PAG.
The QSM was calculated using the phase information of the first echo time of the FLASH acquisition and the method proposed by Schweser et al. (2012). The first echo time was chosen because it had the highest absolute SNR. The coil combination of phase data was done automatically by the scanner vendor software (version VB17). This automatic coil combination results in some minor phase singularities, but are partially accounted by the employed superfast dipole inversion (SDI) approach which, includes a modified SHARP algorithm. The modified SHARP algorithm is described in Schweser et al. (2012). The masking of the data was done using BET which was manually adjusted if the binary mask was too lenient. This was determined by visual inspection on a subject to subject basis. The QSM intensities were normalized by subtracting the mean QSM value of the combined lateral ventricle conjunction masks from the main QSM volume. This normalisation was necessary due to the arbitrary settings of the resonance frequency, use of high pass filtering, and the use of a single echo to calculate the QSM values (Schäfer et al. 2009; Langkammer et al. 2012). To ensure that no gray matter tissue was included in the lateral ventricle masks, the masks were first eroded with a 2 mm Gaussian kernel. All -, -maps, and QSM were calculated in native space.
Results
Inter-rater reliability
All segmentations resulted in a good to excellent mean agreement between the two raters, indicating that it is feasible to identify these subcortical structures in individual space. See Table 1 for the Dice coefficient per structure and age group. There were, however, different effects of age on the inter-rater reliability for these separate structures. For the STR, RN, SN, LV, and third ventricle there were positive correlations between the Dice coefficient and age (STR: r = 0.58, t(104) = 7.22, p < 0.001; RN: r = 0.48, t(104) = 5.57, p ≤ 0.001; SN: r = 0.33, t(104) = 3.59, p = 0.006; third ventricle: r = 0.63, t(51) = 5.75, p < 0.001), indicating that the inter-rater reliability increases with age. This was the opposite for the GPe and the GPi as there was a negative correlation between the Dice coefficient and age (GPe: r = −0.46, t(104) = −5.30, p < 0.001; GPi: r = −0.54, t(104) = −6.62, p < 0.001). There was no significant correlation between the Dice coefficient and age for the STN, PAG, LV, cerebral aqueduct, and fourth ventricle.
Table 1.
The inter-rater reliability coefficient and conjunction volume in mm3 estimates
| Structure | Young | Middle aged | Elderly | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume | Dice coefficient | Volume | Dice coefficient | Volume | Dice coefficient | |||||||
| Mean mm3 | SD | Mean | SD | Mean mm3 | SD | Mean | SD | Mean mm3 | SD | Mean | SD | |
| Intracranial volume | 1545970.0 | 113255.60 | – | – | 1523494.0 | 117079.20 | – | – | 1546127.0 | 17295930.0 | – | – |
| STR | ||||||||||||
| Left | 10128.62‡ | 1038.96 | 0.89 | 0.01 | 8586.88 | 903.45 | 0.9 | 0.01 | 8466.28 | 1334.86 | 0.91 | 0.01 |
| Right | 10064.2‡ | 1051.34 | 0.89 | 0.01 | 8423.03 | 723.4 | 0.89 | 0.01 | 8370.9 | 1506.64 | 0.91 | 0.01 |
| Overall | 10096.41‡ | 1036.78 | 0.89 | 0.01 | 8504.95 | 807.42 | 0.9 | 0.01 | 8418.59 | 1381.72 | 0.91 | 0.01 |
| GPe | ||||||||||||
| Left | 932.47‡ | 124.16 | 0.87 | 0.02 | 972.34 | 115.55 | 0.83 | 0.08 | 1056.35 | 181.07 | 0.85 | 0.02 |
| Right | 904.5‡ | 123.09 | 0.88 | 0.01 | 963.31 | 108.36 | 0.86 | 0.03 | 1007.08 | 143.92 | 0.84 | 0.03 |
| Overall | 918.49‡ | 123.38 | 0.88 | 0.02 | 967.84 | 110.02 | 0.84 | 0.06 | 1033.59 | 160.87 | 0.84 | 0.02 |
| GPi | ||||||||||||
| Left | 366.63‡ | 63.16 | 0.82 | 0.03 | 267.98 | 53.31 | 0.77 | 0.07 | 244.82 | 47.06 | 0.74 | 0.08 |
| Right | 365.11‡ | 57.42 | 0.84 | 0.03 | 286.31 | 63.26 | 0.8 | 0.05 | 248.19 | 90.29 | 0.75 | 0.08 |
| Overall | 365.87‡ | 59.85 | 0.83 | 0.03 | 277.15 | 58.16 | 0.79 | 0.06 | 247.01 | 69.85 | 0.74 | 0.08 |
| RN_1 | ||||||||||||
| Left | 285.05‡ | 49.42 | 0.89 | 0.03 | – | – | – | – | – | – | – | – |
| Right | 276.85‡ | 49.79 | 0.89 | 0.03 | – | – | – | – | – | – | – | – |
| Overall | 280.95 | 49.36 | 0.89 | 0.03 | – | – | – | – | – | – | – | – |
| RN_2 | ||||||||||||
| Left | 290.76 | 40.39 | 0.88 | 0.03 | 260.69 | 35.21 | 0.92 | 0.02 | 239.07 | 33.38 | 0.91 | 0.02 |
| Right | 279.1 | 43.58 | 0.89 | 0.02 | 253.35 | 38.02 | 0.93 | 0.02 | 228.93 | 31.84 | 0.92 | 0.01 |
| Overall | 284.93 | 42.07 | 0.89 | 0.03 | 257.02 | 36.15 | 0.92 | 0.02 | 234.0 | 32.07 | 0.92 | 0.02 |
| STN_1 | ||||||||||||
| Left | 52.83‡ | 16.26 | 0.72 | 0.14 | – | – | – | – | – | – | – | – |
| Right | 59.5‡ | 15.71 | 0.76 | 0.09 | – | – | – | – | – | – | – | – |
| Overall | 56.17‡ | 16.2 | 0.74 | 0.12 | – | – | – | – | – | – | – | – |
| STN_2 | ||||||||||||
| Left | 40.75 | 12.05 | 0.66 | 0.14 | 25.46 | 9.07 | 0.72 | 0.07 | 29.71 | 8.95 | 0.71 | 0.09 |
| Right | 50.07 | 14.53 | 0.71 | 0.14 | 24.99 | 9.19 | 0.7 | 0.09 | 28.72 | 11.46 | 0.68 | 0.1 |
| Overall | 45.41 | 14.04 | 0.68 | 0.14 | 25.22 | 8.96 | 0.71 | 0.08 | 29.22 | 9.99 | 0.69 | 0.09 |
| SN | ||||||||||||
| Left | 223.18‡ | 46.79 | 0.76 | 0.05 | 319.55 | 73.21 | 0.79 | 0.04 | 369.72 | 114.84 | 0.79 | 0.07 |
| Right | 226.33‡ | 50.46 | 0.76 | 0.04 | 313.26 | 77.96 | 0.79 | 0.07 | 331.83 | 97.77 | 0.79 | 0.04 |
| Overall | 224.75‡ | 48.27 | 0.76 | 0.04 | 316.4 | 74.28 | 0.79 | 0.06 | 350.78 | 105.28 | 0.79 | 0.06 |
| PAG | 292.25 | 40.47 | 0.79 | 0.04 | 315.76 | 33.76 | 0.82 | 0.03 | 292.05 | 28.59 | 0.78 | 0.04 |
| LV | ||||||||||||
| Left | 7829.75 | 3367.64 | 0.89 | 0.03 | 10001.19 | 3844.15 | 0.89 | 0.02 | 13728.48 | 3657.5 | 0.91 | 0.02 |
| Right | 7220.83 | 3579.82 | 0.87 | 0.04 | 9579.08 | 3776.74 | 0.87 | 0.05 | 14322.18 | 5622.29 | 0.91 | 0.02 |
| Overall | 7525.29 | 3459.43 | 0.88 | 0.04 | 9790.54 | 3745.54 | 0.88 | 0.04 | 14025.33 | 4611.28 | 0.91 | 0.02 |
| 3rd | 606.47 | 205.54 | 0.69 | 0.07 | 859.32 | 263.74 | 0.76 | 0.06 | 1756.09 | 536.94 | 0.82 | 0.03 |
| Aquaduct | 49.52 | 12.43 | 0.72 | 0.06 | 63.72 | 20.95 | 0.73 | 0.05 | 82.55 | 25.47 | 0.76 | 0.05 |
| 4th | 1514.64 | 521.67 | 0.74 | 0.11 | 1356.6 | 641.71 | 0.75 | 0.07 | 1091.12 | 178.11 | 0.66 | 0.12 |
Rater 1 was constant across structures and participants
STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey, LV lateral ventricle, 3rd third ventricle, Aqueduct cerebral aqueduct, 4th fourth ventricle. _1 rater pair between rater 1 and rater 2; _2 rater pair between rater 1 and rater 3
‡Previously published in Keuken et al. (2014)
The variability in inter-rater reliability between the structures and age ranges could be considered a confounding factor for any further analysis. We addressed this by incorporating the Dice coefficient as a covariate in all further analyses unless stated otherwise.
Effect of different raters
The correlation between the Dice coefficient and age for the STN and RN did not statistically change whether the previously published masks were used or the new parcellation (RN: r = 0.45, t(104) = 5.18, p < 0.001 versus r = 0.48, t(104) = 5.57, p < 0.001; STN: r = −0.18, t(104) = −1.99, p = 0.062 versus r = 0.07, t(104) = 0.75, p = 0.46 respectively). As indicated by the Fisher r-to-z transformation (Steiger 1980), the correlations did not statistically differ (RN: Z = −0.27, p = 0.79; STN: Z = −1.81, p = 0.07). The volumetric effects of age for the RN and the STN were also independent of which masks were used for the young group (RN: r = −0.55, t(106) = −6.73, p < 0.001 versus r = −0.53, t(106) = −6.26, p < 0.001; STN: r = −0.70, t(106) = −9.89, p < 0.001 versus r = −0.67, t(106) = −9.11, p < 0.001, respectively). As indicated by the Fisher r-to-z transformation, the correlations did not statistically differ (RN: Z = −0.2, p = 0.84; STN: Z = −0.41 p = 0.68).
These results indicate that the effect of rater pair is minimal compared to the effect of age since the Dice coefficient and volumetric results with age did not statistically change depending on the rater pair. All results that follow which include the RN and STN masks of the young participants are based on the rater pair who also parcellated the middle-aged and elderly participants.
Effect of aging on volume
In line with previous work (Courchesne et al. 2000; Ge et al. 2002; Mortamet et al. 2005), there was no significant effect of age on the intracranial volume. The STR, GPi, RN, and STN all showed a decrease in volume with age (STR: r = −0.65, t(106) = −8.68, p < 0.001; GPi: r = −0.40, t(106) = −4.44, p < 0.001; RN: r = −0.53, t(106) = −6.26, p < 0.001 STN: r = −0.67, t(106) = −9.11, p < 0.001). However, somewhat unexpectedly, both the GPe and SN showed a positive correlation between volume and age (r = 0.33, t(106) = 3.535, p = 0.007; r = 0.53, t(106) = 6.33, p < 0.001). While positive relations between grey matter volume and age have been previously reported in the literature (Mueller et al. 1998; Salat et al. 2002, 2004; Lemaitre et al. 2012) these are usually interpreted as an artefact of the employed method or measurement error. While we have no reason to assume that the employed MRI sequence and segmentation protocol were biased towards the GPe and SN, the volumetric results are counterintuitive and should be interpreted with caution. In line with previous work (Fjell and Walhovd 2010), the LV, third ventricle and the cerebral aqueduct showed a significant increase in volume with age (LV: r = 0.54, t(106) = 6.51, p < 0.001; third ventricle: r = 0.45, t(53) = 3.56, p = 0.009; cerebral aqueduct: r = 0.52, t(53) = 4.36, p < 0.001), whereas the PAG and the fourth ventricle did not show a significant increase in volume with age. Overall, the total volume of the parcellated ventricle compartments indicated an increase with age (r = 0.47, t(53) = 3.81, p < 0.001). See Fig. 2a for the volumetric results of the gray matter nuclei; Table 1 and Fig. 3a for the average values per age group.
Effect of aging on the location
To test whether aging had an effect on the spatial location of the different gray matter structures, the scores along the first eigenvector of the PCA analysis were correlated with the age of the participants while controlling for the Dice coefficient and the total ventricle volume. The total ventricle volume was included as a covariate since age and ventricle volume were strongly correlated, and ventricular expansion may affect the location of subcortical nuclei. In line with previous work there were several structures that showed a significant effect of age on the spatial location in standard MNI-space (STR: r = 0.29, t(106) = 3.05, p = 0.021; GPe: r = 0.47, t(106) = 5.35, p < 0.001; STN: r = 0.28, t(106) = 2.91, p = 0.031; RN: r = 0.28, t(106) = 2.90, p = 0.032; PAG: r = 0.28, t(106) = 2.94, p = 0.028) indicating the need for the visualization of the individual anatomy in aging populations (Keuken et al. 2013).
Effects of aging on values
All grey matter nuclei showed a positive correlation between the values and age (STR: r = 0.32, t(106) = 3.41, p = 0.006; GPe: r = 0.44, t(106) = 4.97, p < 0.001; GPi: r = 0.47, t(106) = 5.43, p < 0.001; STN: r = 0.50, t(106) = 5.78, p < 0.001; RN: r = 0.60, t(106) = 7.57, p < 0.001; SN: r = 0.33, t(106) = 3.53, p = 0.004; PAG: r = 0.77, t(53) = 8.54, p < 0.001). The non-thresholded values of the STR and PAG also showed a positive correlation with age (STR: r = 0.26, t(106) = 2.73, p = 0.015; PAG: r = 0.77, t(53) = 8.54, p < 0.001). An increase in values is thought to reflect a decrease of myelinisation (Callaghan et al. 2014). See Fig. 2b for the values per structure; Table 2 and Fig. 3b for the average values per age group.
Table 2.
The mean and standard deviation of the values in ms per structure and age group
| Structure | Young | Middle-aged | Elderly | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| STR | ||||||
| Left | 1643.71 | 39.53 | 1703.71 | 71.78 | 1721.40 | 62.53 |
| Right | 1723.51 | 41.30 | 1762.79 | 70.91 | 1774.38 | 73.01 |
| Overall | 1683.61 | 56.79 | 1733.25 | 76.20 | 1747.01 | 71.35 |
| GPe | ||||||
| Left | 1245.14 | 28.61 | 1287.44 | 48.81 | 1328.26 | 64.87 |
| Right | 1277.72 | 30.54 | 1304.99 | 45.35 | 1344.61 | 63.03 |
| Overall | 1261.43 | 33.62 | 1296.22 | 47.09 | 1336.44 | 62.61 |
| GPi | ||||||
| Left | 1223.43 | 32.29 | 1279.28 | 48.88 | 1310.55 | 56.11 |
| Right | 1241.45 | 31.76 | 1284.55 | 41.49 | 1316.10 | 55.39 |
| Overall | 1232.44 | 33.03 | 1281.91 | 44.57 | 1313.33 | 54.16 |
| RN | ||||||
| Left | 1235.49 | 38.80 | 1296.25 | 61.35 | 1319.50 | 30.74 |
| Right | 1225.58 | 34.18 | 1291.36 | 55.81 | 1315.22 | 30.16 |
| Overall | 1230.54 | 36.60 | 1293.80 | 57.60 | 1317.36 | 29.62 |
| STN | ||||||
| Left | 1182.41 | 36.77 | 1215.14 | 74.46 | 1238.13 | 49.13 |
| Right | 1202.12 | 39.10 | 1228.43 | 74.75 | 1292.45 | 39.81 |
| Overall | 1192.26 | 38.92 | 1221.79 | 73.52 | 1265.29 | 51.61 |
| SN | ||||||
| Left | 1303.52 | 43.87 | 1338.69 | 55.87 | 1337.68 | 48.30 |
| Right | 1326.41 | 40.16 | 1353.00 | 68.91 | 1355.96 | 48.97 |
| Overall | 1314.97 | 43.27 | 1345.84 | 61.98 | 1346.82 | 48.12 |
| PAG | 1750.91 | 74.30 | 1907.00 | 84.29 | 1952.60 | 47.68 |
STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey
Effects of aging on values
The STR and RN showed a negative correlation between the values and age (STR: r = −0.71, t(106) = −10.15, p < 0.001; RN: r = −0.49, t(106) = −5.74, p < 0.001). Contrary to these structures, there was a positive correlation between the values and age for the GPi and SN, two of which were found to have an increased volume with age (GPi: r = 0.46, t(106) = 5.28, p < 0.001; SN: r = 0.34, t(106) = 3.65, p < 0.001). The STN, GPe, and PAG did not show a significant correlation between age and values. The values for the non-thresholded STR indicated a negative correlation with age, whereas the PAG still did not show a significant correlation (STR: r = −0.55, t(106) = −6.62, p < 0.001). A change in values is thought to reflect a change in ratio of the contribution of myelin or iron to the measured signal (Deistung et al. 2013; Stüber et al. 2014). See Fig. 2c for the values per structure; Table 3 and Fig. 3c for the average values per age group.
Table 3.
The mean and standard deviation of the values in ms per structure and age group
| Structure | Young | Middle-aged | Elderly | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| STR | ||||||
| Left | 27.97 | 1.98 | 23.42 | 1.64 | 23.38 | 2.70 |
| Right | 28.00 | 1.89 | 23.23 | 1.63 | 22.02 | 2.52 |
| Overall | 27.97 | 1.92 | 23.33 | 1.61 | 22.70 | 2.63 |
| GPe | ||||||
| Left | 12.95 | 1.20 | 13.56 | 2.62 | 20.07 | 15.89 |
| Right | 12.59 | 0.83 | 13.15 | 2.32 | 16.39 | 5.15 |
| Overall | 12.77 | 1.04 | 13.36 | 2.43 | 18.22 | 11.62 |
| GPi | ||||||
| Left | 12.82 | 0.85 | 13.34 | 2.21 | 20.95 | 9.41 |
| Right | 12.65 | 0.92 | 13.07 | 1.57 | 22.26 | 10.86 |
| Overall | 12.74 | 0.88 | 13.20 | 1.88 | 21.61 | 9.88 |
| RN | ||||||
| Left | 17.38 | 1.67 | 14.26 | 1.61 | 14.29 | 1.65 |
| Right | 17.14 | 1.30 | 14.69 | 1.83 | 14.00 | 1.90 |
| Overall | 17.26 | 1.49 | 14.48 | 1.70 | 14.15 | 1.73 |
| STN | ||||||
| Left | 15.11 | 1.60 | 13.39 | 2.46 | 14.41 | 2.78 |
| Right | 14.98 | 1.45 | 13.61 | 2.11 | 14.41 | 2.85 |
| Overall | 15.05 | 1.51 | 13.50 | 2.25 | 14.41 | 2.73 |
| SN | ||||||
| Left | 13.32 | 1.11 | 14.23 | 2.36 | 20.55 | 10.19 |
| Right | 13.12 | 0.94 | 16.00 | 8.20 | 19.37 | 9.22 |
| Overall | 13.22 | 1.03 | 15.12 | 5.99 | 19.96 | 9.44 |
| PAG | 42.31 | 2.84 | 40.85 | 3.50 | 43.92 | 5.73 |
STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey
Effects of aging on the QSM values
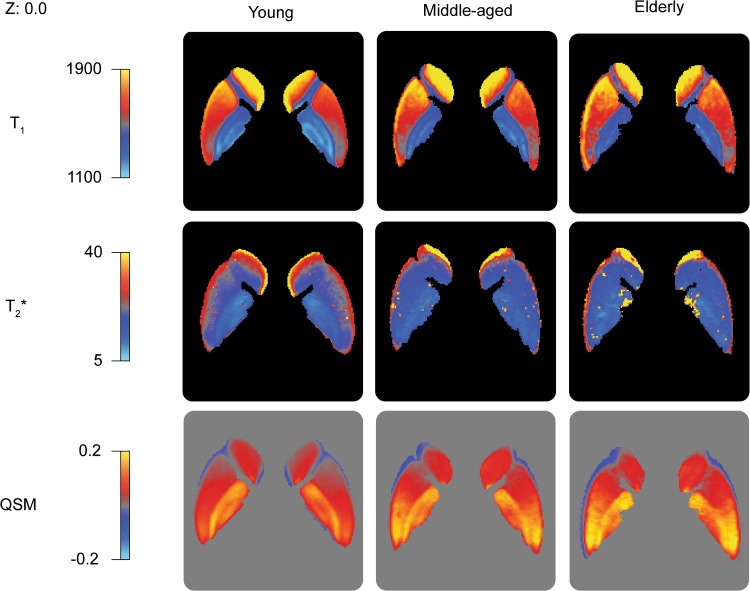
The STR, RN, and SN showed a positive correlation of age with the QSM values indicating an increase of iron concentration (STR: r = 0.43, t(106) = 4.80, p < 0.001; RN: r = 0.45, t(106) = 5.12, p < 0.001; SN: r = 0.37, t(106) = 3.974, p = 0.001). There was no statistically significant relationship between the QSM values and age for the GPe, GPi, STN or PAG. See Fig. 2d for the QSM values per structure; Table 4 and Fig. 3d for the average values per age group. See Fig. 4 for the spatial distribution of mean , , and QSM values within the striatum and globus pallidum per age group.
Table 4.
The mean and standard deviation of the QSM concentration in ppm per structure and age group
| Structure | Young | Middle-aged | Elderly | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| STR | ||||||
| Left | 0.003 | 0.027 | 0.026 | 0.014 | 0.035 | 0.019 |
| Right | 0.010 | 0.029 | 0.025 | 0.011 | 0.035 | 0.019 |
| Overall | 0.007 | 0.028 | 0.025 | 0.012 | 0.035 | 0.019 |
| GPe | ||||||
| Left | 0.111 | 0.038 | 0.120 | 0.015 | 0.130 | 0.044 |
| Right | 0.119 | 0.040 | 0.120 | 0.011 | 0.133 | 0.039 |
| Overall | 0.115 | 0.039 | 0.120 | 0.013 | 0.131 | 0.040 |
| GPi | ||||||
| Left | 0.100 | 0.038 | 0.100 | 0.020 | 0.110 | 0.066 |
| Right | 0.111 | 0.043 | 0.111 | 0.021 | 0.116 | 0.052 |
| Overall | 0.106 | 0.041 | 0.106 | 0.021 | 0.113 | 0.058 |
| RN | ||||||
| Left | 0.025 | 0.036 | 0.074 | 0.027 | 0.083 | 0.014 |
| Right | 0.031 | 0.036 | 0.074 | 0.025 | 0.084 | 0.020 |
| Overall | 0.028 | 0.036 | 0.074 | 0.026 | 0.083 | 0.017 |
| STN | ||||||
| Left | 0.026 | 0.041 | 0.027 | 0.047 | 0.042 | 0.042 |
| Right | 0.029 | 0.046 | 0.034 | 0.025 | 0.029 | 0.029 |
| Overall | 0.027 | 0.043 | 0.030 | 0.037 | 0.036 | 0.036 |
| SN | ||||||
| Left | 0.092 | 0.031 | 0.115 | 0.030 | 0.124 | 0.033 |
| Right | 0.098 | 0.036 | 0.117 | 0.024 | 0.141 | 0.046 |
| Overall | 0.095 | 0.034 | 0.115 | 0.026 | 0.133 | 0.040 |
| PAG | −0.045 | 0.030 | −0.057 | 0.017 | −0.053 | 0.015 |
STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey
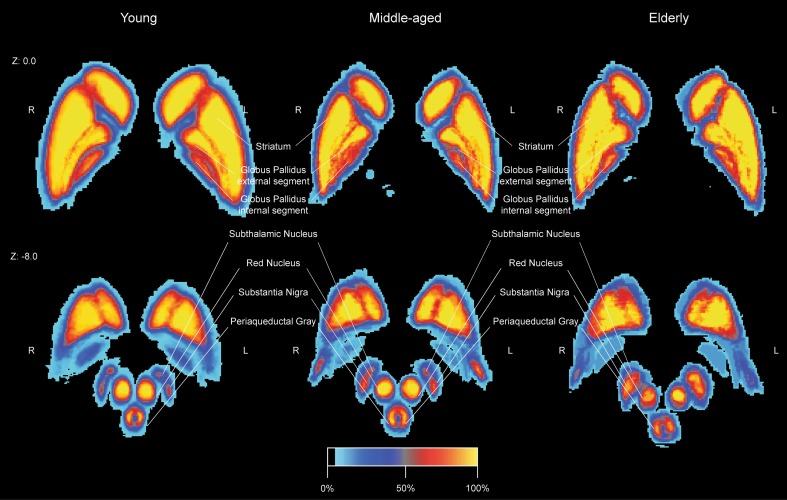
Fig. 4.
The spatial distribution of the mean , , and QSM values within the striatum and globus pallidum per age group at MNI Z coordinate 0. The linearly registered probability atlas was thresholded at 33% overlap
Relationship between qMRI values
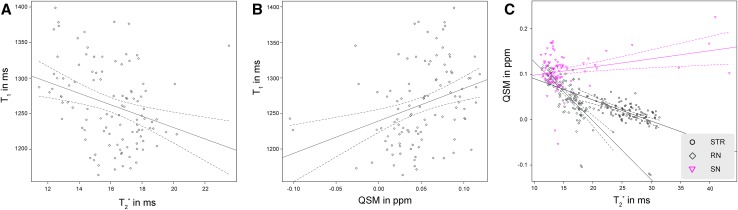
To test whether the , , and QSM values are highly dependent on each other, the different modalities were correlated. For the RN only, the values correlated with the (r = −0.31, t(104) = −3.30, p = 0.009) and QSM values (r = 0.32, t(104) = 3.48, p = 0.005). For the STR and RN there was a negative correlation between the QSM and values (STR: r = −0.57, t(104) = −7.04, p < 0.001; RN: r = −0.70, t(104) = −10.05, p < 0.001), but for the SN, this correlation was positive (r = 0.27, t(104) = 2.91, p = 0.031). See Fig. 5 for the significant correlations between qMRI values per structure.
Fig. 5.
The relationship between qMRI parameters. a The values versus the values in the striatum. b The values versus the QSM values in the striatum. c The QSM values versus the values in the striatum, red nucleus and substantia nigra. The regression lines indicate a significant correlation between qMRI parameters for a given structure. The dotted lines indicate 95% confidence interval. STR striatum, RN red nucleus, RN substantia nigra, QSM quantitative susceptibility mapping
Probability maps
Using the linearly registered probability atlases, the maximum and mean percentage overlaps were calculated following (Diedrichsen et al. 2009). Except for the STN, the maximum percentage overlap was generally high across structures and age groups. Similarly, the mean percentage overlap was lowest for the STN compared to the other included structures. This might indicate either larger anatomical or increased registration variability for the STN compared to the other structures. As neighbouring structures of similar size and shape (RN, SN) did not show this trend, anatomical variability seems more plausible. This would need further testing with larger samples and more elaborate shape analysis to fully answer this question. See Table 5 for the maximum and mean overlap per structure and age group. Given that different registration procedures can result in differences in overlap, one would ideally only use the current probability atlases after using similar normalisation protocols (Diedrichsen et al. 2009; Klein et al. 2009). See Fig. 6 for the linearly registered probability atlases per structure and age group.
Table 5.
The maximum and mean percentage overlap per linearly registered structure and age group
| Structure | Young | Middle-aged | Elderly | |||
|---|---|---|---|---|---|---|
| Maximum | Mean | Maximum | Mean | Maximum | Mean | |
| STR | ||||||
| Left | 100.00 | 42.94 | 100.00 | 47.01 | 100.00 | 46.82 |
| Right | 100.00 | 42.66 | 100.00 | 43.80 | 100.00 | 45.47 |
| GPe | ||||||
| Left | 100.00 | 26.79 | 100.00 | 30.88 | 100.00 | 35.41 |
| Right | 100.00 | 26.26 | 100.00 | 30.10 | 100.00 | 35.22 |
| GPi | ||||||
| Left | 100.00 | 22.09 | 99.14 | 26.52 | 100.00 | 23.89 |
| Right | 99.18 | 21.82 | 100.00 | 25.44 | 100.00 | 23.95 |
| RN | ||||||
| Left | 100.00 | 25.14 | 100.00 | 30.87 | 100.00 | 28.72 |
| Right | 100.00 | 25.22 | 100.00 | 28.37 | 95.90 | 28.00 |
| STN | ||||||
| Left | 65.09 | 9.11 | 49.27 | 8.53 | 61.14 | 14.24 |
| Right | 63.31 | 9.96 | 54.38 | 8.79 | 53.52 | 10.81 |
| SN | ||||||
| Left | 90.42 | 18.10 | 98.22 | 22.93 | 99.40 | 25.84 |
| Right | 78.02 | 16.70 | 89.57 | 20.89 | 88.89 | 22.59 |
| PAG | 95.31 | 19.91 | 99.83 | 24.48 | 93.34 | 21.62 |
STR striatum, GPe globus pallidus externa, GPi globus pallidus interna, RN red nucleus, STN subthalamic nucleus, SN substantia nigra, PAG periaqueductal grey
Fig. 6.
The linearly registered probability atlas in MNI-space per age group. The colour intensity reflects the percentage overlap across the participants per age group. A 100% overlap for the young indicates that 30 participants shared that voxel, whereas a 100% overlap for the elderly indicates that nine participants shared that voxel
Discussion
Using ultra-high field 7T MRI, we show that healthy aging has variable anatomical effects on a number of subcortical structures. In line with previous reports we show that ventricular volume generally increased with age, and that the volume of most subcortical grey matter areas decrease (Barron et al. 1976; Scahill et al. 2003; Terribilli et al. 2011). In addition to volumetric changes the spatial location of several structures was also affected by age. For the STR, GPe, RN, STN, and PAG it was shown that the CoM in MNI space changed with age. This shift in location with age has been previously reported for the STN, using a range of different registration pipelines, but not, to the best of our knowledge, for the STR, GPe, RN, or PAG (Dunnen and Staal 2005; Kitajima et al. 2008; Keuken et al. 2013; Mavridis et al. 2014). These volumetric and positional changes should be taken into account in surgical procedures such as deep brain stimulation (DBS). For instance, one of the frequently targeted DBS structures for Parkinson’s disease is the STN (Deep-Brain Stimulation for Parkinson's Disease Study Group 2001; Follett and Torres-Russotto 2011; Schuepbach et al. 2013). There are several different strategies to determine the location of the stimulation site within the STN, but a prominent procedure is the indirect targeting of the STN using landmarks such as the AC-PC mid commissural point or the RN (Bejjani et al. 2000; Andrade-Souza et al. 2005; Fytagoridis and Blomstedt 2010). Given the changes in volume and location of the RN and STN, one might argue against this procedure of indirect targeting and instead use direct visualization of the STN, ideally with the use of ultra-high field MRI (Cho et al. 2010, 2011; Abosch et al. 2010; Beisteiner et al. 2011). In general, as indicated by the probability atlas, there is a large anatomical variability across the different age groups and structures. These volumetric and positional changes should be taken into account in surgical procedures such as deep brain stimulation (DBS).
In terms of quantitative MRI parameters, we show that for all included subcortical grey matter nuclei, the value gradually increased with age, possibly indicating a loss of myelin or incomplete remyelination (Zivadinov 2007; Stüber et al. 2014; Callaghan et al. 2014; Steiger et al. 2016). The and QSM results were more variable and are thought to reflect different aspects of the iron and myelin content in the tissue (Deistung et al. 2013; Stüber et al. 2014). For instance, a decrease in myelin is thought to be indicated with a lengthening of and an increase of QSM values, whereas an increase of iron is considered to shorten and increase quantitative magnetic susceptibility (Deistung et al. 2013). QSM is thought to mainly reflect ferritin-bound iron in grey matter, with an increase in QSM indicating an increased iron concentration (Langkammer et al. 2012; Zheng et al. 2013; Stüber et al. 2014; Ropele and Langkammer 2016).
There was converging evidence for a potential increase of iron concentration with age within the STR and RN, which showed decreased and increased magnetic susceptibility. In addition, there was a negative correlation between the and QSM values, hinting at an increase of iron. This is in line with a recent meta-analysis indicating an aging effect on the accumulation of iron in the STR and RN (Daugherty and Raz 2013). No statistical significant effect of or QSM were found for the GPe, STN, or PAG. The lack of results for the GPe where not surprising as the reported effect size of age on values in the GP are relatively small and might be difficult to find with the current sample size (Daugherty and Raz 2013). The absence of a significant QSM correlation for the GP and no changes in the PAG were reported previously (Lambert et al. 2013; Acosta-Cabronero et al. 2016). It was surprising to find no significant relationship between the values in the STN and age. This contrasts with previous findings by our group using similar segmentation protocols and more recently by whole brain approaches (Keuken et al. 2013; Betts et al. 2016). As all three studies are based on cross-sectional designs with relative small sample sizes, there is a clear need for large sample longitudinal studies to answer the question whether age influences the values in the STN.
The results for the GPi and SN are more challenging to interpret. For the SN, the increased indicates a potential decrease in myelin content, and the increased magnetic susceptibility shows a potential increase of iron content. The increase in QSM values in the SN has been reported previously (Bilgic et al. 2012; Gong et al. 2015; Acosta-Cabronero et al. 2016). The decrease of myelin for the SN is further supported by the positive correlation between the and QSM values. For the GPi, an increase of was found but no significant age effects were detected for the QSM values. The increase in is more difficult to explain and is in direct contrast to the meta-analysis of Daugherty and Raz (2013). The increase of may arise from a more uniform distribution of iron within the tissue, or may provide additional evidence for the decrease of myelin (Siemonsen et al. 2008; Deistung et al. 2013).
These changes with age in quantitative MRI values have several methodological implications. The change in may well affect the accuracy of a number of automatic parcellation tools, such as FIRST or Freesurfer, which have been used to investigate volumetric changes in the subcortex with aging (e.g., Goodro et al. 2012; Liem et al. 2015). These parcellation tools are solely based on the contrast of a -weighted image (Visser et al. 2016a) and could thus easily be influence by changes in the value of (Jernigan et al. 2001). Previous work by Wenger et al. (2014) and Lorio et al. (2014, 2016b) have indeed shown an age-related bias in automatic segmentation and that certain sequences are less prone to this age effect (Helms et al. 2009).
The change of values might affect the sensitivity in fMRI studies. Since the optimal TE in a gradient-echo EPI sequence is equal to the relaxation time, an effect of aging on the values results in a change of BOLD sensitivity (Ugurbil et al. 2007; Koopmans et al. 2011; Norris 2012). It might be the case that when fMRI data is acquired for two age groups with the same TE this might result in a significant apparent difference which might actually be driven by changes in anatomy but not necessarily in function (e.g., Mell 2009; Eppinger et al. 2013).
Limitations
As with any cross-sectional study there is always the risk for cohort effects. Particularly when a small sample is used, it is not trivial to attribute the observed effects to individual variability or to healthy aging. The number of healthy elderly subjects that underwent all the UHF-MRI scans was relatively low and might be the cause of relatively low correlation coefficients. While this is true, it should be noted that cross-sectional and longitudinal studies generally show similar effects of aging, while the effects are more pronounced in longitudinal studies (Scahill et al. 2003). Another limitation is that the structures were parcellated using only one MRI contrast, which for most structures was shown to change with age. Though unlikely, the changes in , , and QSM values could have resulted in a shift of the perceptual boundaries, influencing the volumetric results, while the underlying true anatomy remained stable. A potential solution for this is to use multiple contrasts simultaneously to inform the parcellation. Recent parcellation tools have indeed shown that the combination of multiple MRI contrasts in a Bayesian framework improve the segmentation of subcortical structures (Kim et al. 2014; Visser et al. 2016b, a; Lorio et al. 2016a).
The estimation of the qMRI values and the relationship with the underlying tissue composition is not trivial (Weiskopf et al. 2015; Ropele and Langkammer 2016). In the current study the values were estimated using a well-characterized and tested MP2RAGE sequence, but it is acknowledged that the resulting maps may contain a residual transmit field bias which would result in less precise measures (Lutti et al. 2014). Nonetheless, the reported values of the GP are similar to a recent 7T multi-site test–retest validation study indicating that the values estimated from the MP2RAGE are stable (Voelker et al. 2016). The values were estimated by fitting a mono-exponential fit on a multi-echo -weighted volume (Chavhan et al. 2009) but it is known that the values are influenced by the choice of TE, number of TE’s, and the actual function used to estimate the values (Yin et al. 2010; Milford et al. 2015). Although different methods were used to estimate , the current values for the STR, GP, SN, and RN resulted in a similar rank ordering as previous 7T MRI work (Khabipova et al. 2015). The same holds for the calculation of QSM values. These values are influenced by a number of variables during both the acquisition phase and the post-processing phase (Haacke et al. 2015; Ropele and Langkammer 2016).
While ultra-high field MRI has several benefits for imaging small subcortical structures it is not without methodological challenges (van der Zwaag et al. 2015). As the field strength increases, the B0 inhomogeneity increases as well resulting in an inhomogeneous tissue contrast (van de Moortele et al. 2005; van der Zwaag et al. 2015). However, the autoshim facility of the 7T MRI scanner used is normally observed to provide a satisfactory shim, and is comparable to other 7T MRI sites (Voelker et al. 2016).
A final limitation is the low number of parcellated subcortical structures, given the total number of structures that are known to exist (Alkemade et al. 2013). We included only a small number of structures for several limiting reasons. The first one is the required time for careful manual delineation of each individual structure. The second, but more important, reason is that we selected only those structures that were easily visible with the contrast and spatial resolution employed. To discriminate more structures such as the thalamic nuclei, it may help to use specific MRI sequences that are tailored to that structure (Tourdias et al. 2014), improve the spatial resolution, or consider post mortem studies (Keren et al. 2015; Forstmann et al. in press). By reducing the voxel size, the partial volume effects (PVE) become less prominent. As the voxel resolution improves, the correction for subject motion becomes increasingly important and needs to be taken into account during the data acquisition (Tisdall et al. 2016). Prospective motion correction would additionally reduce the PVE and result in a higher SNR while allowing for higher spatial resolution (e.g., Stucht et al. 2015; Federau and Gallichan 2016). The need for reducing the PVE is crucial for smaller structures as their surface-area-to-volume ratio is larger (Vos et al. 2011).
Conclusion
Using quantitative ultra-high field 7T MRI, we were able to show variable age-related changes in anatomical features in several subcortical nuclei, as well as age-related changes in underlying MRI parameters that drive typical image contrast. These qMRI changes seem to be driven by different mechanisms: the increase of values indicate a global decrease of myelination across the subcortical structures, whereas some of the structures, and QSM results indicate an iron accumulation with age.
Acknowledgements
The work was supported by a Vidi grant by the Dutch Organization for Scientific Research (NWO) (BUF) and a starter grant from the European Research Council (ERC) (BUF). We would like to thank Domenica Wilfing and Elisabeth Wladimirow for their help in acquiring the data and Rosie Mulray for helpful comments on earlier versions of this manuscript.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 T. Neurosurgery. 2010;67:1745–1756. doi: 10.1227/NEU.0b013e3181f74105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A et al (2016) In vivo MRI mapping of brain iron deposition across the adult lifespan. 36:364–374. doi:10.1523/JNEUROSCI.1907-15.2016 [DOI] [PMC free article] [PubMed]
- Alkemade A, Keuken MC, Forstmann BU. A perspective on terra incognita: uncovering the neuroanatomy of the human subcortex. Front Neuroanat. 2013 doi: 10.3389/fnana.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M, Smith S (2007) Non-linear registration, aka spatial normalisation. Technical Report TR07JA2, Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Department of Clinical Neurology, Oxford University, Oxford, UK. Available at http://www.fmrib.ox.ac.uk/analysis/techrep
- Andrade-Souza YM, Schwalb JM, Hamani C, et al. Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in Parkinson’s disease. Neurosurgery. 2005;56:360–368. doi: 10.1227/01.NEU.0000145797.35968.ED. [DOI] [PubMed] [Google Scholar]
- Aquino D, Bizzi A, Grisoli M, et al. Age-related Iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252:165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- Barron SA, Jacobs L, Kinkel WR. Changes in size of normal lateral ventricles during aging determined by computerized tomography. Neurology. 1976;26:1011–1011. doi: 10.1212/WNL.26.11.1011. [DOI] [PubMed] [Google Scholar]
- Bastin ME, Clayden JD, Pattie A, et al. Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging. 2009;30:125–136. doi: 10.1016/j.neurobiolaging.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Bazin P-L, Weiss M, Dinse J et al (2013) A computational framework for ultra-high resolution cortical segmentation at 7 T. NeuroImage 1–9. doi:10.1016/j.neuroimage.2013.03.077 [DOI] [PubMed]
- Beisteiner R, Robinson S, Wurnig M, et al. Clinical fMRI: evidence for a 7 T benefit over 3 T. NeuroImage. 2011;57:1015–1021. doi: 10.1016/j.neuroimage.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, Bonnet AM, Marsault C, Agid Y, Philippon J, Cornu P (2000) Bilateral subthalamic stimulation for Parkinson’s disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg 92(4):615–625 [DOI] [PubMed]
- Benedetti B, Charil A, Rovaris M, et al. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–539. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, et al. High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R2* measurements at 7 T. NeuroImage. 2016;138:43–63. doi: 10.1016/j.neuroimage.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Bilgic B, Pfefferbaum A, Rohlfing T, et al. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage. 2012;59:2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan MF, Freund P, Draganski B, et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol Aging NBA. 2014;35:1862–1872. doi: 10.1016/j.neurobiolaging.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavhan GB, Babyn PS, Thomas B, et al. Principles, techniques, and applications of -based MR imaging and its special applications1. RadioGraphics. 2009;29:1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Péran P, Caltagirone C, et al. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. NeuroImage. 2009;48:29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Cho ZH, Kim YB, Han JY, et al. New brain atlas—mapping the human brain in vivo with 7.0 T MRI and comparison with postmortem histology: Will these images change modern medicine? Int J Imaging Syst Technol. 2008;18:2–8. doi: 10.1002/ima.20143. [DOI] [Google Scholar]
- Cho ZH, Min HK, Oh SH, et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg. 2010;113:1–9. doi: 10.3171/2010.3.JNS091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ZH, Kim JM, Park SY, et al. Direct visualization of Parkinson’s disease by in vivo human brain imaging using 7.0 T magnetic resonance imaging. Mov Disord. 2011;26:713–718. doi: 10.1002/mds.23465. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, Polimeni JR, Helmer KG, et al. mapping and B0 orientation-dependence at 7 T reveal cyto- and myeloarchitecture organization of the human cortex. NeuroImage. 2012;60:1006–1014. doi: 10.1016/j.neuroimage.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Raz N. Age-related differences in iron content of subcortical nuclei observed in vivo: a meta-analysis. NeuroImage. 2013;70:113–121. doi: 10.1016/j.neuroimage.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deistung A, Schäfer A, Schweser F, et al. Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. NeuroImage. 2013;65:299–314. doi: 10.1016/j.neuroimage.2012.09.055. [DOI] [PubMed] [Google Scholar]
- Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, et al. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dinse J, Härtwich N, Waehnert MD, et al. A cytoarchitecture-driven myelin model reveals area-specific signatures in human primary and secondary areas using ultra-high resolution in-vivo brain MRI. NeuroImage. 2015;114:71–87. doi: 10.1016/j.neuroimage.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Deep-Brain Stimulation for Parkinson's Disease Study Group (2001) Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med 345(13):956–963 [DOI] [PubMed]
- Draganski B, Ashburner J, Hutton C, et al. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ) NeuroImage. 2011;55:1423–1434. doi: 10.1016/j.neuroimage.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnen Den WF, Staal MJ. Anatomical alterations of the subthalamic nucleus in relation to age: a postmortem study. Mov Disord. 2005;20:893–898. doi: 10.1002/mds.20417. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Schuck NW, Nystrom LE, Cohen JD. Reduced striatal responses to reward prediction errors in older compared with younger adults. J Neurosci. 2013;33:9905–9912. doi: 10.1523/JNEUROSCI.2942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federau C, Gallichan D. Motion-correction enabled ultra-high resolution In-vivo 7T-MRI of the brain. PloS One. 2016;11:e0154974–e0154912. doi: 10.1371/journal.pone.0154974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/REVNEURO.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Follett KA, Torres-Russotto D. Deep brain stimulation of globus pallidus interna, subthalamic nucleus, and pedunculopontine nucleus for Parkinson’s disease: Which target? Parkinsonism Realt Disord. 2011;18:S165–S167. doi: 10.1016/S1353-8020(11)70051-7. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Schäfer A, et al. Multi-modal ultra-high resolution structural 7-Tesla MRI data repository. Scient Data. 2014;1:140050–140058. doi: 10.1038/sdata.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, de Hollander G, van Maanen L et al (in press). Towards a mechanistic understanding of the human subcortex. Nat Rev Neurosci [DOI] [PubMed]
- Fukunaga M, Li TQ, van Gelderen P, et al. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Nat Acad Sci. 2010;107:3834–3839. doi: 10.1073/pnas.0911177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fytagoridis A, Blomstedt P. Complications and side effects of deep brain stimulation in the posterior subthalamic area. Stereotact Funct Neurosurg. 2010;88:88–93. doi: 10.1159/000271824. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL (2002) Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. Am J Neuroradiol 23(8):1327–1333 [PMC free article] [PubMed]
- Gong N-J, Wong C-S, Hui ES, et al. Hemisphere, gender and age-related effects on iron deposition in deep gray matter revealed by quantitative susceptibility mapping. NMR Biomed. 2015;28:1267–1274. doi: 10.1002/nbm.3366. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodro M, Sameti M, Patenaude B, Fein G. Age effect on subcortical structures in healthy adults. Psychiatr Res Neuroimaging. 2012;203:38–45. doi: 10.1016/j.pscychresns.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, et al. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging. 2008;29:290–302. doi: 10.1016/j.neurobiolaging.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Liu S, Buch S, et al. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging. 2015;33:1–25. doi: 10.1016/j.mri.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Haase A, Frahm J, Matthaei D, et al. FLASH imaging. Rapid NMR imaging using low flip-angle pulses. J Magn Reson. 1986;67:258–266. doi: 10.1016/j.jmr.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Haber SN, Gdowski MJ (2004) The basal ganglia. In: Paxinos G, Mai JK (ed) The human nervous system, 2nd edn. Academic Press, London, p 676–738
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Helms G, Draganski B, Frackowiak R, et al. Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. NeuroImage. 2009;47:194–198. doi: 10.1016/j.neuroimage.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/S0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Keren NI, Taheri S, Vazey EM et al (2015) Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage 1–11. doi:10.1016/j.neuroimage.2015.03.020 [DOI] [PMC free article] [PubMed]
- Keuken MC, Bazin PL, Schafer A, et al. Ultra-high 7 T MRI of structural age-related changes of the subthalamic. Nucleus. 2013;33:4896–4900. doi: 10.1523/JNEUROSCI.3241-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuken MC, Bazin PL, Crown L, et al. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. NeuroImage. 2014;94:40–46. doi: 10.1016/j.neuroimage.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Khabipova D, Wiaux Y, Gruetter R, Marques JP. A modulated closed form solution for quantitative susceptibility mapping — A thorough evaluation and comparison to iterative methods based on edge prior knowledge. NeuroImage. 2015;107:163–174. doi: 10.1016/j.neuroimage.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Kim J, Lenglet C, Duchin Y, et al. Semiautomatic segmentation of brain subcortical structures from high-field MRI. IEEE J Biomed Health Inform. 2014;18:1678–1695. doi: 10.1109/JBHI.2013.2292858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Korogi Y, Kakeda S, et al. Human subthalamic nucleus: evaluation with high-resolution MR imaging at 3.0 T. Neuroradiology. 2008;50:675–681. doi: 10.1007/s00234-008-0388-4. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig SH. Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med. 1991;20:285–291. doi: 10.1002/mrm.1910200210. [DOI] [PubMed] [Google Scholar]
- Koopmans PJ, Barth M, Orzada S, Norris DG (2011) Multi-echo fMRI of the cortical laminae in humans at 7 T. NeuroImage 1–41. doi:10.1016/j.neuroimage.2011.02.042 [DOI] [PubMed]
- Lambert C, Chowdhury R, Fitzgerald T, et al. Characterizing aging in the human brainstem using quantitative multimodal MRI analysis. Front Hum Neurosci. 2013 doi: 10.3389/fnhum.2013.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage. 2012;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee J, Shmueli K, Fukunaga M, et al. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci USA. 2010;107:5130–5135. doi: 10.1073/pnas.0910222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, et al. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? NBA. 2012;33:617.e1–617.e9. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet C, Abosch A, Yacoub E, et al. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7 T MRI. PloS One. 2012;7:e29153. doi: 10.1371/journal.pone.0029153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu B, Batrachenko A, et al. Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum Brain Mapp. 2013;35:2698–2713. doi: 10.1002/hbm.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F, Mérillat S, Bezzola L, et al. Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. NeuroImage. 2015;108:95–109. doi: 10.1016/j.neuroimage.2014.12.035. [DOI] [PubMed] [Google Scholar]
- Lorio S, Lutti A, Kherif F, et al. Disentangling in vivo the effects of iron content and atrophy on the ageing human brain. NeuroImage. 2014;103:280–289. doi: 10.1016/j.neuroimage.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorio S, Fresard S, Adaszewski S, et al. New tissue priors for improved automated classification of subcortical brain structures on MRI. NeuroImage. 2016;130:157–166. doi: 10.1016/j.neuroimage.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorio S, Kherif F, Ruef A et al (2016b) Neurobiological origin of spurious brain morphological changes: a quantitative MRI study. Hum Brain Mapp n/a–n/a. doi:10.1002/hbm.23137 [DOI] [PMC free article] [PubMed]
- Lutti A, Dick F, Sereno MI, Weiskopf N (2014) Using high-resolution quantitative mapping of R1 as an index of cortical myelination. NeuroImage 1–13. doi:10.1016/j.neuroimage.2013.06.005 [DOI] [PubMed]
- Maniega SM, Hernández MCV, Clayden JD, et al. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. NBA. 2015;36:909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Gruetter R. New developments and applications of the MP2RAGE sequence - focusing the contrast and high spatial resolution R1 mapping. PloS One. 2013;8:e69294–e69211. doi: 10.1371/journal.pone.0069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage. 2010;49:1271–1281. doi: 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Mavridis I, Boviatsis E, Anagnostopoulou S. Stereotactic anatomy of the human subthalamic nucleus: providing coordinates for accurate electrode placement. J Neurol Surg A Cent Eur Neurosurg. 2014;75:289–298. doi: 10.1055/s-0034-1368093. [DOI] [PubMed] [Google Scholar]
- McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI from picture to proton. 1. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Mell T. Altered function of ventral striatum during reward-based decision making in old age. Front Hum Neurosci. 2009 doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milford D, Rosbach N, Bendszus M, Heiland S. Mono-exponential fitting in T2-relaxometry: relevance of offset and first echo. PloS One. 2015;10:e0145255–e0145213. doi: 10.1371/journal.pone.0145255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamet B, Zeng D, Gerig G, Prastawa M, Bullitt E (2005) Effects of healthy aging measured by intracranial compartment volumes using a designed MR brain database. Med Image Comput Comput Assist Interv 8(Pt 1):383–391 [DOI] [PMC free article] [PubMed]
- Mueller EA, Moore MM, Kerr D, et al. Brain volume preserved in healthy elderly through the eleventh decade. Neurology. 1998;51:1555–1562. doi: 10.1212/WNL.51.6.1555. [DOI] [PubMed] [Google Scholar]
- Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? a stereotactic, anatomical and magnetic resonance imaging study. Neuromodul Technol Neural Interface. 2008;11:13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- Norris DG. Spin-echo fMRI: the poor relation? NeuroImage. 2012;62:1109–1115. doi: 10.1016/j.neuroimage.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Okubo G, Okada T, Yamamoto A, et al. MP2RAGE for deep gray matter measurement of the brain: a comparative study with MPRAGE. J Magn Reson Imaging. 2015;43:55–62. doi: 10.1002/jmri.24960. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Persson N, Wu J, Zhang Q, et al. Age and sex related differences in subcortical brain iron concentrations among healthy adults. NeuroImage. 2015;122:385–398. doi: 10.1016/j.neuroimage.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. NeuroImage. 2009;47:493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropele S, Langkammer C (2016) Iron quantification with susceptibility. NMR Biomed 1–9. doi:10.1002/nbm.3534 [DOI] [PubMed]
- Saito N, Sakai O, Ozonoff Al, Jara H. Relaxo-volumetric multispectral quantitative magnetic resonance imaging of the brain over the human lifespan: global and regional aging patterns. Magn Reson Imaging. 2009;27:895–906. doi: 10.1016/j.mri.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Wharton S, Gowland P, Bowtell R. Using magnetic field simulation to study susceptibility-related phase contrast in gradient echo MRI. NeuroImage. 2009;48:126–137. doi: 10.1016/j.neuroimage.2009.05.093. [DOI] [PubMed] [Google Scholar]
- Schenker C, Meier D, Wichmann W, et al. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology. 1993;35:119–124. doi: 10.1007/BF00593967. [DOI] [PubMed] [Google Scholar]
- Schuepbach WMM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage. 2011;54:2789–2807. doi: 10.1016/j.neuroimage.2010.10.070. [DOI] [PubMed] [Google Scholar]
- Schweser F, Deistung A, Sommer K, Reichenbach JR. Toward online reconstruction of quantitative susceptibility maps: Superfast dipole inversion. Magn Reson Med. 2012;69:1581–1593. doi: 10.1002/mrm.24405. [DOI] [PubMed] [Google Scholar]
- Schweser F, Deistung A, Reichenbach JR. Foundations of MRI phase imaging and processing for Quantitative Susceptibility Mapping (QSM) Zeitschrift für medizinische Physik. 2016;26:6–34. doi: 10.1016/j.zemedi.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Siemonsen S, Finsterbusch J, Matschke J, et al. Age-dependent normal values of and T2′ in brain parenchyma. AJNR Am J Neuroradiol. 2008;29:950–955. doi: 10.3174/ajnr.A0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver NC, Good CD, Barker GJ, MacManus DG. Sensitivity of contrast enhanced MRI in multiple sclerosis. Effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain. 1997;120:1149–1161. doi: 10.1093/brain/120.7.1149. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Gronemeyer SA, Taylor JS. Age-related changes in proton T1 values of normal human brain. J Magn Reson Imaging. 1995;5:43–48. doi: 10.1002/jmri.1880050111. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. doi: 10.1037/0033-2909.87.2.245. [DOI] [Google Scholar]
- Steiger TK, Weiskopf N, Bunzeck N. Iron level and myelin content in the ventral striatum predict memory performance in the aging brain. J Neurosci. 2016;36:3552–3558. doi: 10.1523/JNEUROSCI.3617-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber C, Morawski M, Schäfer A, et al. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. NeuroImage. 2014;93:95–106. doi: 10.1016/j.neuroimage.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Stucht D, Danishad KA, Schulze P, et al. Highest resolution in vivo human brain MRI using prospective motion correction. PLoS One. 2015;10:e0133921. doi: 10.1371/journal.pone.0133921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC (2013) R: a language and environment for statistical computing.
- Terribilli DB, Schaufelberger MS, Duran FLS, et al. Age-related gray matter volume changes in the brain during non-elderly adulthood. NBA. 2011;32:354–368. doi: 10.1016/j.neurobiolaging.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall MD, Reuter M, Qureshi A, et al. Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion. NeuroImage. 2016;127:11–22. doi: 10.1016/j.neuroimage.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourdias T, Saranathan M, Levesque IR, et al. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7 T. NeuroImage. 2014;84:534–545. doi: 10.1016/j.neuroimage.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Adriany G, Akgun C, et al. High magnetic fields for imaging cerebral morphology, function, and biochemistry. In: Robitaille P-M, Berliner L, et al., editors. Ultra high field magnetic resonance imaging. New York: Springer; 2007. pp. 285–342. [Google Scholar]
- van de Moortele P-F, Akgun C, Adriany G, et al. B1 destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med. 2005;54:1503–1518. doi: 10.1002/mrm.20708. [DOI] [PubMed] [Google Scholar]
- van der Zwaag W, Schäfer A, Marques JP et al (2015) Recent applications of UHF-MRI in the study of human brain function and structure: a review. NMR Biomed n/a–n/a. doi:10.1002/nbm.3275 [DOI] [PubMed]
- Visser E, Keuken MC, Douaud G, et al. Automatic segmentation of the striatum and globus pallidus using MIST: multimodal image segmentation tool. NeuroImage. 2016;125:479–497. doi: 10.1016/j.neuroimage.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser E, Keuken MC, Forstmann BU, Jenkinson M. Automated segmentation of the substantia nigra, subthalamic nucleus and red nucleus in 7 T data at young and old age. NeuroImage. 2016;139:324–336. doi: 10.1016/j.neuroimage.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker MN, Kraff O, Brenner D et al (2016) The traveling heads: multicenter brain imaging at 7 T. 1–17. doi:10.1007/s10334-016-0541-8 [DOI] [PubMed]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, et al. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, et al. Partial volume effect as a hidden covariate in DTI analyses. NeuroImage. 2011;55(4):1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Mohammadi S, Lutti A, Callaghan MF. Advances in MRI-based computational neuroanatomy. Curr Opin Neurol. 2015;28:313–322. doi: 10.1097/WCO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- Wenger E, Mårtensson J, Noack H, et al. Comparing manual and automatic segmentation of hippocampal volumes: reliability and validity issues in younger and older brains. Hum Brain Mapp. 2014;35:4236–4248. doi: 10.1002/hbm.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall KP, Mackay AL, Graeb DA, et al. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Shah S, Katsaggelos AK. Improved R2* measurement accuracy with absolute SNR truncation and optimal coil combination. NMR Biomed. 2010;23:1127–1136. doi: 10.1002/nbm.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Youdim M, Riederer P, et al. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nichol H, Liu S, et al. Measuring iron in the brain using quantitative susceptibility mapping and X-ray fluorescence imaging. NeuroImage. 2013;78:68–74. doi: 10.1016/j.neuroimage.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R. Can imaging techniques measure neuroprotection and remyelination in multiple sclerosis? Neurology. 2007;68:S72–S82. doi: 10.1212/01.wnl.0000275236.51129.d2. [DOI] [PubMed] [Google Scholar]