Abstract
The ability of dead cells of endophytic Drechslera hawaiiensis of Morus alba L. grown in heavy metals habitats for bioremoval of cadmium (Cd2+), copper (Cu2+), and lead (Pb2+) in aqueous solution was evaluated under different conditions. Whereas the highest extent of Cd2+ and Cu2+ removal and uptake occurred at pH 8 as well as Pb2+ occurred at neutral pH (6–7) after equilibrium time 10 min. Initial concentration 30 mg/L of Cd2+ for 10 min contact time and 50 to 90 mg/L of Pb2+ and Cu2+ supported the highest biosorption after optimal contact time of 30 min achieved with biomass dose equal to 5 mg of dried died biomass of D. hawaiiensis. The maximum removal of Cd2+, Cu2+, and Pb2+ equal to 100%, 100%, and 99.6% with uptake capacity estimated to be 0.28, 2.33, and 9.63 mg/g from real industrial wastewater, respectively were achieved within 3 hr contact time at pH 7.0, 7.0, and 6.0, respectively by using the dead biomass of D. hawaiiensis compared to 94.7%, 98%, and 99.26% removal with uptake equal to 0.264, 2.3, and 9.58 mg/g of Cd2+, Cu2+, and Pb2+, respectively with the living cells of the strain under the same conditions. The biosorbent was analyzed by Fourier Transformer Infrared Spectroscopy (FT-IR) analysis to identify the various functional groups contributing in the sorption process. From FT-IR spectra analysis, hydroxyl and amides were the major functional groups contributed in biosorption process. It was concluded that endophytic D. hawaiiensis biomass can be used potentially as biosorbent for removing Cd2+, Cu2+, and Pb2+ in aqueous solutions.
Keywords: Biosorption, Died biomass, Drechslera hawaiiensis, Heavy metals
In developing countries such as Egypt many industries are operated at smaller units that can produce a huge pollution load which, in many cases, is discharged directly into the environment without any facilities for wastewater remediation [1]. Heavy metals pollution is deleterious problem because of their environmental toxic effects and accretion through the food chain, which results in serious health and ecological problems [2,3]. Heavy metals of concern that are frequently revealed in industrial wastewaters originate from different industries like mining activities, smelting, battery manufacture, metals plating, petroleum refining, paint manufacture, pesticides, pigment manufacture, printing, and photographic industries involve lead, copper, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, silver, gold, and nickel [3,4]. Currently heavy metals pollution in the aquatic system has become a serious threat and of great environmental concern because they are persistent. These metals have negative effects on humans like brain damage, reproductive failures, nervous system failures and tumor formation. Cadmium is linked with renal dysfunction, disruptive lung disease, cancer, and harm of human respiratory systems but high doses of copper lead to anemia, liver and kidney failure, stomach and intestinal agitation and as a probable human carcinogen [5,6]. In recent years, biological methods of metals removal have been recommended as alternative non-hazardous, inexpensive and efficient techniques to conventional approaches of industrial wastewater treatment and possibility of recovering metals from the adsorbing fungal mass. Endophytic fungi has been applied to sequester copper, lead, zinc, nickel, cadmium, gold, silver through several functional chemical groups that might attract and sequester metal ions [1,5,7].
In this study, fungal biomass obtained from endophytic Drechslera hawaiiensis of Morus alba L., was used as an alternate low-cost biosorbent for heavy metal ions Pb2+, Cd2+, and Cu2+ removal from aqueous solutions. The biosorptive performances of this fungal biomass in the heavy metals biosorption processes was examined in batch experiments as a function of the most important operating parameters (initial pH, heavy metals concentration, biosorbent dose, and contact time) in order to establish the optimal conditions for bioprocess operation. The experimental results were modeled using Langmuir and, Freundlich isotherm models in order to highlight the most important characteristics of the biosorption mechanism. The efficiency of live and nonliving cells for removing these metals from real industrial wastewater was evaluated. Moreover the biomass functional groups in D. hawaiiensis implicated in the biosorption process were studied using Fourier Transformer Infrared Spectroscopy (FT-IR) spectral analysis.
MATERIALS AND METHODS
Chemicals and preparation of the stock solutions.
Sodium hydroxide, nitric acid, and metal ions standard were of analytical grade and they were performed according to Saiano et al. [8]. Cu2+, Cd2+, and Pb2+ solution were prepared from Cu(NO3)2, Cd(NO3)2, and Pb(NO3)2 solutions by dilution with deionized distilled water to the desired concentration in measuring flasks and used directly after preparation.
Factory effluents, plant samples, isolation and identification of fungal endophytes.
Collection of wastewater resulted from different industries, their contents of metal ions under study (Cu2+, Cd2+, and Pb2+). Fresh leaves of Morus alba samples, isolation and identification of their fungal endophytes were described in the previous study of El-Gendy et al. [5].
Culture conditions and fungal biomass preparation for biosorption.
Spores (106 CFU/mL) of 10-day-old culture on potato dextrose agar were routinely cultured into a growth medium, yeast malt extract glucose medium composed of yeast extract (5 g/L), malt extract (10 g/L), and glucose (20 g/L) in distilled deionized water and cultivated under static conditions at 30℃ for 10 days in dark and mycelia were taken as byproduct. Thereafter the biomasses were autoclaved at 121℃ for 15 min and then biomass harvested by filtering through a membrane filter, washed with deionized water to remove non-biomass particles and freeze-dried. Dried dead biomass was ground using a mortar and pestle and then used as fungal powder for biosorption studies.
Metal uptake.
Thirty milligrams of fungal biomass were inoculated into 55 mL of such metal ion solution containing 5mg/L of cadmium, copper or lead individually and adjusted pH to 6.0 ± 0.1. The flasks were kept under magnetic stirring, the time dependence of metal ion concentration measured within 3 hr at 30℃, the filtrates were acidified with HNO3 and its contents of each metal ion after each treatment were analyzed after proper digestion and dilution by atomic absorption spectrophotometer. Metal solution without adding biomass was served as control. Removal and uptake of each metal ion was expressed as % and mg/g, respectively. Experiments were carried out in duplicate and average values were recommended. Amount of metal ions (mg) bioadsorped per gram dry biomass was calculated using the following equation: Q = [(C0 – C)/M]V.
Where Q, mg of metal ion bioadsorped per gram of biomass; C0, initial metal ion concentration (mg/L); C, final metal ion concentration (mg/L) after biosorption; M, mass of biomass in the reaction mixture (g); and V, volume of the reaction mixture (L) [9].
Adsorption isotherms.
Two isotherm equations have been tested in the present study, namely Langmuir and Freundlich equations: Langmuir equation represented as qe = Q° bCe/(1 + bCe), where qe is the amount of biosorbed metal ions at time t (mg/g); Ce is the equilibrium concentration (mg/L); Q° (mg/g) and b (L/mg) are the maximum biosorption capacity and energy of adsorption, respectively. Ka = 1/Kd = b, ln Ka = ΔG max/RT (R is the gas constant, 8.314 J/mol K). The essential characteristics of a Langmuir isotherm can be expressed in terms of a dimensionless L constant separation factor called the equilibrium parameter, RL, which is used to predict if an adsorption system is “favorable” or “unfavorable by the following relationship RL = 1/(1 + bC0), where RL and C are the dimensionless constant separation factor or equilibrium parameter and initial metal ions concentration, respectively. The value of RL indicates the shape of isotherm to be either unfavorable (RL > 1) or linear (RL = 1) or favorable (0 < RL < 1) or irreversible (RL = 0).
The Freundlich expression is an empirical equation based on adsorption on a heterogeneous surface. The equation is commonly presented as: qexp = n · Ce · Kf, where qexp is the amount of adsorbed metal ions at time t (mg/g), Ce is the equilibrium concentration (mg/L), Kf (mg/g), and n (g/L) are the equilibrium constants indicative of biosorption capacity and biosorption intensity [9].
Effect of different pH values on the removal and uptake of Cd2+, Cu2+, and Pb2+ by D. hawaiiensis.
Initial pH values of the solutions were set at pH 2, 4, 6, 7, and 8 using nitric acid and sodium hydroxide solutions. The solution containing 5 mg/L metal ions was mixed with 30 mg dried biomass and kept under magnetic stirring for different contact time 10, 30, 60, 120, 180, and 360 min. The biomass was separated by filtration through filter paper Whatman No. 1 (Whatman, Kent, UK) and then filtrate analyzed for remaining cadmium, copper or lead. Maximum sorption data was used to find out optimum pH and the remaining experiment was conducted at the optimum pH.
Effect of initial metal ion concentration on Cd2+, Cu2+, and Pb2+ removal by D. hawaiiensis.
Different initial metal ion concentrations ranged from 10–110 mg/L were fixed with 30 mg biomass to calculate biosorption capacity at optimum pH. The initial and the final concentration were measured by atomic absorption spectrophotometer (AA, Model-M Series; Thermo Scientific, Waltham, MA, USA) for different contact times ranged between 10 and 360 min.
Effect of biomass dose on metal ions removal by D. hawaiiensis.
To find out the metal biosorption capacity of the treated biomass, the dissolved metal was taken at 5 mg/L while the concentration of biomass in the solution used in the range of 5–110 mg, each solution was tested at optimum pH for different contact time ranged between 10 and 360 min.
Metal adsorption from real industrial wastewater.
Adsorption experiments were performed using real industrial wastewater obtained from textile and painting factory effluents. Wastewater was previously filtered with a Millipore 0.45 mm filter and adsorption capacities were evaluated at different pH values (pH 4.0, 6.0, and 7.0 ± 0.1, respectively) using 30mg of biomass in 55mL of the wastewater, the suspension stirred for 3 hr and then the determination of metal content in the solution was performed as described before [8].
FT-IR analysis.
FT-IR was used to determine the vibration frequency groups in the biosorbent. For the IR studies, died dried fungal biomass before and after biosorption were mixed with KBr and then grounded in an agate mortar. The mixture was pressed to form pellets and used in the recording of spectra using Unican Mattson Mod 7000 FT-IR Spectrometer. Correspondences of characteristic peaks in the present study were based on known data from the literatures of Chew et al. [10], Ratnasri and Hemalatha [11], Madani et al. [12], Huang et al. [13], Gu et al. [14], and Barsainya et al. [15].
RESULTS AND DISCUSSION
Adsorption isotherms studies.
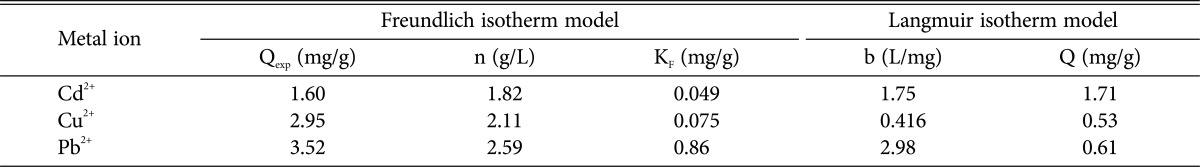
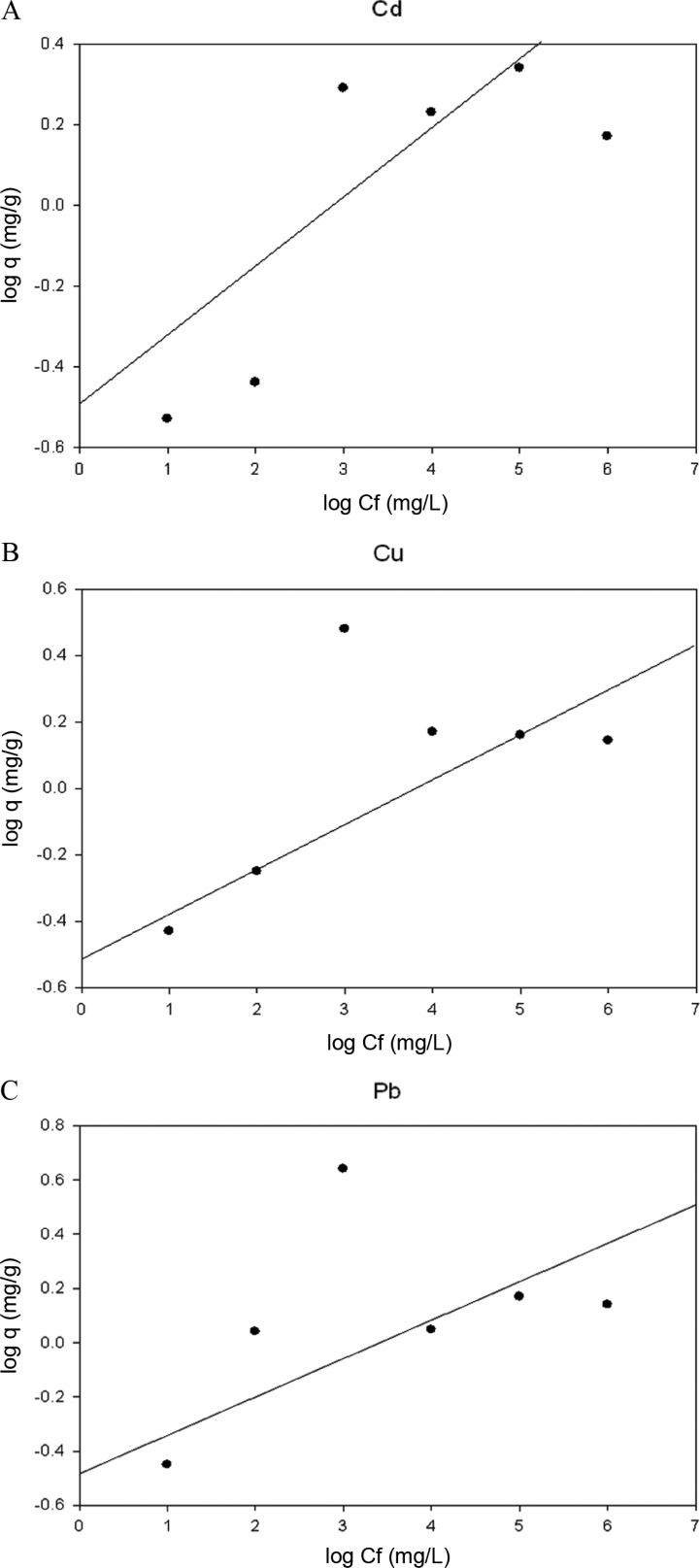
The Langmuir and Freundlich adsorption models were applied for descripting the mathematical biosorption equilibrium of Cd2+, Cu2+, and Pb2+ to dead biomass of D. hawaiiensis (Table 1, Figs. 1 and 2). In Freundlich isotherm model Qexp, n constant, and KF were determined to be (1.60, 2.95, and 3.52 mg/g), (1.82, 2.11, and 2.59 g/L) and (0.049, 0.075, and 0.86 mg/g) for Cd2+, Cu2+, and Pb2+, respectively by died biomass of D. hawaiiensis (Table 1, Fig. 1). Our data show a low correlation between the experimental values and those evaluated by the Freundlich model that may be attributed to the Freundlich model does not predict the surface saturation of the adsorbent after forming a monolayer on the metal as previously reported by Varshney et al. [16] and Verma et al. [6]. On the other hand, the high Langmuir constant b value for Pb2+ (2.98 mg/L) followed by Cd2+ (1.75 mg/L) and Cu2+ (0.416 mg/L) shows a steep desirable beginning of the isotherm, the high binding affinity between the biosorbent and Pb2+ followed by Cd2+ sorbets (metal ions) that result in more stable adsorption product and the adsorption equilibrium data fitted very well to the Langmuir model (Table 1, Fig. 2). Moreover, Q° as well as b constant in Langmuir isotherm model that estimated to be (1.71, 0.53, and 0.61 mg/g) and (1.75, 0.416, and 2.98mg/L) for Cd2+, Cu2+, and Pb2+, respectively reflect the good correlation between the values estimated by the Langmuir model and the experimental data. Thus Langmuir isotherm is created to be in parallel with experimental results as reported by previous studies [17]. Conversely Filipović-Kovačevic et al. [18] and Lokeshwari and Joshi [19] reported that Freundlich model displays better fit to the sorption results of Cr (VI) and they proposes that multilayer sorption takes place on the surface of biomass on fungal mycelium.
Table 1. Langmuir and Freundlich isotherm models constants for biosorption of Cd2+, Cu2+, and Pb2+ from aqueous solution by Drechslera hawaiiensis.
Fig. 1. Application of the Freundlich equation to the adsorption results of Cd2+ (A), Cu2+ (B), and Pb2+ (C) onto Drechslera hawaiiensis biomass.
Fig. 2. Application of the Langmuir equation to the adsorption results of Cd2+ (A), Cu2+ (B), and Pb2+ (C) onto Drechslera hawaiiensis biomass.
Effect of different pH values on removal and uptake of Cd2+, Cu2+, and Pb2+ by D. hawaiiensis.
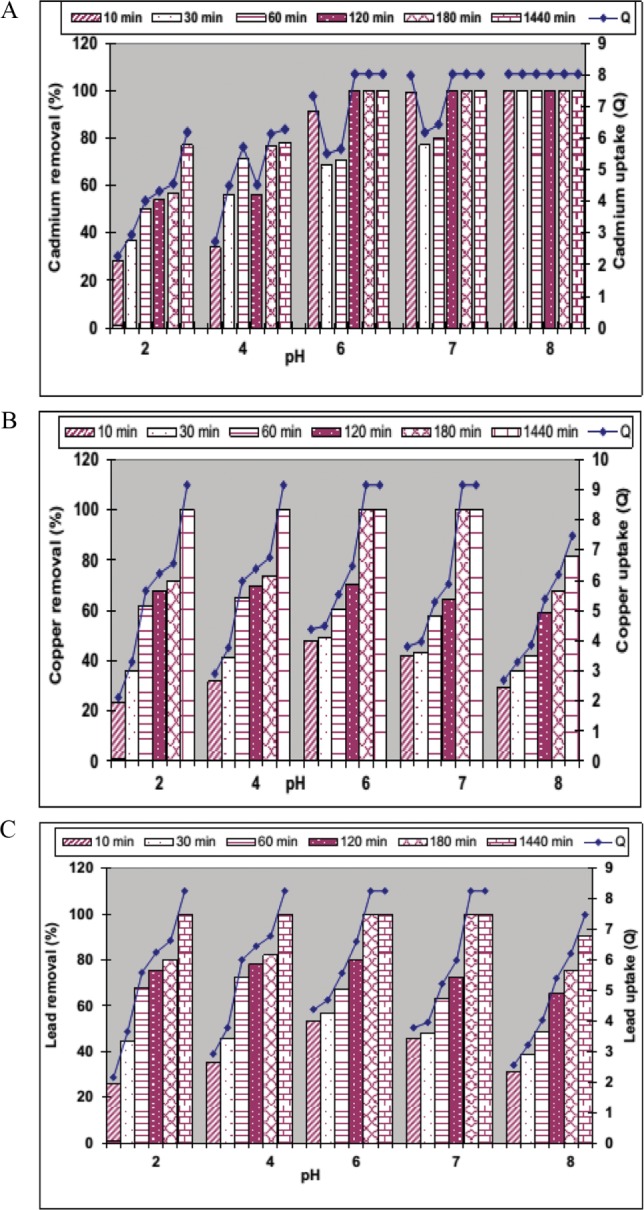
Due to pH is a critical factor in adsorption of metal ions that influences electrostatic binding of ions to corresponding functional groups, the removal and uptake of Cd2+, Cu2+, and Pb2+ by dyed biomass of D. hawaiiensis was studied in batch experiments at varying pH values ranging from pH 2 to pH 8 at different contact times ranged from 10 to 360 min. Overall at lower pH values ranged from 2 to 4 removal and uptake of Cd2+ was decreased (77.16% and 78.3% removal with 6.19, and 6.28 mg/g uptake after 360 min equilibrium time) (Fig. 3A) may be attributed to the competition between hydrogen and metal ions on the sorption sites with an apparent preponderance of hydrogen ions [6]. However, D. hawaiiensis exhibited 100% removal of Cd2+ at pH 6.0, 7.0, and 8.0 with uptake 8.3 mg/g after process time of 120, 120, and 10 min, respectively. These data supporting a correlation between increasing pH and decreasing the time of biosorption process on one hand and supported pH 8 for the best removal and uptake of cadmium at the shortest time by D. hawaiiensis on the other hand (Fig. 3A). Moreover, acidic pH was not fit for removal and uptake of both Cu2+ and Pb2+ due to D. hawaiiensis removed 100% of both metals with uptake equal to 9.16, and 8.26mg/g, respectively at the longest contact time (360 min). Neutral pH (6.0–7.0) supported the best removal of Cu2+ and Pb2+ by the dyed biomass of D. hawaiiensis at the shortest time and it reached a peak (100% removal) with uptake value equal to 9.16, and 8.26 mg/g, respectively with contact time of 180 min for both processes (Fig. 3B and 3C) but at pH 8.0 removal of Cu2+ and Pb2+ was decreased by 32.4% and 24.84% after 180 min as well as 18.4% and 9.54% after 360 min contact times, respectively. Mrudula et al. [4] reported that the pH increase, the competing effect of H3O+ ions decreases because of the lower concentration of H3O+ ions and the positively charged Pb2+ ions could easily adsorb on the available adsorption sites of the adsorbents. An increase in the pH also causes deprotonation of metal ion binding sites, allowing an exchange of H+ with the metal ions in the solution, favoring the binding of cations. In line with our results, maximum biosorption by Aspergillus versicolor, Rhizopus oligosporus, Penicillium purpurogenum, was obtained at pH 6.0 and decreased above pH 6.0 as previously reported by Martínez-Juárez et al. [20] but maximum values of metal ions removal by fungi like Penicillium resedanum, Aspergillus wentii, Alternaria alternata, and Eupenicillium katangense were achieved at pH between 5–8.6 [2,6,21]. Conversely, the acidic pH 2.0 to 4.5 was found to be optimal for biosorption of uranium (VI), cadmium, copper, lead, Fe+3 and Mn+2 ions by Fusarium sp. #ZZF51, R. cohnii, R. arrhizus, P. chrysogenum, Pleurotus mutilus and then declined rapidly with further decrease or increase in the pH [12,22,23,24,25].
Fig. 3. Effect of pHs on removal and uptake of Cd2+ (A), Cu2+ (B) and Pb2+ (C) by Drechslera hawaiiensis at different contact times.
Effect of various concentrations of Cd2+, Cu2+, and Pb2+ on their removal and uptake by D. hawaiiensis biomass.
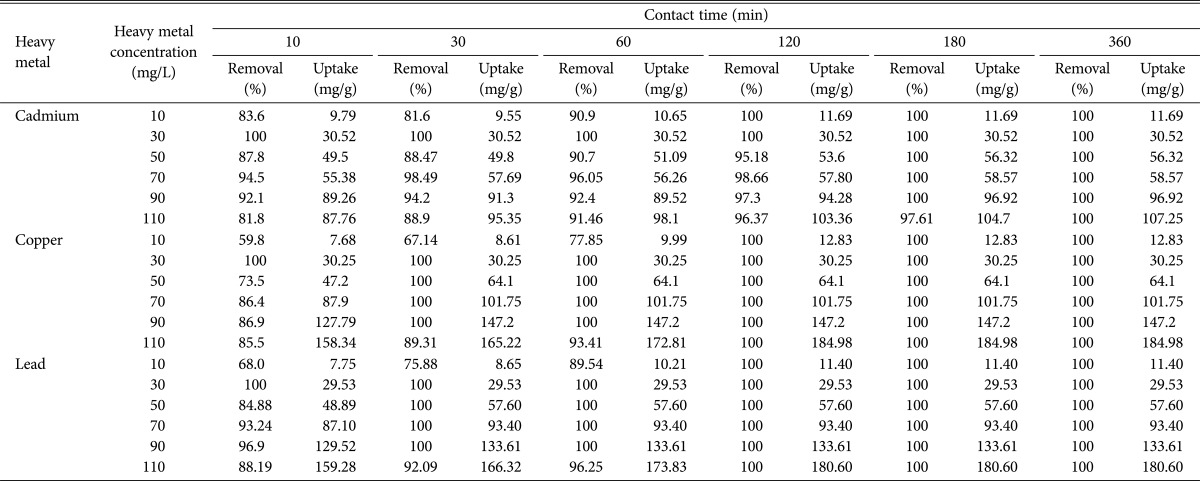
The initial concentration of metal ions in the solution plays a key role as a driving force to overcome the mass transfer resistance between the aqueous and solid phases. D. hawaiiensis biomass totally removed 10 and 30 mg/L of Cd2+ from aqueous solution with uptake equal to 11.69, and 30.52mg/g after 120 and 10 min, respectively and when Cd2+ concentrations reached to 50, 70, and 90 mg/L, the equilibrium time for its Cd2+ removal and uptake capacity from aqueous solution was detected to be 180min for 100% removal and 56.32, 58.57, and 96.92 mg/g uptake of Cd2+, respectively but it needed 360min to totally removing of 110 mg/L from aqueous solution with uptake equal to 107.25mg/g of D. hawaiiensis biomass (Table 2). Furthermore, D. hawaiiensis showed 100% removal of 10 mg/L with 12.83, and 11.4 mg/g uptake of Cu2+ and Pb2+ individually after 120 min contact time, respectively and 100% removal of 50, 70, and 90 mg/L of Cu2+ and Pb2+ individually from the aqueous solutions with uptake of Cu2+ (equal to 64.10, 101.57, and 147.20 mg/g) and Pb2+ (equal to 57.60, 93.40, and 133.61 mg/g), respectively were obtained in the batch mode after 30 min but 100% removal of both metal ions at a concentration of 110 mg/L with uptake calculated to be 184.98 and 180.6 mg/g, respectively were achieved after contact time reached 120 min (Table 2). Thus the dyed biomass D. hawaiiensis proved to be a potent biosorbent for all metal ions under study from aqueous solution at their lower and higher concentrations (ranging from 10 to 110 mg/L per unit dry weight of D. hawaiiensis) (Table 2) at 30℃.
Table 2. Effect of initial cadmium, copper, and lead concentrations on their removal and uptake by Drechslera hawaiiensis at different contact times.
Increasing uptake ability of biosorbent with increasing initial metal concentrations is owing to higher availability of metal ions (Cd2+, Cu2+, and Pb2+) for the sorption. Furthermore, higher initial concentration provides increased driving force to overcome all mass transfer resistance of metal ions between the aqueous and solid phase, resulting in higher probability of collision between metal ions and sorbents [1,26]. Our results are in line with Ratnasri and Hemalatha [11] on biosorption of different metals by isolates of Aspergillus species and Barsainya et al. [15] on Cr(VI) uptake using psychrophilic and mesophilic Penicillium species. Moreover, the uptake capacity by A. niger, P. simplicissimum, T. asperellum, A. niger, and endophytic fungi A. alternata were increased with increasing the initial concentration of heavy metals [6,14,27]. On the other hand, Onn et al. [7] in his work on biosorption by mangrove endophytic fungi and Tanwar et al. [26] reported that there is a decrease of the removal percentage of metal like Cd2+ and Pb2+ with increase in initial metal concentrations from 5–25 mg/L.
Effect of D. hawaiiensis biomass concentration on heavy metals removal and uptake.
The effect of sorbents dose was evaluated as an important parameter to identify the relationship between metal ion sorption and mass of sorbents. The variations of the removal and uptake amount of each metal ion with increasing the adsorbent dosage in Table 3 shows that onto 5 mg of D. hawaiiensis the total removal of Cd2+ and Cu2+ with uptake of 31.46 and 66.0 mg/g, respectively was reported after 360 min of treatment. On the other hand, with increasing of D. hawaiiensis biomass concentrations (30, 50, 70, 90, and 110 mg), the uptake was decreased to 5.24, 3.14, 2.24, 1.74, and 1.43 mg/g for Cd2+ as well as 11, 6.6, 4.71, 3.66, and 3 mg/g for Cu2+, respectively from the aqueous solutions after treatment for 10 min (Table 3). Furthermore, using 5 mg of D. hawaiiensis biomass (after contact time 180 min); 30 mg (after contact time 120 min); 50, 70, 90, and 110 mg (after contact time 10 min) showed 100% removal of Pb2+ with uptake equal to 52.8, 8.8, 5.28, 3.77, 2.93, and 2.4 mg/g, respectively (Table 3).
Table 3. Effect of Drechslera hawaiiensis biomass concentration on removal and uptake of cadmium, copper, and lead.
Consequently, increasing biomass concentrations resulted in a decrease in the removal and uptake of Cd2+, Cu2+ and Pb2+ per unit of D. hawaiiensis dry weight. The same conclusion was obtained on the biosorption by Penicillium cyclopium [24], Trichoderma sp. [10], T. longibrachiatum [25], A. niger [14], endophytic fungi A. alternata [6], Beauveria bassiana [3], mangrove endophytic fungi [7], Nocardiopsis sp. MORSY1948, and Nocardia sp. 2014 [1], they displayed no further increase in adsorption of heavy metals after a certain amount of adsorbent was added because of the higher dose of adsorbents in the solution, the greater availability of exchangeable sites for the ions and the high mass of adsorbent not permitting effective contact between the adsorbent and the metal ions but partial cell aggregation occur at high biomass concentrations lead to decrease of active sites.
Adsorption of heavy metals Cd2+, Cu2+, and Pb2+ from real industrial wastewater by died and active biomass of D. hawaiiensis.
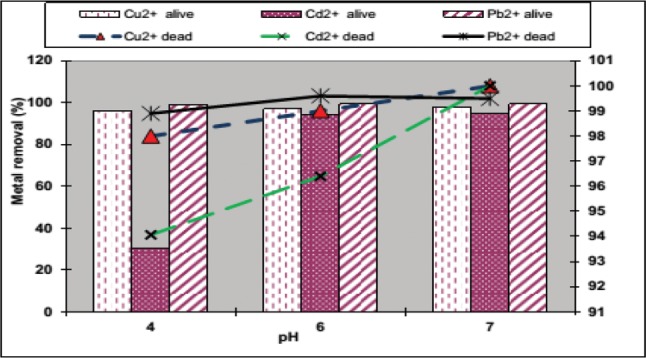
Perusal of results gotten from aforesaid findings was applied to conduct experiment with wastewater of electroplating industry. The biosorption activity (removal and uptake capacities) of Cd2+, Cu2+, and Pb2+ ions from real industrial wastewater was estimated by using live and dead fungal biomass of D. hawaiiensis at three pH values (Fig. 4) under the optimized conditions. The biomass of D. hawaiiensis removed all the three heavy metal ions meaningfully and showed same preference order of Pb2+ > Cd2+ > Cu2+ as was obtained with synthetic solution (Fig. 4). The maximum removal (100%, 100%, and 99.6%) and uptake capacity (0.28, 2.33, and 9.63 mg/g) of Cd2+, Cu2+ and Pb2+, respectively were achieved within 3 hr contact time at pH 7.0, 7.0, and 6.0 by using the dead biomass of D. hawaiiensis compared to the bioaccumulation capacity equal to 94.7%, 98%, and 99.26% and uptake of 0.264, 2.3, and 9.58 mg/g of Cd2+, Cu2+, and Pb2+, respectively detected with the active biomass of the strain under the same conditions (Fig. 4). Then, the bioaccumulation and removal capacity of these heavy metals by dead biomass of the strain was higher than alive biomass in the real industrial wastewater due to the pretreatment of the fungal biomass with heat enhanced the stability, settling property and heavy metal uptake capacities of the biomass as previously reported [3,11,28]. Under optimized conditions slight decrease in heavy metal ions noted in sorption potential from industrial effluents than from synthetic solutions could be due to various impurities present in electroplating industrial effluents in the form of anions i.e., SO42−, NO3−, and Cl− that may compete for binding sites on the fungal cell walls and then reduce uptake of metallic ions from industrial wastewater using fungi [2,29]. Similar results were reported on the removal and uptake of heavy metals by endophytic fungi like mangrove endophytic fungus Fusarium sp. #ZZF51 [22] and endophytic A. alternata isolated from Cupressus torulosa [6].
Fig. 4. Adsorption of Cd2+, Cu2+, and Pb2+ from real industrial wastewater by alive and dead Drechslera hawaiiensis.
FT-IR spectral analysis.
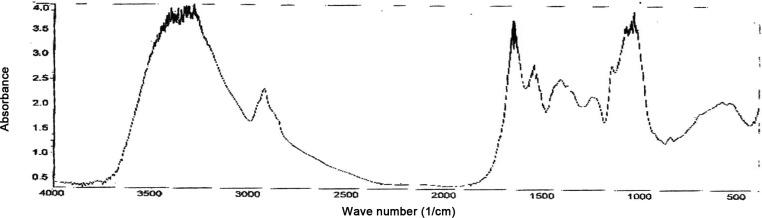
The FT-IR analysis of the D. hawaiiensis is required to recognize the functional groups that played a role in the adsorption of metal ions. Data in Fig. 5 show that major representative bands recorded in biomass involved a broad O-H and N-H stretching vibration band gotten around 3,500–3,200/cm (for intermolecular hydrogen bonding H-bonded OH groups) attributed to the overlapping of O-H and N-H stretching vibrations, the band at 2,930/cm is representative of -CH stretching containing -CH3 and CH2 functional groups attributed to fatty acids found in fungal membrane phospholipids, a pronounced peak at 1,650/cm and 1,500/cm correspond to aromatic C=C, C=O, and/or C=O of conjugated ketones or attributed to the C=O, and C=N of primary and secondary amides stretching but peaks at 1,458/cm and 1,400/cm refer to N-H bending in the amine groups and methyl asymmetric C-H bending, respectively. The peaks located at 1,242/cm, 1,103/cm, 1,075/cm, 1,056/cm, and 1,035/cm can be attributed to P=O asymmetric and symmetric stretching vibrations from phosphate functional groups while the peaks at 1,000/cm to 800/cm may be attributed to the C-O stretching, C-OH and C-O-C groups of alcohols, carboxylic acids, carbohydrates or polysaccharides-like substances. Bands at 678.9–470.6/cm could be correlated to C-C-OH asymmetric bending and C≡C-H functional groups.
Fig. 5. IR spectrum data of Drechslera huawanisis.
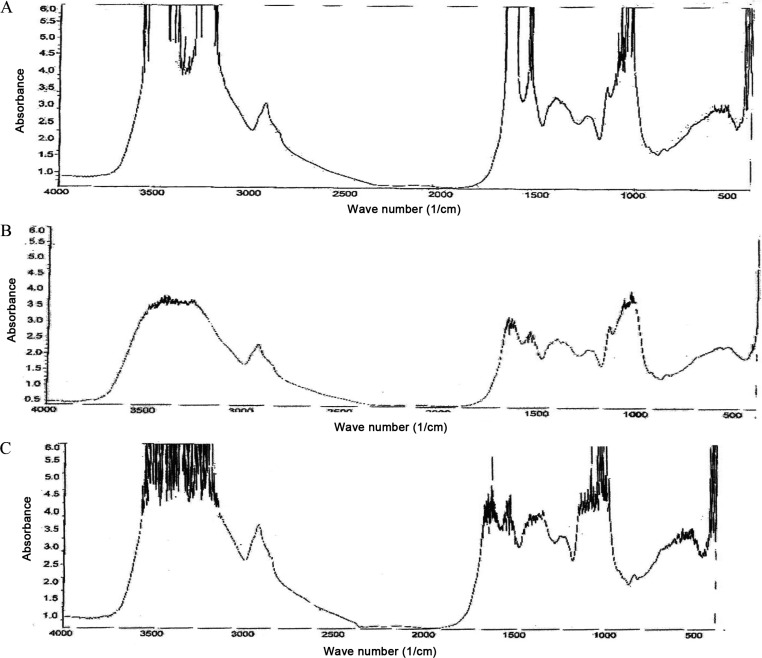
The absorption spectrum of cadmium, copper and lead-loaded fungal biomass individually was compared with that of control biomass. The FT-IR spectroscopic analysis of cadmium, copper and lead loaded biosorbent of D. hawaiiensis (Fig. 6A–6C) exhibited shifted of mentioned peaks in the unloaded biomass (control) especially in the biomass loaded with cadmium followed by lead and copper. Whereas strong asymmetrical stretching bands at 3,500–3,000/cm indicative of hydrogen bonded O-H and -NH groups stretching, 2,800–3,000/cm bands were due to C-H stretching (methyl, methane group). Different functional groups such as CO stretching in carboxyle or amide group (1,760–1,670/cm), N,N-disubstituted amide (1,640–1,680/cm), N-unsubstituted amide (1,650–1,700/cm), N-alkyl aromatic amide (1,595–1,670/cm), and N-unsubstituted aromatic amide (1,595–1,670/cm). Moreover, asymmetric and symmetric stretching vibrations of ionic carboxylic groups (COO−) seemed at 1,575/cm be assigned to the C-O stretching vibration of carboxylic acids and alcohols and these shifts may be attributed to the changes in counter ions linked with carboxylate and hydroxylate anions, suggesting that acidic groups, carboxyl and hydroxyl, are predominant contributors in metal ion uptake. C-N stretching, O-H bending, sulphur and phosphorus compounds (1,000–1,400/cm) were also characterized. The absorption at 929/cm probably corresponded to ring vibrations similar to dioxan (type I) of β-glycosides but band at 885/cm band corresponded to C1-H axial hydrogen bending vibrations in β-sugars. Distinctive bands around 700–400/cm refer to nitro compound and disulfide group, The peak at 579/cm representing O-C-O scissoring but C=O bending vibrations are because of lipids, these results indicated that Cd2+, Cu2+, and Pb2+, compounds were present on the mycelium. The peaks shifted with the attendance of each metal ion proposing an interaction of metals with these functional groups in the biosorption process. Moreover, the main functional groups responsible for a biosorption process were the hydroxyls, carbonyls, carboxyls, phosphate, sulfhydryl, amides, imidazoles, phosphonates, phosphodiester and other functional groups are the sites of metal uptake. Our data were in line with Chew et al. [10], Ratnasri and Hemalatha [11], Madani et al. [12], Huang et al. [13], Gu et al. [14], and Barsainya et al. [15] studies.
Fig. 6. IR spectrum data of Drechslera huawanisis (KBr) after Cd2+ (A), Cu2+ (B), and Pb2+ (C) removal.
It can be concluded that this study validates that the dyed biomass of D. hawaiiensis is an efficient and alternative biomass for removing metals ions in the order Pb2+ > Cd2+ > Cu2+ in terms of highly efficient reliable biosorbing bioagent for effectively removing heavy metals from aqueous environment. There were variances in the positions of the peaks in FT-IR graphs presentation the shifting of all the peaks or bands influenced by the metal ions. A change in peak position in the spectrum of D. hawaiiensis after remediation the sample contain either Pb2+, Cd2+, Cu2+, and real wastewater shows that there are bindings of heavy metal onto the functional groups. Biosorption results fitted well with the Langmuir adsorption isotherm equation and indicated sufficient biosorption by the test fungal strain at varying range of metal ion concentration as well as real industrial wastewater
References
- 1.El-Gendy MM, El-Bondkly AM. Evaluation and enhancement of heavy metals bioremediation in aqueous solutions by Nocardiopsis sp. MORSY1948 and Nocardia sp. Braz J Microbiol. 2016;47:571–586. doi: 10.1016/j.bjm.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shakya M, Sharma P, Meryem SS, Mahmood Q, Kumar A. Heavy metal removal from industrial wastewater using fungi: uptake mechanism and biochemical aspects. J Environ Eng. 2015;142:C6015001. [Google Scholar]
- 3.Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath M, Ahammad SZ. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour Technol. 2016;218:388–396. doi: 10.1016/j.biortech.2016.06.096. [DOI] [PubMed] [Google Scholar]
- 4.Mrudula V, Vijaya T, Mouli KC, Jyothi UN, Aishwarya S, Reddy VD. Novel method for removal of heavy metals by using low cost absorbents. Indo Am J Pharm Res. 2016;6:5472–5480. [Google Scholar]
- 5.El-Gendy MM, Hassanein NA, Ibrahim HA, Abd El-Baky DH. Evaluation of some fungal endophytes of plant potentiality as low-cost adsorbents for heavy metals uptake from aqueous solution. Aust J Basic Appl Sci. 2011;5:466–473. [Google Scholar]
- 6.Verma DD, Bhatt A, Agrawal PK. In-vitro study on bioaccumulation and tolerance of heavy metals by endophytic fungi Alternaria alternata isolated from Cupressus torulosa D.DON. Octa J Environ Res. 2016;4:146–154. [Google Scholar]
- 7.Onn ML, Lim PT, Mujahid A, Proksch P, Müller M. Initial screening of mangrove endophytic fungi for antimicrobial compounds and heavy metal biosorption potential. Sains Malaysiana. 2016;45:1063–1071. [Google Scholar]
- 8.Saiano F, Ciofalo M, Cacciola SO, Ramirez S. Metal ion adsorption by Phomopsis sp. biomaterial in laboratory experiments and real wastewater treatments. Water Res. 2005;39:2273–2280. doi: 10.1016/j.watres.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Bayramoğlu G, Arıca MY. Removal of heavy mercury (II), cadmium (II) and zinc (II) metal ions by live and heat inactivated Lentinus edodes pellets. Chem Eng J. 2008;143:133–140. [Google Scholar]
- 10.Chew AW, Rahman NN, Kadir MO, Chen CC. Dried and wet Trichoderma sp. biomass adsorption capacity on Ni, Cd and Cr in contaminated groundwater; International Conference on Environmental Science and Technology (IPCBEE); 2012 Mar 10-11; Chennai, India. [Google Scholar]
- 11.Ratnasri PV, Hemalatha KP. Studies on biosorption of different metals by isolates of Aspergillus species. IOSR J Pharm Biol Sci. 2015;10:1–5. [Google Scholar]
- 12.Madani A, Chergui A, Selatnia A. Biosorption of Fe+3 and Mn+2 ions from aqueous solution by a Pleurotus mutilus fungal biomass. J Chem Pharm Res. 2015;7:19–26. [Google Scholar]
- 13.Huang F, Dang Z, Guo CL, Lu GN, Gu RR, Liu HJ, Zhang H. Biosorption of Cd (II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium contaminated soil. Colloids Surf B Biointerfaces. 2013;107:11–18. doi: 10.1016/j.colsurfb.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Xu W, Liu Y, Zeng G, Huang J, Tan X, Jian H, Hu X, Li F, Wang D. Mechanism of Cr(VI) reduction by Aspergillus niger: enzymatic characteristic, oxidative stress response, and reduction product. Environ Sci Pollut Res Int. 2015;22:6271–6279. doi: 10.1007/s11356-014-3856-x. [DOI] [PubMed] [Google Scholar]
- 15.Barsainya M, Chandra P, Singh DP. Investigation of Cr (VI) uptake in saline condition using psychrophilic and mesophilic Penicillium sp. Int J Curr Microbiol Appl Sci. 2016;5:274–288. [Google Scholar]
- 16.Varshney R, Bhadauria S, Gaur MS. Biosorption of copper (II) from electroplating wastewaters by Aspergillus terreus and its kinetics studies. Water. 2011;2:142–151. [Google Scholar]
- 17.Vieira DM, Augusto da Costa AC, Henriques CA, Cardoso VL, Pessoa de França F. Biosorption of lead by the brown seaweed Sargassum filipendula-batch and continuous pilot studies. Electron J Biotechnol. 2007;10:368–375. [Google Scholar]
- 18.Filipović-Kovačević Ž, Sipos L, Briški F. Biosorption of chromium, copper, nickel and zinc ions onto fungal pellets of Aspergillus niger 405 from aqueous solutions. Food Technol Biotechnol. 2000;38:211–216. [Google Scholar]
- 19.Lokeshwari N, Joshi K. Biosorption of heavy metal (chromium) using biomass. Global J Environ Res. 2009;3:29–35. [Google Scholar]
- 20.Martínez-Juárez VM, Cárdenas-González JF, Torre-Bouscoulet ME, Acosta-Rodríguez I. Biosorption of mercury (II) from aqueous solutions onto fungal biomass. Bioinorg Chem Appl. 2012;2012:156190. doi: 10.1155/2012/156190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed E, El-Mehalawy RA, El Bah F. Removal of zinc metal from industrial wastewater by some fungi [thesis] Cairo: Faculty of Science, Ain Shams University; 2000. [Google Scholar]
- 22.Yang HB, Tan N, Wu FJ, Liu HJ, Sun M, She ZG, Lin YC. Biosorption of uranium (VI) by a mangrove endophytic fungus Fusarium sp. #ZZF51 from the South China Sea. J Radioanal Nucl Chem. 2011;292:1011–1016. doi: 10.1007/s10967-011-1552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo JM, Xiao X, Luo SL. Biosorption of cadmium (II) from aqueous solutions by industrial fungus Rhizopus cohnii. Trans Nonferrous Met Soc China. 2010;20:1104–1111. [Google Scholar]
- 24.Ianis M, Tsekova K, Vasileva S. Copper biosorption by Penicillium cyclopium: equilibrium and modeling study. Biotechnol Biotechnol Equip. 2006;20:195–201. [Google Scholar]
- 25.Adeogun AI, Kareem SO, Durosanya JB, Balogun ES. Kinetics and equilibrium parameters of biosorption and bioaccumulation of lead ions from aqueous solutions by Trichoderma longibrachiatum. J Microbiol Biotechnol Food Sci. 2012;1:1221–1234. [Google Scholar]
- 26.Tanwar A, Goswami SP, Arora SK, Mathur SP. Biosorption of Cd (II) and Pb (II) ions from aqueous solutions using Carissa carandus. Res J Pharm Biol Chem Sci. 2012;3:614–624. [Google Scholar]
- 27.Iskandar NL, Zainudin NA, Tan SG. Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci (China) 2011;23:824–830. doi: 10.1016/s1001-0742(10)60475-5. [DOI] [PubMed] [Google Scholar]
- 28.El-Sayed MT. The use of Saccharomyces cerevisiae for removing cadmium (II) from aqueous waste solutions. Afr J Microbiol Res. 2012;6:6900–6910. [Google Scholar]
- 29.Javaid A, Bajwa R, Javaid A. Biosorption of heavy metals using a dead macro fungus Schizophyllum commune Fries: evaluation of equilibrium and kinetic models. Pak J Bot. 2010;42:2105–2118. [Google Scholar]