ABSTRACT
Vibrio cholerae is the etiological agent of cholera, an acute intestinal infection in humans characterized by voluminous watery diarrhea. Cholera is spread through ingestion of contaminated food or water, primarily in developing countries that lack the proper infrastructure for proper water and sewage treatment. Vibrio cholerae is an aquatic bacterium that inhabits coastal and estuarine areas, and it is known to have several environmental reservoirs, including fish. Our laboratory has recently described the use of the zebrafish as a new animal model for the study of V. cholerae intestinal colonization, pathogenesis, and transmission. As early as 6 h after exposure to V. cholerae, zebrafish develop diarrhea. Prior work in our laboratory has shown that this is not due to the action of cholera toxin. We hypothesize that accessory toxins produced by V. cholerae are the cause of diarrhea in infected zebrafish. In order to assess the effects of accessory toxins in the zebrafish, it was necessary to develop a method of quantifying diarrheal volume as a measure of pathogenesis. Here, we have adapted cell density, protein, and mucin assays, along with enumeration of V. cholerae in the zebrafish intestinal tract and in the infection water, to achieve this goal. Combined, these assays should help us determine which toxins have the greatest diarrheagenic effect in fish and, consequently, which toxins may play a role in environmental transmission.
IMPORTANCE Identification of the accessory toxins that cause diarrhea in zebrafish can help us understand more about the role of fish in the wild as aquatic reservoirs for V. cholerae. It is plausible that accessory toxins can act to prolong colonization and subsequent shedding of V. cholerae back into the environment, thus perpetuating and facilitating transmission during an outbreak. It is also possible that accessory toxins help to maintain low levels of intestinal colonization in fish, giving V. cholerae an advantage when environmental conditions are not optimal for survival in the water. Studies such as this one are critical because fish could be an overlooked source of cholera transmission in the environment.
KEYWORDS: cholera, enteric pathogens, Vibrio cholerae, zebrafish
INTRODUCTION
Vibrio cholerae is a Gram-negative aquatic bacterium that is responsible for causing cholera, an acute intestinal infection in humans characterized by profuse watery diarrhea. It is typically found in brackish estuaries and along coastal areas. In the environment, it has been found in fish and bird intestinal tracts (1–3) and is known to form biofilms on chitinous substrates (4).
The primary virulence factors are cholera toxin (CTX) and toxin-coregulated pilus (TCP), both of which are required for colonization of the human small intestine (5). It is the action of cholera toxin that is responsible for the voluminous secretory diarrhea, the hallmark of cholera (6). However, V. cholerae also has accessory toxins that are able to cause diarrhea even in the absence of cholera toxin (5, 7, 8). There are at least three major accessory toxins, hemolysin (HlyA), MARTX (RtxA), and hemagglutinin/protease (HA/P) (9). The exact roles accessory toxins play in the life cycle of V. cholerae are not yet fully understood.
The most commonly used mammalian models for cholera, the suckling mouse and adult rabbit ligated loop models, seek to recapitulate what happens in a human host infected with V. cholerae (10, 11). While the suckling mouse model is good for studying colonization, infant mice do not develop diarrhea as a result of infection with V. cholerae. The adult rabbit model is useful for studying enterotoxicity but requires surgical manipulation. Neither model is a natural host for V. cholerae. In recent years, an adult mouse model has been developed to study V. cholerae-induced diarrhea (7, 12). Again, significant manipulation of the animal is required and adult mice do not exhibit a disease state similar to human cholera. The infant rabbit model has been reinvestigated in recent years and found to produce a state closest to human cholera in an animal model (13). A downside of all rabbit and mouse models is that they are not natural V. cholerae hosts. Nonmammalian models of cholera are less frequently used and include Drosophila melanogaster, Caenorhabditis elegans, and Dictyostelium discoideum (14–17).
Our laboratory has developed the zebrafish as a natural host model for the study of V. cholerae infection (18). During colonization experiments with zebrafish, it was observed that within several hours of infection (∼ 6 to 8 h), fish had what appeared to be diarrhea. This was especially pronounced if the inoculum was given with brine shrimp; the fish excretions tended to be orange in color and thus more noticeable. If the inoculum did not include brine shrimp, the fish diarrhea consisted primarily of small white particulates, which made the fish infection water appear turbid.
Also observed was the fact that the water became more and more turbid during time course experiments, again with small white particulates. However, this was not due to bacterial growth in the water. Human cholera patients characteristically produce rice-water stool, so named because it resembles water after washing rice. Human rice water stool is known to contain large amounts of intestinal mucus, both dispersed and in clumps (19). If zebrafish are having diarrhea as a result of V. cholerae infection, it is likely that they too will excrete increased amounts of intestinal mucus. It is well known that infection with V. cholerae induces mucus secretion, specifically mucins, large glycoproteins that are the primary component of mucus (20).
Fish were observed to have diarrhea even when infected with ΔtoxT strains of V. cholerae that do not produce cholera toxin, which suggests that there are other factors responsible for inducing diarrhea in infected fish (18). Accessory toxins are the most likely cause of this noncholera diarrhea. Vaccine studies using live strains of V. cholerae that did not produce cholera toxin still observed diarrhea in human volunteers (21–24). Additionally, non-O1, non-O139 strains, which typically do not produce cholera toxin, have caused outbreaks of noncholera diarrhea.
Since zebrafish are a relatively new, aquatic model system for the study of cholera, there is no previously established method for quantitating pathogenicity or diarrheal volume. In order to assess the effects of cholera accessory toxins on zebrafish, a way to measure fish excretion needed to be developed. It was determined that a combination of optical density measurement, protein assays, mucin assays, and enumeration of excreted V. cholerae together can be used to quantify diarrhea in fish.
RESULTS
Optical density, protein, and mucin levels in the water can be used to quantitate fish diarrhea.
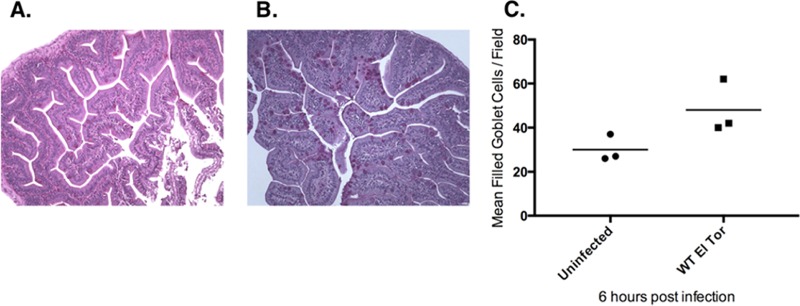
Since mucus is a major component of rice-water stool in human cholera diarrhea, we thought it might also be present in zebrafish diarrhea induced by V. cholerae. To test this hypothesis, we performed histology on sections of infected zebrafish intestines using periodic acid-Schiff hematoxylin (PASH) staining, which stains carbohydrates/mucins. Fish were infected and then sacrificed at 6 h postinfection (hpi), the earliest time point at which diarrhea had been observed. Compared with uninfected control fish, infected zebrafish appeared to have an increased number of mucin-filled goblet cells (Fig. 1). Unfortunately, this approach was not sufficiently quantitative, was very time-consuming, and required precise skill to obtain high-quality histological sections. Since the diarrhea from the fish was being observed in the infection water, we reasoned that perhaps there was a way to test for the presence of mucins excreted into the water rather than localized in the fish intestines.
FIG 1.
V. cholerae infection increases the number of filled goblet cells in the zebrafish intestinal tract. (A and B) Representative PASH sections taken from zebrafish 6 h postinfection, showing more filled goblet cells in V. cholerae-infected fish (B) than in uninfected fish (A). (C) The number of filled goblet cells per field of view (from panels A and B), based on 10 fields of view per fish and three fish per treatment group; the difference between groups was found not to be statistically significant (P = 0.084) by two-tailed t test.
To answer the question of whether mucins could be detected in water, our next approach was to try to stain for mucins within a water sample on a microscope slide. After much trial and error, it was determined that 50 ml of the infection water (out of 200 ml total volume, typically) could be used to pellet the solid materials in the sample, and the pellet was resuspended in 1 to 2 ml of fresh infection water or 1× phosphate-buffered saline (PBS), effectively concentrating it. We could then use this concentrated fish water to perform a cytospin, a centrifugation technique which deposits the solid material onto a slide while spinning off the liquid portion of the sample. Once the sample slides were fixed, they were stained with alcian blue, which stains acidic mucins (like those found in the intestinal tract) blue. This procedure was much faster and required far less technical skill than histology. However, accurate quantitation of the mucin adhered to the slides proved to be problematic. Successful staining of cytospins for mucin demonstrated that mucins in the fish infection water could be detected (data not shown). Since mucins were detectable in a liquid sample, perhaps a liquid assay existed that made reliable quantitation possible.
More trial and error ensued in the search for a liquid mucin assay that reliably produced a standard curve from which the amount of mucin in a sample could be determined. We were able to adapt a microplate periodic acid-Schiff assay to satisfy this basic requirement. This assay is cheap and fast, and the results are reproducible and quantitative.
It was later decided to add optical density measurements, a total protein assay, and bacterial counts to have a more complete snapshot of the composition of diarrhea from fish infected with V. cholerae.
Fish exposed to live V. cholerae have increased levels of excretion compared with control fish.
In order to determine whether the optical density, protein, and mucin assays could be used in concert to detect and quantify diarrhea in fish, we exposed groups of fish to the following: wild-type (WT) El Tor strain E7946 V. cholerae (25), heat-killed (HK) WT V. cholerae, and PBS only. The PBS-only group served as the uninfected control, the HK group served as a mock-infected control, and the WT group was the only one infected with live bacteria.
After 24 h, fish infected with WT V. cholerae exhibited higher levels of optical density, mucin, and protein in their infection water than the uninfected fish or fish exposed to heat-killed WT V. cholerae (Fig. 2). Among multiple experiments that were performed on different days, the relative amounts of cellular debris (as measured by optical density), protein, and mucin were very similar. Not only were the results of the individual assays very similar but there also was substantial agreement among the three assays as well. These data suggest that optical density, protein, and mucin analysis can be used together to quantify excretions of V. cholerae-infected fish.
FIG 2.
Fish infected with wild-type (WT) V. cholerae excrete more than uninfected fish. At 24 hpi, the water from infected fish showed increased optical density (A), total protein (B), and mucin (C) compared to the water from either PBS or heat-killed (HK) control groups. These data were compiled from three experiments. Data shown are means ± standard errors of the means (SEM). One-way ANOVA was performed, followed by Tukey's multiple-comparison test to detect differences between groups. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Fish infected with toxin mutants exhibit diarrhea.
Since fish infected with WT V. cholerae exhibited diarrhea, we next asked how fish would respond to infection with isogenic toxin mutant strains. Fish were infected with the El Tor Δcore mutant, which is missing the genes encoding cholera toxin (ctxAB), accessory cholera enterotoxin (ace), and zonula occludens toxin (zot) (26). Another group of fish was infected with the El Tor Δatt mutant, which contains the Δcore deletions plus a truncated and nonfunctional copy of the MARTX A subunit gene (rtxA) (27). At 24 hpi, the groups infected with the Δcore and Δatt mutants showed levels of optical density, protein, and mucin in the water similar to those of the fish infected with WT V. cholerae (Fig. 3). In addition, there was no difference in the levels of intestinal colonization between fish infected with either WT V. cholerae or the Δcore and Δatt mutants (data not shown). Collectively, these data suggest that some other factor produced by V. cholerae is giving rise to diarrhea in infected zebrafish.
FIG 3.
Infection with multitoxin deletion mutants did not reduce excretion levels below those of WT infection. At 24 hpi, the water from fish infected with both Δcore and Δatt mutants showed similar levels of optical density (A), total protein (B), and mucin (C) as the water from WT V. cholerae-infected fish. These data were compiled from three experiments. Data shown are means ± standard errors of the means (SEM). One-way ANOVA was performed, followed by Tukey's multiple-comparison test to detect differences between groups. †, significant difference from the PBS group (P < 0.0001); ‡, significant difference from the HK group (P < 0.0001); **, significant difference between PBS and HK groups (P ≤ 0.01).
Amount of excretion decreases with colonization levels.
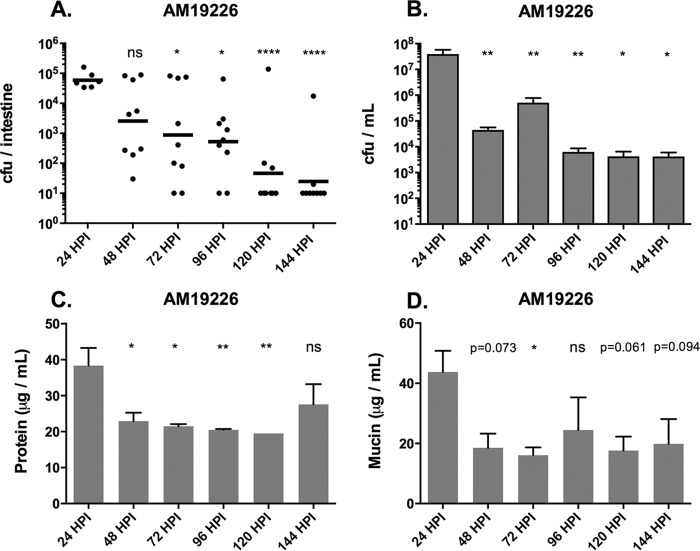
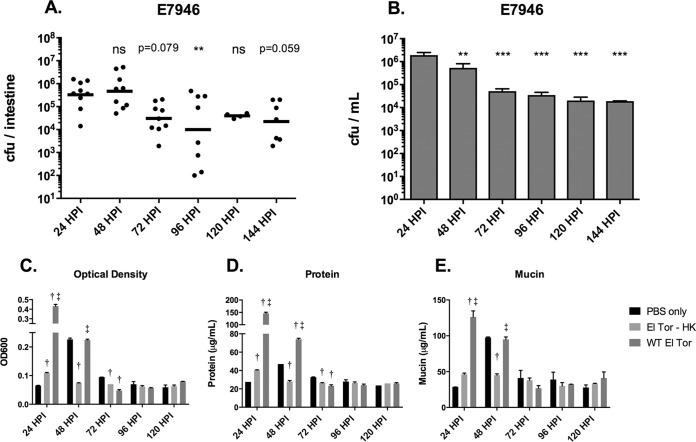
Since zebrafish are highly colonized by V. cholerae at 24 h postinfection and can remain colonized for several days, the next question was whether diarrhea in the fish tracked with colonization levels over time. In addition to the El Tor strain used throughout this study, we also performed time course experiments with AM-19226, a non-O1, non-O139 clinical isolate that lacks TCP and CTX, the primary virulence factors of pandemic V. cholerae. Instead, it possesses a type 3 secretion system (T3SS) as the major virulence factor. AM-19226 was used as a representative of nonpandemic strains found in the environment (28). We observed that in both the El Tor strain and the non-O1, non-O139 strain, excretion levels decreased along with colonization levels over time. Fish infected with AM-19226 have the highest excretion levels in the 24 h following initial infection (Fig. 4C and D), which correlates with the highest bacterial loads in the intestine and excreted into the water (Fig. 4A and B). After 24 h, the amount of bacteria in the water as well as excretion levels of infected fish dramatically decreased, while overall colonization levels gradually declined to the limit of detection over a period of about 4 days (Fig. 4A to D). In contrast, El Tor-infected fish maintained high levels of intestinal colonization, as well as large numbers of bacteria in the water and high levels of excretion, for 48 h postinfection (Fig. 5A to E). We observed an unexplained spike in optical density (OD) and mucin in the PBS control at 48 h in the representative experiment shown in Fig. 5E. It is not clear why this spike appeared, but one possible explanation is that feeding was resumed after 24 hpi. After 48 h, overall excretion levels dropped to those of uninfected and mock-infected fish (Fig. 5C to E). The numbers of bacteria in the water decreased somewhat, as did colonization levels, but remained at a relatively high level throughout the remainder of the 6 days of the experiment (Fig. 5A and B). Taken together, these data show that high colonization levels correspond to high overall excretion levels in the first 24 to 48 h postinfection. Additionally, the El Tor strain appears to have a colonization advantage over the non-O1, non-O139 strain in that it can persist in the fish intestinal tract and in the water for 6 days, and likely longer. The non-O1, non-O139 strain is nearly gone from the fish by 6 days postinfection, although it persists in the water at lower levels.
FIG 4.
AM-19226 intestinal colonization and excretion levels both decrease over time. (A) Intestinal colonization levels of AM-19226 over time. Each dot represents one fish. The horizontal bar indicates the geometric mean bacterial load per fish. (B) The amount of live bacteria remaining in the fish water at each time point. (C and D) Total protein (C) and mucin (D) levels over time. These data were compiled from three experiments. Data shown in panels B to D are means ± standard errors of the means (SEM). One-way ANOVA was performed (on log-transformed data shown in panel A), followed by Dunnett's multiple-comparison test against the 24-h time point as a control. ns, P > 0.1 (P values between 0.05 and 1.0 are stated in panel D); *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001.
FIG 5.
El Tor intestinal colonization and excretion levels both decrease over time. (A) Intestinal colonization levels with El Tor over time. Each dot represents one fish. The horizontal bar indicates the geometric mean bacterial load per fish. (B) The amount of live bacteria remaining in the fish water at each time point. (C to E) Optical density (C), total protein (D), and mucin (E) levels over time. The data shown in panels A and B were compiled from three experiments. The data shown in panels C to E were from one representative experiment. Data shown in panels B to D are means ± SEM. In panels A and B, one-way ANOVA was performed (on log-transformed data shown in panel A), followed by Dunnett's multiple-comparison test against the 24-h time point as a control. ns, P ≥ 1.0 (P values between 0.05 and 1.0 are stated in panel A); *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001. In panels C to E, two-way ANOVA was performed, followed by Tukey's multiple-comparison test to detect differences between groups. †, significant difference from the PBS group (P ≤ 0.05); ‡, significant difference from the HK group (P ≤ 0.05).
DISCUSSION
In this paper, we have shown that zebrafish infected with V. cholerae develop diarrhea, which can be quantified through optical density measurements and protein and mucin assays of fish infection water. Readings from these three assays are consistently elevated in the water from fish infected with live V. cholerae but not in the water of either uninfected fish or mock-infected fish. Together with assessment of intestinal colonization levels and numbers of V. cholerae excreted into the infection water, these simple metrics provide a quantitative overview of zebrafish pathogenesis after V. cholerae infection.
The choice to use the measurement of mucin was based upon histological observations of increased filled goblet cells, together with knowledge of induced mucin production during human infection (20). In Fig. 1, the infected fish appears to have more filled goblet cells than the uninfected fish at 6 h postinfection. This observation is in contrast to other studies which have shown that infected animals have many empty goblet cells (12, 13). This disparity may be due to timing. The other studies sacrificed animals between 12 and 22 h postinfection, whereas our study has only looked at 6 h postinfection. It could simply be that at this time point, diarrhea had not yet fully commenced. This finding is in agreement with optical density, protein, and mucin data that do not demonstrate an increase in excretion at 6 h postinfection (data not shown). It may be more informative to perform future histology and infection water assays at 9, 12, 18, and 24 h postinfection to look at the number of filled versus empty goblet cells along with changes in excretion levels in the water.
It is interesting that while the heat-killed WT El Tor group exhibits far less diarrhea than any of the infected groups, those fish still have more excretion than the PBS control fish. This suggests that some component or components in the heat-killed inoculum induce a mild diarrheagenic reaction. This could be due simply to some structural component of the bacteria themselves, such as lipopolysaccharide (LPS) or flagellin proteins. Rui et al. (29) showed that strains lacking flagellins are no longer reactogenic in rabbits. In addition, LPS alone is known to induce mucin secretion (30).
None of the toxin mutants that we have tested decreased the amount of diarrhea by more than a small margin compared to the wild type. It was not expected that cholera toxin itself would be a primary driving force of diarrhea in the zebrafish. Most environmental or nonpandemic strains of V. cholerae that a fish in the wild would encounter do not carry the cholera toxin phage (CTXΦ) or Vibrio pathogenicity island (VPI), the two major genetic elements responsible for cholera in humans (5). Environmental strains do, however, encode various other virulence factors, like accessory toxins hemolysin (HlyA), repeats-in-toxin–MARTX (RtxA), hemagglutinin protease (HA/P), and a type three secretion system (T3SS) in some strains (31–34). Many of these factors have been implicated in outbreaks of noncholera diarrhea (34–36). Confirming our findings that toxins encoded by the CTXΦ are not the cause of diarrhea in zebrafish, the attenuated vaccine candidate, CVD110 (derived from E7946 El Tor), still caused mild to moderate diarrhea even though it lacked ctxA, ace, zot, and hlyA (24). However, in a more recent study in a C. elegans model, it was shown that CVD109, the immediate parent strain of CVD110 with an intact hlyA gene, was more lethal to worms than CVD110, with a lethality equal to that of wild-type V. cholerae (16).
Infections with both El Tor and non-O1, non-O139 strains result in an increase in fish excretions at 24 h postinfection. While excretion levels themselves drop to uninfected levels after 24 to 48 h, colonization levels with the El Tor strain are decreased but maintained over at least 6 days postinfection and likely longer. Colonization with the non-O1, non-O139 strain gradually decreases over the course of 4 days to almost nothing, commensurate with excretion levels in the water. This could be due to the fact that non-O1, non-O139 strains, although regularly found in the environment, are often not human pathogens and do not carry the virulence genes normally found in pandemic strains, such as El Tor. In addition to the VPI, El Tor strains carry two other pathogenicity islands, VSP-1 and VSP-2 (37, 38), which could play a role in persistent colonization. Perhaps accessory toxins are a mechanism to stay in the infected host, an effect which could be amplified in pandemic strains by the carriage of cholera toxin, VPI genes, and possibly other unknown factors. In V. cholerae-infected humans, El Tor carriers tend to shed bacteria in their stool for a much longer period of time, up to 10 days, than do carriers of the classical strain, which tend to shed bacteria for only about 1.5 days (5, 9). Previous work in our laboratory confirmed this finding in zebrafish infected with V. cholerae. We found that in time course experiments, zebrafish infected with classical strain O395 remained highly colonized only for about 24 h postinfection and were cleared by 72 h postinfection (18). However, as we have also demonstrated in this study, zebrafish infected with the El Tor strain remained robustly colonized for at least 144 h postinfection, or 6 days.
The observation that zebrafish remain highly colonized by El Tor over several days yet only exhibit diarrhea for 48 h suggests that colonization with V. cholerae alone is not sufficient to cause diarrhea, as had been speculated to be the cause of diarrhea in volunteer vaccine studies (24). Colonization persistence in fish could be a mechanism to maximize the transmission of bacteria, particularly in light of the fact that V. cholerae is hyperinfectious, requiring a much lower infectious dose for virulence than normal, for a short time after exiting the host (39, 40). The longer it can persist in the host, the longer it can be shed in the hyperinfectious phenotype, and the more likely it is to be successfully transmitted to a new host, thus possibly initiating or perpetuating a human outbreak of cholera.
In summary, here we have adapted several simple assays in order to begin to assess the effects of V. cholerae accessory toxins in a zebrafish model system. This should allow us to determine which toxins induce the most diarrhea in fish and further elucidate the role accessory toxins play in intestinal colonization and environmental transmission of V. cholerae.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. All bacteria were grown at 37°C in LB broth with streptomycin added to a final concentration of 100 μg/ml.
TABLE 1.
Strains used in this study
| V. cholerae strain | Descriptiona | Source (reference) |
|---|---|---|
| E7946 | O1 serogroup, El Tor (Ogawa) biotype, Strr | Laboratory collection (25) |
| Δcore | E7946 ΔorfU Δace Δzot ΔctxAB (deletions are from the core region of the CTXΦ) | Gift from K. Satchell (26) |
| Δatt | E7946 deletion of the entire CTXΦ, plus the 3′ end of rtxA | Gift from K. Satchell (27) |
| AM-19226 | Non-O1, non-O139 serogroup, Strr | Gift from M. Dziejman (28) |
Strr, streptomycin resistance.
Reagents and materials.
Unless otherwise specified, all reagents, chemicals, and other materials were purchased from Fisher Scientific (Pittsburgh, PA).
Zebrafish.
Adult, wild-type ZDR zebrafish were used in all experiments. The fish were housed in tank water filtered by reverse osmosis and maintained at pH 7.0 to 7.5. Tank water was conditioned with Instant Ocean salt (Aquarium Systems, Mentor, OH) to a conductivity of 400 to 550 μS. The fish were fasted for at least 12 h prior to each experiment. Zebrafish were euthanized in 100 ml of 320 μg/ml Tricaine-S (tricaine methanesulfonate; MS-222; Western Chemical, Ferndale, WA) for a minimum of 25 min. All animal protocols were approved by the Wayne State University IACUC.
Inoculation of zebrafish via immersion.
Bacterial cultures were grown with aeration in LB broth at 37°C for 16 to 18 h. Cells were subsequently washed once and then diluted to the correct concentration in sterile 1× PBS. Bacterial cell densities ranged from 107 to 1010 per beaker (∼5 × 104 to 5 × 107 CFU/ml). Four to five zebrafish per experimental group were placed into a 400-ml beaker with a perforated lid containing 200 ml of sterile infection water (autoclaved tank water). One milliliter of bacterial inoculum then was added to the beaker with fish. The two control groups included fish that were exposed to 1 ml of 1× PBS only and fish that were exposed to 1 ml heat-killed WT V. cholerae (wild-type inoculum boiled at 100°C for 5 min). Each beaker was placed into a glass-front incubator set at 28°C for the duration of the experiment.
Intestinal colonization assessment.
After the specified time point, fish were euthanized as described above. The intestines of each fish were aseptically dissected and placed into homogenization tubes (2.0-ml screw-cap tubes; Sarstedt, Nümbrecht, Germany), 1.5 g of 1.0-mm glass beads (BioSpec Products, Inc., Bartlesville, OK), and 1 ml of 1× PBS and held on ice. Homogenization tubes were loaded into a Mini-Beadbeater-24 (BioSpec Products, Inc.) and shaken at maximum speed for two 1-min cycles, with the samples being incubated for 1 min on ice after both the first and last cycles. Intestinal homogenates from each fish were diluted and plated for enumeration on LB agar plates that contained 100 μg/ml streptomycin and 40 μg/ml X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactopyranoside; Gold Biotechnology, Inc., St. Louis, MO). Plates were incubated overnight at 37°C.
Histology of V. cholerae-infected zebrafish intestines.
Histology was performed as described in reference 18 with the following changes. Fish were sacrificed at 6 h before being placed in fixative. Fixed sections were immersed in 0.5% periodic acid solution (77310-25G; Sigma-Aldrich, St. Louis, MO), immersed in Schiff's reagent (Sigma-Aldrich), rinsed in double-distilled H2O (ddH2O), and then counterstained with hematoxylin (American MasterTech).
Processing fish infection water.
With a 60-ml syringe (BD 309653; Franklin Lakes, NJ), 50 ml of fish infection water was extracted, in duplicate, and put into two 50-ml conical tubes. Tubes were centrifuged at 10,000 rpm for 15 min at 4°C and supernatant was decanted, being careful not to disturb the pellet. Each pellet was resuspended in 2 ml of 1× PBS. Unprocessed water samples can be stored at 4°C for up to 1 week before analysis.
Microtiter PAS assay.
The microtiter PAS assay was adapted from procedures described previously (41). Prior to the procedure, 1 ml of a 50% (wt/vol) periodic acid (Sigma-Aldrich) stock solution was made. This can be stored protected from light at 4°C for up to 1 week. A 96-well plate (Corning Costar 3361; Corning, NY) was loaded with 100 μl/well of the blank (1× PBS), mucin standards (see below), and samples in triplicate. A volume of 50 μl/well of fresh 0.1% periodic acid solution (10 μl of the 50% periodic acid stock added to 5 ml of 7% acetic acid, used immediately after making) was added and mixed by pipetting. The plate was covered in plastic wrap and incubated at 37°C for 1 to 1.5 h. After incubation, the plate was cooled to room temperature before adding 100 μl/well Schiff's reagent (250 ml; 84655; Sigma-Aldrich) and mixed with a pipette. The plate was again covered in plastic wrap and placed on a rocker or shaker for 15 to 40 min or until sufficient color developed. Absorbance was read at 560 nm using a plate reader (Tecan SpectraFluor plus; Männedorf, Switzerland).
Mucin standards.
The following series of mucin standards was made by suspending the appropriate amount of mucin from porcine stomach: type III (M1778-10G; Sigma-Aldrich) in sodium acetate buffer, pH 5.5 (100 mM sodium acetate, 5 mM EDTA, pH 5.5, with glacial acetic acid), at 400 μg/ml, 300 μg/ml, 200 μg/ml, 150 μg/ml, 100 μg/ml, 75 μg/ml, 50 μg/ml, 25 μg/ml, and 10 μg/ml. The mixtures were stored at 4°C.
Protein assay.
One milliliter of Pierce 660-nm protein assay reagent (Thermo Scientific) was combined with 67 μl of each protein standard (see below) or sample, mixed, and incubated at room temperature for 2 min. Absorbance was read at 660 nm on a spectrophotometer (Thermo Fisher Scientific, Waltham, MA) using ddH2O as a blank.
Protein standards.
The following bovine serum albumin (BSA) standards were made from a 1,800 μg/ml BSA stock solution: 1,800 μg/ml, 1,000 μg/ml, 750 μg/ml, 500 μg/ml, 250 μg/ml, 125 μg/ml, 50 μg/ml, and 25 μg/ml. The solutions were stored at 4°C.
Optical density determination.
The optical density at 600 nm (OD600) of 1 ml of each sample was read in a spectrophotometer using 1× PBS as the blank.
Statistical analysis.
Each experiment was performed a minimum of three times on separate occasions, unless otherwise specified in the figure legends. A two-tailed t test was performed for results shown in Fig. 1. In the remaining figures, one-way and two-way analyses of variance (ANOVA) with Tukey's multiple-comparison test or Dunnett's multiple-comparison test against a control were done as described in the figure legends. Analyses were performed using GraphPad Prism 7.0.
ACKNOWLEDGMENTS
We are grateful to Karla Satchell and Michelle Dziejman for their generous gifts of strains used in these experiments.
This work was supported by PHS grants R21AI095520 and R01AI127390 (to J.H.W.), by NIH NIGMS training grant R25GM058905 (to K.C.M.), and by Wayne State University funds.
REFERENCES
- 1.Halpern M, Senderovich Y, Izhaki I. 2008. Waterfowl: the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog 4:e1000173. doi: 10.1371/journal.ppat.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senderovich Y, Izhaki I, Halpern M. 2010. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senderovich Y, Halpern M. 2012. Bacterial community composition associated with chironomid egg masses. J Insect Sci 12:149. doi: 10.1673/031.012.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler SM, Camilli A. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol 3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaper JB, Morris JG Jr, Levine MM. 1995. Cholera. Clin Microbiol Rev 8:48–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmgren J. 1981. Actions of cholera toxin and the prevention and treatment of cholera. Nature 292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 7.Olivier V, Queen J, Satchell KJ. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352. doi: 10.1371/journal.pone.0007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardo MJ, Michalski J, Martinez-Wilson H, Morin C, Hilton T, Osorio CG, Nataro JP, Tacket CO, Camilli A, Kaper JB. 2007. An in vivo expression technology screen for Vibrio cholerae genes expressed in human volunteers. Proc Natl Acad Sci U S A 104:18229–18234. doi: 10.1073/pnas.0705636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier V, Salzman NH, Satchell KJF. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect Immun 75:5043–5051. doi: 10.1128/IAI.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klose KE. 2000. The suckling mouse model of cholera. Trends Microbiol 8:189–191. doi: 10.1016/S0966-842X(00)01721-2. [DOI] [PubMed] [Google Scholar]
- 11.Spira WM, Sack RB, Froehlich JL. 1981. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun 32:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawasvirojwong S, Srimanote P, Chatsudthipong V, Muanprasat C. 2013. An adult mouse model of Vibrio cholerae-induced diarrhea for studying pathogenesis and potential therapy of cholera. PLoS Negl Trop Dis 7:e2293. doi: 10.1371/journal.pntd.0002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie JM, Rui H, Bronson RT, Waldor MK. 2010. Back to the future: studying cholera pathogenesis using infant rabbits. mBio 1:e00047-10. doi: 10.1128/mBio.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blow NS, Salomon RN, Garrity K, Reveillaud I, Kopin A, Jackson FR, Watnick PI. 2005. Vibrio cholerae infection of Drosophila melanogaster mimics the human disease cholera. PLoS Pathog 1:e8. doi: 10.1371/journal.ppat.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Callaghan D, Vergunst A. 2010. Non-mammalian animal models to study infectious disease: worms or fly fishing? Curr Opin Microbiol 13:79–85. doi: 10.1016/j.mib.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Cinar HN, Kothary M, Datta AR, Tall BD, Sprando R, Bilecen K, Yildiz F, McCardell B. 2010. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One 5:e11558. doi: 10.1371/journal.pone.0011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. 2014. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol 80:1710–1717. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 20.Forstner JF, Roomi NW, Fahim RE, Forstner GG. 1981. Cholera toxin stimulates secretion of immunoreactive intestinal mucin. Am J Physiol 240:G10–G16. [DOI] [PubMed] [Google Scholar]
- 21.Silva T, Schleupner MA, Tacket CO, Steiner TS, Kaper JB, Edelman R, Guerrant R. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and Q139 Vibrio cholerae. Infect Immun 64:2362–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tacket CO, Kotloff KL, Losonsky G, Nataro JP, Michalski J, Kaper JB, Edelman R, Levine MM. 1997. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD 111. Am J Trop Med Hyg 56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 23.Tacket CO, Losonsky G, Nataro JP, Comstock L, Michalski J, Edelman R, Kaper JB, Levine MM. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis 172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 24.Tacket CO, Losonsky G, Nataro JP, Cryz SJ, Edelman R, Fasano A, Michalski J, Kaper JB, Levine MM. 1993. Safety and immunogenicity of live oral cholera vaccine candidate CVD 110, a ΔctxA Δzot Δace derivative of El Tor Ogawa Vibrio cholerae. J Infect Dis 168:1536–1540. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 25.Mekalanos JJ. 1983. Duplication and amplification of toxin genes in Vibrio cholerae. Cell 35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DN, Killeen KP, Hack DC, Kenner JR, Coster TS, Beattie DT, Ezzell J, Hyman T, Trofa A, Sjogren MH, Friedlander A, Mekalanos JJ, Sadoff JC. 1994. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis 170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 27.Lin W, Fullner KJ, Clayton R, Sexton JA, Rogers MB, Calia KE, Calderwood SB, Fraser C, Mekalanos JJ. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci U S A 96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, Rahman MH, Heidelberg JF, Decker J, Li L. 2005. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci U S A 102:3465–3470. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rui H, Ritchie JM, Bronson RT, Mekalanos JJ, Zhang Y, Waldor MK. 2010. Reactogenicity of live-attenuated Vibrio cholerae vaccines is dependent on flagellins. Proc Natl Acad Sci U S A 107:4359–4364. doi: 10.1073/pnas.0915164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smirnova MG, Guo L, Birchall JP, Pearson JP. 2003. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 221:42–49. doi: 10.1016/S0008-8749(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 31.Debellis L, Diana A, Arcidiacono D, Fiorotto R, Portincasa P, Altomare DF, Spirli C, de Bernard M. 2009. The Vibrio cholerae cytolysin promotes chloride secretion from intact human intestinal mucosa. PLoS One 4:e5074. doi: 10.1371/journal.pone.0005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivier V, Haines GK, Tan Y, Satchell KJF. 2007. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect Immun 75:5035–5042. doi: 10.1128/IAI.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Queen J, Satchell KJ. 2012. Neutrophils are essential for containment of Vibrio cholerae to the intestine during the proinflammatory phase of infection. Infect Immun 80:2905–2913. doi: 10.1128/IAI.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin OS, Tam VC, Suzuki M, Ritchie JM, Bronson RT, Waldor MK, Mekalanos JJ. 2011. Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae. mBio 2:e00106-11. doi: 10.1128/mBio.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagchi K, Echeverria P, Arthur JD, Sethabutr O, Serichantalergs O, Hoge CW. 1993. Epidemic of diarrhea caused by Vibrio cholerae non-O1 that produced heat-stable toxin among Khmers in a camp in Thailand. J Clin Microbiol 31:1315–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa A, Kato J, Watanabe H, Nair BG, Takeda T. 1990. Cloning and nucleotide sequence of a heat-stable enterotoxin gene from Vibrio cholerae non-O1 isolated from a patient with traveler's diarrhea. Infect Immun 58:3325–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci U S A 99:1556–1561. doi: 10.1073/pnas.042667999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Shea YA, Finnan S, Reen FJ, Morrissey JP, O'Gara F, Boyd EF. 2004. The Vibrio seventh pandemic island-II is a 26.9 kb genomic island present in Vibrio cholerae El Tor and O139 serogroup isolates that shows homology to a 43.4 kb genomic island in V. vulnificus. Microbiology 150:4053–4063. doi: 10.1099/mic.0.27172-0. [DOI] [PubMed] [Google Scholar]
- 39.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamayo R, Patimalla B, Camilli A. 2010. Growth in a biofilm induces a hyperinfectious phenotype in Vibrio cholerae. Infect Immun 78:3560–3569. doi: 10.1128/IAI.00048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilcoyne M, Gerlach JQ, Farrell MP, Bhavanandan VP, Joshi L. 2011. Periodic acid-Schiff's reagent assay for carbohydrates in a microtiter plate format. Anal Biochem 416:18–26. doi: 10.1016/j.ab.2011.05.006. [DOI] [PubMed] [Google Scholar]