Abstract
Background
Most alcoholics experience periods of voluntary alcohol abstinence or imposed alcohol deprivation followed by a return to alcohol drinking. The current study examined whether varenicline (VAR) reduces alcohol intake during a return to drinking after periods of alcohol deprivation in rats selectively bred for high alcohol drinking (the alcohol preferring or “P” rats).
Methods
Alcohol-experienced P rats were given 24-hour access to food and water and scheduled access to alcohol (15% and 30% v/v) for 2 h/d. After 4 weeks, rats were deprived of alcohol for 2 weeks, followed by reaccess to alcohol for 2 weeks, and this pattern was repeated for a total of 3 cycles. Rats were fed either vehicle (VEH) or VAR, in doses of 0.5, 1.0 or 2.0 mg/kg BW, at 1 hour prior to onset of the daily alcohol reaccess period for the first 5 days of each of the 3 alcohol reaccess cycles.
Results
Low dose VAR (0.5 mg/kg BW) reduced alcohol intake during the 5 days of drug treatment in alcohol reaccess cycles 1 and 2. Higher doses of VAR (1.0 mg/kg BW and 2.0 mg/kg BW) reduced alcohol intake during the 5 days of treatment in all 3 alcohol reaccess cycles. The decrease in alcohol intake disappeared with termination of VAR treatment in all alcohol reaccess cycles.
Conclusions
The results demonstrate that VAR decreases alcohol intake during multiple cycles of alcohol reaccess following alcohol deprivation in rats and suggests that it may prevent a return to heavy alcohol drinking during a lapse from alcohol abstinence in humans with AUD.
Keywords: Varenicline, Alcohol, Alcohol-Preferring (P) Rats, Alcohol Deprivation, Alcoholism Pharmacotherapies
Addiction to alcohol is one of the most prevalent addictions in the world and prevention and treatment are of paramount importance. Alcohol use disorder (AUD), which encompasses the prior distinct disorders of alcohol abuse and alcohol dependence, is defined as a problematic pattern of alcohol use leading to clinically significant impairment or distress with at least 2 of 11 symptoms manifested within the past 12 months (American Psychiatric Association, 2013). Several characteristics are associated with AUD such as intake of alcohol that is greater, or over a longer period of time, than is intended, persistent desire to cut down alcohol use, disruption of other life obligations in order to access alcohol, craving of alcohol, and alcohol use that results in poor performance in other facets of life.
Almost all alcoholics go through periods without drinking which are either voluntary or involuntary and the vast majority of alcoholics return to alcohol drinking (Anton et al., 2006; Dawson et al., 2007; Litten et al., 2013; Schuckit et al., 1997). This pattern is colloquially called “falling off the wagon” or clinically called “relapse”. In fact, one of the strongest defining characteristics of alcoholism is alcohol relapse (Dawson et al., 2007). Relapse is motivated by a desire to reduce alcohol withdrawal signs and symptoms which can last anywhere from hours to months but are usually greatest within the first three months following discontinuation of alcohol drinking (American Addiction Centers, 2016). Although relapse is a characteristic feature of alcoholism, definitions of relapse are remarkably varied. A recent review of the literature on AUD relapse concluded that there are at least 25 different definitions of alcohol relapse (Maisto et al., 2016) Relapse ranges from being loosely defined as a return to drinking after alcohol abstinence (voluntary) or deprivation (imposed as in legal constraints) to being very strictly defined as consumption of a given amount of alcohol during a defined period of time. Although the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) refrains from defining alcohol relapse, it is widely accepted that one of the most common characteristics of AUD is a return to drinking after periods of abstinence/deprivation. Relapse rates can vary from 90%, if relapse refers to ingestion of one or more drinks (Edwards and Orford, 1977), to around 50%, if relapse refers to the return to pretreatment drinking levels (Paredes et al., 2008).
Currently only three drugs are FDA approved to treat AUD: disulfiram, acamprosate, and naltrexone. Of these, naltrexone and acamprosate are the most widely used (O’Malley and Froehlich, 2003). Several reviews and meta-analyses have reported that these drugs are only moderately effective for treating alcohol relapse since relapse rates of 83% -86% are reported in individuals treated with acamprosate (Donoghue et al., 2015; Pilling et al., 2011; Rösner et al., 2010; Sinclair et al., 2016) and relapse rates of 83% -92% are reported in individuals treated with naltrexone (Donoghue et al., 2015; Pilling et al., 2011; Sinclair et al., 2016). Identifying new medications for the treatment of AUD is of prime importance.
Varenicline (VAR) is an α4β2 nicotinic acetylcholinergic receptor partial agonist that is marketed as CHANTIX® in the United States and as CHAMPIX® in Europe and elsewhere. VAR was FDA approved for smoking cessation in 2006. Since then, evidence has accumulated that VAR also reduces alcohol intake in both rodents and humans. In rodents, VAR reduces alcohol drinking (Chatterjee et al., 2011; Froehlich et al., 2016, 2017; Lacroix et al., 2017), operant self-administration of alcohol (Wouda et al., 2011), reinstatement of extinguished alcohol seeking (Funk et al., 2016) and flavor-induced alcohol drinking (Steensland et al., 2007). In humans, VAR reduces alcohol consumption (for review see Erwin and Slaton, 2014), in both alcohol dependent (Litten et al., 2013) and alcohol non-dependent individuals (Mitchell et al., 2012) and in both smokers and non-smokers (Litten et al., 2013), although a reduction in consumption is not always seen (Schacht et al., 2014). VAR also reduces alcohol craving (Fucito et al., 2011) and the subjective reinforcing effects of alcohol (“high” or “intoxication”), and increases the likelihood of abstaining from drinking (McKee et al., 2009). VAR is a potentially attractive medication for alcohol relapse because it is effective when administered in clinically relevant doses (Faessel et al., 2006) and it lacks serious side effects in most patients (McKee et al., 2009; Stapleton et al., 2008).
Rodents that are selectively bred for high voluntary alcohol drinking, such as the alcohol-preferring (P) rat line (Froehlich and Li, 1991; Li et al., 1979), are well suited for testing the efficacy of medications with the potential to reduce alcohol intake. We have previously reported that VAR blocks the expression of a genetic predisposition toward high alcohol drinking in alcohol-naïve P rats (Froehlich et al., 2017) and reduces alcohol intake throughout prolonged periods of treatment (Froehlich et al., 2016). The current study examined whether VAR can also decrease alcohol intake in rats during alcohol reaccess following repeated cycles of alcohol deprivation, a condition commonly experienced by individuals with AUD.
MATERIALS AND METHODS
Subjects
Forty six adult male rats from the 78th generation of selective breeding for alcohol preference (P line) were purchased from the Indiana University Alcohol Research Resource Center. At the onset of the study, all rats were between 239–251 days of age. Rats were housed individually in stainless steel hanging cages that were located in an isolated vivarium with temperature controlled at 21±1°C, and with a 12 hour light/dark cycle with dark onset at 1000 hours. Standard rodent chow (Laboratory Rodent Diet #7001, Harlan Teklad, Madison, WI) and water were available ad libitum. All experimental procedures were in compliance with the NIH Guide for the Care and Use of Laboratory Animals in addition to being approved by the Indiana University Institutional Animal Care and Use Committee.
Alcohol Solutions
The 15% (v/v) and 30% (v/v) alcohol solutions were prepared by diluting 95% alcohol (ethanol) with distilled and deionized water. Two different alcohol solutions were used because studies have shown that providing P rats with multiple concentrations of alcohol increases alcohol intake (Bell et al., 2003). The two alcohol solutions were presented concurrently in separate calibrated glass drinking tubes, along with water, and daily fluid intakes were recorded to the nearest milliliter. To prevent the development of a side preference, the positions of the three drinking tubes were rotated daily. Alcohol intake in milliliters was converted from ml alcohol/kg BW to g alcohol/kg BW prior to data analysis.
Alcohol Drinking Induction
All rats were provided with access to food, water, a 15% (v/v) alcohol solution and a 30 % (v/v) alcohol solution. Alcohol access was introduced using a “step-down” procedure as previously described (Froehlich et al., 2013a, b, 2016), and illustrated in Figure 1, to maximize alcohol intake during a daily 2-hour alcohol access period. After alcohol intake stabilized during the daily 2 hour alcohol access period, all rats were given imposed alcohol deprivation for 2 weeks followed by 2 weeks of reaccess to alcohol for 2 hours a day and this cycle of alcohol access followed by alcohol deprivation was repeated for a total of 3 cycles. During alcohol access, rats were given a daily 2-hour concurrent free-choice between 2 concentrations of alcohol (15% and 30% v/v) from 1000 hours (onset of dark) to 1200 hours, 7 days a week. The following indices were recorded: daily alcohol intake (g/kg BW/2 hour) and water intake (ml/kg BW/day), and semiweekly body weight (g) for the duration of the study.
Figure 1.
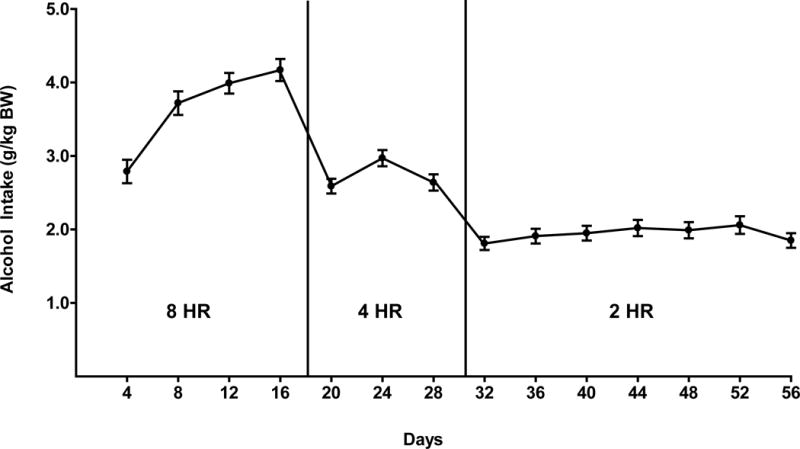
Alcohol intake in male P rats given scheduled access to (15% or 30% v/v) for 8 hours a day, for 17 days followed by 4 hours a day for 11 days, and 2 hours a day for 28 days prior to the initiation of drug treatment. Each point represents the mean ± SEM.
Choice of drug dose
VAR was used in doses of 0.5, 1.0 and 2.0 mg/kg BW. Low doses of VAR are preferable to high doses because low doses are receptor specific and do not alter food consumption in rats. VAR, like many other ligands, interacts with different receptor classes as a depending on the dose administered. The higher the dose, the less selective VAR becomes. In doses above 3.0 mg/kg, VAR may lose specificity for the α4β2 and/or the α7 nicotine acetylcholine receptors (Rollema et al., 2007) and a dose of 3.0 mg/kg VAR has been reported to decrease food intake in rats (O’Connor et al., 2010).
Drug Preparation and Oral Delivery
Varenicline tartrate (VAR) (Pfizer Int., Groton, CT), in doses of 0.5, 1.0, and 2.0 mg/kg BW, was incorporated into flavored, star-shaped pieces of gelatin that were voluntarily consumed by the rats, as previously described (Froehlich et al., 2013a, b, 2016, 2017). The volume of each gelatin star (approximately 1.8 g) was determined by the body weight of each rat on a daily basis. The gelatin star was inserted through a hole in the front of the cage. All of the rats consumed the gelatin star within 1 minute. Cages were checked to confirm that no small pieces of gelatin were dropped. If pieces were dropped, which was rare, they were refed to the rat. Gelatin stars were fed once each day at 1 hour prior to onset of the daily 2-hour alcohol access period because the half-life of VAR in the rat is short (4.0 ± 0.9 hours) (Obach et al., 2006). This oral drug delivery approach can be used for any water soluble drug that is orally active. It is well suited for chronic drug delivery because it eliminates stress and conditioned aversion to handling and eliminates the potential for harming the animal.
Assigning Rats to Treatment Groups
During the week prior to onset of the first alcohol deprivation, baseline alcohol and water intake were calculated for each rat over 5 consecutive days and the rats were ranked in descending order in terms of average daily alcohol consumption. Rats were assigned to drug dose groups (0.0, 0.5, 1.0, or 2.0 mg/kg BW) in a manner that ensured that the groups did not differ in baseline alcohol intake prior to VAR administration as previously described (Froehlich et al., 2013a, b, 2016, 2017).
Experimental Design
After completion of the alcohol step-down procedure and 4 weeks of access to alcohol for 2 hours a day, all rats were given 3 cycles of alcohol drinking and imposed alcohol deprivation and were treated with either VAR or VEH during the first 5 consecutive days of reaccess to alcohol in each of the 3 cycles. Each cycle involved alcohol deprivation for 2 weeks followed by 2 weeks of alcohol reaccess.
The effects of VAR on alcohol drinking were assessed during reaccess to alcohol following each of the 3 alcohol deprivation periods. Rats were treated with VAR or VEH for the first 5 consecutive days of each of the three reaccess cycles. The remaining days of each alcohol reaccess cycle were not accompanied by drug treatment, but alcohol access was maintained at 2 hours a day.
Data Analysis
Alcohol and water intake during the 2-hr daily alcohol reaccess periods were analyzed using 2-way repeated measures (RM) analyses of variance (ANOVAs) (treatment x day, RM on day). When sphericity could not be assumed, as indicated by a significant Mauchly’s test (p<0.05), Greenhouse-Geisser was used to correct the degrees of freedom. Significant main effects and interactions were further analyzed using Dunnett’s pairwise multiple comparisons against a single mean (VEH). To assess the stability of alcohol intake prior to and following deprivation, alcohol intake during the last 5 days prior to onset of alcohol deprivation was compared with alcohol intake on day 1 of alcohol reaccess in the VEH group using a paired student’s t-test.
To determine whether the effect of VAR persisted following termination of treatment in each of the 3 alcohol reaccess cycles, mean alcohol intake prior to alcohol deprivation was compared to mean alcohol intake on the day following termination of VAR treatment using a 1-way ANOVA.
Significance was accepted at p<0.05 (unless otherwise stated) and data are presented as mean ± SEM. Data were analyzed for extreme scores using the Dixon extreme score test with a conservative cutoff of p<0.01. No values were found to be significant outliers.
RESULTS
Alcohol Intake during Alcohol Drinking Induction
When given an 8-hour free-choice between two alcohol solutions (15% v/v and 30% v/v) and water for 17 days, rats of the P line attained an average daily alcohol intake of 4.2 g alcohol/kg BW (Fig. 1). When access to alcohol was reduced to 4 hours a day for 11 days, P rats consumed approximately 3.0 g alcohol/kg BW daily and when access to alcohol was further reduced to 2 hours a day for 27 days prior to initiation of alcohol deprivation, P rats consumed an average of 2.0 g alcohol/kg BW per day. These levels of alcohol consumption agree well with our prior findings in P rats under similar scheduled alcohol access conditions (Froehlich et al., 2013a, b, 2015, 2016).
Effect of VAR on Alcohol and Water Intake Following the First Alcohol Deprivation Cycle
With regard to alcohol intake during the first 5 days of alcohol reaccess, there was a significant effect of treatment F (3, 42) = 9.8, p<0.001, day F (4, 168) = 3.3, p<0.05, and a treatment X day interaction F (12, 168) = 1.8, p<0.05 (Fig. 2). Dunnett’s multiple comparisons against a single mean (VEH) revealed that VAR, in doses of 1.0, and 2.0 mg/kg BW, each reduced alcohol intake (p<0.01, and p<0.001, respectively), and that the lowest dose of VAR (0.5 mg/kg BW) showed a strong trend toward a reduction in alcohol intake (p=0.054) (Fig. 2). Dunnett’s multiple comparisons for each dose against a single mean (VEH) within day, revealed that the lowest dose of VAR (0.5 mg/kg BW), reduced alcohol intake only on day 2 of treatment (p<0.05). A higher dose of VAR, 1.0 mg/kg BW, reduced alcohol intake on days 2–5 of treatment (days 2 and 4; p<0.001; days 3 and 5, p<0.01), and the highest dose of VAR (2.0 mg/kg BW), reduced alcohol intake on days 2–5 of treatment (day 2, p<0.001; days 3, 4, and 5, p<0.01).
Figure 2.

(A) Effect of oral varenicline (VAR) (0.5 1.0 or 2.0mg/kg BW) or vehicle (VEH) on alcohol intake in male P rats given access to alcohol (15% or 30%) for 2 hours per day during the first alcohol reaccess cycle. (B) Effect of VAR or VEH on mean alcohol intake over the 5 days of drug treatment. **p<0.01, and ***p<0.001 versus vehicle. Each point represents mean ± SEM.
With regard to alcohol intake after termination of drug treatment, there was no effect of prior drug treatment indicating that the effect of VAR disappeared following termination of treatment.
With regard to the stability of alcohol intake prior to and following deprivation, when alcohol intake prior to alcohol deprivation (mean of 5 days) was compared to alcohol intake during reaccess to alcohol on day 1, there was no effect of treatment, a significant effect of day, F (1, 42) = 7.4, p<0.01, and no treatment X day interaction (Fig. 2).
With regard to water intake during the first 5 days of the alcohol reaccess period (Greenhouse-Geisser correction, ε=0.83), there was no effect of treatment, a significant effect of day F (3.3, 139.8) = 8.8, p<0.001, and no treatment X day interaction (Table 1).
Table 1.
Effect of Varenicline (VAR) (0.0, 0.5, 1.0, or 2.0 mg/kg BW) on mean 2-hour water intake (ml/kg BW ± SEM/2 h) in each of the 3 alcohol reaccess cycles.
| Treatment | Mean 2-hr water intake during 5 days of VAR treatment | Postdrug day 1 | |
|---|---|---|---|
| Alcohol Reaccess 1 | VEH | 1.86 ± 0.57 | 0.48 ± 0.33 |
| 0.5 mg VAR | 1.39 ± 0.40 | 0.74 ± 0.53 | |
| 1.0 mg VAR | 1.57 ± 0.33 | 0.78 ± 0.55 | |
| 2.0 mg VAR | 1.44 ± 0.22 | 1.50 ± 0.57 | |
|
| |||
| Alcohol Reaccess 2 | VEH | 1.48 ± 0.45 | 3.37 ± 2.89 |
| 0.5 mg VAR | 0.64 ± 0.21 | 1.32 ± 0.48 | |
| 1.0 mg VAR | 1.50 ± 0.35 | 1.61 ± 1.05 | |
| 2.0 mg VAR | 1.63 ± 0.29 | 1.69 ± 0.56 | |
|
| |||
| Alcohol Reaccess 3 | VEH | 1.09 ± 0.18 | 0.95 ± 0.4 |
| 0.5 mg VAR | 1.87 ± 0.31 | 0.77 ± 0.41 | |
| 1.0 mg VAR | 1.58 ± 0.36 | 1.76 ± 0.68 | |
| 2.0 mg VAR | 1.39 ± 0.26 | 2.42 ± 0.60 | |
Effect of VAR on Alcohol and Water Intake Following the Second Alcohol Deprivation Cycle
With regard to alcohol intake during the first 5 days of the alcohol reaccess period, there was a significant effect of treatment F (3, 42) = 6.4, p<0.01, no effect of day, and no treatment X day interaction (Fig. 3). Dunnett’s multiple comparisons against a single mean (VEH) revealed that VAR, in doses of 0.5, 1.0, and 2.0 mg/kg BW, each reduced alcohol intake (p<0.05, p<0.001, and p<0.01, respectively) (Fig. 3).
Figure 3.
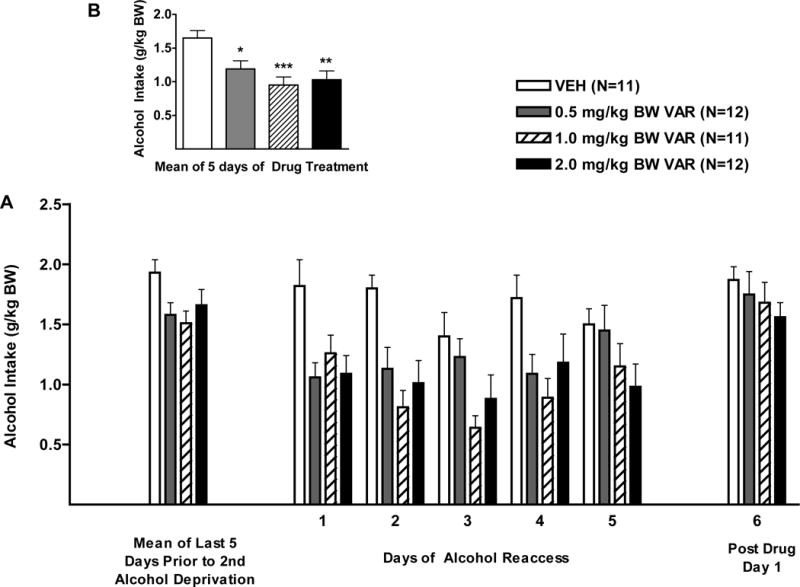
(A) Effect of oral varenicline (VAR) (0.5 1.0 or 2.0mg/kg BW) or vehicle (VEH) on alcohol intake in male P rats given access to alcohol (15% or 30%) for 2 hours per day during the second alcohol reaccess cycle. (B) Effect of VAR or VEH on mean alcohol intake over the 5 days of drug treatment. **p<0.01, and ***p<0.001 versus vehicle. Each point represents mean ± SEM.
With regard to alcohol intake after termination of drug treatment, there was no significant effect of prior treatment, indicating that the effect of VAR disappeared following termination of treatment (Fig. 3).
With regard to the stability of alcohol intake prior to and following deprivation, when alcohol intake prior to alcohol deprivation (mean of 5 days) was compared to alcohol intake during reaccess to alcohol on day 1, there was a significant effect of treatment, F (3, 42) = 5.0, p<0.01, and day F (1, 42) = 21.5, p<0.001, but no treatment X day interaction (Fig. 3A). Dunnett’s multiple comparisons against a single mean (VEH) revealed that, in the VEH-treated group, there was no change in alcohol intake on day 1 of alcohol reaccess when compared to alcohol intake prior to alcohol deprivation (Fig. 3). VAR, in doses of 0.5 and 2.0 mg/kg BW each reduced alcohol intake on day 1 of reaccess when compared to alcohol intake prior to the second deprivation (p<0.01, p<0.001, respectively), indicating a rapid onset of action of VAR (Fig. 3).
With regard to water intake during the first 5 days of the alcohol reaccess period (Greenhouse-Geisser correction, ε=0.71), there was no effect of treatment, day, and no treatment X day interaction (Table 1).
Effect of VAR on Alcohol and Water Intake Following the Third Alcohol Deprivation Cycle
With regard to alcohol intake during the first 5 days of the alcohol reaccess period, there was a significant effect of treatment F (3, 42) = 8.9, p<0.001, and day F (4, 168) = 10.1, p<0.001, but no treatment X day interaction. Dunnett’s multiple comparisons against a single mean (VEH) revealed that VAR, in doses of 1.0, and 2.0 mg/kg BW, each reduced alcohol intake (p<0.001, and p<0.05, respectively) (Fig. 4). Dunnett’s multiple comparisons for each dose against a single mean (VEH) within day, revealed that the lowest dose of VAR (0.5 mg/kg BW) did not reduce alcohol intake on any of the 5 days of drug treatment. A higher dose of VAR (1.0 mg/kg BW) reduced alcohol intake on all 5 days of treatment (days 1, 2, and 4, p<0.05; days 3 and 5, p<0.01), and the highest dose of VAR (2.0 mg/kg BW), reduced alcohol intake on days 4 and 5 of treatment (p<0.01, and p<0.05, respectively).
Figure 4.
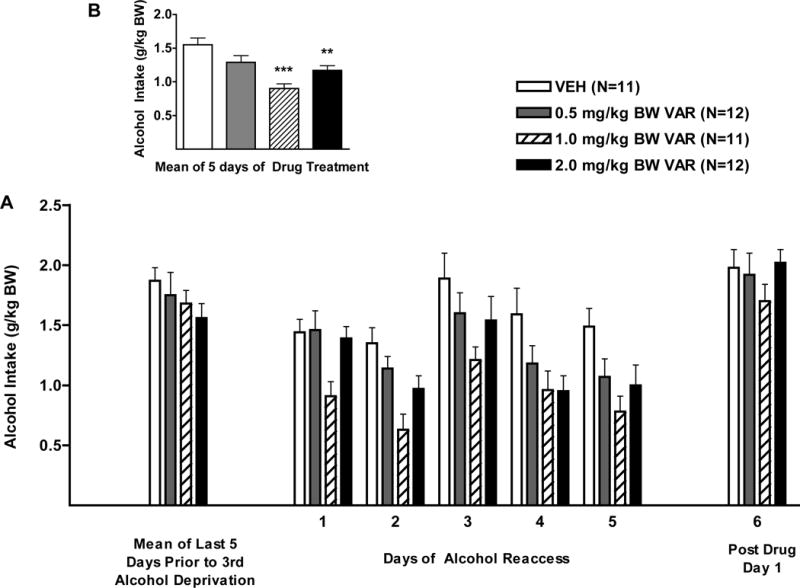
(A) Effect of oral varenicline (VAR) (0.5 1.0 or 2.0mg/kg BW) or vehicle (VEH) on alcohol intake in male P rats given access to alcohol (15% or 30%) for 2 hours per day during the third alcohol reaccess cycle. (B) Effect of VAR or VEH on mean alcohol intake over the 5 days of drug treatment. **p<0.01, and ***p<0.001 versus vehicle. Each point represents mean ± SEM.
With regard to alcohol intake after termination of drug treatment, there was no effect of treatment, a significant effect of day F (1, 41) = 6.5, p<0.05, and no treatment X day interaction. Dunnett’s multiple comparisons against a single mean (VEH) on post drug day 1 revealed that alcohol intake was significantly higher than alcohol intake prior to deprivation (p<0.05).
With regard to the stability of alcohol intake prior to and following deprivation, when alcohol intake prior to alcohol deprivation (mean of 5 days) was compared to alcohol intake during reaccess to alcohol on day 1, there was no effect of treatment, a significant effect of day F (1, 42) = 40.2, p<0.001, and a treatment X day interaction F (3, 42) = 3.1, p<0.05. Dunnett’s multiple comparisons against a single mean (VEH) revealed that, in the VEH-treated group, there was no change in alcohol intake on day 1 of alcohol reaccess when compared to alcohol intake prior to alcohol deprivation (Fig. 4). VAR, in doses of 0.5 and 1.0 mg/kg BW each reduced alcohol intake on day 1 of reaccess when compared to alcohol drinking prior to alcohol deprivation (p<0.05, and p<0.001, respectively) indicating a rapid onset of action of VAR (Fig. 4).
With regard to water intake during the first 5 days of the alcohol reaccess period (Greenhouse-Geisser correction, ε= 0.66), there was no effect of treatment, a significant effect of day F (2.6, 111.2) = 9.1, p<0.001, and no treatment X day interaction (Table 1).
DISCUSSION
Alcohol relapse is a primary concern for individuals struggling with AUD (Edwards and Orford, 1977; Paredes et al., 2008). Currently, there are only three FDA-approved medications for the treatment of AUD: disulfiram, acamprosate and naltrexone (NTX) (Williams, 2005). Disulfiram (Antibuse®) acts by inhibiting the enzyme acetaldehyde dehydrogenase which results in a buildup of acetaldehyde during alcohol drinking which is accompanied by symptoms such as nausea and headache that result in reduced medication compliance (National Center for Biotechnology Information, Disulfiram). Acamprosate (Campral®) has side effects including diarrhea, headache, and nausea which can also result in low medication compliance (Williams, 2005). Naltrexone (Trexan®, Rivia®, Vivitrol®) has fewer side effects than either disulfiram or acamprosate but it is not effective for all alcoholics (Krystal, et al., 2001). Identifying new medications for the treatment of AUD is needed and finding a medication that can prevent a return to heavy drinking during a lapse from alcohol abstinence is of particular importance since repeated relapses can prolong and strengthen withdrawal signs and symptoms which can put an individual at higher risk for subsequent relapse (Becker and Littleton 1996; Booth and Blow, 1993; Koob, 2011).
In the current study varenicline (VAR) lowered alcohol intake during alcohol reaccess following each of 3 alcohol deprivation periods. VAR in doses of 1.0 mg/kg and 2.0 mg/kg reduced alcohol intake during all 3 alcohol reaccess periods and a dose of 0.5 mg/kg reduced alcohol intake during the first 2 alcohol reaccess periods. The mechanism underlying VARs ability to reduce alcohol intake is not clear. It is well known that alcohol increases the release of dopamine (DA) in the nucleus accumbens (NAc) during the first hour following peripheral or central alcohol administration (Di Chiara and Imperato, 1988; Ericson et al., 2009) or following alcohol consumption (Weiss et al., 1993). This increased DA release is thought to mediate the reinforcing (euphoric) effects of alcohol that contribute to alcohol drinking (Di Chiara and Imperato, 1988; Koob, 1992; Samson et al., 1992; Weiss et al., 1993). VAR also releases DA in the NAc during the first hour following central administration, though to a lesser degree than does alcohol, and continues to stimulate DA release for several hours following administration (Ericson et al., 2009). However, interestingly, co-administration of VAR and alcohol into the NAc at the same time inhibits alcohol-induced DA release (Ericson et al., 2009). It is not known whether the ability of VAR to reduce alcohol intake is due to the fact that VAR blocks alcohol-induced DA release during the first hour of brain exposure to alcohol, thus reducing alcohol-induced euphoria and reducing the motivation to drink, or whether the VAR-induced stimulation of DA release at later time intervals acts through a “drug substitution” mechanism to reduce alcohol withdrawal symptoms and craving, and hence the motivation for alcohol drinking. Both of these explanations may be correct depending on the stage of the addiction process being examined since early alcohol drinking, in nonaddicted individuals, is thought to be reinforced by alcohol-induced euphoria while alcohol drinking in alcohol-dependent individuals is thought to be reinforced by alcohol-induced attenuation of withdrawal symptoms and craving.
This study illustrates the potential of VAR to reduce alcohol intake during reaccess to alcohol following alcohol deprivation but potential limitations should be considered when translating the findings. First, the subject population was rats selectively bred for high voluntary alcohol intake (alcohol-preferring or P rats). Therefore, the findings may be more relevant for individuals who are genetically predisposed toward alcohol drinking, that is, people with a family history of alcoholism (family history positive or FHP), than for the general population (for review see Begleiter and Porjesz, 1990; Porjesz and Begleiter, 1991; Rice et al., 1995). Second, side effects are a potential limitation of any medication used to treat AUD. While VAR does not have side effects that are as extensive as are those of antibuse, side effects do exist (headache, nausea, insomnia) (Drovandi et al., 2016) and are relatively comparable to those of other drugs approved for the treatment of AUD such as naltrexone (Srisurapanont and Jarusuraisin, 2005) and acamprosate (Carmen et al., 2004). Early reports of VAR-induced altered behavior, which resulted in a 2015 FDA warning regarding the use of VAR, were not substantiated and the FDA revised the warning in 2016 and stated that VAR does not pose serious mental health risks. Importantly, there appears to be minimal side effects of VAR reported in individuals with AUD (McKee et al., 2009; Mitchell et al., 2012, Verplaetse et al., 2016a, 2016b).
The evidence supporting a role for VAR in the treatment of AUD continues to accumulate. We have previously reported that, in rats, VAR reduces alcohol intake when given over prolonged periods of time (Froehlich et al., 2016), when given alone or in combination with naltrexone (Froehlich et al., 2016, 2017), when given prior to initial alcohol exposure (Froehlich et al., 2017) and, in the current study, when given during repeated cycles of alcohol reaccess following alcohol deprivation. The versatility of VAR in reducing alcohol drinking, in a variety of situations, suggests that VAR may be a flexible pharmacotherapeutic agent for the treatment of AUDs.
Acknowledgments
We thank Dr. Ting-Kai Li and the Indiana Alcohol Research Center for supplying the selectively bred rats used in this study and Pfizer International for providing the varenicline. This work was supported by NIH grants R01 AA021208 (JCF) and P60 AA007611 (JCF).
Footnotes
DR. JANICE C FROEHLICH (Orcid ID: 0000-0003-2920-6937)
There are no conflicts of interests in this manuscript.
References
- American Addiction Centers. Alcohol Withdrawal Treatment, Symptoms and Timeline. 2017 Mar 14; 2017. < http://americanaddictioncenters.org/withdrawal-timelines-treatments/alcohol/>.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Publishing; Washinton DC: 2013. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Becker HC, Littleton JM. The alcohol withdrawal “kindling” phenomenon: clinical and experimental findings. Alcohol Clin Exp Res. 1996;20:121a–124a. doi: 10.1111/j.1530-0277.1996.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Neuroelectric processes in individuals at risk for alcoholism. Alcohol and Alcoholism. 1990;25:251–256. doi: 10.1093/oxfordjournals.alcalc.a044998. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, Mcbride WJ. Effects of concurrent access to single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol Alcohol. 1993;28:593–598. [PubMed] [Google Scholar]
- Carmen B, Angeles M, Ana M, María AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. Partial agonists of the α3β4* neuronal nicotinic acetycholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology. 2011;36:603–615. doi: 10.1038/npp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. Journal of medicinal chemistry. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction. 2015;110:920–930. doi: 10.1111/add.12875. [DOI] [PubMed] [Google Scholar]
- Drovandi AD, Chen CC, Glass BD. Adverse effects cause varenicline discontinuation: A meta-analysis. Current drug safety. 2016;11:78–85. doi: 10.2174/1574886311207040282. [DOI] [PubMed] [Google Scholar]
- Edwards G, Orford J. Management of alcoholism. Lancet. 1977;10:1233–1234. doi: 10.1016/s0140-6736(77)90476-7. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Söderpalm B. The smoking cessation mediation varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–30. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Annals of Pharmacotherapy. 2014;48:1445–1455. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Smith BJ, Gibbs MA, Gobey JS, Clark DJ, Burstein AH. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. J Clin Pharmacol. 2006;46:991–998. doi: 10.1177/0091270006290669. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. FDA Drug Safety Communication: Safety review update of Chantix (varenicline) and risk of neuropsychiatric adverse events. http://www.fda.gov/Drugs/DrugSafety/ucm276737.htm. Published October 24, 2011. Accessed May 11, 2017.
- Froehlich JC, Fischer SM, Dilley JE, Nicholson ER, Smith TN, Filosa NJ, Rademacher LC. Combining varenicline (Chantix) with naltrexone decreases alcohol drinking more effectively than does either drug alone in a rodent model of alcoholism. Alcohol Clin and Exp Res. 2016;40:1961–1970. doi: 10.1111/acer.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Fischer SM, Nicholson ER, Dilley JE, Smith TN, Filosa NJ, Rademacher LC. A Combination of naltrexone + varenicline retards the expression of a genetic predisposition toward alcohol drinking. Alcohol Clin Exp Res. 2017;41:644–652. doi: 10.1111/acer.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2013a;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Fischer SM, Wise B, Rasmussen DD. Prazosin reduces alcohol intake in an animal model of alcohol relapse. Alcohol Clin Exp Res. 2015;39:1538–1546. doi: 10.1111/acer.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res. 2013b;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Li T-K. Animal models of the study of alcoholism: utility of selected lines. J Addict Dis. 1991;10:61–71. doi: 10.1300/J069v10n01_05. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Lo S, Coen K, Lê AD. Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse. Behavioural brain research. 2016;296:157–162. doi: 10.1016/j.bbr.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2011;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk WF, Rosenheck RA, Veterans Affairs Naltrexone Cooperative Study 425 Group Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lacroix F, Pettorelli A, Maddux JMN, Heidari-Jam A, Chaudhri N. Varenicline Reduces Context-Induced Relapse to Alcohol-Seeking through Actions in the Nucleus Accumbens. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-K, Lumeng L, McBride WJ, Waller MB. Progress toward a voluntary oral consumption model of alcoholism. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A double-blind, placebo- controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Roos CR, Hallgren KA, Moskal D, Wilson AD, Witkiewitz K. Do Alcohol Relapse Episodes During Treatment Predict Long-Term Outcomes? Investigating the Validity of Existing Definitions of Alcohol Use Disorder Relapse. Alcoholism: Clinical and Experimental Research. 2016;40:2180–2189. doi: 10.1111/acer.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self- administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology. 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. Disulfiram. 2016 Mar 9; n.d. < https://pubchem.ncbi.nlm.nih.gov/compound/3117#section=Top>.; Varenicline. 2016 Mar 9; n.d. < https://pubchem.ncbi.nlm.nih.gov/compound/170361#section=Information-Sources>.
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, Miller S, Coe JW. Metabolism and disposition of varenicline, a selective α4β2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug metabolism and disposition. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine- receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology. 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. In: Galanter M, editor. Recent Developments in Alcoholism XVI: Research on Alcoholism Treatment. Kluwer Academic/PlenumPublishers; New York, NY: 2003. pp. 217–245. [PubMed] [Google Scholar]
- Paredes A, Gregory D, Rundell OH, Williams HL. Drinking behavior, remission, and relapse: the Rand report revisited. Alcohol Clin Exp Res. 2008;3:3–10. doi: 10.1111/j.1530-0277.1979.tb04758.x. [DOI] [PubMed] [Google Scholar]
- Pfizer. About Chantix. 2015 < http://www.chantix.com/about-chantix>.
- Pilling S, Yesufu-Udechuku A, Taylor C, Drummond C, Guideline Development Group Diagnosis, assessment, and management of harmful drinking and alcohol dependence: summary of NICE guidance. BMj. 2011;342:d700. doi: 10.1136/bmj.d700. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacol. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. The Cochrane Library; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Haraguchi M, Hodge CW. Alcohol Self-Administration: Role of Mesolimbic Dopaminea. Annals of the New York academy of sciences. 1992;654:242–253. doi: 10.1111/j.1749-6632.1992.tb25971.x. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol- dependent individuals. Psychopharmacology. 2014;231:3799–3807. doi: 10.1007/s00213-014-3518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Bucholz KK. Periods of abstinence following the onset of alcohol dependence in 1,853 men and women. J Stud Alcohol. 1997;58:581–589. doi: 10.15288/jsa.1997.58.581. [DOI] [PubMed] [Google Scholar]
- Sinclair JM, Chambers SE, Shiles CJ, Baldwin DS. Safety and tolerability of pharmacological treatment of alcohol dependence: comprehensive review of evidence. Drug safety. 2016;39:627–645. doi: 10.1007/s40264-016-0416-y. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta- analysis of randomized controlled trials. International Journal of Neuropsychopharmacology. 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of Lowering the Dose of Varenicline on Alcohol Self-administration in Drinkers With Alcohol Use Disorders. J Addict Med. 2016a;10:166–173. doi: 10.1097/ADM.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of Varenicline Combined with High-Dose Alcohol on Craving, Subjective Intoxication, Perceptual Motor Response, and Executive Cognitive Function in Adults with Alcohol Use Disorders: Preliminary Findings. Alcohol Clin Exp Res. 2016b;40:1567–1576. doi: 10.1111/acer.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. Journal of Pharmacology and Experimental Therapeutics. 1993;267:250–258. [PubMed] [Google Scholar]
- Williams Steven H. Medications for Treating Alcohol Dependence. American Family Physician. 2005;72:9. 1775–1780. 13 March 2016. < http://www.aafp.org/afp/2005/1101/p1775.html>. [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, Van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


