Abstract
Different endophytes isolated from the seeds of Sophora flavescens were tested for their ability to produce matrine production. Response surface methodology (RSM) was applied to optimize the medium components for the endophytic fungus. Results indicated that endophyte Aspergillus terreus had the ability to produce matrine. The single factor tests demonstrated that potato starch was the best carbon source and the combination of peptone and NH4NO3 was the optimal nitrogen source for A. terreus. The model of RSM predicted to gain the maximal matrine production at 20.67 µg/L, when the potato starch was 160.68 g/L, peptone was 24.96 g/L and NH4NO3 was 2.11 g/L. When cultured in the optimal medium, the matrine yield was an average of 20.63 ± 0.11 µg/L, which was consistent with the model prediction. This study offered an alternative source for the matrine production by endophytic fungus fermentation and may have far-reaching prospect and value.
Keywords: Endophytic fungi, Matrine, Response surface methodology, Sophora flavescens
Due to environmental condition and over-harvesting, some wild medicinal resources of plants are facing with reduction [1]. So, it is important to search for sustainable alternative sources of well-known high value plants. Endophyte is an endosymbiont, which takes the whole or part of its life to reside in the endocellular or intracellular areas of host plants. And, it can protect its hosts from diseases [2,3,4]. Endophyte can hasten growth of hosts and increase their ability of stress resistance [5]. What's more, some of endophytes have the ability to produce the secondary metabolites generally assumed to be produced by the host plants. Therefore, a lot of researchers are interested in discovering endophytes and using microbial fermentation to meet the ever-growing demand for several life-saving drugs derived-from plants resources.
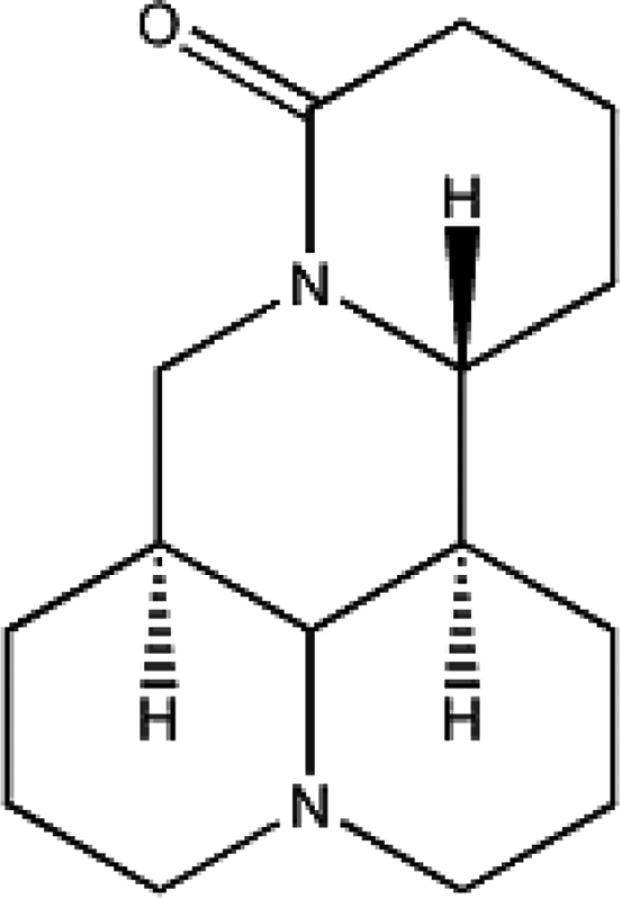
Sophora flavescens (Leguminosae) has been used as an herbal medicine or food ingredients in East Asian countries for thousands of years. With the increase of usage, the resources of S. flavescens gradually decreases. Matrine (Fig. 1), a tetracycloquinolizindine alkaloid, is one of the main active ingredients of S. flavescens, S. alopecuroides, and S. subprostrata [6,7,8]. Matrine has been used clinically in the treatment of gastric cancer, the study of Li et al. [9] showed that matrine inhibited SGC-7901 cell proliferation by changing miRNA expression in a dose-dependent manner (0.5–2.5 mg/mL). Shao et al. [10] demonstrated that treatment with 3mM matrine exerted inhibitory effects on the cell viability of estrogen receptor-positive MCF7 cells, human epidermal growth factor receptor 2-positive BT-474 cells and highly metastatic MDA-MB-231 cells, resulting in 76.4–84.5% reduction in cell numbers. Matrine (4–128 µg/mL) could inhibit human enterovirus 71 infection in human RD cells, treatment with 20 mg/kg also could reduce the mortality of mice upon lethal enterovirus 71 challenge [11]. Additionally it is considered a useful agent in treatment of allergy and central nervous system autoimmunity [12,13].
Fig. 1. The chemical structure of matrine.
Response surface methodology (RSM) is an effective statistical method that can build mathematical models to assess the effects of several factors onto a desired response [14]. RSM combines optimization theory with modern mathematic statistic to reduce the number of experiments needed and evaluate multiple variables and their interaction. RSM can address some drawbacks, such as time consumption and high cost [14]. Box-Behnken design (BBD) of RSM is suitable for fermentation optimization [15]. BBD has been widely used in the optimization of the medium constituents and other fermentation parameters for the secondary metabolites, such as extracellular lipase, curdlan, cordycepin, c-phycocyanin, and extracellular polysaccharide [14,15,16,17].
A study of He [18] reported that endophyte Aspergillus terreus, isolated from the seeds of Sophora flavescens, was able to produce matrine. But he did not quantify matrine yield because A. terreus only produced trace amount of matrine. That offered a new source for the matrine production by endophytic fungus fermentation and may have far-reaching prospect and value. So in present study, we attempt to isolate the endophyte A. terreus and optimize the medium components of it using RSM to increase yield of matrine.
MATERIALS AND METHODS
Reagents and instruments
The reference standard of matrine were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). SSC800 high-speed centrifuge (Hebei DECO Machinery Co. Ltd., Xingtai, China); LRH-250-Z incubator shaker (medical equipment factory in Guangdong, China); peptone, yeast extract (analytical grade, Beijing AoBoXing Bio-Tech Co. Ltd., Beijing, China); agar (analytical grade, Beijing AUKS Technology Co. Ltd., Beijing, China); KH2PO4, MgSO4 · 7H2O, NH4NO3, (NH4)2SO4 (analytical grade, Beijing Chemical Works, Beijing, China); sucrose, maltose, glucose (analytical grade, Aladdin Industrial Corporation, Shanghai, China); methanol (chromatographic grade, Sinopharm Chemical Reagent Co. Ltd., Shanghai, China); triethylamine (chromatographic grade, Shanghai Feather Flower Bio-Tech Co. Ltd., Shanghai, China).
Endophyte separation
Healthy S. flavescens were collected from Shandong Provinces, China, in October 2014. The endophytes were isolated from the seeds of S. flavescens according to He [18] described with some modification. Specific protocol as follows: the seeds were sterilized by sequential immersion in 70% (v/v) ethanol for 60s and 0.1% (v/v) HgCl2 for 180 sec, and three times rinsed with sterile water. The successful sterilization process was confirmed by a plated final wash onto potato dextrose agar (PDA; potato 200 g/L, dextrose 20 g/L, agar 15 g/L) medium and plates were incubated at 30℃ for 5 days. If no microbial growth was found, the seeds were used for further analysis. Then cut them into 0.3 cm2 small pieces, and plated on fresh PDA medium with streptomycin (50 U/mL) at 25℃ for 10 days. The growing mycelia were transferred and purified on PDA medium by picking mycelia tip, repeatedly above operations to obtain the pure strains. The isolated strains were incubated and the ingredients of fermentation broths were identified by high-performance liquid chromatography (HPLC), respectively. The isolated strains were saved on PDA medium at 4℃ respectively and the slants were subcultured every 3 mon. Identification of endophyte which had the ability to produce matrine was carried out by morphological characteristics (scanning optical microscope, Jiangnan Optical Instrument Factory, Nanjing, China) and internal transcribed spacer (ITS) sequences of rDNA. The rDNA ITS region of the endophyte was cloned and sequenced, and the characteristic of ITS sequence was performed by Shanghai GeneCore Bio Technologies Co. Ltd. (Shanghai, China).
Shake flask clture
Inoculum was prepared by transferring a few of endophyte A. terreus mycelia blocks on PDA medium to an erlenmeyer (250 mL) with 100 mL seed medium (glucose 20 g/L, peptone 5 g/L, yeast extract 5 g/L, MgSO4 · 7H2O 0.5 g/L, KH2PO4 0.5 g/L). Seeds were cultured at 25℃ on a rotary shaker incubator at 150 rpm for 6 days. The fermentation culture was carried out with orbital agitation at 25℃ for 15 days in 500-mL erlenmeyer flask with 200 mL of original nutrient solution (potato 200 g/L, glucose 20 g/L). And, the inoculum size was 5% (v/v). Each experiment repeated three times.
Analytical methods
The fermentation broth was centrifuged at 10,000 ×g for 15 min to separate mycelia and supernatant. The supernatant was concentrated under vacuum to 1/50 of the original volume. The condensed supernatant was applied to measure matrine yield using a waters series HPLC system equipped with a 2996 photodiode array detector. Chromatographic separation was carried out on a ZORBAX Eclipse XDB C18 column (250 × 4.6 mm i.d.; particle 5-µm, Agilent Technologies, Palo Alto, CA, USA). The mobile phase was done according to Li and Wang [19], to be specific, it was composed of 0.01 mol/L KH2PO4 buffer-methanol-triethylamine in the ratio 94 : 6 : 0.01 (v/v/v). Flow rate was 1.0 mL/min, detection wave length was 208 nm and the column temperature was maintain at 40℃. The chromatographic peak of matrine was identified bycomparing the retention time with matrine reference standard.
RSM for optimizing medium constituents
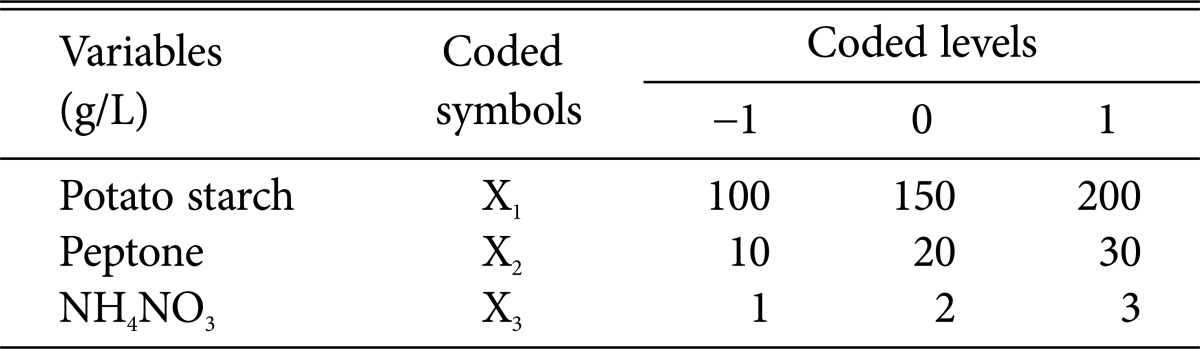
The variables (potato, peptone and NH4NO3) were selected to study their concentration and their interaction effect on matrine yield using BBD of RSM. The software Design-Expert 8.0.5b trial was used in experimental design, date analysis, and quadratic equation construction. The range of independent variables and their levels were presented in Table 1. The independent variables and their levels were chosen based on the results of our pre-experiments. The response value was matrine production. Triplicates at the center (-1, 0, and 1) of the experimental design were intended to estimate the pure error sum of squares. About the statistical calculation, the independent variables were coded according to the following formula.
| (1) |
Where xi was the coded values of Xi, while Xi represented the real value of an independent variable, X0 represented the real value of independent variables on the center point and ΔXi was the step change value.
Table 1. Variables and experiments design levels for RSM.
RSM, response surface methodology.
The behavior of the system was explained by the following polynomial model equation:
| (2) |
Where Y was predicted response value, β0 was intercept term, βi was linear term, βii was squared term, βij was interaction, Xi and Xj were the coded level of independent variables.
The statistics analysis of the polynomial equation was performed to evaluate analysis of variance (ANOVA). Fisher's F-test and the coefficient of determination R2 were applied to express the goodness of fit of the polynomial model. The surface and contour plots expressed the fitted polynomial equation, which could show the relationship between experimental level of each independent and the response to deduce the optimal conditions [20,21].
RESULTS AND DISCUSSION
HPLC chromatogram of matrine
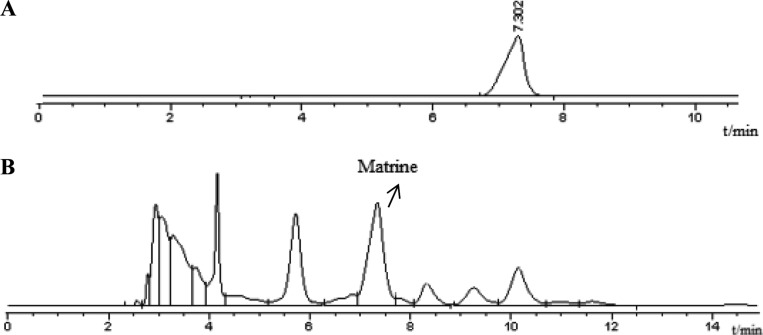
With the report of Stierle et al. [22] on taxol production by endophyte Taxomyces andreanae, a host of researchers were interested in bioprospecting of endophytes for secondary metabolites and got some breakthrough [23,24,25]. In present study, five strains were isolate from the seeds of S. flavescens. The isolated strains were incubated and the ingredients of fermentation broths were identified by HPLC, respectively. Results indicated that only one of strains could have the ability to produce matrine. Fig. 2A and 2B showed that typical chromatograms of reference standard and fermentation broth sample, respectively. The retention time of matrine was 7.302 min. Comparison the chromatograms of sample with standard indicated that there was matrine production present in fermentation broth of A. terreus. Endophyte A. terreus had the ability to produce the secondary metabolites generally assumed to be produced by the host S. flavescens.
Fig. 2. Chromatograms of matrine standard (A) and fermentation broth sample (B).
Identification of endophyte A. terreus
Identification of endophyte which had the ability to produce matrine was carried out by morphological characteristics and ITS sequences of rDNA. The centre of the strain colony was yellow, while the edge was white. The fruiting bodies of the endophyte had several aerial mycelium with diaphragm. There were lots of branch hyphae with linear conidia chains, and the spores were round. The ITS sequence was BLAST matched with GenBank database on homology, and the similarity with ribosomal rDNA of A. terreus was 100%, which indicated that the endophyte had the ability to produce matrine was A. terreus. He [18] made the same conclusion according to the analysis of ITS, 16S rDNA sequences and morphological method.
Optimization of carbon source and nitrogen source
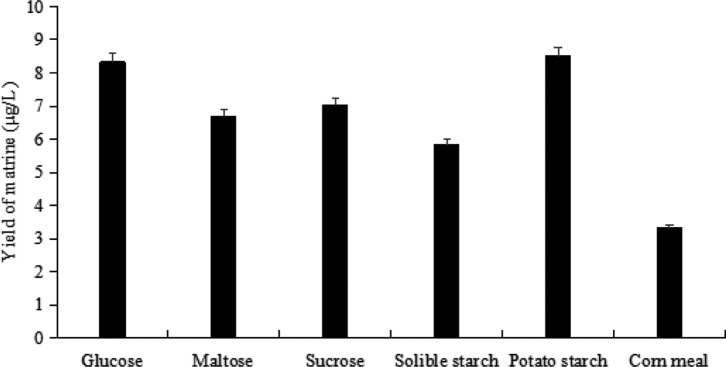
Different strains have different living habits. Carbon source and nitrogen source, two major ingredients of medium, play significant roles in synthesis of secondary metabolites and the growth of cells [26]. Their types and concentration have effect on yield of secondary metabolites [27,28]. So, the optimum carbon source and nitrogen source for matrine yield secreted by endophyte A. terreus were screened using single factor tests. In this study, glucose (Aladdin Industrial Corporation), maltose (Aladdin Industrial Corporation), sucrose (Aladdin Industrial Corporation), soluble starch (Tianjin Dingshengxin Chemical Industry Co. Ltd., Tianjin, China), potato starch and corn meal (Shandong Zhenhua Biotechnology Co., Ltd., Binzhou, China) (50 g/L, respectively) were applied to elect the optimum carbon source for endophyte A. terreus. The yield of matrine was assayed and the results were exhibited in Fig. 3, which indicated that all kinds of carbon sources could increase matrine production. But their influences on matrine yield were different. Potato starch and glucose were better suited for A. terreus to produce matrine than others. From the perspective of economic, potato starch was used as optimal carbon source in following experiment.
Fig. 3. Effect of different carbon source (50 g/L, respectively) on matrine yield.
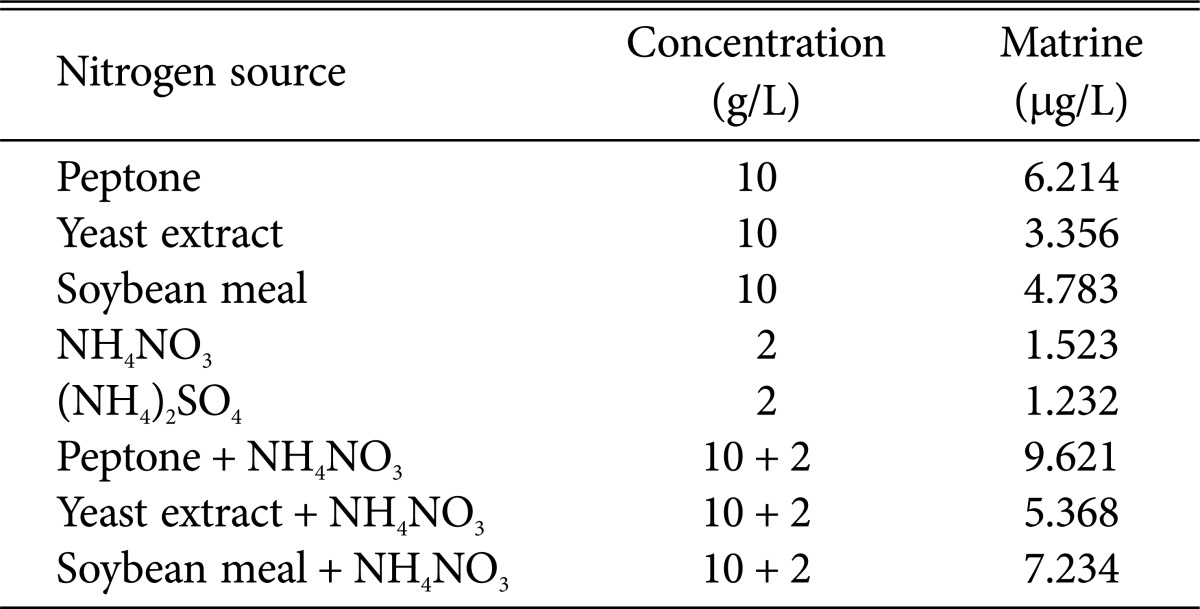
In order to search for the optimal nitrogen source for A. terreus, organic nitrogen source (peptone, yeast extract, soybean meal [Beijing AoBoXING Bio-Tech Co., Ltd.]), inorganic source (NH4NO3, (NH4)2SO4 [Beijing Chemical Works]) or the combination were performed and the results were shown in Table 2. The combination of peptone and NH4NO3 was preferable for matrine production. A considerable body of evidence indicated that the combination of organic source and inorganic source was more suitable for strains to produce secondary metabolites [29,30,31]. So, the combination of NH4NO3 and peptone were used in following experiments.
Table 2. Comparison of nitrogen source for matrine production.
A host of studies increased the yield of secondary metabolites by optimizing the formula of media. Zong et al. [29] selected peptone, (NH4)2SO4 and glucose as the most influential parameters to optimize the medium constituents for ε-poly-L-lysine. Under the optimal culture condition, the value of ε-poly-L-lysine was 1.75 times than that of original medium. Tanyol et al. [32] reported that peptone, ammonium sulphate and sunflower oil cake (carbon source) were the most influential parameters in lipase production, the maximum lipase activity of 10.8 U/L was obtained when sunflower oil cake was 11.10% (w/v), peptone was 1.18% (w/v), ammonium sulphate was 0.83% (w/v). A report by Wang et al. [20] indicated that potato, glucose, and wheat bran were the most influential parameters in the production of extracellular polysaccharide from Agaricus blazei Murrill. A 1.79-fold increase in production was obtained under the optimal medium.
Optimizing medium components by RSM
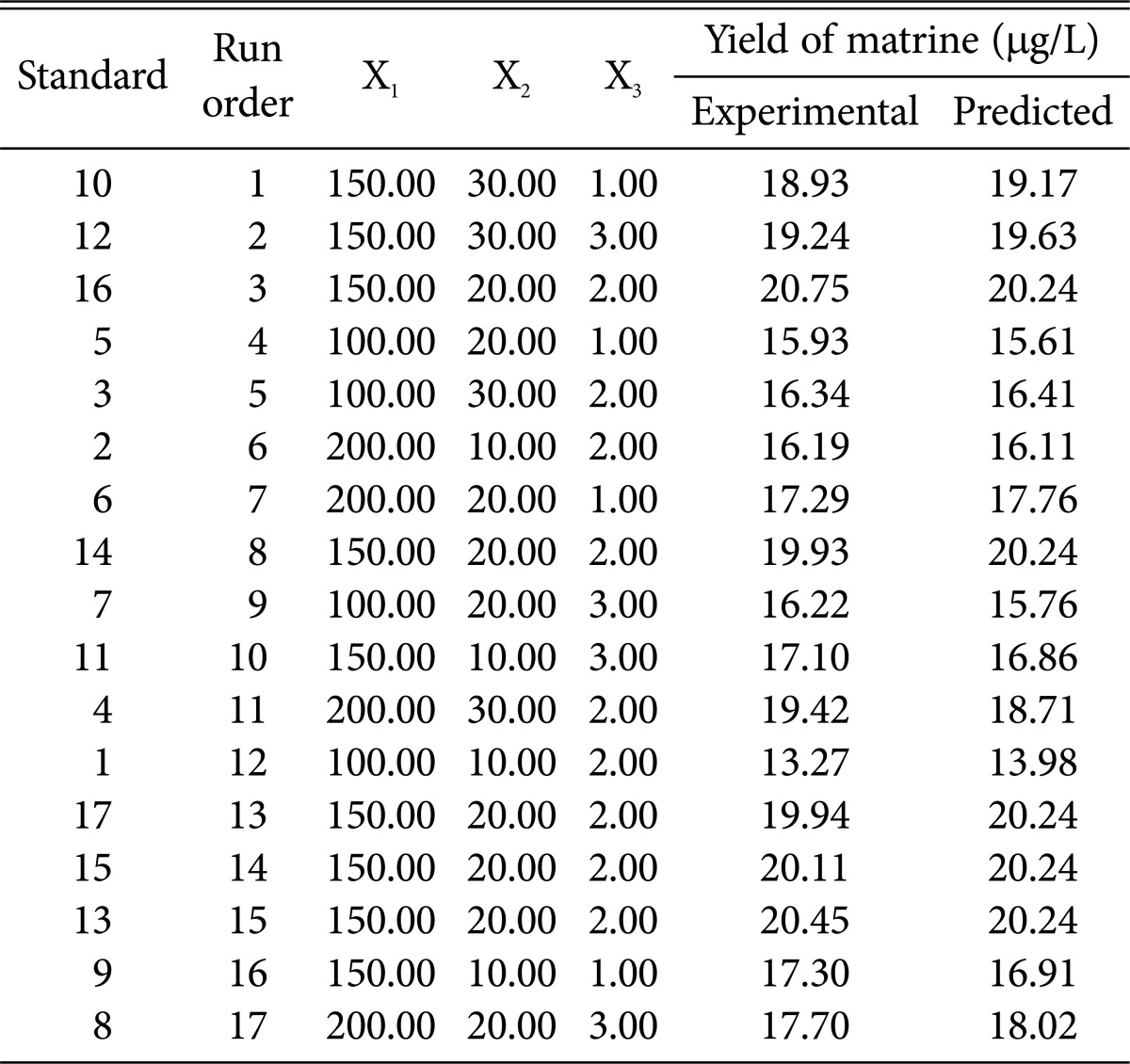
RSM is an effective optimization technology and it has been widely used in many fields [33,34,35], especially in biological engineering. In this study, the BBD of RSM was used to estimate the influence of the individual factors including potato starch content (X1), peptone treatment (X2), NH4NO3 addition (X3) and their interaction effects on matrine production. In the 17 experiments of BBD, the experimental and predicted values of matrine under different treatment conditions were presented in Table 3.
Table 3. Design and experimental results of the Box-Behnken design.
The predicted response Ymatrine for matrine production in terms of coded factors was expressed as follow:
| (3) |
Where Ymatrine was the respone variable of matrine, and X1, X2 and X3 were the concentration of potato starch, peptone and NH4NO3, respectively.
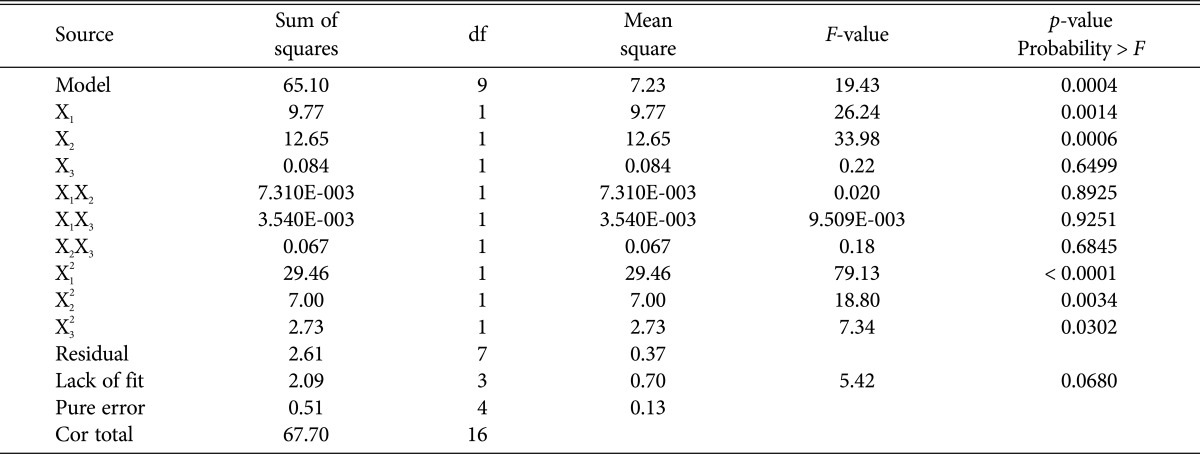
Table 4 showed the ANOVA for response surface quadratic model and the statistical significance of the regression model, which indicated that the model was highly significant for the matrine production, as was evident from F-value (model = 19.43) with a very low probability value (p > F) [36]. The p-value less than 0.05 declared that model term was significant. The values of R2 (0.9615) and adjusted R2 (0.9120) were closer to 1 and they also implied the high efficacy of the Eq. (3). Adequate precision ratio, used to measure the ratio of signal to noise, was generally desirable to be more than 4 [37]. Adequate precision ratio of 13.364 indicated an adequate signal and could be used to navigate the design space. The coefficient of variation value was 3.39% and indicated a great precision and accuracy of this study. Above all, the regression model for matrine production was a good prediction of the experimental results and the factor effects were real.
Table 4. Analysis of variance (ANOVA) for the fitted quadratic polynomial model for optimization of matrine production.
R2 = 0.9615; adj-R2 = 0.9120.
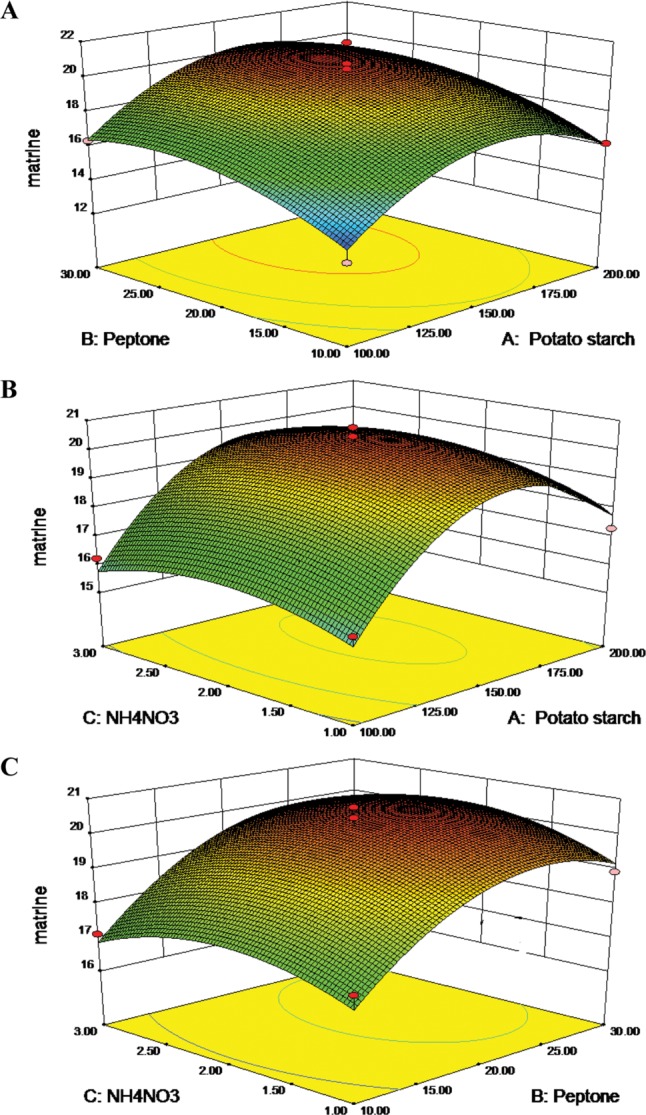
Fig. 4A, 4B, and 4C represented the three dimension surface (3D-surface) plot and two dimensional response projection (2D-projection) as a function of potato starch addition, peptone addition and NH4NO3 treatment on matrine yield. The 3D-surface and 2D-projection intuitively showed the response over a region of independent variables and the relationship between experimental levels of each factor. Fig. 4A showed the effect of potato starch addition and peptone treatment on matrine yield. The 3D-surface showed evidence that marine yield increased upon increasing potato starch addition and peptone treatment. A maximal matrine production was obtained when the potato starch was 160.68 g/L and peptone was 24.96 g/L. Afterwards, the matrine production decreased with potato starch and peptone increase. Fig. 4B showed that increasing the content of NH4NO3 to more than 2.11 g/L caused shrink of matrine yield. A quadratic effect of peptone and NH4NO3 in the response was observed in Fig. 4C. The curves of Fig. 4C bear some similarity to that in Fig. 4A and 4B, respectively. Thus, the best content of X1, X2, and X3 were found to be 160.68 g/L, 24.96 g/L, and 2.11 g/L, respectively. The predicted matrine yield obtained from the model using the optimal medium was 20.67 µg/L. The average result of verification tests was 20.63 ± 0.11 µg/L, which was 1.67 times higher than that of initial media (potato 200 g/L, glucose 20 g/L).
Fig. 4. The 3D-plot and 2D-projection of response surface represent the interaction between two factors in matrine production (µg/L) by keeping the other two variables constant: potato starch and peptone (g/L) (A), potato starch and NH4NO3 (g/L) (B), peptone and NH4NO3 (g/L) (C), (the yellow and red dot represent design points below predicted value and above predicted value, respectively).
With S. flavescens over harvesting, the wild resources of S. flavescens could not meet the ever-growing demand. A. terreus, an endophytic fungus harboring seeds of S. flavescens, had the ability to produce matrine, which may be the alternative source of S. flavescens and have far-reaching prospect and value.
The yield of matrine produced by A. terreus was very little, further studies were required to optimize the other culture conditions (PH, temperature, precursors, mineral ion, oxygen supply, and light condition) or obtain mutations by a high-energy (~MeV) proton beam to increase the yield of matrine [38]. The biosynthetic pathway of matrine secreted by A. terreus also will be clarified in the days ahead.
In this study, endophyte A. terreus which had the ability to produce matrine was isolated from the seeds of S. flavescens. Which offered an alternative source for matrine production by endophytic fungus fermentation. The single factor tests demonstrated that potato starch was the best carbon source and the combination of peptone and NH4NO3 was the optimal nitrogen source for A. terreus. The model of RSM predicted to gain the maximal matrine production at 20.67 µg/L, when the potato starch was 160.68 g/L, peptone was 24.96 g/L and NH4NO3 was 2.11 g/L. The average result of three verified experiments was 20.63 ± 0.11 µg/L, which was consistent with the model prediction.
ACKNOWLEDGEMENTS
This work was supported by the Foundation of National Natural Science Foundation of China (81573606) and (81373968), Jinan Science and Technology Development Program (201303055) and Project of Shandong Province Higher Educational Science and Technology Program (J14LK61).
References
- 1.Venugopalan A, Potunuru UR, Dixit M, Srivastava S. Effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Bioresour Technol. 2016;206:104–111. doi: 10.1016/j.biortech.2016.01.079. [DOI] [PubMed] [Google Scholar]
- 2.Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 3.Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 4.Yang SX, Wang HP, Gao JM, Zhang Q, Laatsch H, Kuang Y. Fusaroside, a unique glycolipid from Fusarium sp., an endophytic fungus isolated from Melia azedarach. Org Biomol Chem. 2012;10:819–824. doi: 10.1039/c1ob06426f. [DOI] [PubMed] [Google Scholar]
- 5.Wu B, Wu L, Ruan L, Ge M, Chen D. Screening of endophytic fungi with antithrombotic activity and identification of a bioactive metabolite from the endophytic fungal strain CPCC 480097. Curr Microbiol. 2009;58:522–527. doi: 10.1007/s00284-009-9361-7. [DOI] [PubMed] [Google Scholar]
- 6.Küçükboyaci N, Özkan S, Adigüzel N, Tosun F. Characterisation and antimicrobial activity of Sophora alopecuroides L. var. alopecuroides alkaloid extracts. Turk J Biol. 2011;35:379–385. [Google Scholar]
- 7.Guo Z, Zhang L, Song C, Zhang X. Molecularly imprinted solid-phase extraction of matrine from radix Sophorae tonkinensis. Analyst. 2011;136:3016–3022. doi: 10.1039/c1an15281e. [DOI] [PubMed] [Google Scholar]
- 8.Fu Q, Fang Q, Feng B, Sun S, Du W, Amut E, Xiao A, Chang C. Matrine-imprinted monolithic stationary phase for extraction and purification of matrine from Sophorae flavescentis Ait. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:894–900. doi: 10.1016/j.jchromb.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Xie S, Liu X, Wu H, Lin X, Gu J, Wang H, Duan Y. Matrine alters microRNA expression profiles in SGC-7901 human gastric cancer cells. Oncol Rep. 2014;32:2118–2126. doi: 10.3892/or.2014.3447. [DOI] [PubMed] [Google Scholar]
- 10.Shao H, Yang B, Hu R, Wang Y. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-κB signaling pathway. Oncol Lett. 2013;6:517–520. doi: 10.3892/ol.2013.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Xiu J, Zhang X, Zhang L, Yan K, Qin C, Liu J. Antiviral effect of matrine against human enterovirus 71. Molecules. 2012;17:10370–10376. doi: 10.3390/molecules170910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XM, Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J Allergy Clin Immunol. 2009;123:297–306. doi: 10.1016/j.jaci.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kan QC, Zhu L, Liu N, Zhang GX. Matrine suppresses expression of adhesion molecules and chemokines as a mechanism underlying its therapeutic effect in CNS autoimmunity. Immunol Res. 2013;56:189–196. doi: 10.1007/s12026-013-8393-z. [DOI] [PubMed] [Google Scholar]
- 14.Rafigh SM, Yazdi AV, Vossoughi M, Safekordi AA, Ardjmand M. Optimization of culture medium and modeling of curdlan production from Paenibacillus polymyxa by RSM and ANN. Int J Biol Macromol. 2014;70:463–473. doi: 10.1016/j.ijbiomac.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Jia J, Yang X, Wu Z, Zhang Q, Lin Z, Guo H, Lin CS, Wang J, Wang Y. Optimization of fermentation medium for extracellular lipase production from Aspergillus niger using response surface methodology. Biomed Res Int. 2015;2015:497462. doi: 10.1155/2015/497462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiapeng T, Yiting L, Li Z. Optimization of fermentation conditions and purification of cordycepin from Cordyceps militaris. Prep Biochem Biotechnol. 2014;44:90–106. doi: 10.1080/10826068.2013.833111. [DOI] [PubMed] [Google Scholar]
- 17.Singh NK, Parmar A, Madamwar D. Optimization of medium components for increased production of C-phycocyanin from Phormidium ceylanicum and its purification by single step process. Bioresour Technol. 2009;100:1663–1669. doi: 10.1016/j.biortech.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 18.He L. Isolation and identification of the antagonistic substance of the endophytic fungi from Sophora flavescens and its function mechanism. Shenyang: Shenyang Agricultural University; 2011. [Google Scholar]
- 19.Li K, Wang H. Simultaneous determination of matrine, sophoridine and oxymatrine in Sophora flavescens Ait. by high performance liquid chromatography. Biomed Chromatogr. 2004;18:178–182. doi: 10.1002/bmc.308. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Li G, Zhang W, Han C, Xu X, Li YP. The protective effect of Agaricus blazei Murrill, submerged culture using the optimized medium composition, on alcohol-induced liver injury. Biomed Res Int. 2014;2014:573978. doi: 10.1155/2014/573978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu CH, Engelmann NJ, Lila MA, Erdman JW., Jr Optimization of lycopene extraction from tomato cell suspension culture by response surface methodology. J Agric Food Chem. 2008;56:7710–7714. doi: 10.1021/jf801029k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 23.Macías-Rubalcava ML, Hernández-Bautista BE, Jiménez-Estrada M, González MC, Glenn AE, Hanlin RT, Hernández-Ortega S, Saucedo-García A, Muria-González JM, Anaya AL. Naphthoquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry. 2008;69:1185–1196. doi: 10.1016/j.phytochem.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Arora D, Sharma N, Singamaneni V, Sharma V, Kushwaha M, Abrol V, Guru S, Sharma S, Gupta AP, Bhushan S, et al. Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine. 2016;23:1312–1320. doi: 10.1016/j.phymed.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Han Y, Xiao J, Li L, Guo L, Jiang X, Kong L, Che Y. Chlorotheolides A and B, spiroketals generated via diels-alder reactions in the endophytic fungus Pestalotiopsis theae. J Nat Prod. 2016;79:2616–2623. doi: 10.1021/acs.jnatprod.6b00550. [DOI] [PubMed] [Google Scholar]
- 26.Xiao Z, Wang X, Huang Y, Huo F, Zhu X, Xi L, Lu JR. Thermophilic fermentation of acetoin and 2,3-butanediol by a novel Geobacillus strain. Biotechnol Biofuels. 2012;5:88. doi: 10.1186/1754-6834-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Liu Y, Di Z, Han C, Liu Z. The strategies for increasing cordycepin production of Cordyceps militaris by liquid fermentation. Fungal Genom Biol. 2016;6:134. [Google Scholar]
- 28.Mao XB, Eksriwong T, Chauvatcharin S, Zhong JJ. Optimization of carbon source and carbon/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Process Biochem. 2005;40:1667–1672. [Google Scholar]
- 29.Zong H, He Y, Zhan Y, Du J, Feng F, Li D. Optimization of medium constituents for ε-poly-L-lysine fermentation with response surface methodology. J Food Sci. 2010;75:M552–M556. doi: 10.1111/j.1750-3841.2010.01820.x. [DOI] [PubMed] [Google Scholar]
- 30.Mao XB, Zhong JJ. Significant effect of NH4 + on cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2006;38:343–350. [Google Scholar]
- 31.Masuda M, Urabe E, Sakurai A, Sakakibara M. Production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2006;39:641–646. [Google Scholar]
- 32.Tanyol M, Uslu G, Yönten V. Optimization of lipase production on agro-industrial residue medium by Pseudomonas fluorescens (NRLL B-2641) using response surface methodology. Biotechnol Biotechnol Equip. 2015;29:64–71. doi: 10.1080/13102818.2014.991635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shende D, Sidhu GK. Response surface methodology to optimize enzyme-assisted aqueous extraction of maize germ oil. J Food Sci Technol. 2016;53:3282–3295. doi: 10.1007/s13197-016-2303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momen SB, Siadat SD, Akbari N, Ranjbar B, Khajeh K. Applying central composite design and response surface methodology to optimize growth and biomass production of Haemophilus influenzae type b. Jundishapur J Microbiol. 2016;9:e25246. doi: 10.5812/jjm.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raza A, Li F, Xu X, Tang J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int J Biol Macromol. 2016;94(Pt A):335–344. doi: 10.1016/j.ijbiomac.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Murthy MS, Swaminathan T, Rakshit SK, Kosugi Y. Statistical optimization of lipase catalyzed hydrolysis of methyloleate by response surface methodology. Bioprocess Eng. 2000;22:35–39. [Google Scholar]
- 37.Feng YL, Li WQ, Wu XQ, Cheng JW, Ma SY. Statistical optimization of media for mycelial growth and exopolysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem Eng J. 2010;49:104–112. [Google Scholar]
- 38.Das SK, Masuda M, Hatashita M, Sakurai A, Sakakibara M. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Lett Appl Microbiol. 2008;47:534–538. doi: 10.1111/j.1472-765X.2008.02456.x. [DOI] [PubMed] [Google Scholar]