Abstract
The Wnt/β-catenin signaling pathway is critical for the establishment of organizer and embryonic body axis in Xenopus development. Here, we present evidence that Xenopus Rap2, a member of Ras GTPase family, is implicated in Wnt/β-catenin signaling during the dorsoventral axis specification. Ectopic expression of XRap2 can lead to neural induction without mesoderm differentiation. XRap2 dorsalizes ventral tissues, inducing axis duplication, organizer-specific gene expression and convergent extension movements. Knockdown of XRap2 causes ventralized phenotypes including shortened body axis and defective dorsoanterior patterning, which are associated with aberrant Wnt signaling. In line with this, XRap2 depletion inhibits β-catenin stabilization and the induction of ectopic dorsal axis and Wnt-responsive genes caused by XWnt8, Dsh or β-catenin, but has no effect on the signaling activities of a stabilized β-catenin. Its knockdown also disrupts the vesicular localization of Dsh, thereby inhibiting Dsh-mediated β-catenin stabilization and the membrane recruitment and phosphorylation of Dsh by frizzled signaling. Taking together, we suggest that XRap2 is involved in Wnt/β-catenin signaling as a modulator of the subcellular localization of Dsh.
Keywords: dishevelled, Rap2 GTPase, Wnt-β catenin signaling, Xenopus laevis
Introduction
The Wnt family of secreted glycoproteins regulates many biological processes including cell proliferation, cell polarity and embryonic patterning (Wodarz and Nusse, 1998; Moon et al, 2002). The Wnt/β-catenin signaling pathway, which is highly conserved from Drosophila to vertebrates, has been studied in greatest detail. This signaling is initiated by Wnt ligands binding to serpentine frizzled receptors and LRP5/6 coreceptors. These ligand–receptor–coreceptor complexes activate intracellular protein dishevelled (Dsh), leading to the inhibition of a cytoplasmic β-catenin destruction complex, which is composed of the scaffolding protein Axin, glycogen synthase kinase-3β (GSK-3β) and adenomatous polyposis coli (APC) tumor suppressor. The inhibition of this Axin complex allows cytoplasmic β-catenin to accumulate in the nucleus and bind to TCF/LEF transcription factors to stimulate the transcription of Wnt target genes. In the absence of Wnt signal, GSK-3β phosphorylates β-catenin, which earmarks it for rapid degradation by the ubiquitin system.
In Xenopus embryos, the Wnt/β-catenin signaling is essential for proper dorso-ventral axis specification (Harland and Gerhart, 1997; Moon and Kimelman, 1998). During cortical rotation of the egg, Dsh and GBP (GSK-3-binding protein) are translocated from the vegetal pole to the dorsal side, resulting in the local accumulation of β-catenin and the establishment of the Spemann's organizer on the dorsal region (Miller et al, 1999; Weaver et al, 2003). In knockdown experiments, depletion of maternal β-catenin or GBP causes a loss of dorsal axial structures (Yost et al, 1998; Heasman et al, 2000). Moreover, ventral ectopic expression of XWnt8, Dsh, β-catenin or dominant-negative GSK-3 induces the formation of a secondary dorsal axis (He et al, 1995). The subcellular localization of Dsh has a pivotal role in the regulation of Wnt signaling. Dsh is associated with vesicle-like structures in Xenopus embryos and mammalian cells (Miller et al, 1999; Capelluto et al, 2002). In the presence of frizzled, Dsh is redistributed to the plasma membrane. The DIX domain of Dsh is responsible for its punctuate localization (Rothbächer et al, 2000). Mutation of the vesicle-binding motif in the DIX domain disrupts vesicular association of Dsh, thereby leading to the inhibition of Dsh phosphorylation and its ability to stabilize β-catenin and induce a secondary dorsal axis (Capelluto et al, 2002). These results indicate that the vesicular localization of Dsh is critical for the Wnt/β-catenin signaling. However, little is known regarding the nature of Dsh-associated punctuate structures and how this localization of Dsh is regulated.
Rap proteins belong to the Ras family of small GTPases. These proteins constitute two subfamilies (Rap1 and Rap2), and each family contains two isoforms (A and B) showing 95 and 90% identity for Rap1 and Rap2, respectively. Similar to other Ras-related GTPases, Rap proteins function as binary switches by cycling between two interconvertible states: a GDP-bound inactive and a GTP-bound active form. Rap1, which contains the same effector domain as Ras, antagonizes Ras in many aspects. For example, overexpression of Rap1 is capable of reverting the phenotype of Ras-transformed NIH 3T3 fibroblasts (Kitayama et al, 1989). In Drosophila, Rap1 has been shown to regulate normal morphogenesis in the ovary, the eye disk and the embryo (Asha et al, 1999). In addition, Rap1 regulates cell adhesion, adherens junction positioning and integrin activation (Knox and Brown, 2002; McLeod et al, 2004). In contrast to Rap1, no cellular function has yet been attributed to Rap2.
In the current study, we provide evidence that Xenopus Rap2 is implicated in the dorsal axis specification during early development. We also demonstrate that XRap2 functions as a regulator of the subcellular localization of Dsh in Wnt/β-catenin signaling.
Results
Cloning and expression pattern of Xenopus Rap2 GTPase
Using a PCR-based approach, we obtained two Xenopus homologs encoding Rap2A and Rap2B (GenBank accession numbers: XRap2A, AY608667; XRap2B, AY392502) whose amino-acid sequences are highly conserved compared to human and mouse proteins (Supplementary Figure 1). Temporally, they are expressed both maternally and zygotically throughout early development (Supplementary Figure 2). During the cleavage and blastula stages, XRap2 is expressed in the animal and vegetal hemispheres of the embryo. In particular, its transcripts in vegetal cells are detected as punctate perinuclear staining. At the beginning of gastrulation, the intense expression of XRap2 is visible in the dorsal blastopore lip. Analysis of dissected embryos revealed that it was the cells of the deep layers of the lip that expressed XRap2 messages most abundantly at the early gastrula stage. By the midgastrula stage, XRap2 is expressed in the lateral regions of the presumptive neural plate and in a broad anterior domain of mesendoderm. At later stages, it is restricted to the eye primordia, the roof of the neural tube, the anterior endoderm and head region.
XRap2 has dorsalizing and neuralizing activities
To study the function of XRap2, we first analyzed its biological activities in Xenopus ectoderm. Injection of XRap2 RNA induces Xnr3, siamois and chordin, which are the target genes of Wnt/β-catenin signaling pathway (McKendry et al, 1997; Wessely et al, 2001), while we failed to detect other markers for mesoderm or endoderm in the early gastrula stage explants (Figure 1A). XRap2 also increases the early neural marker, zic-3, and decreases epidermal markers, msx-1 and epidermal keratin, suggesting the ability of XRap2 to inhibit BMP signaling. By stage 27, the cement gland and the neural markers NCAM, OtxA and XAG-1 are induced in XRap2-expressing animal caps (Figure 1B). The neural gene induced is of the anterior character, as it expresses a forebrain–midbrain marker (OtxA) but not a spinal cord marker (HoxB9). This neural induction by XRap2 occurs in the absence of mesoderm formation (α-globin and muscle actin). The pan-endodermal marker, endodermin (EDD), was also induced in XRap2-injected explants. The induction of cement gland and neural markers in ectoderm in the absence of mesoderm might be the result of antagonizing endogenous BMP signaling (Wilson and Hemmati-Brivanlou, 1995). To test whether BMP-4 can over-ride the effects of XRap2 in animal caps, we coinjected XRap2 and BMP-4 RNAs with the result that the latter could counteract the induction of NCAM, OtxA and XAG-1 by the former (Figure 1C). These indicate that XRap2 antagonizes the epidermal induction and neural inhibition activity of BMPs in ectoderm. Given the above data, the inhibitory effects of XRap2 on BMP signaling could be achieved by expressing secreted BMP-4 antagonists such as Xnr3 and chordin through the Wnt/β-catenin signaling (Wessely et al, 2001). Supporting this possibility, synergism between XRap2 and a small dose of activin was also observed (Figure 1D). Combination of suboptimal levels of activin and XRap2 mRNA has synergistic effects on the induction of mesodermal markers, leading to even cerberus, an anterior endodermal marker, being activated, which is similar to the action of XWnt8 (Christian et al, 1992). Overall, although XRap2 has no mesoderm-inducing activity of its own, it may modify the competence of animal cap cells to respond to mesoderm-inducing signals.
Figure 1.
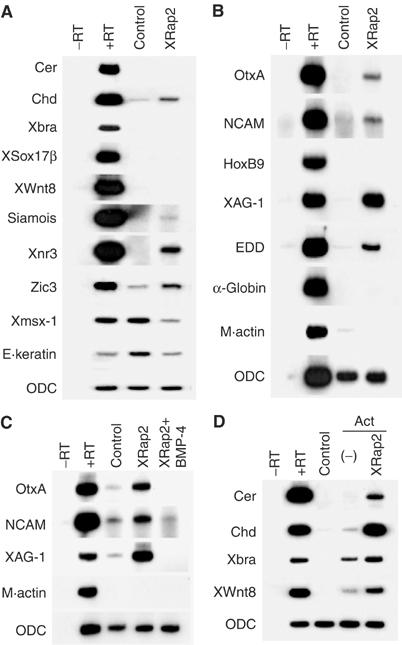
Characterization of XRap2's activity in Xenopus ectoderm. (A–D) Four-cell stage embryos were microinjected into the animal region with 100 pg XRap2, 2 ng BMP-4 or a combination as indicated. Animal caps were dissected at stage 8.5, cultured until stage 10.5 (A, D) or stage 27 equivalent (B, C) and analyzed by RT–PCR for expression of marker genes indicated. In (D), the Activin protein (Act) was added at 5 ng/ml to the animal caps at stage 8.5. ODC, a loading control; −RT, minus reverse transcription control sample.
In order to test the effects of XRap2 misexpression on mesoderm patterning, we analyzed the XRap2-injected embryos at the tadpole stage. Overexpression of XRap2 in two ventrovegetal blastomeres of four-cell stage embryos duplicated the dorsal axis (40%, n=75) (Figure 2A), which contained ectopic gut lumen, muscle and neural tissues but lacked a head and notochord (data not shown). Ventral misexpression of XRap2 also caused the ventral marginal zone (VMZ) explants to undergo convergent extension movements similar to the dorsal marginal zone (DMZ) explants (Figure 2C–E). The dorsalizing activity of XRap2 was further analyzed at the molecular level using VMZ explants from XRap2-injected embryos (Figure 2F). At the gastrula stage, ectopic expression of XRap2 induced the organizer-specific marker, chordin, and the anterior endodermal markers, cerberus and hex, in the VMZ explants. Later, the neural and dorsal mesodermal markers NCAM, collagen II and muscle actin were induced in VMZ explants expressing XRap2. Together, the induction of neural fate and dorsal mesoderm in XRap2-expressing VMZ strongly suggests that it acts to dorsalize the mesoderm.
Figure 2.
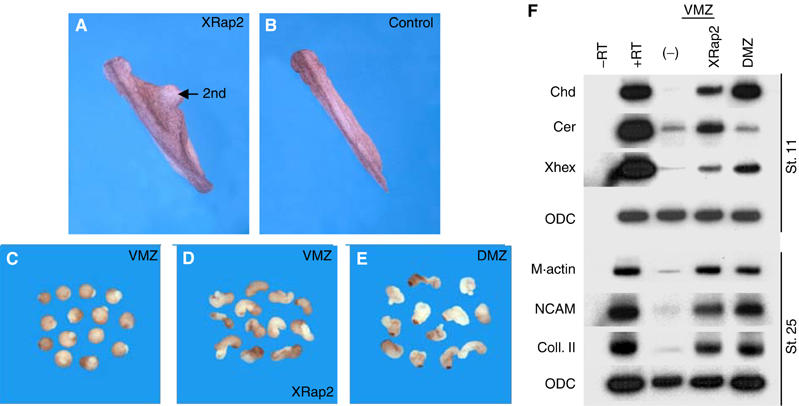
XRap2 dorsalizes ventral mesoderm. (A) Ventral injection of XRap2 RNA or DNA (150 pg) induced a secondary axis (40%, n=75). (B) Uninjected control embryo at stage 28. 2nd indicates an induced secondary axis. (D) XRap2 caused VMZ explants to undergo convergent extension movements and become elongated. In contrast to VMZ controls (C), DMZ explants elongate extensively (E). (F) RT–PCR analysis of the induction of marker genes by ventral injection of XRap2. (−), uninjected VMZ control; DMZ, uninjected DMZ control.
Organizer-specific genes are activated in ectoderm by high doses of the mesoderm inducers, activin and nodal-related factors (Zorn et al, 1999). Thus, we tested whether XRap2 could be induced by these activin-like signals. XRap2 as well as cerberus was strongly induced by activin, Xnr-1 and Smad2, a downstream effector of activin-like signaling (Supplementary Figure 3A). In contrast, XWnt8 and β-catenin activated the expression of Xnr3 but not XRap2. In control experiments, Xnr3 transcripts were also detectable in animal tissues injected with high doses of activin or Xnr-1. Therefore, XRap2 is a downstream target of activin-like mesoderm inducers but not of Wnt signaling.
Upon stimulation by activin-like inducers, Smad2 and Smad4 interact with a nuclear factor, FAST-1, affecting downstream transcriptional responses (Chen et al, 1997). We examined if XRap2 is induced by activin in a FAST-dependent manner. As shown in Supplementary Figure 3B, the induction of XRap2 by activin was inhibited by coinjection of a transcriptional repressor form of FAST-1 (FAST-EnR). In control experiments, FAST-EnR also blocked the activation of goosecoid and chordin by activin. Taken together, XRap2 expression is activated by activin-like inducers in a Smad2/FAST-dependent fashion.
XRap2 knockdown causes ventralization
To determine the loss-of-function phenotype of XRap2, we used an antisense morpholino (MO) oligonucleotide to knock down XRap2 function in the embryo. Because the highly homologous XRap2A and XRap2B are both expressed, we designed MOs for both XRap2A and XRap2B. The MO designed against each XRap2 isoform inhibited the production of its respective Myc-tagged XRap2 without affecting actin protein level, which serves as a specificity control (Figure 3A).
Figure 3.
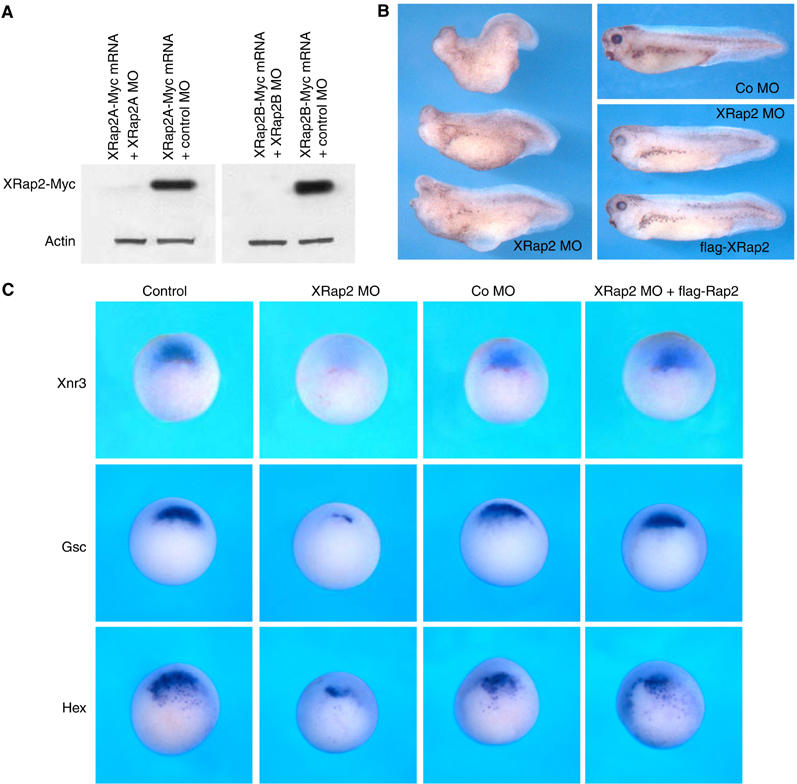
Knockdown of XRap2 leads to ventralization. (A) XRap2 MO inhibits specifically the translation of its cognate mRNA. C-terminally Myc-tagged XRap2 RNA (100 pg) was injected with XRap2 MO (40 ng) or control MO (40 ng) in the animal region of four-cell stage embryos, and animal caps isolated at early gastrula stages were subjected to Western blotting. Actin serves as a specificity control. (B) XRap2 knockdown results in embryos with severe anterior truncations and shortened body axis. These ventralized embryos were rescued by coinjected flag-XRap2 RNA. XRap2 MO (20 ng per blastomere) with or without flag-XRap2 RNA (200 pg) was injected into two dorsal blastomeres of four-cell stage embryos. Co MO, control MO. (C) XRap2 MO, but not control MO, inhibits organizer-specific expression of Xnr3, goosecoid (Gsc) and hex. XRap2 inhibition was rescued by flag-XRap2. Four-cell stage embryos were injected into two dorsal blastomeres with XRap2 MO (40 ng), control MO, flag-XRap2 (100 pg) or a combination as indicated, cultured until stage 10.5 and analyzed by whole-mount in situ hybridization for expression of the indicated markers. The embryos are shown in vegetal view, with dorsal region at the top. Control, uninjected embryos at stage 10.5.
When both MOs were coinjected (the mixed MOs will be designated XRap2 MO hereafter) into two dorsal blastomeres at the four-cell stage, embryos developing with severe anterior truncations (30%, n=123) or microcephaly and eye defects (55%, n=123) were observed (Figure 3B). This phenotype was efficiently rescued by flag-tagged XRap2 mRNA, which cannot bind to its MO and is resistant to translational inhibition (Supplementary Figure 3C). To test the requirement of XRap2 in early embryonic dorsalization, we examined early dorsal markers in XRap2 MO-injected embryos by in situ hybridization. XRap2 MO, but not control MO, inhibited the expression of Xnr3, goosecoid and Hex in the dorsal region at the early gastrula stage, and the suppression was rescued by coinjection of flag-XRap2 RNA (Figure 3C). Together, these results suggest that loss of XRap2 function leads to impaired dorsalization, resulting in ventralized phenotypes seen at later stages of development.
XRap2 is required for Wnt/β-catenin signaling pathway
As described above, dorsal injection of XRap2 MO inhibited the expression of Xnr3, an early direct target of Wnt/β-catenin signaling (McKendry et al, 1997), suggesting its possible requirement for this signaling. To assess a possible function for XRap2 in Wnt signaling, we first investigated whether XRap2 synergizes with XWnt8 in axis duplication. Interestingly, coinjection of XRap2 and a suboptimal dose of XWnt8 increased the frequency of partial secondary axes from 0% (n=65) to 25% (n=45) (Supplementary Figure 3D). We further tested whether complete secondary axis induction by ectopic activation of Wnt/β-catenin signaling could also be inhibited by coinjected XRap2 MO. Axis duplication caused by XWnt8, β-catenin or Dsh (data not shown) was blocked by coinjection of XRap2 MO (Figure 4A–D), confirming that XRap2 function is indispensable for Wnt/β-catenin signaling pathway. Consistently, the induction of siamois and Xnr3 by XWnt8 or β-catenin was specifically inhibited in ectodermal explants from embryos injected with XRap2 MO (Figure 4E, lanes 5 and 8), suggesting that XRap2 functions parallel to or downstream of β-catenin in Wnt signaling. In order to determine clearly the position of XRap2 in the pathway, we asked whether VP16-TCF3, an activated form of TCF factor that can activate transcription without associating with β-catenin (Vonica and Gumbiner, 2002), could rescue the inhibition by XRap2 MO of expression of target genes, which was induced by β-catenin. Coinjection of VP16-TCF3 factor increased significantly the expression of siamois and Xnr3 reduced by XRap2 MO (Figure 4E, lane 9). Thus, the overall results suggest that XRap2 functions parallel to β-catenin in Wnt signaling.
Figure 4.
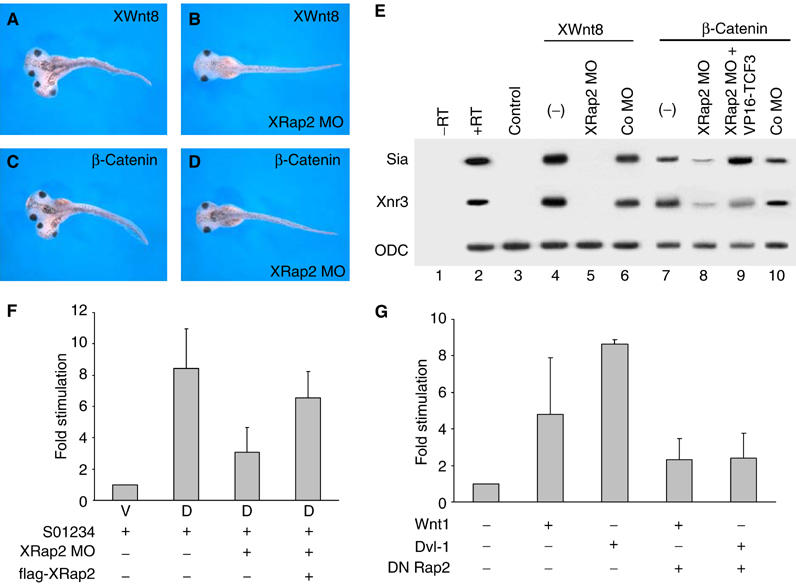
XRap2 is required for Wnt/β-catenin signaling. (A–D) XRap2 MO blocks the induction of a complete secondary axis by XWnt8 or β-catenin. Four-cell stage embryos were injected into one ventral vegetal blastomere with RNA or MO as indicated. (A) Injection of 10 pg XWnt8 mRNA (complete axis: 85%, n=34). (B) Coinjection of 10 pg XWnt8 mRNA and 40 ng XRap2 MO (complete axis: 23%, n=34). (C) Injection of 100 pg β-catenin mRNA (complete axis: 81%, n=40). (D) Coinjection of 100 pg β-catenin mRNA and 40 ng XRap2 MO (complete axis: 45%, n=35). (E) XRap2 MO, but not control MO, inhibits the induction of siamois and Xnr3 by XWnt8 or β-catenin. This inhibition was rescued by VP16-TCF3, an activated form of TCF3 factor. (F) Luciferase assays in Xenopus embryos injected with β-catenin/TCF factor-dependent reporter DNA (S01234), XRap2 MO and flag-XRap2 RNA. V, ventral region; D, dorsal region. (G) TOPFLASH reporter assay in 293T cells. The amount of transfected DNA is as follows: mouse Wnt1, 5 ng; mouse dishevelled-1 (Dvl-1), 50 ng; dominant-negative Xenopus Rap2 (S17N Rap2), 100 ng.
To analyze whether XRap2 directly activates Wnt/β-catenin-dependent transcription, we tested the effect of XRap2 depletion on the transcriptional activity of the luciferase reporter driven by the siamois promoter, which is responsive to the β-catenin/Tcf-3 complex (Brannon et al, 1997). We injected the S01234 siamois reporter gene into the dorsal blastomeres of four-cell stage embryos, with and without XRap2 MO, and then dorsal or ventral explants isolated at early gastrula stages were used to examine its activity. The siamois promoter was activated to higher levels on the dorsal side than the ventral side (Figure 4F), responding to the endogenous Wnt/β-catenin activity present in the dorsal marginal region of Xenopus early embryos (Larabell et al, 1997). However, the dorsal activation of the siamois promoter was impaired when coinjected with XRap2 MO compared with the reporter injected alone. This reduction was to some extent rescued by coinjected flag-XRap2 RNA. These results suggest a specific requirement of XRap2 for the activation of the siamois promoter by Wnt/β-catenin signaling.
To examine whether the action of Rap2 is conserved in higher vertebrates, we evaluated the Rap2-directed modulation of Wnt/β-catenin signaling in mammalian cells. We used the TOPFLASH luciferase reporter plasmid in 293T cells as a readout for Wnt/β-catenin signaling activation. Expression of Wnt1 gave an approximate five-fold increase in luciferase activity, which was less compared to Dvl-1, which activated the reporter activity >8-fold in these assays (Figure 4G). Cotransfection of a dominant-negative Rap2 (S17N Rap2) reduced significantly the increase in β-catenin/TCF-dependent transcription induced by Wnt1 or Dvl-1. These results indicate that Rap2 is a conserved positive factor in Wnt/β-catenin signaling.
Stabilization and nuclear localization of endogenous β-catenin protein are well-established measures of Wnt/β-catenin signaling (Yost et al, 1996; Larabell et al, 1997). We next investigated the impact of XRap2 depletion on the steady-state levels of total β-catenin proteins. Coinjection of XRap2 MO decreased the steady-state levels of ectopic myc-tagged β-catenin protein expressed from injected RNA in animal cap cells (Figure 5A). In addition, elevated levels of endogenous β-catenin protein were observed in animal cap cells expressing Xenopus Wnt8 (Figure 5B). And this increase was significantly inhibited by XRap2 depletion. Consistent with the requirement of XRap2 for the accumulation of β-catenin, its overexpression enhanced nuclear localization of endogenous β-catenin in animal cap cells (Figure 5C and D). Moreover, XRap2 knockdown inhibited the nuclear accumulation of β-catenin caused by exogenous XWnt8 RNA (Figure 5E–G). Taken together, these results suggest that XRap2 is essential for the stabilization and nuclear accumulation of β-catenin induced by Wnt signaling.
Figure 5.
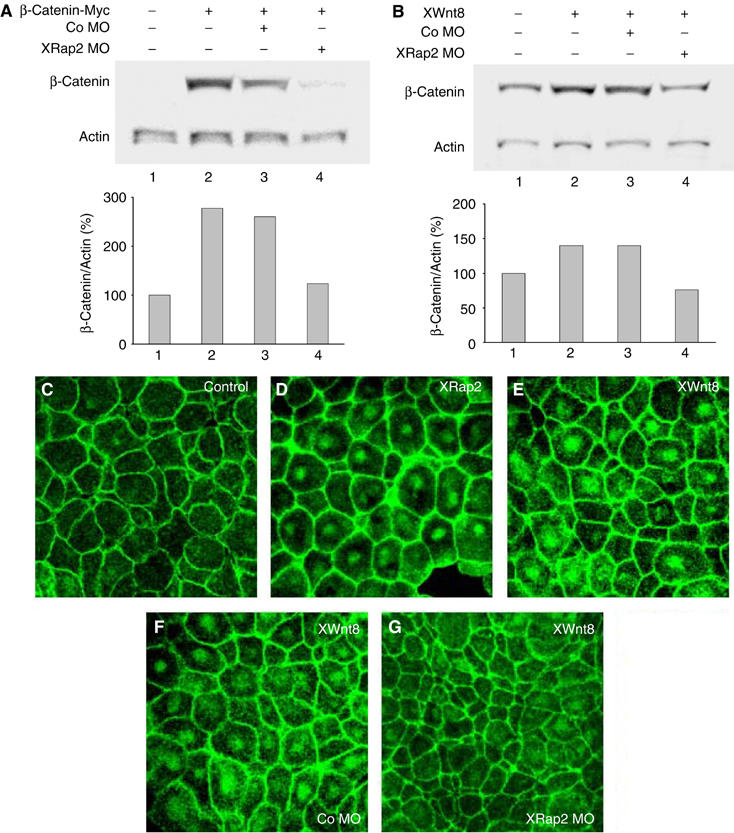
XRap2 regulates the steady-state levels of β-catenin. (A) XRap2 MO (40 ng) decreases the levels of exogenous myc-β-catenin expressed from injected RNA (100 pg) in animal cap cells. (B) An increase in the level of β-catenin induced by XWnt8 (20 pg) is impeded by XRap2 MO. (C–G) XRap2 is required for the nuclear accumulation of endogenous β-catenin in animal cap cells (AC). (C) Uninjected control AC, (D) XRap2-injected AC, (E) XWnt8-injected AC, (F) AC injected with XWnt8 and Co MO and (G) AC injected with XWnt8 and XRap2 MO. Four-cell stage embryos were injected into the animal region with the indicated reagents (XRap2 RNA, 150 pg; XWnt8 RNA, 2 ng; XRap2 MO, 40 ng; Co MO, 40 ng). Animal caps dissected from late blastulae were stained with anti-β-catenin antibody.
XRap2 affects the stability of β-catenin by regulating the subcellular localization of dishevelled
Next, we determined the subcellular localization of XRap2 by confocal microscopy using a green fluorescent protein (GFP)-fused version of XRap2. Interestingly, XRap2 staining is localized predominantly in the plasma membrane of animal cap cells, and to a lesser extent in various subcellular regions with punctate appearance resembling vesicular structures (Figure 6A). This led us to investigate the possible role of XRap2 in the regulation of subcellular distribution of Dsh, a cytoplasmic component of Wnt signaling, since as demonstrated above, XRap2 is required for Wnt signaling and its punctate staining in the cytoplasm is similar to Dsh's association with cytoplasmic vesicle-like structures (Figure 6B). First, we tested the effects of XRap2 depletion on the subcellular localization of Dsh. When coexpressed with XRap2 MO, GFP-Dsh does not exhibit a punctate pattern and instead is distributed diffusely throughout the cytoplasm (Figure 6C), whereas coinjected control MO has no effect on the pattern of subcellular distribution of Dsh (Figure 6D). We further examined whether XRap2 and Dsh colocalize with each other. Normally, ectopic GFP-Dsh is distributed in a punctate fashion at various subcellular sites of animal cap cells (Figure 6B) as described previously (Miller et al, 1999; Rothbächer et al, 2000). This subcellular localization of GFP-Dsh is similar to that observed for endogenous Dsh in animal cap cells (Miller et al, 1999). Intriguingly, GFP-Dsh localizes to the plasma membrane when coexpressed with XRap2 (Figure 6E), reminiscent of the pattern caused by experimental conditions in which Dsh is coexpressed with Axin or frizzled receptor (Yang-Snyder et al, 1996; Fagotto et al, 1999). Note that the GFP-Dsh translocated to the membrane colocalizes with XRap2 (Figure 6G). Since stabilization of β-catenin is lost when Dvl2 is defective in vesicle targeting (Capelluto et al, 2002), we next examined whether XRap2 knockdown could block the stabilization of β-catenin caused by Dsh. As shown in Figure 6H, greater levels of β-catenin were detected in Dsh-expressing animal cap cells relative to those in uninjected cells, and this elevation could be inhibited by coinjection of XRap2 MO, suggesting the essential role of XRap2-mediated vesicle targeting of Dsh in the stabilization of β-catenin.
Figure 6.
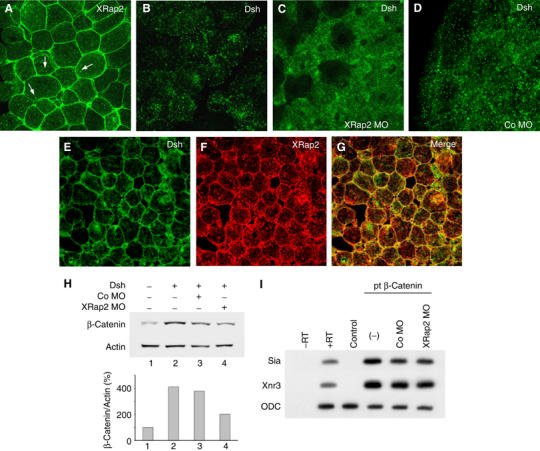
XRap2 is required for the subcellular localization of Dsh. (A, B) The subcellular localizations of ectopic GFP-XRap2 (A) and GFP-Dsh (B) in animal cap cells. Arrows point to the localization of XRap2 in cytoplasmic vesicle-like structures. (C, D) XRap2 MO, but not control MO, disrupts the punctate localization of ectopic GFP-Dsh in animal cap cells. (E–G) Coexpression of myc-XRap2 and GFP-Dsh. XRap2 translocates Dsh to the plasma membrane. (E) Dsh localization, (F) XRap2 localization and (G) merged images (yellow signals correspond to the colocalization of GFP-Dsh and myc-XRap2). (A–G) Four-cell stage embryos were injected into the animal region with the reagents indicated. Animal caps were cut from late blastulae or early gastrulae, immediately fixed and mounted for GFP-tagged proteins (A–D) or subjected to a standard immunostaining procedure (E–G). The amount of injected reagents is as follows: GFP-XRap2, 150 pg; myc-XRap2, 150 pg; GFP-Dsh, 500 pg; XRap2 MO, 40 ng; Co MO, 40 ng. (H) Western blot showing the steady-state levels of β-catenin in animal halves from embryos injected with Dsh (2 ng) alone or in combination with XRap2 MO (40 ng) or control MO. Actin was used as a loading control. This is a representative result from four independent experiments. (I) XRap2 MO has no effect on the ability of pt β-catenin, a stabilized mutant of β-catenin, to induce target genes in animal caps. The amount of injected reagents is as follows: pt β-catenin, 100 pg; XRap2 MO, 40 ng; Co MO, 40 ng.
If XRap2 affects β-catenin stability by regulating the subcellular localization of Dsh, a stabilized β-catenin could be insensitive to the inhibitory effects of XRap2 depletion on Wnt signaling, while wild-type β-catenin could become easily unstable in the absence of XRap2, as shown in Figure 5. To test this possibility, we examined the effects of XRap2 MO on the ability of pt β-catenin, a stabilized form that lacks the phosphorylation sites required for its degradation (Yost et al, 1996), to induce ectopic axis or target genes. Ventral expression of pt β-catenin RNA (50 pg) induced ectopic complete body axis in 70% of the injected embryos (n=51; figures not shown). As expected, coinjection of XRap2 MO had no significant effects on the duplication of complete axis caused by pt β-catenin (65%, n=56). Consistently, XRap2 depletion did not inhibit the induction of siamois or Xnr3 by pt β-catenin in animal cap cells (Figure 6I). Overall, these results support that XRap2 becomes involved in the stabilization of β-catenin by modulating the subcellular localization of Dsh in Wnt signaling.
We further investigated whether XRap2 MO affects the translocation ability of Dsh upon coexpression of frizzled receptors. As described previously (Yang-Snyder et al, 1996; Kinoshita et al, 2003), in the presence of frizzled (Fz) receptors such as rat Fz-1 and Xenopus Fz-7, GFP-Dsh relocalizes predominantly to the plasma membrane (Figure 7B and E). However, coexpression of XRap2 MO blocks this translocation, leading to the less obvious accumulation at the membrane and diffuse distribution in the cytoplasm of GFP-Dsh (Figure 7C and F), indicating that XRap2 is also required for the translocation of Dsh caused by frizzled receptors.
Figure 7.
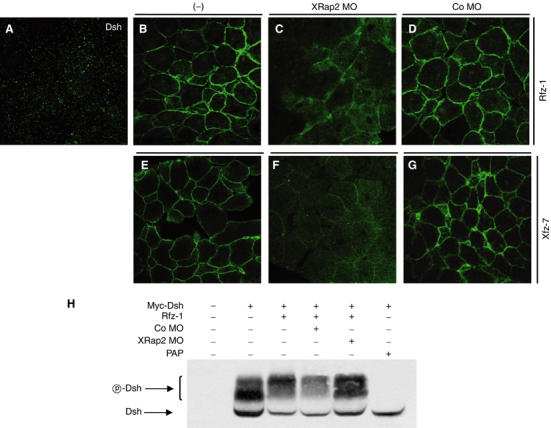
XRap2 is required for the translocation and phosphorylation of Dsh induced by frizzled signaling. (A) Ectopic GFP-Dsh is distributed in a punctate fashion in animal cap cells. XRap2 MO inhibits the relocalization of GFP-Dsh to the plasma membrane caused by rat Fz-1 (B, C) or Xenopus Fz-7 (E, F). Co MO has no effect on Dsh localization (D, G). (−), control without MO injection. (H) XRap2 depletion inhibits the phosphorylation of Dsh by Rfz-1 signaling. For phosphatase treatment, the immunoprecipitate from early gastrulae injected with myc-Dsh was incubated with potato acid phosphatase (PAP). (A–H) Four-cell stage embryos were injected into the animal pole region with RNAs, MO or a combination as indicated. Animal caps were cut from late blastulae and immediately fixed or subjected to Western blot. The subcellular localization of GFP-Dsh was analyzed by confocal microscopy. The amount of injected reagents is as follows: GFP-Dsh, 500 pg; rat Fz-1, 500 pg; Xenopus Fz-7, 500 pg; Myc-Dsh, 2 ng; XRap2 MO, 40 ng; Co MO, 40 ng.
Dsh is phosphorylated upon activation of the Wnt/β-catenin signaling pathway (Yanagawa et al, 1995; Peters et al, 1999). Phosphorylation of Dsh is correlated with its vesicular association and translocation to the membrane (Rothbächer et al, 2000; Capelluto et al, 2002). Thus, we tested whether XRap2 MO affects the phosphorylation state of Dsh. Myc-tagged Dsh mRNA was injected with or without XRap2 MO in four-cell stage embryos and then, animal cap explants isolated at around stage 10 were subjected to SDS–polyacrylamide gel electrophoresis (SDS–PAGE). Three bands were observed in Dsh-injected explants (Figure 7H). Immunoprecipitated Myc-Dsh treated with acid phosphatase migrates as a single band, suggesting that two bands of higher molecular weight represent mobility-shifted, phosphorylated Dsh and the lowest molecular weight band represents nonphosphorylated Dsh. In the absence of rat Fz-1 signaling, the lower band of the two phosphorylated bands was more intense than the upper one. Coinjection of rat Fz-1 made the upper phosphorylated band much more intense, with a concomitant decrease in the levels of the lower phosphorylated and nonphosphorylated bands, indicating the hyperphosphorylation of Dsh by rat Fz-1 signaling. However, coinjection of XRap2 MO inhibited this hyperphosphorylation, recovering to some extent the levels of the lower phosphorylated and nonphosphorylated Dsh. These results indicate a role for XRap2 in the phosphorylation of Dsh, further suggesting that XRap2 is required for the signaling from frizzled receptor to Dsh.
Discussion
In Xenopus early development, Wnt/β-catenin signaling is important for the dorsal axis specification (Harland and Gerhart, 1997; Moon and Kimelman, 1998). In this study, we present evidence that XRap2, as a positive component of Wnt/β-catenin signaling, is implicated in dorsal axis formation. Ventral expression of XRap2 induces dorsalized phenotypes, which includes axis duplication, convergent extension movement and dorsal gene expression. In the context of the whole embryo, specific depletion of XRap2 resulted in the ventralized embryos developing with severe anterior truncation and shortened body axis. These gain-of-function and loss-of-function results show that XRap2 is required for dorsal axis specification of Xenopus early embryos.
The XRap2-depleted embryos are reminiscent of those caused by experimental conditions that lead to an increase of BMP signaling and/or decrease of Wnt/β-catenin signaling in the dorsal region of early embryos (He et al, 1995; Suzuki et al, 1997; Heasman et al, 2000). In line with this, the results presented here support that XRap2 exhibits the dorsalizing activity through Wnt/β-catenin signaling. First, XRap2 synergized with XWnt8 in secondary axis induction. In ectoderm, XRap2 is able to induce siamois and Xnr3, direct targets of Wnt signaling, and neural genes, which is similar to the action of XWnt8, Dsh or β-catenin (Baker et al, 1999; Wessely et al, 2001). Furthermore, unlike mesendoderm inducers such as activin and nodal, XRap2 has no mesoderm–inducing activity of its own in ectoderm but can respecify mesoderm differentiation from ventral to dorsal fates. Like XWnt8 and a dominant-negative GSK-3 (Christian et al, 1992; He et al, 1995), XRap2 sensitizes ectoderm, augmenting the ability of activin-like signal to induce mesoderm. On the other hand, some aspects of XRap2's activity are similar to those that BMP antagonists such as chordin and noggin share: partial axis duplication, induction of anterior neural markers in ectoderm and enhancement of activin-mediated dorsal mesoderm induction. Consistently, overexpression of BMP-4 counteracted the neural induction by XRap2, suggesting an antagonistic relationship between XRap2 and BMP signaling. However, these features could be indirect effects of XRap2, since it can induce on its own BMP inhibitors including chordin and Xnr3 (Figure 1A). These inhibitors are expressed at the blastula stage in a Wnt/β-catenin signaling-dependent manner, thereby enabling anterior neural genes to be induced in the absence of mesoderm (Wessely et al, 2001). Thus, it is possible that XRap2-induced BMP antagonists may render the ectodermal cells more susceptible in responding to activin signaling by inhibiting BMP signaling in animal caps (Figure 1D). The relatively weak activity of XRap2 in axis duplication could be due to insufficient levels of XRap2 RNA used here. Indeed, injection of higher doses of its RNA caused nonspecific toxic effects leading to death (data not shown). Our loss-of-function data corroborate a direct function of XRap2 in Wnt signaling. Dorsal injection of XRap2 MO inhibited the endogenous expression of Xnr3, an early dorsal target gene of Wnt/β-catenin signaling. XRap2 depletion also attenuated significantly the promoter activity of reporter genes, which is dependent on β-catenin/TCF factors. Consistently, XRap2 MO blocks the ectopic induction of target genes and dorsal axis by XWnt8, β-catenin or Dsh. Finally, XRap2 is required for the nuclear accumulation of β-catenin induced by XWnt8, a well-established measure of the activation of Wnt signaling, and regulates the steady-state levels of β-catenin protein. Taking together, we conclude that XRap2 functions as an essential component of Wnt/β-catenin signaling to specify the dorsal axis.
Recent works reveal that the targeting of Dsh to cytoplasmic vesicular structures, which is mediated by its DIX domain, is critical for Wnt/β-catenin signaling (Capelluto et al, 2002; Weitzel et al, 2004). Mutation of the vesicle-binding motif within the DIX domain disrupted punctate localization and phosphorylation of Dsh, rendering it incapable of eliciting cell fate decisions through the Wnt/β-catenin signaling (Rothbächer et al, 2000; Capelluto et al, 2002). XRap2 knockdown impeded the vesicular localization of ectopic GFP-Dsh and β-catenin stabilization and axis duplication caused by XWnt8 or Dsh, suggesting its implication in Wnt/β-catenin signaling as a regulator of the subcellular localization of Dsh. Although XRap2 seemingly functions parallel to or downstream of β-catenin in Wnt signaling (Figure 4), no significant effects that XRap2 depletion had on the ability of a stabilized β-catenin to induce ectopic axis and target genes (Figure 6) support that XRap2 affects β-catenin stabilization by acting to target Dsh to cytoplasmic vesicular structures. Despite this function of XRap2, it did not interact with Dsh in co-immunoprecipitation assay (data not shown). While the nature of Dsh-containing vesicular structures remains unknown, it seems likely that XRap2 modulates the vesicular localization of Dsh through other means, not physical association.
Overexpression of XRap2 relocates Dsh to the plasma membrane (Figure 6), which is similar to the effects of Axin or frizzled (Yang-Snyder et al, 1996; Fagotto et al, 1999), and its depletion blocks the membrane translocation of Dsh induced by frizzled receptors (Figure 7). These suggest the essential role of XRap2 in the membrane recruitment of Dsh in Wnt/frizzled signaling. In Xenopus embryos, a few frizzled receptors can induce the membrane translocation and phosphorylation of Dsh, but not axis duplication when ectopically expressed (Rothbächer et al, 2000), suggesting no correlation between the membrane localization and phosphorylation of Dsh and Wnt/β-catenin signaling. However, several lines of evidence support that stabilization of β-catenin through Wnt/β-catenin signaling involves the membrane targeting and phosphorylation of Dsh. Indeed, casein kinase I regulates the signaling specificity of Dsh through phosphorylation, ultimately directing it down the Wnt/β-catenin pathway from the planar cell polarity pathway (Peters et al, 1999; Cong et al, 2004a). In addition, mutation of the residues of frizzled that are crucial for the membrane recruitment of Dsh disrupts the signaling activities of this receptor in Wnt/β-catenin pathway (Umbhauer et al, 2000; Cong et al, 2004b). In this respect, it is possible that the membrane localization of Dsh caused by XRap2 is closely linked to its axis-inducing activity. Recent evidence indicates that Wnt-induced Dsh phosphorylation depends on only frizzled receptors (González-Sancho et al, 2004), whereas β-catenin stabilization is induced by LRP/Arrow coreceptors independently of frizzled (Brennan et al, 2004). Wnt signaling, however, can be fully stimulated in a Dsh-dependent manner by oligomerizing frizzled and LRP receptors (Cong et al, 2004b). During this event, Dsh may act as a shuttle to recruit Axin to the plasma membrane, which leads to Axin inhibition or degradation and β-catenin stabilization (Mao et al, 2001; Cliffe et al, 2003; Tamai et al, 2004). In light of this model, Dsh phosphorylation and translocation induced by frizzled, which are alone insufficient to activate the β-catenin signaling, could facilitate the LRP-dependent signals leading to β-catenin stabilization. Possibly, the membrane targeting of Dsh by XRap2 or Fz-8 (Rothbächer et al, 2000) may result in inhibition of Axin or synergize with endogenous LRP-dependent signals that lead to its axis-inducing activity in ventral blastomeres of Xenopus embryos. The molecular mechanism of these events awaits further investigation.
The phenotypes of XRap2 knockdown could result from the failure of dorsal mesodermal cells to migrate during gastrulation. Indeed, the membrane localization of Dsh induced by XRap2 suggests its possible involvement in noncanonical Wnt signaling, which is crucial for the regulation of gastrulation movements (Veeman et al, 2003). A recent study shows that mammalian Rap2 is implicated in the activation of integrin in inside-out signaling in lymphocytes (McLeod et al, 2004). In Xenopus embryos, the function of integrin is required for fibronectin (FN) assembly and radial intercalation of cells in the blastocoel roof, which are also essential for normal gastrulation movements (Marsden and DeSimone, 2001). Moreover, integrin can mobilize Dsh to the plasma membrane in dorsal mesodermal cells (Marsden and DeSimone, 2001). These findings raise the possibility that the function of XRap2 depends on integrin activation in Xenopus embryos. However, XRap2 depletion in blastocoel roof region does not inhibit FN fibril assembly (Supplementary Figure 4A). In addition, the membrane localization of Dsh caused by XRap2 could not be blocked by coexpression of a dominant-negative integrin receptor (HAβ1) (Supplementary Figure 4B). These imply that XRap2 is not involved in integrin activation required for normal FN assembly and the membrane targeting of Dsh by XRap2 occurs independently of integrin activation. The phenotype of XRap2-depleted embryos, ultimately, appears distinct from that caused by inhibiting the function of integrin or FN matrix. The defects in gastrulation movements of Xenopus embryos, which include delayed blastoporal closure, kinked or stout phenotypes, spina bifida or microcephaly, appear to be due to misregulation of cell adhesion and/or cell polarity in migrating dorsal cells. While mammalian Rap1 has been known to regulate cadherin-mediated cell–cell adhesion (Price et al, 2004), it remains unknown whether Rap2 has a similar function. Given the phenotypes of XRap2 knockdown embryos, it is probable that Rap2 is also involved in the regulation of cell behaviors in noncanonical Wnt signaling. For future analysis, it will be significant to correlate this possible role of XRap2 with its signaling activities demonstrated in this study.
Materials and methods
Embryo and explants
Developmental stages of embryos were determined according to Nieuwkoop and Faber (1994). For animal cap assays, four-cell stage embryos were microinjected into the animal pole region of all blastomeres with RNA, MO or a combination as indicated. Animal cap explants were cut from late blastulae, cultured until stage 10.5 or 27 and analyzed by RT–PCR. For VMZ assays, RNA or DNA was injected into two ventro-vegetal blastomeres of four-cell stage embryos. VMZ explants were dissected from early gastrulae and cultured until stage 11 or 25 for RT–PCR analysis or until stage 20 for elongation assay.
Plasmids, RNA synthesis and morpholino oligos
For the expression in Xenopus embryos, the entire coding region of XRap2A or XRap2B was cloned into the pCS2+, pCSKA, flag-pCS2+, EGFP-pCS2+ or Myc-pCS2+ vector. The dominant-negative version of XRap2B was generated by changing the amino acid at position 17 from S to N. The coding region of Xdsh was also cloned into the EcoRI site of the EGFP-pCS2+ vector for GFP tagging. Capped mRNAs were synthesized from linearized plasmids using the mMessage mMachine kit (Ambion). XRap2, MT-XRap2, MT-β-catenin, MT-pt β-catenin, Xfz-7, Rfz-1 and VP16-TCF3 constructs were linearized with NotI and mRNA was synthesized using the SP6 mMessage mMachine kit. flag-XRap2, EGFP-XRap2, EGFP-Dsh, Xnr-1 and XWnt8 constructs were linearized with Asp718 and mRNA was synthesized using the SP6 mMessage mMachine kit. The other constructs were linearized and mRNA was synthesized as follows: Dsh and Smad2 (linearized with XbaI and transcribed with SP6), BMP4 (linearized with EcoRI and transcribed with T7), ActivinβB (linearized with EcoRI and transcribed with SP6) and FAST-ENR (linearized with SacII and transcribed with SP6). Antisense MO oligos were obtained from Gene Tools. The MO oligo sequences were as follows: XRap2A MO, 5′-GACCACCACCTTATACTCGCGCATC-3′; XRap2B MO, 5′-CAACTACTTTGTATTCCCGCATGGC-3′; control MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′.
In situ hybridization and RT–PCR analysis
In situ hybridization was performed with digoxigenin (DIG)-labeled probes as described previously (Harland, 1991; Henry et al, 1996). An antisense in situ probe against XRap2 was generated by linearizing the pBS-KS-XRap2 construct with HindIII and transcribing with the T3 RNA polymerase.
RT–PCR assays were carried out as described (Choi and Han, 2002). Primers for XRap2A were (forward) 5′-ATCAAGCCCATGAGGGACCA-3′ and (reverse) 5′-TCCGGTTGGGCTGCATAGTT-3′, and those for XRap2B were 5′-GTAGTTGTCCTTGGATCAGGCG-3′ and 5′-TGACCAGGCTGTAGACGAGGAT-3′. Primers for cerberus, Xbra, XSox17β, XWnt-8, siamois, Xnr-3, NCAM, HoxB9, XAG-1, EDD, α-globin, chordin, collagen type II and goosecoid were as described in Dr De Robertis' homepage (http://www.hhmi.ucla.edu/derobertis/index.html). Primers for ODC, muscle actin, Zic3, OtxA and epidermal keratin (http://www.xenbase.org/xmmr/Marker_pages/primers.html) were as previously described.
Western blotting and luciferase assay
For Western blot analysis, animal cap explants were homogenized in Triton X-100 lysis buffer (20 mM Tris, 1% Triton X-100, 140 mM NaCl, 10% glycerol, 1 mM EGTA, 1.5 mM MgCl2, 1 mM DTT, 1 mM sodium vanadate, 50 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin). Equal amounts of protein were separated by 10 or 6% SDS–PAGE electrophoresis. Western blots were performed according to standard protocol with anti-β-catenin (1:1000, Santa Cruz), anti-myc (1:1000, Santa Cruz) and anti-actin (1:1000, Santa Cruz) antibodies. For phosphatase treatment, the immunoprecipitates were washed with PAP buffer (40 mM MOPS, pH 5.5, 1 mM MgCl2, 50 mM NaCl, 1 mM PMSF) and treated with 0.4 U of PAP (Sigma) at 37°C for 1 h.
For luciferase assay in Xenopus embryos, injections were made in the DMZ or VMZ of four-cell stage embryos with 40 pg of reporter gene (S01234, Wnt-responsive siamois promoter) with or without XRap2 MO (40 ng) and flag-XRap2 RNA (100 pg). Dorsal or ventral explants, which contain marginal and vegetal regions, were cut at stage 10 and then separated into three pools of six explants each for assay in triplicate. For TOPFLASH reporter assays, HEK 293T cells were transfected in 24-well dishes by using Lipofectamine Plus (Invitrogen), and all transfections were normalized to 200 ng DNA per well with pCS2+ vector. Each well received 20 ng of TOPFLASH reporter plasmid (Upstate) and 0.5 ng of Renilla luciferase plasmid (as an internal standard). Approximately 24 h after transfection, all cells were lysed, and luciferase activity assays were performed according to the manufacturer's protocol with the Dual-Luciferase Reporter Assay System (Promega). Experiments were repeated three times. Figure 4F and G shows a single representative result.
Fluorescence microscopy
The subcellular localization of proteins was monitored using an assay previously described (Miller et al, 1999). Four-cell stage embryos were injected into the animal pole region of all blastomeres with mRNAs as indicated. At blastula to early gastrula stages, animal caps were dissected, fixed in 4% paraformaldehyde in PBS for 2 h, rinsed in PBS and directly mounted for GFP-tagged proteins. Alternatively, they were incubated in PBSTB (PBS, 0.1% Triton X-100 and 2% BSA) to block nonspecific binding, followed by a standard immunostaining procedure. Image analysis was performed using a confocal laser-scanning microscope (Zeiss). The antibodies for immunofluorescence were polyclonal anti-myc (1:500 dilution, Santa Cruz) or polyclonal anti-β-catenin (1:200 dilution, Santa Cruz) primary antibodies and fluorescein isothiocyanate (FITC)- labeled (1:160 dilution, Sigma) or rhodamine-labeled (1:300 dilution, Sigma) goat anti-rabbit secondary antibodies.
Supplementary Material
Supplementary Figure
Supplementary Figure
Supplementary Figure
Supplementary Figure
Acknowledgments
We thank Randall Moon, Ken Cho, Doug Melton, Chris Wright, Malcolm Whitman, David Kimelman, Makoto Asashima, Doug DeSimone, Cheol-Hee Kim, Alin Vonica and Jongkyeong Chung for generous gifts of reagents. We are also grateful to Ken Cho for critical reading of the manuscript and helpful comments. This work was supported by the Advanced Basic Research Laboratory Program (R14-2002-012-01001-0) of the KOSEF and Brain Korea 21 project.
References
- Asha H, de Ruiter ND, Wang MG, Hariharan IK (1999) The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO J 18: 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM (1999) Wnt signaling in Xenopus embryos inhibits Bmp-4 expression and activates neural development. Genes Dev 13: 3149–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan K, González-Sancho JM, Castelo-Soccio LA, Howe LR, Brown AMC (2004) Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize β-catenin independently of frizzled proteins. Oncogene 23: 4873–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D (1997) A β-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11: 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DGS, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M (2002) The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419: 726–729 [DOI] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M (1997) Smad4 and FAST-1 in the assembly of activin-response factor. Nature 389: 85–89 [DOI] [PubMed] [Google Scholar]
- Choi S-C, Han JK (2002) Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol 244: 342–357 [DOI] [PubMed] [Google Scholar]
- Christian JL, Olson DJ, Moon RT (1992) XWnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J 11: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M (2003) A role of Dishevelled in relocating Axin to the plasma membrane during Wingless signaling. Curr Biol 13: 960–966 [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H (2004a) Casein kinase Iɛ modulates the signaling specificities of dishevelled. Mol Cell Biol 24: 2000–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H (2004b) Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115 [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F (1999) Domains of Axin involved in protein–protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 145: 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AMC (2004) Wnt proteins induces dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize β-catenin. Mol Cell Biol 24: 4757–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM (1991) In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol 36: 685–695 [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J (1997) Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol 13: 611–667 [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB (1995) Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374: 617–622 [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C (2000) β-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol 222: 124–134 [DOI] [PubMed] [Google Scholar]
- Henry GL, Brivanlou IH, Kessler DS, Hemmati-Brivanlou A, Melton DA (1996) TGFβ signals and a prepattern in Xenopus laevis endodermal development. Development 122: 1007–1015 [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N (2003) PKCδ is essential for dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev 17: 1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M (1989) A ras-related gene with transformation suppressor activity. Cell 56: 77–84 [DOI] [PubMed] [Google Scholar]
- Knox AL, Brown NH (2002) Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 295: 1285–1288 [DOI] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT (1997) Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 136: 1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH III, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell 7: 801–809 [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW (2001) Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development 128: 3635–3647 [DOI] [PubMed] [Google Scholar]
- McKendry R, Hsu S-C, Harland RM, Grosschedl R (1997) Lef-1/TCF proteins mediate Wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev Biol 192: 420–431 [DOI] [PubMed] [Google Scholar]
- McLeod SJ, Shum AJ, Lee RL, Takei F, Gold MR (2004) The Rap GTPases regulates integrin-mediated adhesion, cell spreading, actin polymerization, and Pyk2 tyrosine phosphorylation in B lymphocytes. J Biol Chem 279: 12009–12019 [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder J, Bates RL, Moon RT (1999) Establishment of the dorsal–ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol 146: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of Wnt signaling through β-catenin. Science 296: 1644–1646 [DOI] [PubMed] [Google Scholar]
- Moon RT, Kimelman D (1998) From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. BioEssays 20: 536–545 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J (1994) Normal Table of Xenopus laevis (Daudin), pp 163–188. New York: Garland Publishing [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM (1999) Casein kinase I transduces Wnt signals. Nature 401: 345–350 [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL (2004) Rap1 regulates E-cadherin-mediated cell–cell adhesion. J Biol Chem 279: 35127–35132 [DOI] [PubMed] [Google Scholar]
- Rothbächer U, Laurent MN, Deardorff MA, Klein PS, Cho KWY, Fraser SE (2000) Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J 19: 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Chang C, Yingling JM, Wang X, Hemmati-Brivanlou A (1997) Smad5 induces ventral fates in Xenopus embryos. Dev Biol 184: 402–405 [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X (2004) A mechanism for Wnt coreceptor activation. Mol Cell 13: 149–156 [DOI] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL (2000) The C-terminal cytoplasmic Lys-Thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signaling. EMBO J 19: 4944–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM (2002) Zygotic wnt activity is required for brachyury expression in the early Xenopus laevis embryo. Dev Biol 250: 112–127 [DOI] [PubMed] [Google Scholar]
- Weaver C, Farr GH III, Pan W, Rowning BA, Wang J, Mao J, Wu D, Li L, Larabell CA, Kimelman D (2003) GBP binds kinesin light chain and translocates during cortical rotation in Xenopus eggs. Development 130: 5425–5436 [DOI] [PubMed] [Google Scholar]
- Weitzel HE, IIIies MR, Byrum CA, Xu R, Wikramanayaka AH, Ettensohn CA (2004) Differential stability of β-catenin along the animal–vegetal axis of the sea urchin embryo mediated by dishevelled. Development 131: 2947–2956 [DOI] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschläger M, Pera EM, De Robertis EM (2001) Neural induction in the absence of mesoderm: β-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol 234: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A (1995) Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376: 331–333 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of wnt signaling in development. Annu Rev Cell Dev Biol 14: 59–88 [DOI] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R (1995) The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev 9: 1087–1097 [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai C, Moon RT (1996) A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol 6: 1302–1306 [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH III, Pierce SB, Ferkey DM, Chen MM, Kimelman D (1998) GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93: 1031–1041 [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT (1996) The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443–1454 [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB (1999) Anterior endomesoderm specification in Xenopus by Wnt/β-catenin and TGF-β signaling pathways. Dev Biol 209: 282–297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure
Supplementary Figure
Supplementary Figure
Supplementary Figure


