Abstract
Background
Chronic neuropathic pain, including painful peripheral polyneuropathy and post-herpetic neuralgia, affects 6.9–10% of the general population.
Methods
In this article, we present current treatment recommendations on the basis of a selective review of the literature.
Results
Neuropathic pain does not respond consistently to classic non-opioid analgesic drugs and is better treated with co-analgesic, antidepressant, and anticonvulsant drugs and topical agents. Under certain conditions, however, neuropathic pain can be treated with opioids, even chronically. It was concluded in a large-scale meta-analysis that tricyclic antidepressants, selective serotonin-norepinephrine reuptake inhibitors, and calcium-channel anticonvulsants are the drugs of first choice, with a number needed to treat (NNT) of 3.5–7.7 for a 50% reduction of pain. An analysis of all studies yielded an estimated publication bias of 10%. Treatment planning must include adequate consideration of the patient’s age and comorbidities, concomitant medication, and potential side effects.
Conclusion
Drugs are now chosen to treat neuropathic pain independently of the cause and symptoms of the pain. Topical agents are used only to treat peripheral neuropathy. The utility of a treatment approach based on the patient’s symptoms and pathological mechanisms was recently demonstrated for the first time in a randomized trial. The goal of current research is to facilitate treatment planning on the basis of the clinical phenotype.
Pain is called neuropathic when it arises as the direct result of a disease or lesion of the central and/or peripheral somatosensory nervous system (1). The classic neuropathic pain syndromes include post-herpetic neuralgia, painful peripheral neuropathy, pain after traumatic nerve lesions, and pain due to damage of the spinal cord or brain (box 1). Patients with neuropathic pain often complain of spontaneous burning pain, painful sensitivity to touch, and pain attacks. The prevalence of chronic neuropathic pain in the general population is 6.9–10% (2). Up to 34% of persons with diabetes mellitus suffer from painful diabetic peripheral neuropathy (3).
BOX 1. Examples of neuropathic pain syndromes.
Peripheral neuropathic pain
Painful neuropathy (e.g., diabetic, alcoholic, or post-chemotherapeutic)
Radiculopathy
Traumatic nerve lesion
Post-mastectcomy, -thoracotomy, or -herniotomy syndrome (these may also be mixed neuropathic-nociceptive pain syndromes)
Central neuropathic pain
After a stroke
After a spinal cord injury
In multiple sclerosis
Mixed pain
Subgroups of patients with chronic back pain
Complex regional pain syndrome (CRPS, Sudeck’s dystrophy)
Subgroups of patients with cancer-related pain
The distinction of neuropathic from nociceptive pain is important, as these two types of pain differ fundamentally in their underlying mechanisms and therefore also in their responses to different drugs (4). Nociceptive pain arises from the “physiological” stimulation of nociceptors in an individual with an intact afferent somatosensory system (4). The causative pathological process lies in the tissue. This type of pain is predominant, for example, in osteoarthritis and rheumatoid arthritis. Aside from neuropathic and nociceptive pain, pain can also be a symptom of psychiatric or psychosomatic disease (5).
In this review, we present the basic principles of chronic neuropathic pain and the drugs used to treat it. We will not discuss trigeminal neuralgia here because of the special pathophysiological, clinical, and therapeutic considerations associated with it. (For more information on this subject, see the relevant guideline of the German Neurological Society [Deutsche Gesellschaft für Neurologie, DGN]).
Learning objectives
This article should enable readers to
understand why neuropathic pain is treated with antidepressant and anticonvulsant drugs and topical agents,
know the drugs of first choice, and
know what algorithm to follow as a guide to treatment.
Definition.
Pain is called neuropathic when it arises as the direct result of a disease or lesion of the central and/or peripheral somatosensory nervous system.
Methods
This review is based on pertinent articles retrieved by a selective search of the literature on the treatment of chronic neuropathic pain, including national guidelines and current meta-analyses (AWMF search term, “neuropathischer Schmerz”; PubMed search terms, “neuropathic pain,” “treatment,” “therapy,” “review,” “meta-analysis,” “trial”).
The mechanisms of neuropathic pain as targets for pharmacotherapy
Nerve damage has been shown to alter the neuro-physiological properties of afferent neurons (4). Spontaneous ectopic activity arises, damaged axons degenerate and regenerate, and there is heightened sensitivity to afferent stimuli. These phenomena manifest themselves clinically as spontaneous pain, thermal hyperalgesia, and pain attacks (4). Ectopic activity is induced and maintained by a number of factors, including voltage-gated neuronal sodium channels and transient receptor potential (TRP) channels (4). These channels can be modulated with drugs such as carbamazepine, lidocaine, and capsaicin, with resulting relief of pain (6).
The term “central sensitization” refers to neuronal hyperexcitability that is found mainly in the spinal cord (7). Its clinical manifestations are intensified spontaneous pain, mechanical allodynia, and hyperalgesia. Central sensitization can be modulated with drugs including gabapentin, pregabalin, and opioids, with resulting relief of pain (6).
Nociceptive impulse transmission in the spinal cord is physiologically modulated by a descending system (4).
Inhibition of the reuptake of these neurotransmitters from the synaptic cleft through the action of antidepressant drugs leads mainly to an intensification of the analgesic effect (6).
The concept of mixed pain
Central sensitization.
The term “central sensitization” refers to neuronal hyperexcitability that is found mainly in the spinal cord. Its clinical manifestations are intensified spontaneous pain, mechanical allodynia, and hyperalgesia.
The presence of a neuropathic pain component does not preclude the simultaneous presence of a nociceptive component (e.g., in diabetes mellitus: a patient can have nociceptive pain from a foot ulcer and, at the same time, painful diabetic peripheral neuropathy or cancer-related pain) (8). An estimated 16–25% of patients with back pain (with or without leg pain) have pain of both nociceptive and neuropathic origin (9). This combination has been termed “mixed pain” (box 1)—a concept that has not been validated to date by any clinically applicable gold standard. In any patient who might have either or both types of pain, evidence for neuropathic pain should be sought by meticulous history-taking and physical examination, as the proper analgesic treatment will depend on the particular type of pain that is present: opioid and non-opioid analgesics for nociceptive pain, appropriate drugs (cf. treatment recommendations) for neuropathic pain, and, possibly, a combination of both types of medication for mixed pain.
Diagnosis and classification
Mixed pain.
Pain with simultaneously present nociceptive and neuropathic components is called mixed pain.
Clear diagnostic criteria for neuropathic pain were issued in 2008 by the Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP) (1) on the basis of a revised definition of the entity. According to these criteria, neuropathic pain is definitely present when:
the pain has a neuroanatomically plausible distribution (corresponding to a peripheral or central territory of innervation or representation),
the history suggests a lesion or underlying disease that can damage the somatosensory system, and
both (1) and (2) have been securely demonstrated either clinically or by ancillary testing.
The kinds of tests available for the third criterion above include electrophysiological studies (neurography, evoked potentials) and neuroimaging studies (computed tomography, magnetic resonance imaging). For more detailed information on the diagnostic evaluation of neuropathic pain, see the German guideline on this subject (10). Neuropathic pain is distinguished from nociceptive pain on clinical grounds by the presence of hyperalgesia (increased intensity of pain) and allodynia (pain induced by ordinarily non-painful stimuli) in response to a mechanical and/or thermal stimulus. These positive symptoms are often (11) seen in combination with negative symptoms that reflect a lesion of the somatosensory system, e.g., hypesthesia.
The origin of neuropathic pain can be classified as either peripheral (e.g., peripheral neuropathy) or central types (e.g., stroke or multiple sclerosis) on the basis of the history, physical examination, and further testing if necessary. Some patients have both peripheral and central neuropathic pain. The systemic pharmacotherapy of neuropathic pain is the same regardless of its origin.
A meta-analysis of drug trials performed to date and publication bias
Clinical features of neuropathic pain.
Neuropathic pain is distinguished from nociceptive pain on clinical grounds by hyperalgesia and allodynia in response to a mechanical and/or thermal stimulus.
The most comprehensive and up-to-date meta-analysis on the treatment of chronic neuropathic pain to date appeared in Lancet Neurology in early 2015 and included 229 randomized, double-blind, placebo-controlled trials (6). It yielded the following conclusions:
Wide variations in trial methods, size (patient numbers), and quality make it difficult to compare the utility of older and newer drugs (Table).
The number needed to treat (NNT) of all first-line drugs, i.e., the number of patients who would need to be treated with a given drug so that one of them, on average, would experience a reduction of pain by at least 50%, lies in the range 3.5–7.7. No recommendation can be given for the preferential use of any particular first-line drug over any other (Table) (6).
The treatment recommendations are the same regardless of the etiology of the pain (12).
Table 1. The pharmacotherapy of neuropathic pain: number of trials, number of patients, number needed to treat, evidence levels (GRADE [27]), and common side effects (modified from [6]).
| Number of trials | Number of patients | Number needed to treat [95% CI] | Evidence level (GRADE) | Examples of common side effects (may vary depending on drug and manufacturer) | |
| Tricyclic antidepressants | 15 | 948 | 3.6 [3.0; 4.4] | High | Drowsiness, fatigue, dizziness, hypotension, weight gain |
| Serotonin-norepinephrine reuptake inhibitors | 10 | 2541 | 6.4 [5.2; 8.4] | High | Nausea, dry mouth, somnolence, headache |
| Pregabalin | 25 | 5940 | 7.7 [6.5; 9.4] | High | Drowsiness, somnolence, peripheral edema, weight gain |
| Gabapentin | 14 | 3503 | 7.2 [5.9; 9.1] | High | Somnolence, dizziness |
| Tramadol | 6 | 741 | 4.7 [3.6; 6.7] | Intermediate | Dizziness, nausea |
| High-potency opioids | 7 | 838 | 4.3 [3.4; 5.8] | Intermediate | Sedation, dizziness, headache, constipation, nausea, itch |
| Capsaicin 8% patch* | 6 | 2073 | 10.6 [7.4; 18.8] | High | Pain or erythema at the site of application |
*Only peripheral neuropathic pain. CI, confidence interval. Only evidence of high or intermediate quality was considered in the construction of this table
It should be pointed out, however, that these conclusions are based in part on assumptions of efficacy across pain syndromes that were made only by analogy. This methodological approach may have put some drugs at a disadvantage in the final assessment. For example, a review of the use of cannabinoids yielded a more positive evaluation than the meta-analysis did, though a need for further trials was mentioned in the review (13). As for some other drugs, such as carbamazepine, there is agreement that the available evidence does not clearly support a general recommendation for their use (14).
The meta-analysis also included a statistical estimate of the effect of publication bias (i.e., the tendency of trials with negative findings to remain unpublished), according to which the therapeutic benefit of drugs against neuropathic pain is likely to have been overstated by 10%. This small effect does not negate the treatment recommendations derived from the meta-analysis.
The fundamentals of treatment
Pain should be treated at once if it impairs the patient’s functioning in everyday life. The treatment options should be discussed clearly with the patient to prevent excessively high expectations and possible disappointment (box 2). Drugs can lessen neuropathic pain by 30–50% (6). Complete freedom from pain often cannot be achieved. For all types of drug, 20–40% of patients either experience less than 30% pain reduction (so-called “non-responders”) or have intolerable side effects (6). The choice of drug is independent of the etiology of neuropathic pain (12, 15– 18), but some drugs have not been tested or approved for pain of some etiologies.
BOX 2. Realistic goals for the treatment of neuropathic pain.
Reduction of pain by > 30–50%
Improved sleep
Improved quality of life
Maintenance of social activities and relationships
Recovery and maintenance of the ability to work
To improve compliance, patients should also be informed about the following before the treatment is begun:
Realistic treatment goals.
30–50% pain reduction
Better sleep
Better quality of life
Maintenance of social activity
Recovery and maintenance of ability to work
The substance classes to be used as analgesic or co-analgesic agents
The potential side effects and interactions, including impairment of attention, concentration, and ability to drive a motor vehicle
The temporal course of drug administration until the final dose is reached, and the often delayed onset of the therapeutic effect (e.g., days to weeks for antidepressant and anticonvulsant drugs).
It should be borne in mind in treatment planning that the approval status of the individual active substances may vary from one manufacturer to another. Moreover, drug treatment can be combined at any time with non-pharmacological treatments, and indeed should be if indicated. These treatments include physiotherapy, psychotherapy, and transcutaneous electrical nerve stimulation (TENS) (10).
Substance classes
Treatment options.
Drug treatment can be combined at any time with non-pharmacological treatments, and indeed should be if indicated. These treatments include physiotherapy, psychotherapy, and transcutaneous electrical nerve stimulation (TENS).
In the following sections, we list the substance classes and active agents whose use is recommended by the German Neurological Society in its current S1 guideline and by the Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP) in its meta-analysis. We indicate whenever the recommendations found in these two publications differ. We do not list the scientific evidence for the use of each drug in particular pain syndromes, as the trial data do not show the utility of differential treatment based on the underlying syndrome. We also present results from newer trials that came to our attention through our review of the literature. All of the recommendations given here are based on the findings of randomized, placebo-controlled, double-blind trials. The dosing recommendations may differ from those given in the manufacturers’ informational material for physicians and reflect the authors’ personal experience. We will not discuss the topic of manufacturer-specific approval status for particular active substances; where relevant, this should be checked by the prescribing physician.
Anticonvulsant drugs that act on neuronal calcium channels
Gabapentin
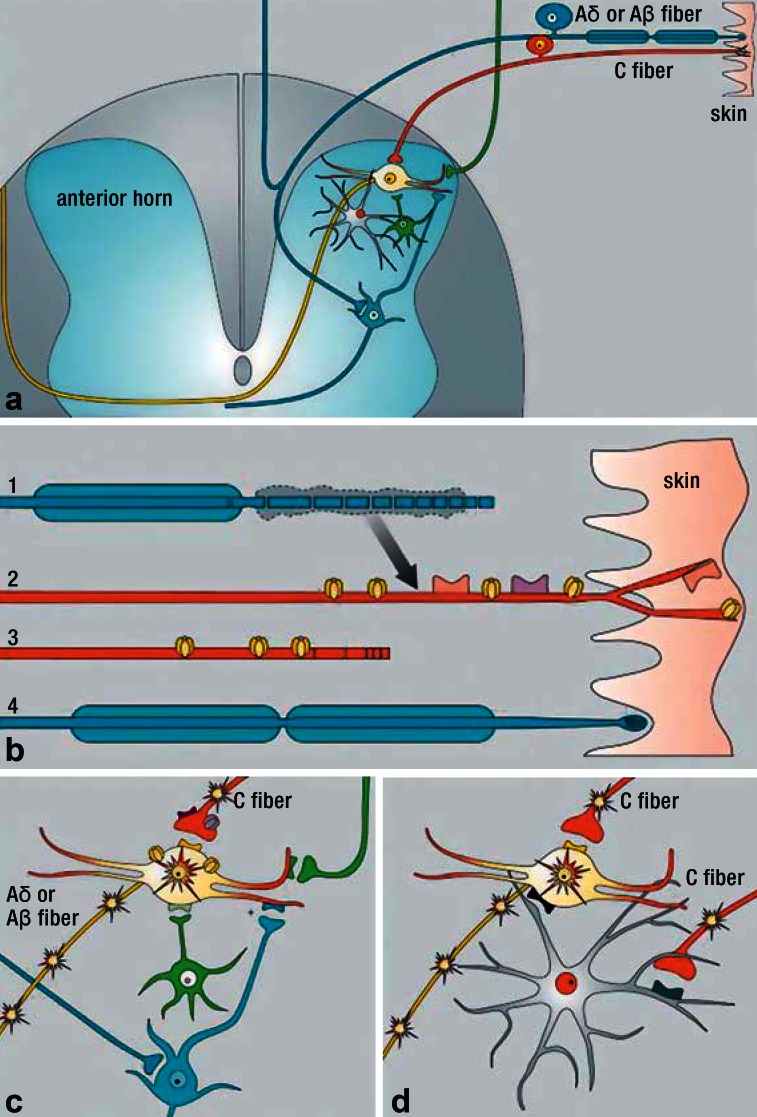
Mechanism of action: Gabapentin is presumed to act on the a2-d-subunit, thereby lessening the activating calcium influx of central neurons (Figure).
Figure 1.
Pathophysiological mechanisms of neuropathic pain (modified from Baron et al., Lancet Neurol 2010 [4])
Schematic diagram of a cross-section of the spinal cord.
Peripheral changes at primary afferent nociceptive neurons after a partial nerve lesion lead to peripheral sensitization. Some axons are damaged and degenerate (axons 1 and 3), while others remain intact (skin, axons 2 and 4). The expression of sodium channels is increased on damaged neurons (axon 3) as a consequence of the lesion. Furthermore, proalgesic substances such as nerve growth factor (NGF) are released in the setting of Wallerian degeneration (arrow) and trigger the expression of channels and receptors (e.g., sodium channels, TRPV1 receptors, and adrenoreceptors) on uninjured nociceptive fibers.
Spontaneous activity in C-nociceptors leads to spinal cord hyperexcitability (star in yellow neuron) and thereby clinically to mechanical allodynia (i.e., the activation of nociceptive pathways by normally non-painful stimuli) and mechanical hyperalgesia. Several presynaptic (opioid receptors, calcium channels) and postsynaptic structures (glutamate receptors, AMPA/kinase receptors, sodium/5HT receptors, GABA receptors, sodium channels) are involved in central sensitization. Inhibitory interneurons and the descending inhibitory system (green neurons) are dysfunctional after nerve lesions, and this leads to the disinhibition or facilitation of spinal cord dorsal horn neurons and to further central sensitization.
Peripheral nerve injury activates spinal cord glial cells (gray cells) via chemokines (e.g., CCL2) that act on chemokine receptors.
Activated microglia further sensitize WDR neurons by releasing cytokines and growth factors (e.g., tumor necrosis factor a, brain-derived neurotrophic factor) and increasing glutamate concentrations.
AMPA, aminomethylphosphonic acid; CCL2, chemokine (C-C motif) ligand 2; GABA, ?-aminobutyric acid; TRPV1, transient receptor potential V1; WDR, wide dynamic range
Dosing: The initial dose is 100 mg tid. The dose can be raised by 3 × 100 mg every three days until a total of 1200–2400 mg/d in three divided doses is reached. The maximum dose is 3600 mg/d. The dose must be adjusted in patients with impaired renal function.
Patient compliance.
To improve compliance, patients should be informed before the treatment is begun about the substance classes to be used, potential side effects and interactions, the temporal course of drug escalation, and the often delayed onset of the effect.
Recommendation: Gabapentin is recommended in both of the main publications as a first-line drug for the treatment of chronic neuropathic pain.
Pregabalin
Mechanism of action: Pregabalin binds to the a2-d-subunit of the voltage-dependent calcium channel of peripheral and central nociceptive neurons, lessening the activating calcium influx.
Dosing: The initial dose is 25, 50, or 75 mg once or twice per day and can be escalated by 50–75 mg every three or four days up to a maximum dose of 600 mg/d in two divided doses. The dose must be adjusted in patients with impaired renal function.
Recommendation: Pregabalin is recommended in both of the main publications as a first-line drug for the treatment of chronic neuropathic pain.
Antidepressant drugs
Tricyclic antidepressants (TCA) and selective serotonin-norepinephrine reuptake inhibitors (SSNRI) have both antidepressant and analgesic effects. The doses of TCA that are used to treat pain are generally lower than the effective dose for the treatment of depression.
This is not true of SSNRI, however. Their analgesic effect is derived from a potentiation of the descending nociceptive inhibitory pathways by presynaptic inhibition of the reuptake of serotonin and norepinephrine, two monoaminergic neurotransmitters. TCA also block voltage-dependent sodium channels and have sympatholytic properties.
Tricyclic antidepressants (TCA)
Dosing: Antidepressants must be given in an individually titrated dose depending on their therapeutic effect and side effects. The initial dose is 10 or 12.5 mg, or else 25 mg in a time-release preparation, given either at night (for sedating TCA) or in the morning (for drugs that have a stimulating effect).
Dose escalation: The dose can be raised by 10–25 mg every three to five days, up to a recommended maximum analgesic dose of 75 mg/d. Depending on the active substance, the drug can be given once a day as a time-release preparation or else in two or three divided doses.
Recommendation: TCA are recommended in both of the main publications as first-line drugs for the treatment of chronic neuropathic pain.
Antidepressants.
The doses of tricyclic antidepressants that are used to treat pain are generally lower than the effective dose for the treatment of depression.
Selective serotonin and norepinephrine reuptake inhibitors (SSNRI)—example: duloxetine
Dosing: The initial dose is 30 mg every morning. Dose escalation should take place over a period of 7–14 days. The target dose to be achieved initially should be 60 mg, and the maximal dose is 120 mg once per day in the morning.
Recommendation: Duloxetine is recommended in both of the main publications as a first-line drug for the treatment of chronic neuropathic pain. It has been approved in Germany for the treatment of painful diabetic polyneuropathy. Venlafaxine has not been approved for the treatment of pain in Germany.
The treatment of neuropathic pain with opioids is controversial; cf. the German S3 LONTS guideline on the long-term treatment of pain of non-malignant origin with opioids (19, 20). In the LONTS guideline, the following statements are made:
Diabetic neuropathy: “Opioid analgesics should be offered as a treatment option for 4 to 12 weeks.”
Post-herpetic neuralgia: “Opioid analgesics can be offered as a treatment option for 4 to 12 weeks.”
Phantom pain, radiculopathy, non-diabetic polyneuropathy, and pain after spinal cord injury: “Opioid analgesics should be offered as a treatment option for 4 to 12 weeks.”
Long-term opiate treatment (for 6 months or more): “Opioid analgesics can be offered as a long-term treatment option to patients with […] chronic neuropathic pain (neuropathies of various causes, post-herpetic neuralgia) who have experienced a clinically relevant reduction of pain and/or subjective physical impairment, with few or no adverse side effects, when treated with such drugs for a limited time (4–12 weeks).”
These recommendations accord with those of the DGN and the NeuPSIG/IASP: both of the latter groups also recommend low- and high-potency opioids for the treatment of chronic neuropathic pain, though only as second-line treatment (low-potency opioids) or third-line treatment (high-potency opioids) under some circumstances.
Opioid analgesics
Selective serotonin and norepinephrine reuptake inhibitors.
Duloxetine is recommended as a first-line drug for the treatment of chronic neuropathic pain. It has been approved in Germany for the treatment of painful diabetic neuropathy.
Mechanisms of action: Opioids act as agonists primarily at the µ opioid receptor. They are classified as either weak (low-potency) or strong (high-potency), depending on their intrinsic activity at the receptor. Tramadol exerts an additional effect on the descending pain-suppressing system by inhibiting the reuptake of norepinephrine and serotonin.
Therapeutic procedure: Current evidence does not support a recommendation to use opioids preferentially. “High-potency opioids are indicated only when there is an interdisciplinary consensus that the pain is resistant to curative approaches or to non-opioid drug treatment, and that low-potency opioids are ineffective or insufficiently effective. Opioids should be given as a long-acting preparation (time-release oral formulations or transdermal systems).” (10) The lowest effective dose must be found by slow titration, starting from a low dose (exception: transdermal systems are not suitable for a dose-finding procedure of this kind). High-potency opioids should not be given to opioid-naive patients as primary treatment. Monitoring for hepatic and renal toxicity with appropriate laboratory tests is recommended despite the low risk. Even under controlled opioid therapy, there is a risk of physical dependency; therefore, a psychiatric and/or psychotherapeutic evaluation is indicated before opioid treatment is begun in any patient with a history of a mental disorder.
Recommendation: Opioids can be used effectively against neuropathic pain when drugs of other types have not been effective or a more rapid onset of pain relief is needed. When opioid therapy is initiated, low-potency opioids should be given first (box 3).
BOX 3. Treatment algorithm for chronic neuropathic pain.
The following treatment algorithm is based, as regards pharmacotherapy, on the recommendations of the German Neurological Society in its S1 guideline, which are substantially the same as those found in the meta-analysis, except for treatment with topical agents (6, 10). The algorithm is intended to be universally applicable to pain of the following types:
Peripheral and/or central neuropathic pain
The neuropathic pain component of a mixed pain syndrome, independently of etiology (exception: topical agents only for peripheral neuropathy).
Step 1: Diagnostic evaluation (basis of treatment planning)
Diagnosis of definite/possible neuropathic pain?
Diagnosis of mixed neuropathic/nociceptive pain?
Tests needed to secure the diagnosis (cf. guideline on the diagnostic evaluation of neuropathic pain)?
Step 2: Causally directed treatment options
Has the cause of the underlying disturbance in the nervous system been definitively identified?
If not, then investigate the etiology further (neurological diagnostic testing; cf. guidelines on peripheral neuropathy, etc.).
If it has been, then exhaust the options for causally directed treatment (e.g., optimize glycemic control, surgically decompress compressed nerves, etc.).
Step 3: Determine the indication for pharmacotherapy
If causally directed therapy is ineffective, insufficiently effective, or unavailable, then early, adequate drug treatment is indicated and should be initiated at once if the patient is suffering from pain to a degree that interferes with his or her everyday life.
Step 4: Treatment planning
Assess previous pain treatments with regard to agent(s) used, dose, and duration (high enough dose for long enough?)
Consider comorbidities and cross-check against potential side effects
Consider concomitantly taken drugs and cross-check against potential interactions
Consider any known drug intolerance
Take the patient’s wishes into account with respect to the avoidance of particular side effects
Step 5: Patient information
Jointly formulate and agree on treatment goals
Determine the drug(s) to be used and explain their use as analgesics to the patient (this improves compliance [23])
Explain potential side effects and interactions and how they can be avoided
Explain the criteria for efficacy and inefficacy (possible latency of effect, planned duration of treatment, need for titration)
Step 6: Pharmacotherapy (based on the recommendations of the German Neurological Society)
The drugs of first choice are:
Tricyclic antidepressants [TCA]
Selective serotonin-norepinephrine reuptake inhibitors [SSNRI] (duloxetine)
Anticonvulsants (gabapentin, pregabalin)
High-dose capsaicin patch
Lidocaine patch
For intense pain, or when a rapid onset of effect is necessary: consider the indications for the additional administration of a low-potency (or, if necessary, high-potency) opioid
For mixed neuropathic and nociceptive pain: consider combination therapy with a non-opioid analgesic drug or opioid drug together with a TCA, SSNRI, anticonvulsant drug, or topical agent.
Step 7: Assessment of the response to treatment (response, partial response, no response)
Prerequisites for assessing the response to treatment: attainment of the target dose at which a therapeutic effect can be expected (cf. target doses for particular drugs, above) and regular ingestion of the target dose for at least ca. 2 weeks. The most common errors are underdosing and too short duration of treatment. Combination therapy is often needed.
Pain reduction to < 3 on the NRS (the Numerical Rating Scale, with 11 levels ranging from 0 = no pain to 10 = worst imaginable pain): continue monotherapy, consider indication for combination therapy where appropriate
Pain reduction by = 30%, but pain intensity = 4 on the NRS: combine with an additional drug of first choice
Pain reduction by < 30% and pain intensity = 4 on the NRS: the drug appears to be ineffective—switch to another drug of first choice
Check for side effects (clinical toleration of the drug; e.g., serum biochemical tests, ECG, inspection of the skin).
If intolerable side effects arise that prevent the patient from taking an effective dose of medication, switch to another drug.
If the patient is taking a clinically effective dose but continues to suffer from intolerable side effects, first lower the dose. Depending on the effects and side effects after this is done, try either switching the drug or starting combination therapy with a low dose of the original drug (even though there is no clear evidence supporting the efficacy of the latter option).
If the pain relief is still inadequate, consult a pain specialist or refer the patient to a pain center.
Step 8: The end of treatment
No data are available from clinical trials to help determine the optimal timing of dose reduction or of the discontinuation of a drug. If the patient experiences adequate pain relief over a relatively long period of time, a gradual, stepwise reduction of the dose (as in dose escalation, only backwards) can be tried at any time, particulaly because spontaneous remission of pain is possible. Most patients, however, will need to continue taking medication against neuropathic pain.
µ-opioid receptor agonist norepinephrine reuptake inhibitors
Mechanism of action: Drugs in this class (abbreviated MOR-NRI) exert a combined analgesic effect, acting as agonists at the µ receptor while simultaneously inhibiting the reuptake of norepinephrine. They do not inhibit serotonin reuptake to any major extent.
Recommendation: In clinical trials, tapentadol relieved the pain of diabetic neuropathy significantly better than placebo (6, 21). It should be used in the same way as the other morphine agonists among the high-potency opioids.
Topical treatments
Treatment options.
If the underlying lesion in the nervous system is of uncertain cause, a diagnostic evaluation should be performed to identify the etiology of the pain.
Lidocaine patch
Mechanism of action: Lidocaine patches developed especially for the treatment of pain prevent the generation of pathological nerve excitation by blocking sodium channels. Unlike conventional lidocaine patches, they do not cause cutaneous hypesthesia.
Dosing: Initial dose: apply 1–3 patches (700 mg/patch, 10 × 13 cm) to the painful area for 12 hours, with an application-free interval of at least 12 hours thereafter. The patch can be cut to a smaller size if indicated.
Drugs of first choice.
Tricyclic antidepressants
Selective serotonin-norepinephrine reuptake inhibitors (duloxetine)
Anticonvulsants (gabapentin, pregabalin)
Opioids.
Opioids can be used effectively against neuropathic pain when drugs of other types have not been effective or a more rapid onset of pain relief is needed.
Escalation: The maximum dose (3 patches in 24 hours) can be given at the first application. The patch should only be applied to intact skin.
Recommendation: In its S1 guideline, the German Neurological Society designates lidocaine patches as first-line treatment (as either monotherapy or combination therapy) against post-herpetic neuralgia, because of their favorable side-effect profile. According to the meta-analysis, lidocaine patches are a second-line option for the treatment of painful peripheral neuropathy.
High-dose capsaicin patches
µ-opioid receptor agonist norepinephrine reuptake inhibitors.
These drugs act as agonists at the µ receptor and simultaneously inhibit the reuptake of norepinephrine. They do not inhibit serotonin reuptake to any major extent.
Mechanisms of action: Capsaicin is a vanilloid receptor (TRPV1) agonist. A single application of a high-dose patch (8%) leads to reversible degeneration of nociceptive afferent fibers in the skin. Cutaneous innervation with nociceptive afferent fibers renormalizes in approximately 90 days (22).
Dosing: Up to 4 high-dose capsaicin patches (8%) (179 mg/patch, 14 × 20 cm) can be used in a single application for 30 or 60 minutes (on the feet or other parts of the body, respectively). They should be applied directly to the painful area of the skin. According to the manufacturers’ instructions, capsaicin patches cannot be used on the head or face and should only be applied to intact skin. There should be an interval of at least 90 days before any second application.
Recommendation: High-dose capsaicin patches are listed in the German guideline as a first-line treatment for the monotherapy or combination therapy of peripheral neuropathic pain. They are listed in the meta-analysis as a second-line option for the the treatment of peripheral neuropathic pain.
Lidocaine patches.
Lidocaine patches developed especially for the treatment of pain prevent the generation of pathological nerve excitation by blocking sodium channels. Unlike conventional lidocaine patches, they do not cause cutaneous hypesthesia.
The treatment of neuropathic pain in the elderly
According to the PRISCUS list of potentially inappropriate medications for elderly patients, published in 2010 (24), selective serotonin reuptake inhibitors (SSRI) or mirtazapine should be used in the elderly in preference to tricyclic antidepressants because the latter increase the risk of falls, delirium, and thromboembolic events. Nonetheless, SSRI and mirtazapine are not recommended for the treatment of neuropathic pain, as outlined above.
The concept of individualized, mechanism-oriented treatment
The assignment of different mechanisms to the different clinical manifestations of neuropathic pain has led to the concept of mechanism-oriented treatment (25). It is postulated that a determination of the individual patient’s clinical pain “phenotype” will enable identification of the underlying pathophysiological mechanisms and thus of the drug that is most likely to relieve the symptoms.
Pursuant to this concept, the German Research Network on Neuropathic Pain (Deutsche Forschungsverbund Neuropathischer Schmerzen, DFNS; www.neuropathischer-schmerz.de) has issued the LoGa classification of clinical manifestations (11). “LoGa” stands for loss and gain, i.e., negative and positive symptoms. The classification is based on the physical examination of sensation in the area of the patient’s pain.
Pharmacotherapy in the elderly.
Selective serotonin reuptake inhibitors or mirtazapine are not recommended as alternatives to tricyclic antidepressants for elderly patients with neuropathic pain.
The relevance of this classification for individualized symptom- and mechanism-oriented treatment was demonstrated recently in a randomized, placebo-controlled trial (26). Further trials are needed to validate this concept. If it should indeed prove useful, individualized treatment based on the patient’s symptoms will be possible in the near future.
Mechanism-oriented treatment.
In this concept, it is postulated that a determination of the individual patient’s clinical pain “phenotype” will enable identification of the underlying pathophysiological mechanisms and thus of the drug that is most likely to relieve the symptoms.
Supplementary Material
Case illustration
A 78-year-old woman presented with pain in the right breast. She said she had developed increasingly severe burning pain in the breast overnight, feeling like a raw wound. In the morning, she noted skin changes as well. Her past medical history included glaucoma, arterial hypertension, and depression. Her current medications were eye drops, amlodipine 10 mg qd, and citalopram 20 mg qd. The physical examination revealed a skin rash in a right T5 and T6 dermatomal distribution.
Herpes zoster (shingles) was diagnosed, and virostatic therapy with brivudine was initiated and continued
for three days. Because of her acute herpetic pain (with score of 5 on the 0–10 point NAS), a consequence of the neurocutaneous infection, metamizole 500 mg qid was prescribed as well. She returned two days later complaining of worse pain (NAS 7–8). As it is known that intense viral activity and intense acute herpetic pain both predict the development of post-herpetic neuralgia, metamizole was stopped and a tilidine/naloxone time-release preparation was prescribed instead, at an initial dose of 100 mg bid, with escalation to 200 mg bid. Tramadol was not prescribed because of the risk of additive serotonin reuptake inhibition in combination with citalopram, and a tricyclic antidepressant was not prescribed because of a possible additive anticholinergic effect in combination with the anti-glaucoma drug. This treatment lowered the intensity of pain to NAS 2–3, which the patient found tolerable.
At her planned follow-up visit four weeks after onset, her skin was well healed, with visible scars. Physical examination revealed mild allodynia to touch and a gradated superficial hypesthesia (“anesthesia dolorosa”) of the skin, both evidence of neu ropathic pain. Pregabalin was prescribed at a dose of 25 mg bid with escalation to 150 mg bid, and tilidine/naloxone was tapered off over two weeks.
She continued to take pregabalin. Three months after the rash had healed, her pain had lessened to NAS 2, but she had developed a marked allodynia to touch, so that she could only wear a loosely-fitting blouse. The elapsed time now allowed the diagnosis of post-herpetic neuralgia according to its strict definition. Combination therapy was begun with the addition of a lidocaine patch, which was applied for 12 hours per day. This led to a satisfactory reduction
of the spontaneous pain and allodynia.
Three months later, gradual lowering of the pregabalin dose led to worsening of the pain only when the dose dropped below 150 g/d. Thus, the patient’s medication could be retitrated during the first year after the emergence of the condition, in accordance with the commonly observed (partially) remitting course of post-herpetic neuralgia. An attempt to discontinue the lidocaine patch entirely was unsuccessful, but the interval between applications could be prolonged to two days. The combination therapy was continued as long-term maintenance therapy.
Further information on CME. This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 11 December 2016, and earlier CME units until the dates indicated:
The Diagnosis, Differential Diagnosis, and Treatment of Sarcoidosis” (Issue 33–34/2016) until 13 November 2016,
The Diagnosis and Treatment of Nail Disorders” (Issue 29–30/2016) until 16 October 2016,
Breastfeeding and Complementary Feeding” (Issue 25/2016) until 18 September 2016.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the most appropriate answer.
Question 1
How is neuropathic pain defined?
Neuropathic pain is pain that arises periodically while a neuroma forms.
Neuropathic pain is pain that arises as the direct consequence of a disease or lesion of the central and/or peripheral somatosensory nervous system.
Neuropathic pain is a periodically arising, unilateral pain of the musculoskeletal system.
Neuropathic pain is a paroxysmal, strictly unilateral, extremely severe type of headache that is felt mainly behind the eye and affects men and women in a ratio of 3:1.
Neuropathic pain is pain that arises during palpation of the abdomen when the examiner’s hand is suddenly pulled away.
Question 2
A patient presents to you with a complex regional pain syndrome. Which of the following is a correct designation of this patient’s pain syndrome?
Phantom pain
Purely central neuropathic pain
Mixed pain
Purely peripheral neuropathic pain
Purely nociceptive pain
Question 3
What is a realistic goal for the treatment of neuropathic pain?
Recovery and maintenance of the ability to work
10% pain reduction at most
Long-term total freedom from symptoms without analgesic medication
Complete regeneration of damaged nerve cells through electrical nerve stimulation
Short-term pain relief through physiotherapy
Question 4
Which of the following are common side effects of gabapentin?
Headache, sedation
Dry mouth, hypotension
Extrapyramidal movement disorders
Exhaustion and anorexia
Daytime sleepiness and dizziness
Question 5
What, in particular, should the patient be told about before drug treatment is begun, in order to improve compliance?
The drug’s approval status
The drug’s performance-improving effect
The possible delay before the drug takes effect
The frequency of administration
The cost of treatment
Question 6
What percentage of patients fail to respond adequately to treatment when all of the pharmacotherapeutic options have been exhausted?
0–20%
20–40%
40–60%
60–80%
80–100%
Question 7
Which of the following is the main clinical manifestation of central sensitization (i.e., hyperexcitability of the spinal cord)?
Chronic phantom pain
Decompression of nerve compression
Better sleep
Lumbar fusion
Intensified spontaneous pain
Question 8
What is the clinical result of spontaneous activity in C nociceptors?
Central sensitization
Reduction of the activating calcium influx
Mechanical allodynia and hyperalgesia
Inhibition of norepinephrine uptake
Hypesthesia
Question 9
Which of the following is a drug of first choice for the treatment of chronic neuropathic pain?
Non-opioid analgesics
Mirtazapine
Tetracyclic antidepressants
Beta-adrenoceptor antagonists
Tricyclic antidepressants
Question 10
When, as a rule, should a drug for the treament of neuropathic pain be entirely discontinued?
When pain is relieved by more than 30%
After three weeks of treatment
After a successful drug taper
When mild side effects arise
When pain is reduced to < 3 on the Numerical Rating Scale (NRS)
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. Binder has served as a paid consultant for Pfizer, Grünenthal, Astellas, and Mundipharma. He has received reimbursement of meeting participation fees and of travel and accommodation expenses from Astellas, Pfizer, and Grünenthal. He has been paid for the preparation of continuing medical education events by Astellas, Pfizer, and Grünenthal. He has received financial support from Grünenthal and Pfizer for a research project that he initiated.
Prof. Baron has served as a paid consultant for Pfizer, Genzyme, Grünenthal, Mundipharma, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Novartis, Bristol Myers-Squibb, Biogenidec, AstraZeneca, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals, Seguris, Teva, Genentech, and Galapagos.
He has received payment for authorship and co-authorship of publications on topics related to that of this article from Pfizer, Genzyme, Grünenthal, Mundipharma, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Desitin, Teva Pharma, Bayer-Schering MSD, Seguris, Novartis, Bristol Myers-Squibb, Biogenidec, AstraZeneca, Merck, Abbvie, Daiichi Sankyo, and Glenmark Pharmaceuticals.
He has received reimbursement of meeting participation fees and of travel and accommodation expenses, as well as lecture honoraria, from Pfizer, Genzyme, Grünenthal, Mundipharma, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Desitin, Teva Pharma, Bayer-Schering, MSD, and bioCSL.
He has received payment for carrying out clinical trials on behalf of Grünenthal and Pfizer. He has received financial support from Pfizer, Genzyme, Grünenthal, and Mundipharma for a research project that he initiated.
References
- 1.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 2.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Alleman CJ, Westerhout KY, Hansen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res Clin Pract. 2015;109:215–225. doi: 10.1016/j.diabres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 5.Wasner G, et al. S1-Leitlinie Diagnostik Neuropathischer Schmerzen Aus: Leitlinien für Diagnostik und Therapie in der Neurologie, Herausgegeben von der Kommission „Leitlinien“ der Deutschen Gesellschaft für Neurologie. In: Diener HC, Weimar C, editors. Stuttgart: Thieme Verlag; 2012. [Google Scholar]
- 6.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185–190. doi: 10.1007/s11916-009-0032-y. [DOI] [PubMed] [Google Scholar]
- 9.Beith ID, Kemp A, Kenyon J, Prout M, Chestnut TJ. Identifying neuropathic back and leg pain: a cross-sectional study. Pain. 2011;152:1511–1516. doi: 10.1016/j.pain.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Baron R, Binder A, Birklein F, et al. S1 Leitlinie Pharmakologisch nicht interventionelle Therapie chronisch neuropathischer Schmerzen. www.awmf.org/uploads/tx_szleitlinien/030-114l_S1_Pharmakologische_Therapie_chronisch_neuropathischer_Schmerzen_2012_1.pdf. (last accessed on 11 July 2016). [Google Scholar]
- 11.Maier C, Baron R, Tolle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndrome. Pain. 150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Finnerup NB, Sindrup SH, Jensen TS. Recent advances in pharmacological treatment of neuropathic pain F1000. Med Rep. 2 doi: 10.3410/M2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29:7–14. doi: 10.11607/ofph.1274. [DOI] [PubMed] [Google Scholar]
- 14.Wiffen PJ, Derry S, Moore RA, Kalso EA. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;4 CD005451. doi: 10.1002/14651858.CD005451.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol; 2010;17 doi: 10.1111/j.1468-1331.2010.02999.x. 1113-e88. [DOI] [PubMed] [Google Scholar]
- 16.Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 17.Haanpaa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmerzgesellschaft D, Gesellschaft DD, et al. (DEGAM) DGfAuF S3 - Leitlinie „Langzeitanwendung von Opioiden bei nicht tumorbedingten Schmerzen - „LONTS“. www.awmf.org/uploads/tx_szleitlinien/145-003l_S3_LONTS_2015-01.pdf. (last accessed on 11 July 2016) [Google Scholar]
- 20.Hauser W, Bock F, Engeser P, Tolle T, Willweber-Strumpfe A, Petzke F. Long-term opioid use in non-cancer pain. Dtsch Arztebl Int. 2014;111:732–740. doi: 10.3238/arztebl.2014.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37:2302–2309. doi: 10.2337/dc13-2291. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy WR, Vanhove GF, Lu SP, et al. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteer. J Pain. 2010;11:579–587. doi: 10.1016/j.jpain.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Matthes J, Albus C. Improving adherence with medication: a selective literature review based on the example of hypertension treatment. Dtsch Arztebl Int. 2014;111:41–47. doi: 10.3238/arztebl.2014.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5:209–227. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- 26.Demant DT, Lund K, Vollert J, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155:2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Schunemann H. [Integrative assessment of evidence in healthcare: the GRADE system] Z Evid Fortbild Qual Gesundhwes. 2009;103:261–268. doi: 10.1016/j.zefq.2009.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case illustration
A 78-year-old woman presented with pain in the right breast. She said she had developed increasingly severe burning pain in the breast overnight, feeling like a raw wound. In the morning, she noted skin changes as well. Her past medical history included glaucoma, arterial hypertension, and depression. Her current medications were eye drops, amlodipine 10 mg qd, and citalopram 20 mg qd. The physical examination revealed a skin rash in a right T5 and T6 dermatomal distribution.
Herpes zoster (shingles) was diagnosed, and virostatic therapy with brivudine was initiated and continued
for three days. Because of her acute herpetic pain (with score of 5 on the 0–10 point NAS), a consequence of the neurocutaneous infection, metamizole 500 mg qid was prescribed as well. She returned two days later complaining of worse pain (NAS 7–8). As it is known that intense viral activity and intense acute herpetic pain both predict the development of post-herpetic neuralgia, metamizole was stopped and a tilidine/naloxone time-release preparation was prescribed instead, at an initial dose of 100 mg bid, with escalation to 200 mg bid. Tramadol was not prescribed because of the risk of additive serotonin reuptake inhibition in combination with citalopram, and a tricyclic antidepressant was not prescribed because of a possible additive anticholinergic effect in combination with the anti-glaucoma drug. This treatment lowered the intensity of pain to NAS 2–3, which the patient found tolerable.
At her planned follow-up visit four weeks after onset, her skin was well healed, with visible scars. Physical examination revealed mild allodynia to touch and a gradated superficial hypesthesia (“anesthesia dolorosa”) of the skin, both evidence of neu ropathic pain. Pregabalin was prescribed at a dose of 25 mg bid with escalation to 150 mg bid, and tilidine/naloxone was tapered off over two weeks.
She continued to take pregabalin. Three months after the rash had healed, her pain had lessened to NAS 2, but she had developed a marked allodynia to touch, so that she could only wear a loosely-fitting blouse. The elapsed time now allowed the diagnosis of post-herpetic neuralgia according to its strict definition. Combination therapy was begun with the addition of a lidocaine patch, which was applied for 12 hours per day. This led to a satisfactory reduction
of the spontaneous pain and allodynia.
Three months later, gradual lowering of the pregabalin dose led to worsening of the pain only when the dose dropped below 150 g/d. Thus, the patient’s medication could be retitrated during the first year after the emergence of the condition, in accordance with the commonly observed (partially) remitting course of post-herpetic neuralgia. An attempt to discontinue the lidocaine patch entirely was unsuccessful, but the interval between applications could be prolonged to two days. The combination therapy was continued as long-term maintenance therapy.