Abstract
Background
Cerebral amyloid angiopathy (CAA) is a degenerative vasculopathy that is classically associated with lobar intracerebral or sulcal hemorrhage. Its prevalence is estimated at 30% in the seventh decade and 50% in the eighth and ninth decades. In this review, we summarize the risks linked to CAA with respect to the treatment and prevention of stroke.
Methods
This review is based on pertinent publications retrieved by a selective search employing the terms “amyloid cerebral angiopathy,” “stroke,” “intracerebral bleeding,” and “acute stroke therapy.”
Results
Among patients given systemic lytic treatment for stroke, those who have microhemorrhages tend to have a higher risk of treatment-associated brain hemorrhage. In a meta-analysis, 70% of patients who sustained a hemorrhage after thrombolytic therapy were found to have CAA, compared to only 22% in a control population. Patients with cerebral hemorrhages have microhemorrhages more commonly than patients with transient ischemic attacks (TIA) or infarcts. This was observed among persons under treatment with vitamin K antagonists (odds ratio, 2.7) or platelet aggregation inhibitors (odds ratio, 1.7). Moreover, the apolipoprotein E2 allele is associated with a higher incidence of intracerebral hemorrhage (ICH) under oral anticoagulation. Strict treatment of arterial hypertension can lower the risk of ICH in persons with probable CAA by 77%. On the other hand, the use of statins after a lobar ICH increases the risk for a clinically manifest recurrent hemorrhage from 14% to 22%.
Conclusion
In patients with CAA, arterial hypertension should be tightly controlled. On the other hand, caution should be exercised in prescribing oral anticoagulants or platelet aggregation inhibitors for patients with CAA, or statins for patients who have already sustained a lobar ICH.
Cerebral amyloid angiopathy (CAA) is characterized histopathologically by amyloid fibrils in the small to middle-sized blood vessels—usually the arteries—of the brain. These amyloid fibrils trigger degenerative changes that destroy the vascular architecture, with consequences that include the formation of microaneurysms, fibrinoid necrosis, vascular occlusion, and concentric splitting of the vessel wall. The apolipoprotein (Apo)E4 allele is a risk factor for CAA (1). Patients who are ApoE4 heterozygotes are at higher risk of CAA and of a more extreme form of CAA than those without the ApoE4 allele. The probability of CAA, and of an extreme form of it, is even higher in ApoE4 homozygotes. There is a positive correlation between the presence of the ApoE2 allele and the occurrence of hemorrhage in CAA (2).
CAA generally occurs sporadically in older individuals; the familial forms are very rare (3). The prevalence of CAA increases with age. It is low in patients below the age of 55. Autopsy studies suggest a prevalence of about 30% in 60- to 69-year-olds and over 50% in the age range from 70 to 89 years (4). Given current demographic changes, the prevalence of CAA may be expected to continue rising (5). Typically, CAA manifests as lobar intracerebral hemorrhage. Other possible clinical correlates are subarachnoid or intraventricular hemorrhage. In about 10% of all cases of primary intracerebral hemorrhage (ICH), CAA is regarded as a possible cause (6). Where there is evidence of atypical, hence usually lobar bleeding, the probability rises to 30%–70% (5).
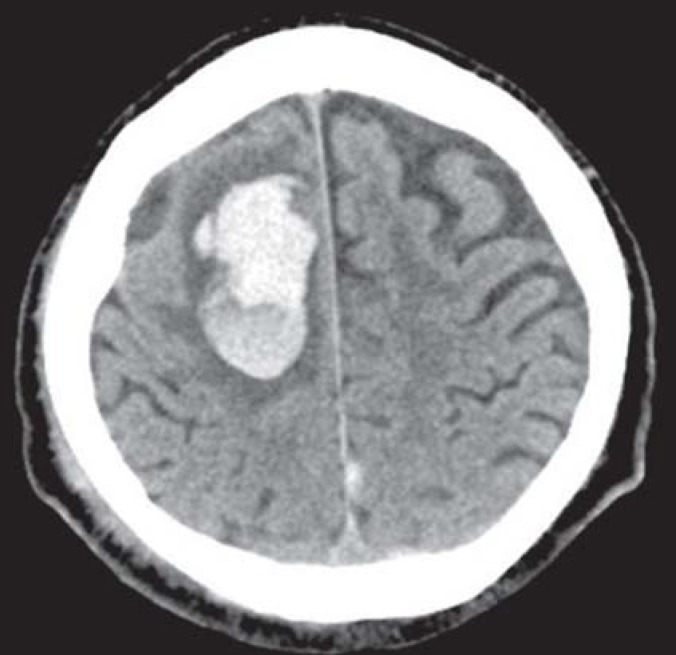
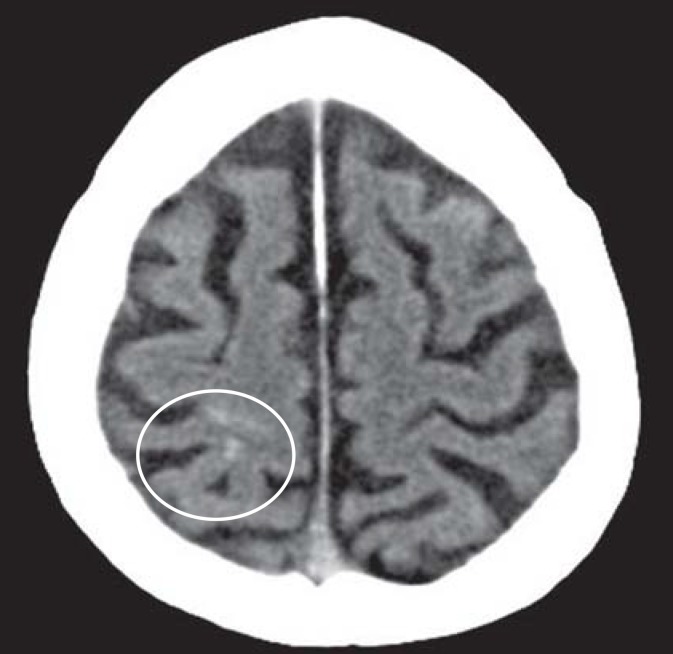
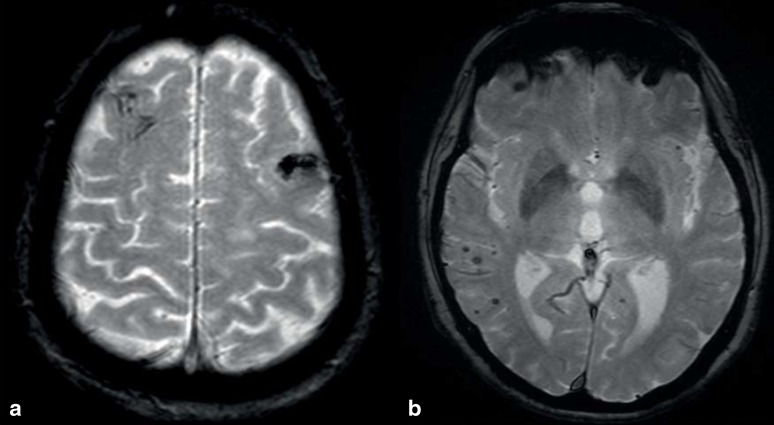
A confirmed diagnosis of CAA can only be made on the basis of biopsy or autopsy evidence of amyloid deposits in the cerebral blood vessels. CAA must be included in the differential diagnosis for older patients with spontaneous lobar or nontraumatic sulcal hemorrhage (Figures 1 and 2). In routine clinical practice, a “probable” CAA can be diagnosed on the basis of clinical and imaging findings in accordance with the modified Boston criteria (box) (7). More recent improvements in magnetic resonance imaging (MRI) technology have further improved our ability to detect traces of bleeding (6). When lobar macro- and microbleeds have been demonstrated and other causes have been excluded, the diagnosis should be classed as probable even in the absence of histopathological confirmation (box) (7). Microbleeds are a perivascular accumulation of hemosiderin-laden macrophages that have developed as a consequence of extravasation of erythrocytes from small blood vessels (8). On MRI the microbleeds appear as small round foci 2 to 5 mm in diameter, showing as hypointensity in the gradient echo sequence (T2*-weighting) (8) (figure 3).
Figure 1.
Cranial computed tomogram of a 69-year-old patient with cerebral amyloid angiopathy, showing lobar hemorrhage in an atypical location
Figure 2.
Cranial computed tomogram of a 79-year-old patient with cerebral amyloid angiopathy, showing sulcal hemorrhage (cortical, nontraumatic subarachnoid hemorrhage)
BOX. Modified Boston criteria for hemorrhage related to cerebral amyloid angiopathy (7).
-
Definite CAA (full postmortem examination demonstrating:)
Lobar, cortical, or corticosubcortical hemorrhage
Severe CAA with vasculopathy
Absence of other diagnostic lesion
-
Probable CAA with supporting pathology (clinical data and pathologic tissue demonstrating:)
Lobar, cortical, or corticosubcortical hemorrhage
Some degree of CAA in specimen
Absence of other diagnostic lesion
-
Probable CAA (clinical data and MRI or CT demonstrating:)
Multiple hemorrhages restricted to lobar, cortical, or corticosubcortical regions (cerebellar hemorrhage allowed) or
Single lobar, cortical, or corticosubcortical hemorrhage and focal (= 3 sulci) or disseminated (= 4 sulci) superficial hemosiderosis
Age = 55 years
Absence of other cause of hemorrhage or superficial siderosis
-
Possible CAA (clinical data and MRI or CT demonstrating:)
Single lobar, cortical, or corticosubcortical hemorrhage or
Focal (= 3 sulci) or disseminated (= 4 sulci) superficial hemosiderosis
Age = 55 years
Absence of other cause of hemorrhage or superficial siderosis
CT, computed tomography; MRI, magnetic resonance imaging; CAA, cerebral amyloid angiopathy;
Systematic treatment of arterial hypertension, the most important risk factor for ICH, has led to a recent drop in the incidence of ICH in patients under the age of 75 (9). In contrast to this, the incidence of ICH in patients over the age of 75 years with lobar bleeds has risen. There is much evidence to suggest that the age-dependent incidence of CAA plays an important part in this. The present article describes the special features of CAA in relation to the current treatment options in the management and secondary prevention of acute stroke.
For this purpose, a selective literature search of the PubMed and Medline databases was carried out for publications dated between 1990 and 2015, using the search terms “amyloid cerebral angiopathy,” “stroke,” “intracerebral bleeding,” and “acute stroke therapy.”
Thrombolysis
Intravenous thrombolytic therapy is regarded as an important step forward in the management of acute ischemic stroke, and has therefore come into wide use since its introduction in the 1990s. Intracranial bleeding is the worst complication of thrombolytic therapy. In 2.4% to 10% of cases, symptomatic ICH occurs within 24 to 36 hours of thrombolysis and can be disabling or even fatal, depending on its extent (10).
The main risk factors for an ICH after thrombolysis are:
Advanced age
High blood pressure
Hyperglycemia
Higher stroke severity score
Early signs of the infarct on cranial CT
A longer interval between the onset of symptoms and thrombolytic therapy
Extensive damage to the white matter (so-called vascular leukoencephalopathy or leukoaraiosis) (11).
Apart from the last, all the factors listed are associated with an increased risk of bleeding into the primary ischemic area. However, it is known from the NINDS study that in 20% of patients who suffer a thrombolysis-related ICH, the bleeding occurs outside the region of primary ischemia (12). In addition, studies on thrombolysis in patients with myocardial infarction have shown that where cerebral hemorrhage occurs as a complication, in 15% to 38% of cases multifocal bleeds occur (13, e1, e2). In addition to leukoaraiosis, cerebral microbleeds are a plausible explanation for distant or multilocular bleeds. In a retrospective analysis of 570 patients who received lysis therapy after suffering ischemic stroke, the risk of symptomatic ICH was twice as high (although still not significantly different) in those with microbleeds on MRI (5.8%) compared to those without microbleeds (2.7%) (14). A meta-analysis of this and other studies confirmed this trend (15). In addition, the meta-analysis demonstrated a significant relationship between increasing numbers of microbleeds and raised risk of ICH after thrombolysis. The association was particularly strong in patients who showed more than ten microbleeds. In a prospective study of over 700 patients, no significant correlation was identified between microbleeds and the occurrence of symptomatic ICH after thrombolysis (e3). In several studies, multilocular bleeds or bleeds outside the primary ischemic area were attributed to CAA (16, e4– e6).
No data from prospective analyses of the population with CAA are available. Studies on the relationship between microbleeds and ICH after thrombolysis did not include the distribution patterns of microbleeds and so failed to differentiate between hypertensive hemorrhages and those caused by CAA. A meta-analysis of pathologic-anatomic studies of thrombolysis-related hemorrhages demonstrated the presence of CAA in 70% of cases. This contrast with a CAA prevalence of 22% in an unselected population in the same age range (17). In a transgenic mouse model of CAA, in comparison with the wild type, an increased risk of ICH after thrombolysis was observed (18).
In case of occlusions of larger vessels, e.g., the proximal middle cerebral artery, mechanical thrombectomy in combination with systemic lysis has been shown to be superior to lytic therapy alone. In a meta-analysis of four positive studies of thrombectomy, the rate of symptomatic ICH in the intervention group was identical to that in the control group (19, e7). Although there is no specific analysis for the patient group with CAA, the results suggest a high level of certainty regarding the risk of ICH. This means that this technique can be used to treat large-vessel occlusions in patients with CAA, even if only a small subset of all stroke patients (4% to 10%) are potential candidates for this form of therapy (e8).
Taking all the studies on this topic together, the accumulated evidence is still insufficient to justify withholding thrombolytic therapy from a patient with known CAA and acute ischemic stroke, so long as all the general contraindications are observed.
Anticoagulation
Among the undesired effects of oral anticoagulation, ICH is the most feared complication as it has the highest associated morbidity and mortality. Since both CAA and atrial fibrillation are age-associated diseases, it must be assumed that these two diseases overlap. In studies of oral anticoagulation using vitamin K antagonists in patients with atrial fibrillation, the ICH rates are in the range of 0.1% to 2.5% per year (20). One hospital-based study from Cincinnati showed a rise in anticoagulation-related hemorrhage from 5% in 1988 to 17% in 1999 (21). In the over-80 age group, during the same time period the incidence of ICH increased by more than 140% (21). A connection between anticoagulation-related ICH and CAA is suggested by the finding in a large autopsy study that CAA was present in 7 out of of 11 cases where lobar bleeding had occurred during treatment with vitamin K antagonists (22), whereas CAA was not shown in a single case of nonlobar bleeding (22). In addition, ApoE2 allele, a genetic risk factor for ICH in the presence of CAA, is present significantly more often in patients who develop ICH during treatment with vitamin K antagonists than in those receiving the same treatment without experiencing an ICH (22, e9). Another pathological study also showed a correlation between the ApoE2 allele and oral anticoagulation on the one hand and increased risk of ICH on the other (e10). In that study, 56% of patients with the ApoE2 allele had an ICH, versus only 20% of those who did not have the ApoE2 allele. A meta-analysis showed that microbleeds occur significantly more often in patients with cerebral hemorrhage than in patients with transient ischemic attack (TIA) or infarct (23). This correlation was stronger in patients being treated with vitamin K antagonists (odds ratio: 2.7) than in those being given antiplatelet drugs (odds ratio: 1.7) (23). These observations suggest that microbleeds increase the risk of vitamin-K-antagonist-associated ICH.
The new oral anticoagulants (NOACs) carry a lower risk of ICH than do vitamin K antagonists (24). A prospective study has shown that NOACs—in contrast to vitamin K antagonists—do not increase the number of microbleeds (e11). To what extent NOACs bring about lower rates of ICH in patients with CAA over the long term remains unclear.
For patients with atrial fibrillation and in whom long-term oral anticoagulation is contraindicated, interventional left atrial appendage closure is a possible therapeutic option. A meta-analysis on this topic suggests that left atrial appendage closure is at least equivalent to oral anticoagulation with a vitamin K antagonist in terms of reducing the incidence of stroke (all causes) and systemic embolism (25). The incidence of cerebral hemorrhage is significantly lower after atrial appendage closure. To what extent this conclusion can be extrapolated to patients with CAA, however, remains unclear. One consideration to be borne in mind is that, after the intervention, patients need treatment with a vitamin K antagonist and acetylsalicylic acid (ASA) for 45 days, and the periprocedural risks (stroke, hemorrhage, cardiac arrhythmias) are not negligible.
Antiplatelet drugs
For over 30 years, ASA has been successfully used as an antiplatelet drug in the prevention of cardiovascular disease. In addition to gastrointestinal bleeding, regarded as the main undesired effect of this drug, intracerebral bleeding can also occur. A meta-analysis of studies of primary prevention showed only a slightly increased risk of ICH, with an odds ratio of 1.4 (26). In another meta-analysis, which included studies of both primary and secondary prevention, the odds ratio for ICH, at 1.84, was higher, but not statistically significantly so (e12). Bleeds during ASA treatment occur predominantly in lobar locations (e13). Microbleeds, too, are seen more often in patients on antiplatelet drugs than in those not taking such drugs (27). Microbleeds with a lobar distribution are more frequent during treatment with ASA than during treatment with other antiplatelet drugs (27). Microbleeds occurring during antiplatelet treatment are more often associated with ICH than with cerebral ischemia (23).
The antiplatelet drug clopidogrel has a different mechanism of action and inhibits platelet function for longer than does ASA. In a retrospective analysis, the prevalences of clopidogrel-related and ASA-related ICH respectively were shown to be comparable (28). Data from the Rotterdam study show that clopidogrel leads to a higher rate of microbleeds (29). Once cardiovascular risk factors and cardiovascular medication are taken into account, a significant association can be shown with microbleeds in deep subcortical and infratentorial locations, but not with those in lobar locations. The combination of clopidogrel with ASA increased the incidence of fatal bleeds compared to ASA monotherapy (odds ratio: 1.35) (30, e14). In a first prospective study on the risk of hemorrhage during endovascular intervention, no increase in risk of ICH was observed in patients with microbleeds in comparison to those without microbleeds (31). A limitation of the study was that the patients showed a mean of 2.3 microbleeds, and only four patients with more than five microbleeds were included.
Antihypertensive drugs
Arterial hypertension is a main risk factor for both ischemic and hemorrhagic stroke. In both primary (32) and secondary prevention (33), rigorous treatment of arterial hypertension has been shown to reduce rates of ICH. Subgroup analysis in the PROGRESS study showed that, for patients with a probable diagnosis of CAA, active treatment in comparison to placebo reduced the risk of further bleeds by 77% (34). A similar result was shown by the working group of Biffi et al. in 2015 (35), so care should also be taken to ensure rigorous blood pressure control after ICH in patients with CAA. Target values to be aimed at in treating hypertension are a systolic pressure below 140 mmHg and a diastolic pressure below 90 mmHg (35).
Statins
In the field of secondary prevention, good, robust data exist to show that statin therapy reduces the incidence of cardiovascular events in patients with cardiovascular risk factors (36). However, an inverse correlation between serum cholesterol concentration and the occurrence of ICH has also been shown (37). The SPARCL study, which demonstrated a positive effect of statin treatment in patients with a history of stroke or TIA, also showed an increased incidence of ICH in the statin arm compared to the placebo arm of the study (38). Post-hoc analysis confirmed that the risk of ICH was particularly increased when the index event for enrolment in the study was an ICH (39). A retrospective study in patients with an ICH suggested that patients being treated with statins showed more microbleeds, particularly cortico-subcortical microbleeds (e15). Multivariate analysis showed that both age and statin therapy were associated with cortico-subcortical microbleeds. This observation can be regarded as indicating a possible association with CAA. Using a decision model, it was calculated that statin therapy in patients with lobar ICH increases the recurrence rate from 14% to 22% (40). This calculation applied even to patients with lobar ICH and a history of cardiovascular events. In patients with hypertension-related ICH, only a small, nonsignificant reduction in recurrence rate was seen (36).
Taken together, these data suggest that statins increase the risk of a recurrence after ICH. When deciding whether to continue or stop statin treatment after an ICH, therefore—in addition to distinguishing between lobar and hypertension-related ICH—the clinician should take into account a prehistory of myocardial or cerebral infarction.
Figure 3.
Cranial magnetic resonance images (T2*-weighted) of two patients with cerebral amyloid angiopathy.
(a) Residues of sulcal hemorrhage (tram-track sign) and stigmata of older parenchymal hemorrhage and
(b) multiple temporo-occipitally accentuated microbleeds are seen.
Key Messages.
Arterial hypertension is an important risk factor for intracerebral hemorrhage in patients with cerebral amyloid angiopathy, and should therefore be rigorously treated.
Since both antiplatelet drugs and oral anticoagulation with vitamin K antagonists increase the risk of intracranial hemorrhage many times over, a careful risk–benefit analysis must be carried out.
Statin therapy also increases the hemorrhage risk in patients with CAA. In patients with CAA who have already experienced intracerebral hemorrhage, statins should only be given after very careful consideration of the therapeutic options.
To what extent patients with CAA and proven atrial fibrillation may benefit from treatment with a new oral anticoagulant (NOAC) or left atrial appendage closure is not conclusively known at present.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Block has received lecture fees from Bayer, Pfizer, and Böhringer Ingelheim.
Dr. Dafotakis has received lecture fees from Daiichi Sankyo and Pfizer.
References
- 1.Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am J Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- 2.McCarron MO, Nicoll JA. High frequency of apolipoprotein E epsilon 2 allele is specific for patients with cerebral amyloid angiopathy-related haemorrhage. Neurosci Lett. 1998;247:45–48. doi: 10.1016/s0304-3940(98)00286-9. [DOI] [PubMed] [Google Scholar]
- 3.Revesz T, Holton JL, Lashley T, et al. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada M. Cerebral amyloid angiopathy: an overview. Neuropathology. 2000;20:8–22. doi: 10.1046/j.1440-1789.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 5.Block F. [Zerebrale Amyloidangiopathie: Cerebral amyloid angiopathy] Nervenarzt. 2011;82:202–206. doi: 10.1007/s00115-010-3035-3. [DOI] [PubMed] [Google Scholar]
- 6.Yeh SJ, Tang SC, Tsai LK, Jeng JS. Pathogenetical subtypes of recurrent intracerebral hemorrhage: designations by SMASH-U classification system. Stroke. 2014;45:2636–2642. doi: 10.1161/STROKEAHA.114.005598. [DOI] [PubMed] [Google Scholar]
- 7.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a field guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovelock CE, Molyneux AJ, Rothwell PM. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007;6:487–493. doi: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 10.Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. J Neurol Neurosurg Psychiatry. 2008;79:1093–1099. doi: 10.1136/jnnp.2007.133371. [DOI] [PubMed] [Google Scholar]
- 11.Lansberg MG, Albers GW, Wijman CIrca. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 12.The NINDS rt-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 13.Kase CS, Pessin MS, Zivin JA, et al. Intracranial hemorrhage after coronary thrombolysis with tissue plasminogen activator. Am J Med. 1992;92:384–390. doi: 10.1016/0002-9343(92)90268-g. [DOI] [PubMed] [Google Scholar]
- 14.Fiehler J, Albers GW, Boulanger JM, et al. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38:2738–2744. doi: 10.1161/STROKEAHA.106.480848. [DOI] [PubMed] [Google Scholar]
- 15.Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke. 2013;8:348–356. doi: 10.1111/j.1747-4949.2012.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendlebury WW, Iole ED, Tracy RP, Dill BA. Intracerebral hemorrhage related to cerebral amyloid angiopathy and t-PA treatment. Ann Neurol. 1991;29:210–213. doi: 10.1002/ana.410290216. [DOI] [PubMed] [Google Scholar]
- 17.McCarron MO, Nicoll JA. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 2004;3:484–492. doi: 10.1016/S1474-4422(04)00825-7. [DOI] [PubMed] [Google Scholar]
- 18.Winkler DT, Biedermann L, Tolnay M, et al. Thrombolysis induces cerebral hemorrhage in a mouse model of cerebral amyloid angiopathy. Ann Neurol. 2002;51:790–793. doi: 10.1002/ana.10210. [DOI] [PubMed] [Google Scholar]
- 19.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 20.Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on thrombosis. Europace. 2011;13:723–746. doi: 10.1093/europace/eur126. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–121. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 22.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947–951. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 23.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 24.Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–2391. doi: 10.1161/CIRCULATIONAHA.112.115410. [DOI] [PubMed] [Google Scholar]
- 25.Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801–1807. doi: 10.1161/01.STR.0000174189.81153.85. [DOI] [PubMed] [Google Scholar]
- 27.Vernooij MW, Haag MD, van der Lugt A, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. doi: 10.1001/archneurol.2009.42. [DOI] [PubMed] [Google Scholar]
- 28.Cordina SM, Hassan AE, Ezzeddine MA. Prevalence and clinical characteristics of intracerebral hemorrhages associated with clopidogrel. J Vas Interv Neurol. 2009;2:136–138. [PMC free article] [PubMed] [Google Scholar]
- 29.Darweesh SK, Leening MJ, Akoudad S, et al. Clopidogrel use is associated with an increased prevalence of cerebral microbleeds in a stroke-free population: the Rotterdam Study. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000359. e000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palacio S, Hart RG, Pearce LA, et al. Effect of addition of clopidogrel to aspirin on stroke incidence: meta-analysis of randomized trials. Int J Stroke. 2015;10:686–691. doi: 10.1111/ijs.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soo YO, Siu DY, Abrigo J, et al. Risk of intracerebral hemorrhage in patients with cerebral microbleeds undergoing endovascular intervention. Stroke. 2012;43:1532–1536. doi: 10.1161/STROKEAHA.111.626853. [DOI] [PubMed] [Google Scholar]
- 32.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 33.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 34.Arima H, Tzourio C, Anderson C, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41:394–396. doi: 10.1161/STROKEAHA.109.563932. [DOI] [PubMed] [Google Scholar]
- 35.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314:904–912. doi: 10.1001/jama.2015.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 37.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 38.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70:2364–2370. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- 40.Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573–579. doi: 10.1001/archneurol.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Uglietta JP, O’Connor CM, Boyko OB, Aldrich H, Massey EW, Heinz ER. CT patterns of intracranial hemorrhage complicating thrombolytic therapy for acute myocardial infarction. Radiology. 1991;181:555–559. doi: 10.1148/radiology.181.2.1924804. [DOI] [PubMed] [Google Scholar]
- E2.Wijdicks EF, Jack CR Jr####. Intracerebral hemorrhage after fibrinolytic therapy for acute myocardial infarction. Stroke. 1993;24:554–557. doi: 10.1161/01.str.24.4.554. [DOI] [PubMed] [Google Scholar]
- E3. Turc G, Sallem A, Moulin S, et al. Microbleed status and 3-month outcome after intravenous thrombolysis in 717 patients with acute ischemic stroke. Stroke. 2015;46:2458–2463. doi: 10.1161/STROKEAHA.115.009290. [DOI] [PubMed] [Google Scholar]
- E4. Ramsay DA, Penswick JL, Robertson DM. Fatal streptokinase-induced intracerebral haemorrhage in cerebral amyloid angiopathy. Can J Neurol Sci. 1990;17:336–341. doi: 10.1017/s0317167100030705. [DOI] [PubMed] [Google Scholar]
- E5.Sloan MA, Price TR, Petito CK, et al. Clinical features and pathogenesis of intracerebral hemorrhage after rt-PA and heparin therapy for acute myocardial infarction: the Thrombolysis in Myocardial Infarction (TIMI) II Pilot and Randomized Clinical Trial combined experience. Neurology. 1995;45:649–658. doi: 10.1212/wnl.45.4.649. [DOI] [PubMed] [Google Scholar]
- E6.Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. 2006;37:556–561. doi: 10.1161/01.STR.0000196942.84707.71. [DOI] [PubMed] [Google Scholar]
- E7.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- E8.Fiehler J, Gerloff C. Mechanical thrombectomy in stroke. Dtsch Arztebl Int. 2015;112:830–836. doi: 10.3238/arztebl.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947–951. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- E10.McCarron MO, Nicoll JA, Ironside JW, Love S, Alberts MJ, Bone I. Cerebral amyloid angiopathy-related hemorrhage. Interaction of APOE epsilon2 with putative clinical risk factors. Stroke. 1999;30:1643–1646. doi: 10.1161/01.str.30.8.1643. [DOI] [PubMed] [Google Scholar]
- E11.Saito T, Kawamura Y, Sato N, et al. Non-vitamin k antagonist oral anticoagulants do not increase cerebral microbleeds. J Stroke Cerebrovasc Dis. 2015;24:1373–1377. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.018. [DOI] [PubMed] [Google Scholar]
- E12.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280:1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- E13.Wong KS, Mok V, Lam WW, et al. Aspirin-associated intracerebral hemorrhage: clinical and radiologic features. Neurology. 2000;54:2298–2301. doi: 10.1212/wnl.54.12.2298. [DOI] [PubMed] [Google Scholar]
- E14.Palacio S, Hart RG, Pearce LA, Benavente OR. Effect of addition of clopidogrel to aspirin on mortality: systematic review of randomized trials. Stroke. 2012;43:2157–2162. doi: 10.1161/STROKEAHA.112.656173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Haussen DC, Henninger N, Kumar S, Selim M. Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage. Stroke. 2012;43:2677–2681. doi: 10.1161/STROKEAHA.112.657486. [DOI] [PubMed] [Google Scholar]