Abstract
Background
We examined the association between salivary mitochondrial DNA (mtDNA) copy number and chronic fatigue combined with depression and insomnia.
Methods
This cross-sectional study included 58 healthy adults with moderate to severe fatigue (Brief Fatigue Inventory [BFI] ≥4) for longer than 6 months. Subjects were classified as those without combined symptoms, with either depression (Beck Depression Inventory [BDI] ≥13) or insomnia (Pittsburgh Sleep Quality Index [PSQI] ≥5), or with both depression and insomnia. Salivary mtDNA copy number was measured by real-time quantitative polymerase chain reaction. The association was evaluated using a general linear model.
Results
About 76% of participants had either depression or insomnia as additional symptoms. These subjects were predominately female, drank more alcohol, and exercised less than those without combined symptoms (P<0.05). The group with both depression and insomnia exhibited significantly higher BFI and lower mtDNA copy number than those without combined symptoms (P<0.05). After adjusting for confounding factors, significant negative associations between mtDNA copy number and usual fatigue were found in the group without combined symptoms, whereas the negative associations in the group with combined symptoms were attenuated. BDI and PSQI were not associated with mtDNA copy number.
Conclusion
Chronic fatigue is negatively associated with salivary mtDNA copy number. Salivary mtDNA copy number may be a biological marker of fatigue with or without combined symptoms, indicating that a separate approach is necessary.
Keywords: Mitochondrial DNA, Mental Fatigue, Depressive Disorder, Sleep Disorders
INTRODUCTION
Fatigue is often indicated by feelings of general exhaustion, tiredness, and reduced energy levels.1) People experiencing fatigue are likely to have additional symptoms, such as mood changes, cognitive deficits, and sleep disturbances in their daily life.2) In the general population, the prevalence of fatigue and related psychological symptoms is 5.6%.3) Addressing these symptom combinations may improve recovery from stressful situations,4) and differentiating treatment according to the particular symptom combination is more likely to be successful.2) Furthermore, fatigue with additional symptoms has been associated with a number of chronic diseases, including arthritis, asthma, and anemia. 5) The annual total health care costs of patients with combined fatigue symptoms was greater than those without combined symptoms ($14,462 versus $9,971).2)
Fatigue is a manifestation of decreased efficiency of cellular energy systems, primarily in the mitochondria.6) Mitochondrial dysfunction or damage results in a decrease of mitochondrial adenosine triphosphate (ATP) production rates.7) The number of mitochondrial DNA (mtDNA) copies are indicative of mitochondrial biogenesis and reflect mitochondrial function.8) Decreased mtDNA copy numbers have been reported in aging, type 2 diabetes, cancer, and neurodegenerative disease.8)
In previous studies, depleted mtDNA, resulting in mitochondrial malfunction, has been implicated in neuropsychiatric disease.8),9) Peripheral blood mtDNA content (mtDNA/genomicDNA ratio) in a fatigued patient was similar to that of a healthy control.10) In addition, depressive symptoms in a young patient were not associated with leukocyte mtDNA copy number in blood.11) However, leukocyte mtDNA copy number in blood and clinically stable depressive disorder are negatively associated.12) Recently, an association between psychological adversity, as well as disease, and mtDNA copy number was reported in physically healthy young adults.13) As described above, studies thus far have yielded discrepant findings. Moreover, the association between salivary mtDNA copy number and chronic fatigue with additional symptoms has not been investigated. In non-malignant patients, mtDNA mutations were more efficiently detected in buccal epithelial cells than blood samples.14) Salivary samples can be easily, noninvasively, and efficiently acquired.15) Therefore, they are suitable DNA sources for screening or preventive purposes.
Our aim in this study was to examine the association between salivary mtDNA copy number from buccal epithelial cells, and chronic fatigue with or without depression or insomnia.
METHODS
1. Study Subjects
Sixty Korean adults who visited the fatigue clinic of Gangnam Severance Hospital, Yonsei University College of Medicine with moderate to severe fatigue for longer than 6 months were recruited between July and November 2013. All participants were assessed for eligibility using the following criteria: age ≥20 years, Brief Fatigue Inventory-Korean version (BFI-K) score ≥4 (moderate to severe fatigue), no pregnancy or lactation, no medical history of malignancy, and no acute or chronic severe illness (history of common cold, acute gastroenteritis, uncontrolled diabetes, uncontrolled hypertension, liver or renal disease, gout, or renal calculi). Participants were excluded for oral vitamin supplement intake, abnormal laboratory tests (white blood cell count, hemoglobin, creatinine, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, thyroid stimulating hormone [TSH]), or urinalysis in the preceding 3 months.
The institutional review board of Gangnam Severance Hospital, Yonsei University College of Medicine, approved this study, and written informed consent was obtained from each participant (#3-2012-154). The original clinical trial is registered at ClinicalTrials.gov (NCT01926132) and was permitted by the Korean Ministry of Food and Drug Safety (20130091445).
2. Study Variables
We measured height, weight, and blood pressure. Weight (kg) and height (cm) were measured using standardized scales and stadiometers while wearing light clothing. Body mass index (BMI) was calculated as body weight divided by the height squared (kg/m2). All physical measurements were made twice and analyses were performed on averaged values. All participants answered questionnaires about comorbidities, medication history, smoking status (never smoked, ex-smoker, or current smoker), alcohol intake (never drink, or more than once per week), physical exercise of at least 30 min/d three times per week, and coffee consumption (per week).
We measured fatigue using the BFI-K, which consists of nine questions. Fatigue and its interference with daily living were scored by patients on a scale from 0 to 10, which ranged from ‘no fatigue’ to ‘fatigue as bad as you can imagine.’ The internal consistency of the Korean version was very high (Cronbach's α coefficient=0.96), and validity was confirmed by factor analysis.16) The BFI-K was evaluated for fatigue ‘now,’ ‘usual’ fatigue, and ‘worst’ fatigue during the last 24 hours. Aspects evaluated included general activity, mood, walking ability, normal work (both outside of the home and daily chores), relationships with other people, and enjoyment of life.17) Since BFI-K scores for ‘usual’ fatigue are 4.07±2.27 in the general Korean population,1) only participants with a BFI-K score greater than four were included in the study. We assessed depression and sleep quality, using a self-evaluation questionnaire. The Beck Depression Inventory (BDI) is one of the most widely used self-reporting instruments for measuring the severity of depression. Each symptom is rated on a 4-point intensity scale, and scores are added to produce a total within the range 0 to 63. The BDI is useful in screening depression in the general population, as well as measuring the severity of depression among psychiatric patients.18) The Pittsburgh Sleep Quality Index (PSQI) is used worldwide to evaluate sleep quality during the previous month. The Korean version of the PSQI has previously been tested for reliability (Cronbach's α coefficient=0.84), sensitivity (0.943), and specificity (0.844).19) Depression was defined as a total BDI score of more than 13 points, while insomnia was defined as more than five points on the PSQI.
3. Measurement of Mitochondrial DNA Copy Number
Buccal epithelial cells of all participants were collected from saliva, after rubbing oral mucosa against teeth. Saliva samples were washed by centrifuging at 1,200× g for 3 minutes in phosphate-buffered saline. DNA components were extracted using the Tissue Spin Mini Kit (Nucleogen, Ansan, Korea). The quantity and quality of DNA were measured with an Epoch spectrophotometer (BioTek, Winooski, VT, USA). The mtDNA content was measured by a real-time quantitative polymerase chain reaction (qPCR) for mitochondrial encoded nicotinamide adenine dinucleotide dehydrogenase 1 (MT-ND1), which was normalized to hemoglobin beta (HBB), encoded by nuclear DNA. Using the CFX96 Real-time System (Bio-Rad, Singapore, Singapore), qPCR was performed. The 15-µL reaction mixture contained 10 ng of template DNA, 1X Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) and 7.5 pmol of primers. Thermal cycling conditions consisted of 1 cycle at 95℃ for 60 seconds, followed by 35 cycles of: 95℃ for 5 seconds, 61℃ for 10 seconds, and 72oC for 20 seconds. After PCR, a dissociation curve analysis was performed from 61℃ to 95℃ (in 0.5℃ increments) to confirm specific amplification. To assess the efficiency of all primer pairs, the standard curves were derived from seven serial dilutions of reference DNA at 40, 20, 10, 5, 2.5, 1.25, and 0.625 ng, using the Bio-Rad CFX Manager version 1.6 software (Bio-Rad). Primers for MT-ND1 were 5'-GACCCTACTTCTAACCTCCCTGT-3' and 5'-TAGGAGGTGTATGAGTTGGTCGT-3'. HBB primers were 5'-ACCCAAGAGTCTTCTCTGTCTCCA-3' and 5'-TCTGCCGTTACTGCCCTGTG-3'. We also screened the University of California, Santa Cruz database (http://genome.ucsc.edu/) to confirm that the primer sequences were unique without any repeats. The threshold cycle number (Ct) indicates the fractional Ct, which is automatically determined for each primer. The ΔCt represented the relative abundance, calculated as {Ct (ND1)-Ct (HBB)}. The mtDNA was quantified using the equation: 2×2−ΔCt.20) All experiments contained reference DNA and were repeated at least three times. Each experiment was normalized against serial dilutions of a control DNA sample.21)
4. Statistical Analysis
To evaluate the relationship between mtDNA copy number and the characteristics measured using BFI-K, BDI, and PSQI, we compared these variables according to the tertile distribution of the log-transformed mtDNA copy number. All analyses used a logarithmically transformed mtDNA copy number.
Clinical characteristics, BFI-K, BDI, PSQI, and mtDNA copy number for the three groups (1) without combined symptoms, (2) with either insomnia or depression, and (3) with both insomnia and depression of fatigue patients were compared by Kruskal-Wallis or chi-squared test.
To assess the association between mtDNA copy number and fatigue according to combined symptoms, the percent difference (95% confidence interval) of mtDNA copy number per one point increase in BFI-K, BDI, or PSQI was calculated using a general linear model. The percent difference of mtDNA copy number was calculated by multiplying the value of (exponentiated β coefficient–1) by 100. Age, sex, BMI, smoking status, alcohol intake, physical exercise, and comorbidities were adjusted.
All analyses were performed using IBM PASW Statistics ver. 21.0 software (IBM Corp., Armonk, NY, USA), and a P-value less than 0.05 was considered statistically significant.
RESULTS
After excluding two participants with uncontrolled anemia and corticosteroid usage, 58 subjects were analyzed in total. All subjects had moderate to severe fatigue, but did not receive any treatment for depression or insomnia.
Characteristics of study subjects, including BFI-K, BDI, and PSQI of individuals grouped into tertiles of log-transformed mtDNA copy number, are shown in Supplementary table 1. The lowest tertile had a higher BFI-K score than the other groups, but with no significant difference, except in walking ability (P=0.04). The BDI, PSQI, and clinical characteristics did not differ between the tertile groups.
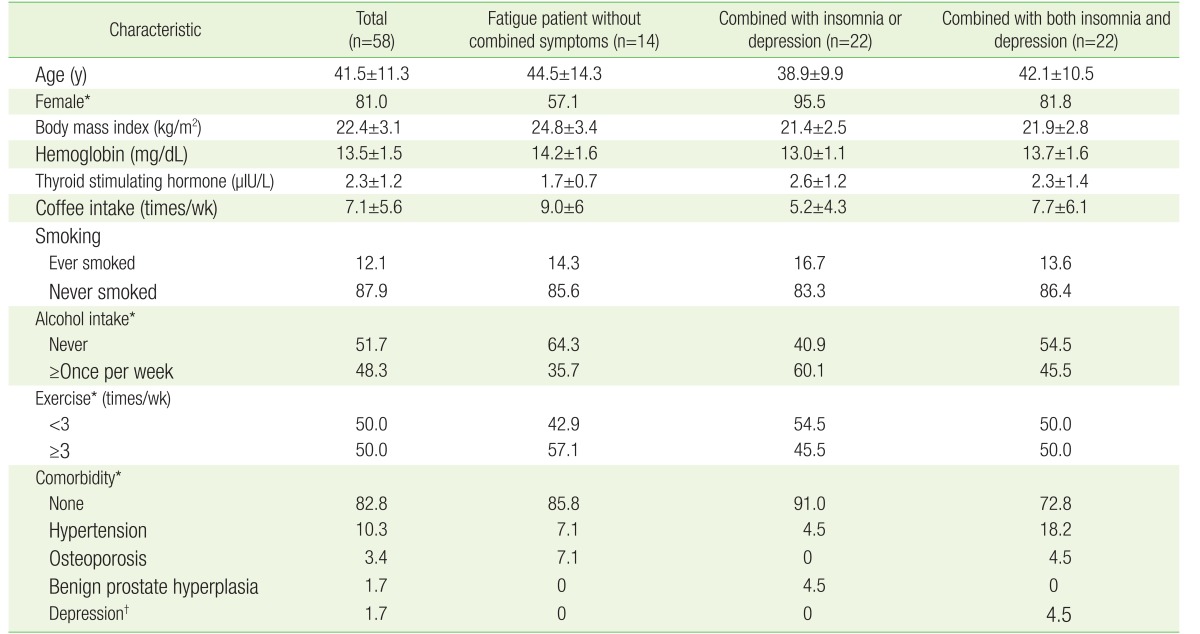
Table 1 shows the characteristics of the study subjects. About 76% of participants had depression or insomnia as an additional symptom. Up to 38% of participants had both insomnia and depression. Fatigue patients with multiple symptoms were predominately female and had a higher alcohol intake per week than those without multiple symptoms. Less frequent exercise in fatigue patients with multiple symptoms was also observed. The study population consisted of relatively healthy subjects. A few subjects had a non-serious illness such as hypertension, osteoporosis, or benign prostate hyperplasia. One subject had a depressive disorder by doctor's diagnosis, but was not receiving any medication. No difference was observed between the three groups with regard to BMI, hemoglobin, TSH, coffee intake, or smoking habits.
Table 1. Baseline characteristics of study population.
Values are presented as mean±standard deviation or %.
*P<0.05 between the three groups was evaluated. †By doctor's diagnosis, but no medication.
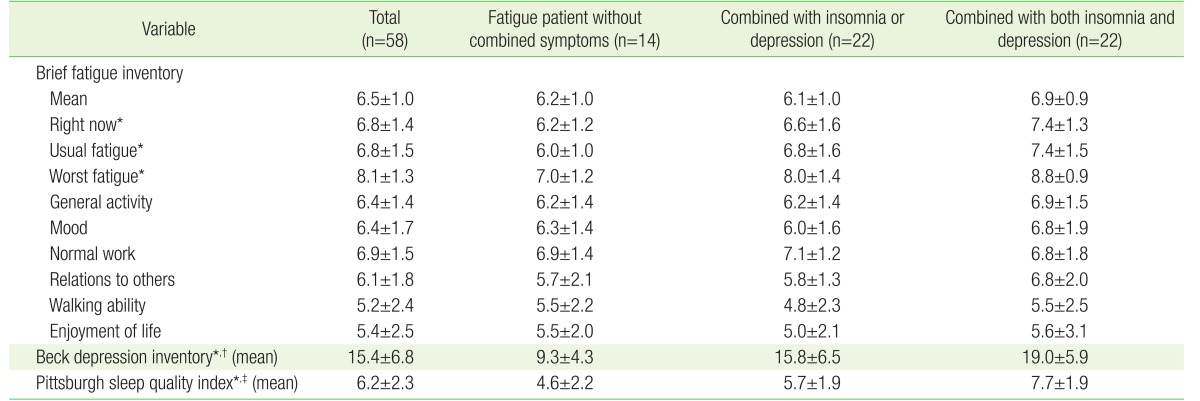
The distribution of BFI-K, BDI, and PSQI according to the combination of symptoms is shown in Table 2. Fatigue patients with both depression and insomnia had a higher overall fatigue score. In particular, they showed significantly higher levels of fatigue ‘now,’ ‘usual’ fatigue, and ‘worst’ fatigue during the last 24 hours than patients without combined symptoms.
Table 2. Distribution of fatigue, depression, and sleep quality according to study groups.
Values are presented as mean±standard deviation.
*P-value <0.05 between group without combined symptom and group with both insomnia and depression. †P-value <0.05 between group without combined symptom and group with one of the combined symptoms. ‡P-value <0.05 between group with one of the combined symptoms and with both insomnia and depression.
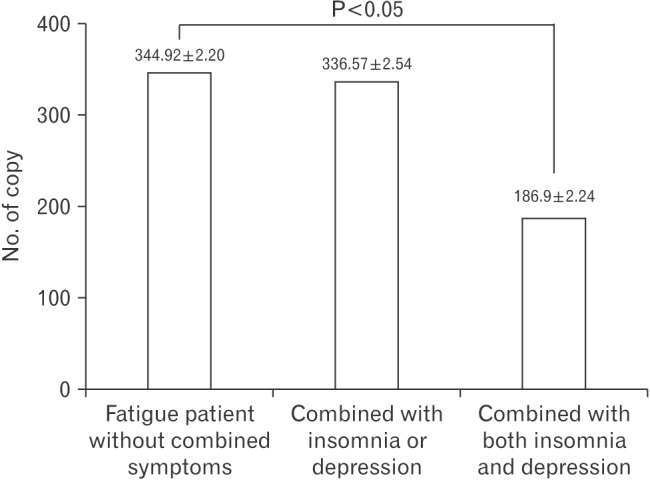
Figure 1 shows the variation in salivary mtDNA copy number in fatigue patients depending on the combination of symptoms. Fatigue patients with both insomnia and depression had a lower copy number of mtDNA than those without combined symptoms (P<0.05).
Figure 1. Comparison of mtDNA copy number in chronic fatigue patients with different combinations of symptoms.
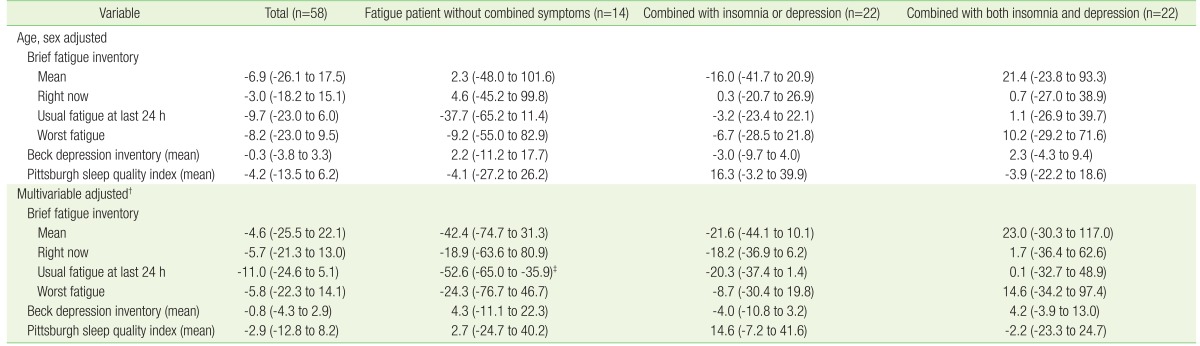
The relationship between mtDNA copy number and BFI-K, BDI, and PSQI is shown in Table 3. After adjusting for age and sex, a negative association was found between mtDNA copy number and BFI-K, BDI, and PSQI. However, the negative association was not significant. Moreover, the association between fatigue, depression, and sleep quality, and mtDNA copy number disappeared in the combined symptoms group. After adjusting for age, sex, BMI, smoking status, alcohol intake, physical exercise, and comorbidities, fatigue patients without combined symptoms displayed significant negative associations between mtDNA copy number and usual fatigue within the preceding 24 hours. The negative association in fatigue patients with depression or insomnia was attenuated. BDI and PSQI in fatigue patients with insomnia and/or depression had no consistent associations with mtDNA copy number.
Table 3. Percent difference (95% confidence interval)* of mtDNA copy number per one point increase in Brief Fatigue Inventory, Beck Depression Inventory, and Pittsburgh Sleep Quality Index.
Values are presented as percent difference (95% confidence interval).
*β coefficients (95% confidence interval) for log-transformed mtDNA copy number per one point increase in Brief Fatigue Inventory, Beck Depression Inventory, and Pittsburgh Sleep Quality Index were calculated by general linear model. Then, percent difference of mtDNA copy number was calculated by multiplying 100 to the value of (exponentiated β coefficient-1). †Adjusted by age, sex, body mass index, smoking status, alcohol intake, exercise, and comorbidity. ‡P<0.05.
DISCUSSION
In this study, a negative association between salivary mtDNA copy number and ‘usual’ fatigue during the preceding 24 hours was observed in chronic fatigue patients without combined symptoms, although subjects with combined symptoms had a higher BFI score and lower mtDNA copy number. In other words, the negative association between mtDNA copy number and BFI was attenuated in patients with combined symptoms.
Mitochondrial dysfunction has been investigated as a potential contributor to fatigue.22) The association between mitochondrial biogenesis and fatigue could be explained by reactive oxygen species, as mitochondrial components are known to be susceptible to damage by them.23) However, based on measurements of mtDNA content, literature reviews have observed inconsistent associations between mitochondrial dysfunction and fatigue.22) As an example, the peripheral blood mtDNA content (mtDNA/genomicDNA ratio) in a fatigued patient was similar to that of a healthy control.10) However, in this study, the negative association between salivary mtDNA copy number and usual fatigue level in chronic fatigue patients was confirmed with a salivary sample.
This result has to be interpreted carefully, because the negative association between fatigue and mtDNA copy number is attenuated in fatigue patients with combined symptoms. In fact, a net positive association was observed in fatigue patients with combined symptoms, although it was not statistically significant, because of positive or null associations between BDI or PSQI, and BFI-K. Additionally, fatigue patients with both insomnia and depression had a lower mtDNA copy number than those without combined symptoms. A suggested explanation is that the fatigue scores of the patients with both insomnia and depression were higher than the scores of other groups.
The associations between fatigue and mtDNA copy number can be explained by neurotransmitter pathways.24) Depression is affected by various neuronal circuits,25) but is primarily regulated by the serotonergic system (5-hydroxytryptamine, 5-HT). Insomnia is caused by dysregulation of the sleep center, which also involves serotonin receptors.24) Fatigue is mediated by the limbic cortex and locus coeruleus, part of the reticular activating system that controls attention and concentration,24) and normal fatigue is confirmed to have a positive association with serotonin receptor 2A.26) However, if the 5-HT receptor is downregulated for a prolonged period, receptor numbers and affinity are consequently reduced, as has been confirmed using a specific radio-ligand and positron emission tomography.27) Consistent with this suggestion, the serotonergic effect in chronic fatigue patients without depression is the opposite of that observed in neuroendocrine studies of patients with major depression.28) Therefore, the level of serotonergic receptors in chronic fatigue would be decreased with combined symptoms, via adaptive neurobiological changes.27) The serotonergic system affects peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), the master regulator of mitochondrial biogenesis.29),30) A 5-HT receptor agonist consequently affects PGC-1α levels, mitochondrial protein ATP synthase β expression, and mtDNA copy number.29),30) Therefore, in chronic fatigue patients with both depression and insomnia, although they have a higher fatigue level, the inverse associations between mtDNA copy number and BFIs can be attenuated, due to up-regulation of the 5-HT receptor from depressive mood or sleep disturbance. Therefore, the association between mtDNA copy number and chronic fatigue may be different depending on the symptom combination. This finding aids understanding of inconsistent associations between mitochondrial dysfunction and fatigue.
In Chang et al.,12) patients with major depressive disorders had decreased leukocyte mtDNA copy numbers compared to control subjects, and an increase in oxidative damage of mtDNA. These findings are inconsistent with our study, which showed that depressive symptoms did not produce any significant association between mtDNA copy number and BDI. This discrepancy can be explained by the fact that reduced mtDNA copy number in major depressive disorders, but not depressive mood, may be a product of downregulation by reactive oxygen species, if exposure is prolonged.12)
Since chronic fatigue is likely not a single illness with a unique etiology, but rather a heterogeneous spectrum of illnesses, developing targeted treatments will require distinctions between subtypes and a customized approach.31) Trying to identify biomarkers of chronic fatigue may be helpful; however, until recently, the biomarkers related to potential etiologies, such as mitochondrial dysfunction, were ambiguous. 31) A major aim of this study is to suggest characteristic biological features of chronic fatigue types, depending on symptom combination. In particular, salivary mtDNA copy number could be considered as a biological marker of fatigue with/without combined symptoms in screening or pre-clinical settings.
Our study has some limitations. Firstly, as this was a cross-sectional study, it was not possible to establish the exact pathophysiology linking mtDNA copy number, chronic fatigue, and combined symptoms. Secondly, we did not measure mtDNA copy number of controls without fatigue symptoms, 5-HT receptor binding affinity, or oxidative damage of mtDNA to explain the pathogenesis of decreased mtDNA copy number in our fatigued subjects. This study presents intriguing results, and further research is needed to fully understand the association between mtDNA and fatigue, and for generalizability or cut-off value. Thirdly, the participant was generally healthy. Therefore, it must be careful when these results were expanded to people with chronic illness or who were taking medication. Further study is needed to evaluate the association in the context of comorbidities, smoking, or alcohol intake. Lastly, because chronic fatigue likely originates from various etiologies, the questionnaire was limited in its ability to prove the associations between subjective symptoms and biological measurements. Furthermore, although many biological studies in chronic fatigue have been conducted, it is difficult to find correlations between biological markers and subjective self-reported symptoms. Nonetheless, improvements in the understanding of chronic fatigue, including these combined symptoms, could provide more objective support for diagnosis and choice of therapy.
In conclusion, chronic fatigue is negatively associated with salivary mtDNA copy number. The negative association between mtDNA copy number and chronic fatigue in patients without comorbid depression and insomnia was sustained; however, the negative association of chronic fatigue with mtDNA copy number in those with comorbid depression and insomnia was attenuated. Considering the relationship between copy number and chronic fatigue, salivary mtDNA may have a role as a diagnostic biological marker for an individualized approach to treating this life-altering disease.
Acknowledgments
We thank Unimed Pharm Inc., Seung Hoon Shin Ph.D., TCM Bio, and Professor Yun-mi Song for their support and technical assistance.
Footnotes
CONFLI CT OF INTEREST: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Supplementary table 1 can be found via http://pdf.medrang.co.kr/KJFM/2017/038/KJFM038-04-06_Supple0.pdf.
Characteristics, fatigue, depression, and sleep quality of study subjects, grouped into tertiles according to log-transformed mitochondrial DNA copy number
References
- 1.Yun YH, Lee MK, Chun HN, Lee YM, Park SM, Mendoza TR, et al. Fatigue in the general Korean population: application and normative data of the Brief Fatigue Inventory. J Pain Symptom Manage. 2008;36:259–267. doi: 10.1016/j.jpainsymman.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Robinson RL, Stephenson JJ, Dennehy EB, Grabner M, Faries D, Palli SR, et al. The importance of unresolved fatigue in depression: costs and comorbidities. Psychosomatics. 2015;56:274–285. doi: 10.1016/j.psym.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Wong WS, Fielding R. The co-morbidity of chronic pain, insomnia, and fatigue in the general adult population of Hong Kong: prevalence and associated factors. J Psychosom Res. 2012;73:28–34. doi: 10.1016/j.jpsychores.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Kallestad H, Jacobsen HB, Landro NI, Borchgrevink PC, Stiles TC. The role of insomnia in the treatment of chronic fatigue. J Psychosom Res. 2015;78:427–432. doi: 10.1016/j.jpsychores.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26:140–144. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 6.Cohen BH, Gold DR. what we know so far. Cleve Clin J Med 200. Cleve Clin J Med. 2001;68:625–626. 629–642. doi: 10.3949/ccjm.68.7.625. [DOI] [PubMed] [Google Scholar]
- 7.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009;36:125–131. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattal O, Budur K, Vaughan AJ, Franco K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Marrero J, Cordero MD, Saez-Francas N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, et al. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013;19:1855–1860. doi: 10.1089/ars.2013.5346. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Tang J, Li Z, Li H, Liao Y, Tang Y, et al. Leukocyte mitochondrial DNA copy number in blood is not associated with major depressive disorder in young adults. PLoS One. 2014;9:e96869. doi: 10.1371/journal.pone.0096869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CC, Jou SH, Lin TT, Lai TJ, Liu CS. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PLoS One. 2015;10:e0125855. doi: 10.1371/journal.pone.0125855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;66:17–20. doi: 10.1016/j.exger.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakupciak JP, Maragh S, Markowitz ME, Greenberg AK, Hoque MO, Maitra A, et al. Performance of mitochondrial DNA mutations detecting early stage cancer. BMC Cancer. 2008;8:285. doi: 10.1186/1471-2407-8-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham JE, Maranian MJ, Spiteri I, Russell R, Ingle S, Luccarini C, et al. Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med Genomics. 2012;5:19. doi: 10.1186/1755-8794-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun YH, Wang XS, Lee JS, Roh JW, Lee CG, Lee WS, et al. Validation study of the korean version of the brief fatigue inventory. J Pain Symptom Manage. 2005;29:165–172. doi: 10.1016/j.jpainsymman.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Lee JI, Kim SH, Tan AH, Kim HK, Jang HW, Hur KY, et al. Decreased health-related quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health Qual Life Outcomes. 2010;8:101. doi: 10.1186/1477-7525-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn HM, Yum TH, Shin YW, Kim KH, Yoon DJ, Chung KJ. A standardization study of the Beck Depression Inventory in Korea. J Korean Neuropsychiatr Assoc. 1986;25:487–502. [Google Scholar]
- 19.Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803–812. doi: 10.1007/s11325-011-0579-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang YC, Lee WC, Liao SC, Lee LC, Su YJ, Lee CT, et al. Mitochondrial DNA copy number correlates with oxidative stress and predicts mortality in nondiabetic hemodialysis patients. J Nephrol. 2011;24:351–358. doi: 10.5301/JN.2010.5816. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Filler K, Lyon D, Bennett J, McCain N, Elswick R, Lukkahatai N, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croteau DL, Bohr VA. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem. 1997;272:25409–25412. doi: 10.1074/jbc.272.41.25409. [DOI] [PubMed] [Google Scholar]
- 24.Lin SY, Stevens MB. The symptom cluster-based approach to individualize patient-centered treatment for major depression. J Am Board Fam Med. 2014;27:151–159. doi: 10.3122/jabfm.2014.01.130145. [DOI] [PubMed] [Google Scholar]
- 25.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Smith AK, Dimulescu I, Falkenberg VR, Narasimhan S, Heim C, Vernon SD, et al. Genetic evaluation of the serotonergic system in chronic fatigue syndrome. Psychoneuroendocrinology. 2008;33:188–197. doi: 10.1016/j.psyneuen.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Cleare AJ, Messa C, Rabiner EA, Grasby PM. Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C]WAY-100635. Biol Psychiatry. 2005;57:239–246. doi: 10.1016/j.biopsych.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Cleare AJ, Bearn J, Allain T, McGregor A, Wessely S, Murray RM, et al. Contrasting neuroendocrine responses in depression and chronic fatigue syndrome. J Affect Disord. 1995;34:283–289. doi: 10.1016/0165-0327(95)00026-j. [DOI] [PubMed] [Google Scholar]
- 29.Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG. 5-hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J Pharmacol Exp Ther. 2010;332:632–639. doi: 10.1124/jpet.109.159947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 31.Fischer DB, William AH, Strauss AC, Unger ER, Jason L, Marshall GD, Jr, et al. Chronic fatigue syndrome: the current status and future potentials of emerging biomarkers. Fatigue. 2014;2:93–109. doi: 10.1080/21641846.2014.906066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics, fatigue, depression, and sleep quality of study subjects, grouped into tertiles according to log-transformed mitochondrial DNA copy number