Clinical trials of preexposure prophylaxis (PrEP) using antiretroviral medications have demonstrated success in preventing HIV infection among at-risk populations. Oral PrEP regimens containing the nucleotide and nucleoside reverse transcriptase inhibitors tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) are highly effective among adherent study participants [1–4]. Studies of dosing patterns from clinical trials indicate increased PrEP efficacy is associated with greater adherence to oral daily dosing regimens [5,6]. However, measuring adherence for persons taking oral PrEP regimens is challenging and has relied primarily on self-report [7]. Laboratory methods of determining adherence to antiretroviral drug regimens involve mass spectrometry analyses of accumulated drug metabolites in hair or blood specimens [8,9]. These assays provide reliable measures of cumulative drug exposure predictive of protection against HIV, yet are technologically complex and expensive. The availability of rapid, inexpensive, and noninvasive methods to assess adherence to PrEP regimens would allow for opportunities to provide timely feedback regarding individual adherence. TDF and FTC are metabolized and excreted primarily in urine [10], and urine tenofovir (TFV) has recently been used to indicate PrEP adherence in an individual using chewed Truvada (TDF/FTC) [11]. Therefore, we sought to determine whether urine might provide a specimen type amenable to development of noninvasive methods to measure PrEP drug exposure.
Urine and peripheral blood were collected from 23 MSM on day 8 after taking daily oral Truvada (Gilead Sciences, Inc., Foster City, California, USA) for 7 consecutive days, and 10 MSM not taking Truvada as controls. TFV and FTC were measured in blood plasma and urine specimens by liquid chromatography mass spectrometry using methodology similar to that previously described with a lower limit of quantification of 10 ng/ml [12]. Standard curves were prepared by spiking solutions of known TFV and FTC concentration into normal human urine diluted 1 : 100 in 0.2% formic acid. Participant urine specimens were diluted 1 : 100 in 0.2% formic acid to obtain measurements within the linear range of the standard curve. Intracellular metabolites tenofovir diphosphate and FTC triphosphate were measured in methanol extracts of peripheral blood mononuclear cells (PBMC) as previously described [13].
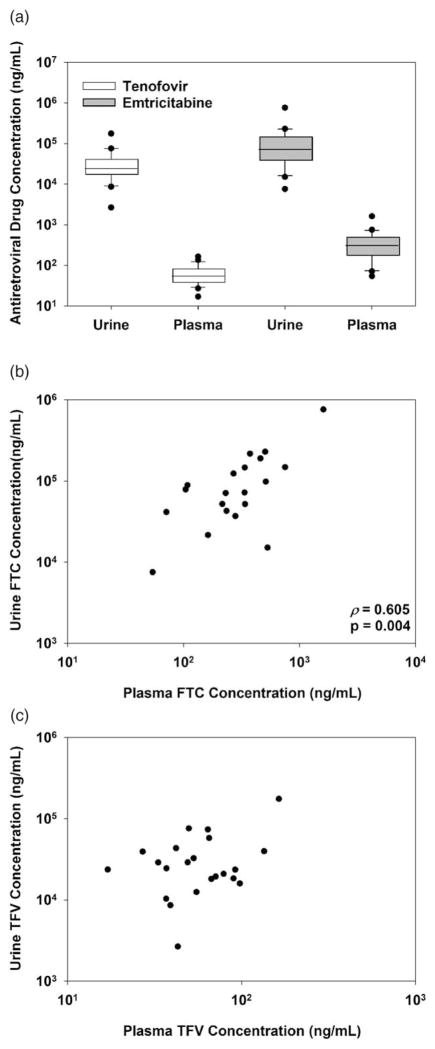
Urine TFVand FTC were detected in specimens from 22 of 23 participants prescribed daily oral Truvada and none of the 10 control participants. Blood plasma and PBMC specimens collected from the person without detectable urine TFV and FTC did not contain detectable TFV, FTC, or metabolites. Urine TFV (median: 24 000 ng/ml, range: 3000–176 000 ng/ml) and FTC (median: 72 000 ng/ml, range: 8000–761 000 ng/ml) concentrations for persons with detectable drug were much greater than plasma TFV (median: 54 ng/ml, range: 17–164 ng/ml) and FTC (median: 280 ng/ml, range: 11–1610 ng/ml) concentrations (Fig. 1a). The median ratio of urine to paired plasma concentrations for each antiretroviral drug was variable yet extremely high (TFV median: 443 : 1, range: 62 : 1–1523 : 1; FTC median: 308 : 1, range: 28 : 1–2316 : 1). Urine TFV and FTC concentrations correlated with each other (Spearman’s rank order ρ =0.625, P=0.002). Additionally, urine FTC correlated with plasma FTC concentrations (Spearman’s rank order ρ =0.605, P=0.004, Fig. 1b), but urine TFV did not correlate with plasma TFV concentrations (ρ =0.106, P>0.05, Fig. 1c). Urine TFV and FTC concentrations did not correlate with corresponding intracellular metabolites in PBMCs (P>0.05, data not shown).
Fig. 1. TFV and FTC concentrations in urine compared to plasma following seven days of oral Truvada.
TFV (white boxes) and FTC (gray boxes) concentrations were measured in urine and plasma specimens collected from MSM (a). Urine FTC concentrations correlate with plasma concentrations in paired specimens (b). TFV urine concentrations were compared with plasma concentrations in paired specimens (c). FTC, emtricitabine; TFV, tenofovir.
In this study of persons prescribed daily oral Truvada, we observed complete concordance in detection of drugs between urine and plasma, indicating detection of TFV and FTC in urine predicts detection in plasma. Similarly, absence of detectable urine drug in one study participant appears to predict lack of adherence to study medication as TFV, FTC, or their metabolites were not detected in additional specimens. We measured TFV and FTC in diluted urine specimens allowing for potential use of urine as a noninvasive specimen requiring minimal preparation prior to analysis. Microgram concentrations of TFV and FTC in urine were markedly greater than plasma concentrations observed here or in saliva as previously reported [14,15], and may allow for use of less sensitive methods for detection of antiretroviral medications. Rapid diagnostic tests are currently used to screen urine for drugs such as cocaine and opiates with limits of detection as low as 25 ng/ml [16] that are far lower than the TFV or FTC concentrations observed here. Although development of rapid detection methods able to specifically distinguish nucleoside analogs from endogenous nucleosides may be technologically challenging, high concentrations of antiretroviral drugs in urine could allow for less specific methodology to detect drug presence. Urine FTC, but not TFV, concentrations correlated to those observed in plasma, which may reflect differing metabolic properties, such as the shorter plasma half-life of FTC compared with TFV [10] or renal expression of transporters organic anion transporter 1 and multidrug resistance-associated protein 4 specifically involved in TFV excretion [17], but also suggest quantitation of urine FTC may predict plasma FTC concentrations. Urine drug concentrations did not correlate with intracellular metabolites in PBMCs that have markedly longer half-lives than those observed in plasma and accumulate to steady state levels indicative of protection [8,10]. Therefore, similar to plasma, urine may not provide accurate measurement of cumulative drug exposure, but may provide utility in detection of recent drug exposure.
The study was limited to a cross-sectional analysis of urine drug concentrations for MSM prescribed Truvada for seven days, indicative of persons adherent to daily oral PrEP dosing. Although participants were asked to take their final dose the night prior to their study visit, data were not collected regarding the time between the previous dose and specimen collection. Therefore, we were unable to define the length of time between a previous dose and lack of detection in urine. Additionally, information regarding fluid intake and urine osmolality was not collected, which may affect absolute concentrations of antiretroviral drugs. Although urine may not provide adherence measures predictive of protection similar to drug metabolites in blood, it may provide a noninvasive indicator of recent exposure similar to plasma. As oral PrEP dosing regimens continue to be evaluated in clinical trials and implemented in clinical practice, adherence to prescribed dosing is critical for PrEP efficacy. Development of rapid measures of adherence to PrEP regimens using noninvasive specimens such as urine can be used to provide individual feedback regarding adherence.
Acknowledgments
The authors thank the study participants for their time and commitment. The authors also thank Helen Koenig for helpful discussions. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention or the Department of Health and Human Services.
Financial support for this study was provided by the United States Centers for Disease Control and Prevention.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention or the Department of Health and Human Services.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. ANRS IPERGAY Study Group. Ondemand preexposure prophylaxis in men at high risk for hiv-1 infection. N Engl J Med. 2015;373:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66:340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS. 2016;11:10–17. doi: 10.1097/COH.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68:13–20. doi: 10.1097/QAI.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TRUVADA. (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use, package insert. Gilead Sciences Inc; CA, USA: Mar, 2016. [Google Scholar]
- 11.Lalley-Chareczko L, Clark D, Zuppa AF, Moorthy G, Conyngham C, Mounzer K, et al. A case study of chewed Truvada® for PrEP maintaining protective drug levels as measured by a novel urine tenofovir assay. Antivir Ther. 2017 doi: 10.3851/IMP3151. [DOI] [PubMed] [Google Scholar]
- 12.Kuklenyik Z, Martin A, Pau CP, Garcia-Lerma JG, Heneine W, Pirkle JL, et al. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J Chromatogr Sci. 2009;47:365–372. doi: 10.1093/chromsci/47.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Kuklenyik Z, Martin A, Pau CP, Holder A, Youngpairoj AS, Zheng Q, et al. Online coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J chromatogr B, Analyt Technol Biomed Life Sci. 2009;877:3659–3666. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 14.de Lastours V, Fonsart J, Burlacu R, Gourmel B, Molina JM. Concentrations of tenofovir and emtricitabine in saliva: implications for preexposure prophylaxis of oral HIV acquisition. Antimicrob Agents Chemother. 2011;55:4905–4907. doi: 10.1128/AAC.00120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonsart J, Saragosti S, Taouk M, Peytavin G, Bushman L, Charreau I, et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial. J Antimicrob Chemother. 2017;72:478–485. doi: 10.1093/jac/dkw412. [DOI] [PubMed] [Google Scholar]
- 16.Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Damron KS, et al. Protocol for accuracy of point of care (POC) or in-office urine drug testing (immunoassay) in chronic pain patients: a prospective analysis of immunoassay and liquid chromatography tandem mass spectometry (LC/MS/MS) Pain Physician. 2010;13:E1–E22. [PubMed] [Google Scholar]
- 17.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]