Abstract
Objective
Initial management strategies of chronic subdural hematoma (cSDH) are controversial, and range from bedside twist-drill or burr hole drainage, to craniotomy with membranectomy (CWM). We aim to (1) perform a meta-analysis of the available data on the outcomes of CWM for treatment of cSDH in published English-language literature, and (2) evaluate collective outcomes of CWM with respect to morbidity, mortality, and recurrence rates.
Methods
A search of English-language literature performed in PubMed, Ovid, and Cochrane databases using keywords (“subdural hematoma” or “chronic subdural hematoma”) and (“membrane” or “membranectomy”) from inception to December 2016 was conducted. Studies reporting outcomes of CWM in cSDH were included. Mortality, morbidity, follow-up duration, and recurrence rate data were extracted and analyzed. Pooled estimates and confidence-intervals (CIs) were calculated for all outcomes using a random-effects model.
Results
Of 301 articles found, 17 articles containing 5369 patients met our eligibility criteria. Mean follow-up duration ranged from 1–30.8 months. Collective mean mortality and morbidity rates were 3.7% and 6.9%, respectively (95% CI 2–5.4% and 2.1–11.6%; p<.001 and p=.004). The collective mean recurrence rate was 7.6% (95% CI: 5%–10.2%; p<.001).
Conclusions
Clinical data on outcomes of CWM in cSDH are limited to single institutional analyses, with considerable variation in recurrence rates and follow-up time. The rates we reported are comparable to the 5% mortality and 3–12% morbidity rates, and lower than the 10–21% recurrence rate in the literature for burr holes or craniotomy without membranectomy. This meta-analysis provides an in-depth analysis of available data and reviews reported outcomes.
Keywords: subdural hematoma, membranectomy, neurosurgery, TBI, traumatic brain injury, craniotomy
Introduction
Initial management strategies of chronic subdural hematoma (cSDH) are controversial, and range from medical to surgical interventions. Surgical interventions vary from bedside twist-drill (TD) or burr-hole (BH) drainage, to craniotomy with drain insertion, irrigation, and/or membranectomy (i.e. resection or fenestration of the subdural inner and/or outer membranes).1 Putnam and Cushing, in 1926, championed craniotomy with outer membrane (CWM) removal in cSDH treatment, despite up to 30% craniotomy mortality.2 However, CWM is currently only used under conditions of subdural re-accumulation, solid hematomas, or following suboptimal postoperative cortical re-expansion.3 In its place, BH drainage 4 has become the primary treatment for cSDH, and has been suggested to be superior to CWM due to lower reoperation rates and better postoperative outcomes.5 The adjunctive use of continuous catheter drainage following BH or TF craniotomy further improved cSDH outcomes 6, particularly when compared to craniotomy alone.7,8,9,10,11
At present, the surgical management of encapsulated subdural hematoma remains unclear as to whether or not the inner membrane should be removed. Arutyunov12, Gorbatsevich and Shustin 13, Gomez 14, and Umbach 15 recommended that all capsular membrane components (i.e. both the outer and inner membrane) be resected, as this may allow for brain re-expansion and reduce the post-operative potential subdural space. However, others 16,17,18,19 have recommended simple burr hole evacuation without the need for a craniotomy. To date, a meta-analysis investigating the outcomes associated with CWM has not been published. In this study, we aim to perform a meta-analysis of the available data and evaluate the collective outcomes of craniotomy with membranectomy for treatment of cSDH in the published English-language literature with respect to morbidity, mortality, and recurrence rates.
Material and Methods
A thorough search of published English-language literature was performed in PubMed, Ovid, and Cochrane databases using the keywords (“subdural hematoma” or “chronic subdural hematoma”) and (“membrane” or “membranectomy”) from database inception to December 2016. Each manuscript was reviewed independently by two authors for relevance. The senior author acted as the final mediator if disagreements for inclusion occurred. This study was exempt from Institutional Review Board evaluation due to its investigations of published literature and non-involvement of human subjects. This study was conducted in agreement with the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement.20
A total of 301 articles were identified and reviewed (Figure 1). Abstracts were screened and case reports, review papers, and studies that didn’t specify which patients underwent craniotomy with membranectomy were excluded. 17 articles remained and the full text of each article was independently assessed by two of the authors. Articles were included if they reported treatment outcomes of craniotomy with membranectomy in cSDH patients. Demographic, lesion characteristics and grading, mortality, morbidity, follow-up duration, and recurrence rate data were extracted and analyzed.
Figure 1.
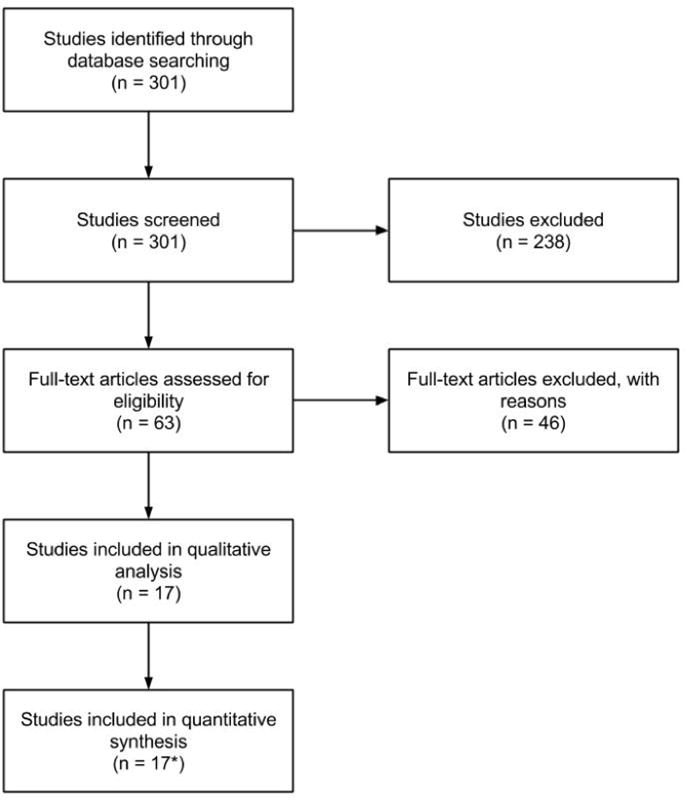
Flow diagram of study selection.
*12 studies were included in the quantitative analysis of recurrence rates, 8 studies were included in the quantitative analysis of morbidity rates, and 14 studies were included in the quantitative analysis of mortality rates.
The primary outcome variables investigated were morbidity, mortality, and recurrence rates. In each study, morbidity was defined as a major complication, disability, or poor health following surgical intervention, and mortality was defined as death secondary to cSDH or its sequelae. Recurrence was defined as new blood visualized on brain imaging following surgical intervention.
cSDH grading was based on the scheme proposed by Markwalder 3, which defined grade 0 as no neurological deficits; grade 1 as patient alert and oriented with mild symptoms and no neurological deficits; grade 2 as drowsiness or disorientation with variable neurological deficits; grade 3 as stupor with response to noxious stimuli and severe focal neurological signs; grade 4 as coma with no motor response or decerebrate or decorticate posturing. Good pre- and postoperative results were considered grades 0–2 and poor pre- and post-operative results were grades 3–4. Statistical analyses were performed using OpenMeta[Analyst] (Brown University, Rhode Island, USA) using the random effect model. The pooled estimates and 95% confidence intervals (CI) were calculated for primary outcome variables.
Results
After careful examination of the published articles, 17 met our eligibility criteria and were used for qualitative and quantitative analyses containing 5369 patients studied during 1964–2016. A tabulation of the included studies with demographic information and sample size is presented in Table 1. The mean sample size was 568 (range: 34–2275), age ranged from 6–100 years, and the reported mean follow-up duration ranged from 1–30.8 months. Table 1 also presents data on study length, intervention type, number of patients being reoperated on, Glasgow coma score, and presenting symptoms.
Table 1.
Details of published studies on outcomes of craniotomy with membranectomy for chronic subdural hematoma.
| First Author, Published Year | Institution | Length of Study (years) | Total Sample Size | Intervention Type (n) | Mean age in years (range) | Female (%) | Number of patients with reoperation (%) | Glasgow Coma Score (n) | Presenting symptoms (n) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Kayaci et al., 201437 | Recep Tayyip Erdogan University | 7 | 252 | CTIM (144) BCOMI (108) |
68.8 ± 1.5 67.6 ± 1.4 |
27/144 (19%) 30/108 (28%) |
N/A | >12(114) < 12 (30) > 12 (90) <12(18) |
Mild-moderate head trauma (102/144) Preexisting medical conditions (51/144) Mild-moderate head trauma (84/108) Preexisting medical conditions (39/108) |
Preexisting conditions include HTN, DM, and ischemic heart disease (n = 60, 54, and 42 in both groups respectively). Most common symptoms overall were cognitive deficits (64%), hemiparesis (46%), and motor dysphasia (24%). |
| Callovini et al., 201438 | Santo Spirito Hospital | 8 | 34 | CR (34) | 71 (17-89) | 13/34 (38%) | 2/34 (6%) | N/A | Headache (14) Altered consciousness (9) Hemisyndrome (15) Aphasia (6) Gait disturbance (9) Seizure (1) |
N/A |
| Lee et al., 200430 | University of Cologne, Germany | 4 | 172 | CR (134) BH (38) |
69 (27-92) | 54/134 (40%) 13/38 (34%) |
25/134 (19%) 6/38 (16%) |
N/A | Headache (67) Hemiparesis (61) Altered consciousness (50) |
BH group used two different BHs without membraneectomy (n=38) |
| Svien and Gelety, 196439 | Mayo Clinic | 5 | 69 | CR (19) | 50 (6-75) | 18/69 (26%) | 7/19 (37%) | N/A | Headache, Papilledema, bilateral cSDH | BH patients had no membranectomy |
| Tyson et al., 198040 | University of Virginia School of Medicine | 6 | 48 | CR (7) | †20–86 | N/A | N/A | N/A | N/A | Reoperation occurred 4–8 days postoperatively in four patients with BHs, which were reopened and 50–100 ml of fluid was evacuated. In two cases, a craniotomy was then performed. |
| Sambasivan, 199741 | Medical College Hospital | 30 | 2275 | CR (2215) BH (60) |
N/A | 407/2275 (18%) | 11/60 (18%) | N/A | Conscious w/pain (338) Behavioral disturbance (404) Seizures (275) Stroke (670) Coma (343 |
2215 patients underwent a subtemporalis marsupialization. |
| Mondorf et al., 200942 | Hannover Nordstadt Hospital, Klinikum Hannover | 5 | 193 | CR (151) BH (42) |
72.5 (26-97) | 80/193 (41% | 3/193 (2%) | N/A | Hemiparesis (112) Altered consciousness (70) Aphasia (46) |
14 deaths due to non-cSDH related pathology |
| Kim et al., 201143 | Seoul Medical Center, Seoul, Korea | 8 | 317 | CR (58) BH (259) |
59.4 63.7 ± 16.9 |
12/58 (21%) 68/259 (26%) |
12/58 (21%) 23/259 (9%) |
N/A | Headache(133) Disturbance of consciousness (89) Motor weakness (68) Gait disturbance (37) Disturbance of consciousness (46) Headache (34) Motor deficits (25) Dysphasia (13) |
N/A |
| Tanikawa et al., 200144 | Nagoya City University School of Medicine, Japan | 3 | 49 | CR (16) BH (33) |
70.3 ± 9.3 69.3 ± 14.9 |
N/A | 0/16 (0%) 4/33 (12%) |
N/A | Headache or hemiparesis | N/A |
| Rocchi et al., 200745 | University of Rome La Sapienza, Italy | 8 | 243 | CR (14) | 62.1 (41-76) | 7/14 (50%) | 9/14 (64%) | 10 (7) 11 (2) 12 (2) 13 (3) |
Hemiparesis (12) Aphasia (6) Hyposthenia (2) |
N/A |
| Gelabert-Gonzalez et al., 20 0546 | Unversity of Santiago de Compostela, Spain | 22 | 1000 | CR (1000) | 72.7 ± 11.4 (12-100) | 372/1000 (37%) | 61/1000 (1%) | N/A | Behavioural Distrubance (285) Headache (251) Hemisyndrome (248) Seizures (126) Aphasia (73) Coma (37) Incidental (1) |
N/A |
| Mohame d, 200947 | Gezera Hospital, Egypt | 12 | 39 | CR (39) | 61 (51-73) | 14/39 (36%) | 0/39 (0%) | > 13 (39) | Contralateral hemiparesis (35) Seizure (1) Headache and fluctuating consciousness (39) Diabetes (3) Hypertensive (2) |
N/A |
| Unterhof er et al., 201648 | Medical University of Innsbruck, Austria | 2 | 52 | CR (52) | 72 (48-89) | 13/52 (25%) | 14/52 (27%) | N/A | Headache (29) | 52 patients but 57 cSDHs |
| Regan et al., 20 1 549 | 3 | 123 | CR (58) BH (61) |
68 72 |
19/58 (33%) 25/61 (41%) |
14/58 (24%) 4/61 (7%) |
§13.6 §14.5 |
N/A | N/A | |
| Van Der Veken et al., 201450 | University Hospital UZ Brussel, Belgium | 6 | 131 | CR (126) | 73.4 | 40/126 (32%) | 11/126 (9%) | N/A | Motor deficit (36) Altered consciousness (33) Headache(23) Dysphasia (15) Gait disturbance (14) Seizure (5) |
N/A |
| White et al., 20 1 051 | Institute of Neurological Sciences, Southern General Hospital-Glasgow, United Kingdom | 3 | 268 | CR (116) BH (130) |
73 63 |
N/A | N/A | 50/116 show improvement 56/130 show improvement |
N/A | N/A |
| Ernestus et al., 199752 | Department of Neurosurgery, University of Cologne-Cologne, Germany | 3 | 104 | CR (10) BH (94) |
*69 (22-94) | 35/104 (34%) | 1/8 (13%) 17/92 (18%) |
N/A | Headache(39) Psychomotor disturbance (21) Seizure (6) Hemisyndrome (39) Aphasia (21) Anisocoria (10) |
BH = Burr Hole Craniostomy |
Abbreviations: BCOMI = burr hole with craniotomy and membrane incision; BH = burr hole; CR = craniotomy; CTIM = catheterization and tearing of inner membrane; NA = not available; HTN = hypertension; DM = diabetes mellitus.
Median was reported instead of mean
Range reported instead of mean
Average GCS reported
Table 2 presents the outcomes of cSDH resection via CWM. The reported mean time from injury to operation was 1–4.4 months, and the post-operative hospital stay ranged from 1–71 days. cSDH grade ranged from one to four. In all studies, the type of membranectomy (e.g. outer vs. inner membranectomy) was extracted when possible. Additionally, the specific surgical intervention was documented. Of note, in 16 of the 17 studies examined, all patients who underwent CWM also had placement of a postoperative drain (as noted in Table 2). In all studies reviewed, no mention of attrition rate was found. The meta-analysis revealed the collective mean mortality rate to be 3.7% (95% CI 2–5.4%; p<.001) (Figure 2). The collective mean morbidity rate was 6.9% (95% CI 2.1–11.6%; p=.004) (Figure 3). The collective mean recurrence rate was 7.6% (95% CI: 5%–10.2%; p<.001) (Figure 4).
Table 2.
Treatment outcomes of craniotomy with membranectomy for chronic subdural hematoma.
| First Author, Published Year | Intervention Type (n) | Average time from injury to operation | CSDH Grade (n) | Outer vs. Inner membranectomy | Drainage | Average follow up in months | Mortality (%) | Morbidity (%) | Recurrence (%) | Post-op duration in days | Major Complications (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kayaci et al., 201437 | CTIM (144) BCOMI (108) |
N/A | 1 (84) 2 (42) 3 (12) 4 (6) 1 (60) 2 (30) 3 (12) 4 (6) |
Inner Outer |
CSD (144) CSD (108) |
12 | N/A | N/A | 0/144 (0%) 9/108 (8%) |
7 ± 0.1 8.8 ± 0.2 |
N/A |
| Callovini et al., 201438 | CR (34) | N/A | 0 (19) 1 (5) 2 (7) 3 (3) |
Outer | CSD (34) | N/A | 1/34 (3%) | 6/34 (18%) | 2/34 (6%) | N/A | Subarachnoid hemorrhage (1) Stroke (2) Hygroma (1) Cardiopulmonary (2) |
| Lee et al., 200430 | CR (134) BH (38) |
N/A | 0 (41) 1 (77) 2 (43) 3 (2) 4 (9) |
N/A | CSD (134) CSD (38) |
N/A | 8/172 (5%) | 0/172 (0%) | N/A | N/A | Subdural empyema (2) |
| Svien and Gelety, 196439 | CR (19) | 4–7 weeks (*10 days – 1 year) |
N/A | N/A | Penrose drain (19) | 3 | 5/69 (7%) | 3/69 (4%) | 0 (0%) | N/A | N/A |
| Tyson et al., 198040 | CR (7) | 4.4 months | N/A | N/A | CSD (7) | 22.5 | 0 (0%) | 1/7 (14%) | N/A | N/A | Severely disability (1) |
| Sambasiv an, 199741 | CR (2215) BH (60) | N/A | N/A | Inner N/A |
CSD (2215) CSD (60) |
18 | 11/2215 (.5%) 2/60 (3%) |
N/A | 8/2215 (.4%) 11/60 (18%) |
N/A | N/A |
| Mondorf et al., 200942 | CR (151) BH (42) |
N/A | N/A | Inner | CSD (151) CSD (42) |
N/A | 7/151 (5%) 1/42 (2%) |
0/193 (0%) | 42/151 (28%) 6/42 (14%) |
N/A | Seizures (14) |
| Kim et al., 201143 | CR (58) BH (259) |
N/A | 0 (24) 1 (28) 2 (4) 3 (0) 4 (2) 0 (202) 1 (26) 2 (5) 3 (5) 4 (21) |
Outer and inner N/A |
subdural CSD (58) CSD (259) |
6 | 2/58 (3%) 21/259 (8%) |
6/58 (10%) 38/259 (15%) |
12/58 (21%) 23/259 (9%) |
Small CR: 35.6 ± 16.5 Large CR: 33.1 ± 14.5 BH: 35.2 ± 21.9 |
ICH (1) Seizures (2) Pneumonia (3) Infection, pneumocephalus, epidural hematoma, & seizure (9) Pneumonia & sepsis (29) |
| Tanikawa et al., 200144 | CR (16) BH (33) |
N/A | 0 (12) 1 (3) 2 (1) 0 (21) 1 (6) 2 (4) 3 (1) |
Outer and intrahematomal N/A |
CSD (16) CSD (33) |
6 | 0/16 (0%) 1/33 (3%) |
N/A | 0/16 (0%) 4/33 12%) |
16.8 ± 3.6 22.4 ± 15.1 |
N/A |
| Rocchi et al., 200745 | CR (14) | 3.5 weeks | N/A | N/A | CSD (14) | 30.6 | 0/14 (0%) | 0/14 (0%) | 0/14 (0%) | 12 | 0 |
| Gelabert-Gonzalez et al., 200546 | CR (1000) | N/A | N/A | N/A | CSD (1000) | N/A | 21/1000 (2%) | 196/1000 (20%) | 61/1000 (6%) | 7.9 (range: 3–51) | Epilepsy (62) Intracranial hypotension (9) Subdural empyema (7) ICH (4) Peneumocephalus (2) Pneumonia (22) Thromboembolic complications (8) Cardiac issues (11) Sepsis (10) |
| Mohamed, 200947 | CR (39) | N/A | N/A | Outer | Subgaleal suction drainage (39) | 3 | 0 (0%) | 0 (0%) | N/A | N/A | Agitation and delirium (5) 47Seizure (2) 47Chest infection (3) |
| Unterhofe r et al., 201648 | CR (52) | 6.5 weeks (*1–20 weeks) |
0 (12) 1 (29) 2 (15) |
Inner (28) | Subdural Jackson-Pratt (52) | 3–6 weeks | 0 (0%) | N/A | 17/57 (30%) | N/A | Acute subdural bleeding (2) Acute subdural hematoma (1) Seizure (1) |
| Regan et al., 201549 | CR (58) BH (61) |
N/A | N/A | Outer | Subdural CSD (58) Subdural CSD (61) |
No long term follow-up | 4/58 (7%) 2/61 (3%) |
N/A | N/A | 10.3 7.3 |
CR (32/58) Seizure (3) Acute stroke (4) New onset arrhythmia (1) Acute intracranial hemorrhage (10) Deep vein thrombosis/pulmonary embolism (1) Myocardial infarction (1) Respiratory failure (3) Pneumonia (4) Uncomplicated UTI (3) Superficial wound infection (1) BH (13/61) Seizure (1) Acute stroke (2) New onset arrhythmia (1) Acute intracranial hemorrhage (2) Deep vein thrombosis/pulmonary embolism (2) Myocardial infarction (1) Respiratory failure (1) Uncomplicated UTI (2) Cellulitis (1) |
| Van Der Veken et al., 201450 | CR (126) | N/A | 0 (72) 1 (29) 2 (5) 3 (3) 4 (0) |
Outer | Subdural Jackson Pratt (126) | 123.5 weeks (2.6–440 weeks) |
17/126 (13%) | N/A | 11/126 (9%) | 15.4 ± 12.5 | 43/126 Pulmonary infection (24) UTI (9) Decubitus wound (1) Deep venous thrombosis (1) Wound infection (2) Seizure (4) Pneumocephalus (1) Intracerebral hemorrhage (1) |
| White et al., 201051 | CR (116) BH (130) |
N/A | N/A | N/A | CSD (116) CSD (130) |
3 | 20/116 (17%) 10/130 (8%) |
N/A | 23/116 (20%) 23/130 (18%) |
N/A | CR Seizure (10) Subdural empyema (3) BH Seizure (12) Subdural empyema (2) |
| Ernestus et al., 199752 | CR (10) BH (94) |
N/A | 0 (5) 1 (2) 2 (1) 0 (38) 1 (30) 2 (15) 3 (9) |
N/A | CSD (10) CSD (94) |
N/A | 2/10 (20%) 2/94 (2%) |
N/A | N/A | Median: 14 days range:1–71 days | N/A |
Abbreviations: BCOMI = burr hole with craniotomy and membrane incision; CR = craniotomy; ICH = intracerebral hematoma; NA = not available; CSD = closed system drainage.
Range reported
Figure 2.
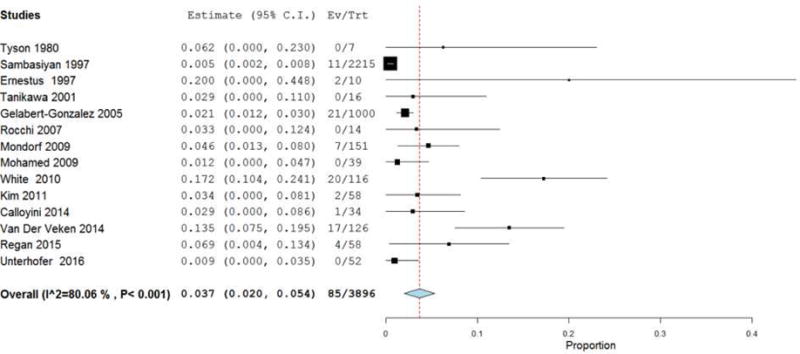
Forest plot demonstrating a 3.7% overall mortality rate with each line representing the 95% confidence interval. Boxes represent mortality rates in that study with its size correlating to the study’s effect size.
Figure 3.
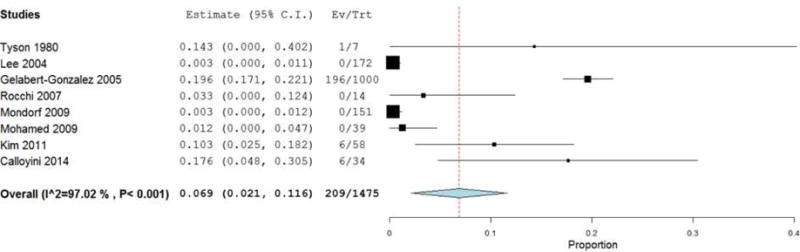
Forest plot demonstrating a 6.9% overall morbidity rate with each line representing the 95% confidence interval. Boxes represent morbidity rates in that study with its size correlating to the study’s effect size.
Figure 4.
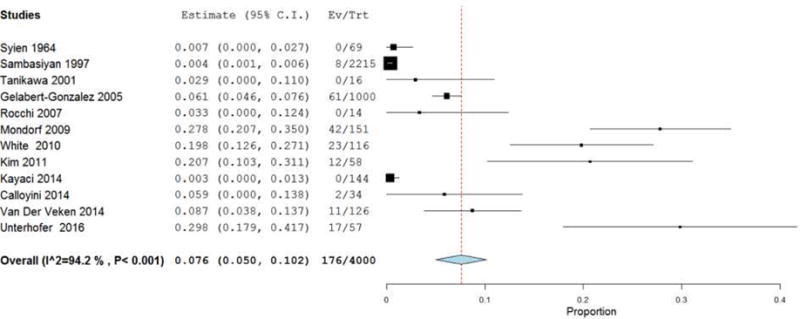
Forest plot demonstrating a 7.6% overall recurrence rate with each line representing the 95% confidence interval. Boxes represent recurrence rates in that study with its size correlating to the study’s effect size.
Discussion
In this study, we analyzed the outcomes of CWM treatment of cSDH on 5369 patients between 1964 and 2016. The ultimate treatment goals for cSDH is complete evacuation with no recurrence following surgical evacuation, while mitigating mortality and morbidity related to the natural history of cSDH and complications secondary to surgical intervention. CWM met these benchmarks for treating cSDH, with lower recurrence rates and similar mortality and morbidity rates to minimally invasive methods over a broad follow-up duration ranging from 1–30.8 months and similar treatment side effects to minimally invasive interventions (i.e. burr hole evacuation). Similar or higher morbidity and mortality rates, and uniformly higher recurrence rates have been reported for burr hole evacuation of cSDH.21,22,1 The mortality following cSDH evacuation ranged from 0% to 20% in the studies included in this meta-analysis, with a collective mortality rate of 3.7% (95% CI 2–5.4%; p<.001). Morbidity rates were generally higher in the included studies, which corresponds with the 6.9% collective morbidity rate we found (95% CI 2.1–11.6%; p=.004). The majority of studies did not report breakdowns of outcome based on cSDH grade, or standard deviations for age and hospital stay and a meta-analysis of these variables could not be conducted. Furthermore, some studies solely investigated craniotomy with membranectomy or mini-craniotomy with membranectomy while others included burr-hole drainage, and therefore all data extracted had to be broken down by intervention type.
Chronic subdural hematomas are a complex neurosurgical entity with increasing prevalence given the increase in the population at risk. In addition, the use of newer anticoagulant therapy for cardiac and cerebrovascular issues increases the likelihood of the development of a cSDH from minor TBI. The optimal treatment for this has been debated for many years and there are many series describing the main treatment modalities of burr holes and craniotomies.23,24,25,26 Although specific clinical parameters dictate the unique surgical intervention for each individual patient, we recommend the establishment of refined decision-making criteria to guide surgical intervention in cSDH patients. At our institution, the decision-tree used to stratify patients to BH drainage or cSDH is depicted in Figure 5, and is based on surgical paradigms proposed in the cSDH literature.1,3,5,9,11,23,30 We have focused in this meta-analysis on the experience with CWM, and our analysis was restricted to papers that described CWM. One of the limitations is that this was frequently poorly defined as to whether the membranectomy was of the outer subdural membrane or the inner subdural membrane.
Figure 5.
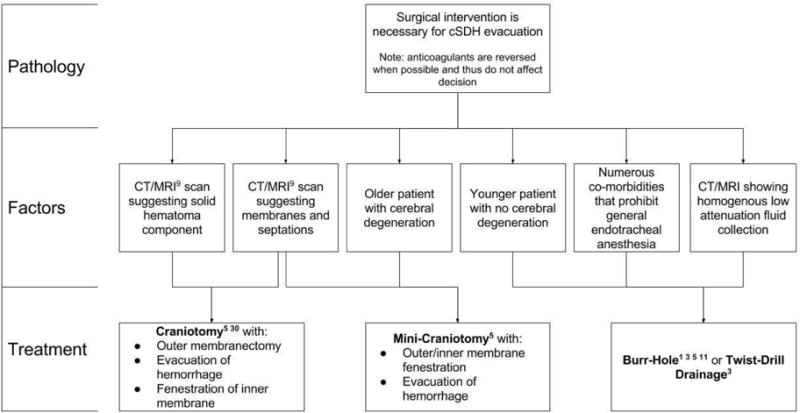
Decision tree for the type of chronic subdural hematoma (cSDH) evacuation once surgical treatment has been deemed necessary.
These are different pathologically. The outer membrane is adherent to the dura and vascular in nature with ample amounts of vEGF.27,28,29 “Stripping” these membranes has been frowned upon in the recent literature because of the tendency for bleeding to occur at the edges from the dura that is exposed.30 The inner membrane is usually thin and translucent and avascular. This can be adherent to the underlying arachnoid over the cortical surface. When this is microdissected off of the arachnoid it may be fenestrated. This allows the underlying brain to re-expand. Also, there are frequently blood break-down products sequestered between the arachnoid and this membrane. Where possible, in our analysis we identified whether membranectomy referred to the outer or inner subdural membranes. The outer cSDH membrane is highly vascularized and exudation from macrocapillaries is critical in cSDH enlargement.31 Fenestration or complete resection of the outer membrane may mitigate rebleeding following cSDH resection, and may facilitate intracranial fluid efflux via the recently elucidated dural lymphatics.32,33 Furthermore, fluid flux through the glymphatic34,35 pathway has been implicated in a variety of intracranial homeostatic and pathological mechanisms, and membranectomy may facilitate the egress and reabsorption of cSDH contents by cortical glymphatic and dural lymphatic pathways.
With an aging population in the United States, the incidence of cSDH is expected to increase. As such, procedures which result in low recurrence rates can positively influence the overall outcomes of cSDH patients. One meta-analyses of 34,829 patients reported 3.5–4% mortality, 7–11% morbidity, and 10.7–11% recurrence rates overall.36 We demonstrate that CWM results in lower recurrence rates on average, than alternative interventions including craniotomy or BH drainage without membranectomy. Furthermore, the morbidity and mortality rates of CWM are comparable to those reported in the literature for cSDH drainage regardless of intervention type. As such, CWM may decrease the likelihood of cSDH recurrence and secondary intervention, while exhibiting similar morbidity and mortality profiles as other interventions reported in the literature.
There were a number of limitations in this study. The heterogeneous methodology of the published papers in reporting their outcomes limited the ability to perform meta-analysis on all variables. Despite this heterogeneity, each study’s methodology was evaluated to determine if differences exist. Heterogeneity of the type of membranectomy (i.e. fenestration or complete resection), and variability of the surgical intervention is a limitation, as studies which utilized a craniotomy or a mini-craniotomy with a membranectomy (regardless of the extent of the membranectomy) were included in the meta-analysis. Several studies had a small sample size, and breakdown of data per patient type was not available. Furthermore, in 16 of the 17 papers included in this meta-analysis, all CWM patients received a postoperative drain. As such, the exact contribution of the membranectomy compared to the postoperative drain in cSDH recurrence remains unclear, and requires further inquiry. This meta-analysis can lay a foundation for future studies to recruit more patients and evaluate differences in surgical intervention with respect to different patient demographics and disease characteristics. In addition, all studies evaluated were case series that did not have control groups. This is an inherent limitation that constrains the conclusions that can be drawn regarding CWM treatment for cSDH. Nonetheless, analysis of the available data in the literature on CWM treatment of cSDH undoubtedly supports the efficacy of this surgical intervention, and offers patients with clinically significant resolution of symptoms while minimizing morbidity, mortality, and recurrence rates. This is the first study to perform a meta-analysis of CWM treatment outcomes for cSDH.
Conclusions
We present data on the outcomes of cSDH resection via CWM in 5369 patients studied during 1964–2016. The meta-analysis revealed the collective mean mortality, morbidity, and recurrence rates to be 3.7%, 6.9%, and 7.6%, respectively. The mortality and morbidity rates we report are comparable to the 3.5–5% mortality and 3–12% morbidity rates for burr holes or craniotomy without membranectomy reported in the literature. However, the 7.6% recurrence rate we report is lower than the 10–21% recurrence rate reported in the literature for burr holes or craniotomy without membranectomy. This suggests that CWM yields a lower likelihood of cSDH recurrence and secondary intervention, while exhibiting similar morbidity and mortality profiles as other interventions reported in the literature.
Acknowledgments
Source of funding: RS is funded in part by an MSTP Grant from the NIH (T32-GM08620)
Abbreviations
- CWM
craniotomy with membranectomy
- cSDH
chronic subdural hematoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial interests: None
Portions of this work were presented in poster form at the 2017 American Association of Neurological Surgeons (AANS) Annual Scientific Meeting, Los Angeles, CA
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clinical Neurology and Neurosurgery. 2005;107(3):223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Putnam T, Cushing H. Chronic subdural hematoma: Its pathology, its relation to pachymeningitis hemorrhagica and its surgical treatment. Archives of Surgery. 1925;11(3):329–393. [Google Scholar]
- 3.Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54(5):637–645. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 4.Pevehouse BC, Bloom WH, Mc KW. Ophthalmologic aspects of diagnosis and localization of subdural hematoma. An analysis of 389 cases and review of the literature. Neurology. 1960;10:1037–1041. doi: 10.1212/wnl.10.11.1037. [DOI] [PubMed] [Google Scholar]
- 5.Svien Hendrik J, Gelety Joseph E. On the Surgical Management of Encapsulated Subdural Hematoma. Journal of Neurosurgery. 1964;21(3):172–177. doi: 10.3171/jns.1964.21.3.0172. [DOI] [PubMed] [Google Scholar]
- 6.Tabaddor Kamran, Shulman Kenneth. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. Journal of Neurosurgery. 1977;46(2):220–226. doi: 10.3171/jns.1977.46.2.0220. [DOI] [PubMed] [Google Scholar]
- 7.Camel Mark, Grubb Robert LJ. Treatment of chronic subdural hematoma by twist-drill craniostomy with continuous catheter drainage. Journal of Neurosurgery. 1986;65(2):183–187. doi: 10.3171/jns.1986.65.2.0183. [DOI] [PubMed] [Google Scholar]
- 8.Reinges MHT, Hasselberg I, Rohde V, Küker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. Journal of Neurology, Neurosurgery & Psychiatry. 2000;69(1):40–47. doi: 10.1136/jnnp.69.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi Kazuo, Maeda Keiichirou, Iijima Akira, Usui Masaaki, Okada Yoshihumi, Kirino Takaaki. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. Journal of Neurosurgery. 1997;87(6):870–875. doi: 10.3171/jns.1997.87.6.0870. [DOI] [PubMed] [Google Scholar]
- 10.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma—burr hole drainage versus burr hole irrigation. Surgical Neurology. 57(6):405–409. doi: 10.1016/s0090-3019(02)00720-6. [DOI] [PubMed] [Google Scholar]
- 11.Markwalder TM, Seiler RW. Chronic subdural hematomas: to drain or not to drain? Neurosurgery. 1985;16(2):185–188. doi: 10.1227/00006123-198502000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Arutiunov AI. Subdural encapsulated hematomas, their clinical aspects and surgical treatment. Vopr Neirokhir. 1961;25:16–21. [PubMed] [Google Scholar]
- 13.Gorbatsevich AB, Shustin VA. On diagnosis and surgical treatment of chronic subdural hematoma. Vopr Neirokhir. 1961;25:21–23. [PubMed] [Google Scholar]
- 14.Gomez F. One hundred cases of subdural hematoma from 1930 to 1955 at the Henry Ford Hospital. Henry Ford Hosp Med Bull. 1957;5(1):35–46. [PubMed] [Google Scholar]
- 15.Umbach W. Treatment of chronic intradural hematoma. Langenbecks Arch Klin Chir Ver Dtsch Z Chir. 1957;287:666–669. [PubMed] [Google Scholar]
- 16.Echlin FA, Sordillo SV, Garvey TQ., Jr Acute, subacute, and chronic subdural hematoma. J Am Med Assoc. 1956;161(14):1345–1350. doi: 10.1001/jama.1956.02970140001001. [DOI] [PubMed] [Google Scholar]
- 17.Freed CG, Boyd HR. Subdural hematoma: review of 106 cases. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1960;57:51–55. [PubMed] [Google Scholar]
- 18.Husby J. Subdural hygroma in association with chronic subdural haematoma in adults. Acta Chir Scand. 1960;119:453–454. [PubMed] [Google Scholar]
- 19.Levy LF. Subdural haematoma. East Afr Med J. 1958;35(7):345–356. [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Santarius T, Kirkpatrick PJ, Ganesan D, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. The Lancet. 374(9695):1067–1073. doi: 10.1016/S0140-6736(09)61115-6. [DOI] [PubMed] [Google Scholar]
- 22.Kansal R, Nadkarni T, Goel A. Single versus double burr hole drainage of chronic subdural hematomas. A study of 267 cases. Journal of Clinical Neuroscience. 2010;17(4):428–429. doi: 10.1016/j.jocn.2009.07.109. [DOI] [PubMed] [Google Scholar]
- 23.Weigel R, Krauss JK, Schmiedek P. Concepts of neurosurgical management of chronic subdural haematoma: historical perspectives. Br J Neurosurg. 2004;18(1):8–18. doi: 10.1080/02688690410001660418. [DOI] [PubMed] [Google Scholar]
- 24.Smely C, Madlinger A, Scheremet R. Chronic subdural haematoma–a comparison of two different treatment modalities. Acta Neurochir (Wien) 1997;139(9):818–825. doi: 10.1007/BF01411399. discussion 825–816. [DOI] [PubMed] [Google Scholar]
- 25.Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: Preliminary results of using dexamethasone. Br J Neurosurg. 2005;19(4):327–333. doi: 10.1080/02688690500305332. [DOI] [PubMed] [Google Scholar]
- 26.Santarius T, Hutchinson PJ. Chronic subdural haematoma: time to rationalize treatment? Br J Neurosurg. 2004;18(4):328–332. doi: 10.1080/02688690400004845. [DOI] [PubMed] [Google Scholar]
- 27.Hohenstein A, Erber R, Schilling L, Weigel R. Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma. 2005;22(5):518–528. doi: 10.1089/neu.2005.22.518. [DOI] [PubMed] [Google Scholar]
- 28.Shono T, Inamura T, Morioka T, et al. Vascular endothelial growth factor in chronic subdural haematomas. J Clin Neurosci. 2001;8(5):411–415. doi: 10.1054/jocn.2000.0951. [DOI] [PubMed] [Google Scholar]
- 29.Hong HJ, Kim YJ, Yi HJ, Ko Y, Oh SJ, Kim JM. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematoma. Surg Neurol. 2009;71(2):161–165. doi: 10.1016/j.surneu.2008.01.023. discussion 165–166. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61(6):523–527. doi: 10.1016/j.surneu.2003.10.026. discussion 527–528. [DOI] [PubMed] [Google Scholar]
- 31.Shim YS, Park CO, Hyun DK, Park HC, Yoon SH. What are the causative factors for a slow, progressive enlargement of a chronic subdural hematoma? Yonsei Med J. 2007;48(2):210–217. doi: 10.3349/ymj.2007.48.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucchieri F, Farina F, Zummo G, Cappello F. Lymphatic vessels of the dura mater: a new discovery? J Anat. 2015;227(5):702–703. doi: 10.1111/joa.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almenawer SA, Farrokhyar F, Hong C, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014;259(3):449–457. doi: 10.1097/SLA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 37.Kayaci S, Kanat A, Koksal V, Ozdemir B. Effect of inner membrane tearing in the treatment of adult chronic subdural hematoma: a comparative study. Neurol Med Chir (Tokyo) 2014;54(5):363–373. doi: 10.2176/nmc.oa.2013-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callovini GM, Bolognini A, Callovini G, Gammone V. Primary enlarged craniotomy in organized chronic subdural hematomas. Neurol Med Chir (Tokyo) 2014;54(5):349–356. doi: 10.2176/nmc.oa2013-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svien HJ, Gelety JE. On the Surgical Management of Encapsulated Subdural Hematoma. A Comparison of the Results of Membranectomy and Simple Evacuation. J Neurosurg. 1964;21:172–177. doi: 10.3171/jns.1964.21.3.0172. [DOI] [PubMed] [Google Scholar]
- 40.Tyson G, Strachan WE, Newman P, Winn HR, Butler A, Jane J. The role of craniectomy in the treatment of chronic subdural hematomas. J Neurosurg. 1980;52(6):776–781. doi: 10.3171/jns.1980.52.6.0776. [DOI] [PubMed] [Google Scholar]
- 41.Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418–422. doi: 10.1016/s0090-3019(97)00188-2. [DOI] [PubMed] [Google Scholar]
- 42.Mondorf Y, Abu-Owaimer M, Gaab MR, Oertel JM. Chronic subdural hematoma–craniotomy versus burr hole trepanation. Br J Neurosurg. 2009;23(6):612–616. doi: 10.3109/02688690903370297. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011;50(2):103–108. doi: 10.3340/jkns.2011.50.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanikawa M, Mase M, Yamada K, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien) 2001;143(6):613–618. doi: 10.1007/s007010170067. discussion 618–619. [DOI] [PubMed] [Google Scholar]
- 45.Rocchi G, Caroli E, Salvati M, Delfini R. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 2007;67(4):374–380. doi: 10.1016/j.surneu.2006.08.066. discussion 380. [DOI] [PubMed] [Google Scholar]
- 46.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed EH. Chronic subdural haematoma treated by craniotomy, durectomy, outer membranectomy and subgaleal suction drainage. Personal experience in 39 patients. British Journal of Neurosurgery. 2003;17(3):244–247. doi: 10.1080/0268869031000153134. [DOI] [PubMed] [Google Scholar]
- 48.Unterhofer C, Freyschlag CF, Thome C, Ortler M. Opening the Internal Hematoma Membrane Does Not Alter the Recurrence Rate of Chronic Subdural Hematomas: A Prospective Randomized Trial. World Neurosurg. 2016;92:31–36. doi: 10.1016/j.wneu.2016.04.081. [DOI] [PubMed] [Google Scholar]
- 49.Regan JM, Worley E, Shelburne C, Pullarkat R, Watson JC. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One. 2015;10(1):e0115085. doi: 10.1371/journal.pone.0115085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Der Veken J, Duerinck J, Buyl R, Van Rompaey K, Herregodts P, D’Haens J. Mini-craniotomy as the primary surgical intervention for the treatment of chronic subdural hematoma–a retrospective analysis. Acta Neurochir (Wien) 2014;156(5):981–987. doi: 10.1007/s00701-014-2042-8. [DOI] [PubMed] [Google Scholar]
- 51.White M, Mathieson CS, Campbell E, Lindsay KW, Murray L. Treatment of chronic subdural haematomas – a retrospective comparison of minicraniectomy versus burrhole drainage. Br J Neurosurg. 2010;24(3):257–260. doi: 10.3109/02688691003675218. [DOI] [PubMed] [Google Scholar]
- 52.Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3):220–225. doi: 10.1016/s0090-3019(97)80031-6. [DOI] [PubMed] [Google Scholar]


