Abstract
With the potential spread of bovine spongiform encephalopathy to people as a variant Creutzfeldt–Jakob disease (CJD), it becomes critical to identify cells in the periphery that carry infection. Initial work with scrapie agents suggested that B cells were central vectors for neuroinvasion. Subsequent studies indicated that B cells played an indirect role by promoting the development of follicular dendritic cells (FDCs) that accumulate abnormal prion protein (PrP). The mechanism for the role of FDCs, however, has not been clear. To further dissect potential B cell functions that contribute to neuroinvasion, we inoculated a CJD agent into mutant mice that (i) lacked B cells, (ii) had B cells unable to secrete Ig, or (iii) could secrete only IgM. Remarkably, all these mice developed disease with practically indistinguishable incubation times. The demonstration that neither immune complexes nor B cells were required for neuroinvasion from the periphery mandates a reanalysis of the accepted view of the essential role of B cells and FDC in these infections. Moreover, immune complexes were not required for the accumulation of pathologic PrP on the surface of FDCs, suggesting that PrP can bind to FDCs autonomously or by means of another factor. Wild-type mice had incubation times ≈50 days less than all mutant mice at the same peripheral doses, indicating that an intact immune system may increase agent uptake and delivery, but this condition is not essential. Specifically, the evidence to date suggests that IgG may enhance pivotal agent interactions with migratory myeloid cells.
Keywords: prion, amyloid, peripheral spread, immunoglobulin, myeloid cells
In a natural setting, Creutzfeldt–Jakob disease (CJD) and scrapie infections occur via a peripheral route. Therefore, the cellular and molecular mechanisms underlying eventual infection of the brain are of considerable interest. It is clear that these infectious agents replicate in the periphery, and that lymphoid tissues and white blood cells can carry the infectious agent (1–4). In addition, the immune system could affect clearance of the agent and/or influence the tropism or accumulation of infectivity. Accordingly, a number of recent studies have tried to pinpoint cells involved in agent replication and invasion of the central nervous system by using a series of mice deficient in various immune system-related cell types or molecules. Although these studies have confirmed a role for the immune system, interpretations have been complicated by the fact that most of the knockouts have complex phenotypes, lacking more than a single immune system cell or function.
Initially, it was proposed that B cells were essential for brain infection because B cell-deficient mice were “resistant” to scrapie, whereas mice lacking in T cells or follicular dendritic cells (FDCs) (tumor necrosis factor receptor knockout) remained susceptible, with rapid disease (5). However, this interpretation was tempered by the fact that mice with prion protein (PrP)-deficient B cells supported peripheral infection and neuroinvasion (6). These data suggested that infection of B cells per se was not required, and that B cells were unlikely to be the vector for transmission to the brain. Thus, an indirect mechanism for the contribution of B cells in scrapie pathogenesis seemed likely—for example, by promoting the development of FDCs or by means of Ab effects on other immune cells. Because it was already known that abnormal PrP accumulated in spleen FDCs (7), FDCs seemed likely candidates for agent propagation. How immobile FDCs, found only in secondary lymphoid tissues, could promote neuroinvasion has not yet been explained.
Pursuing this idea, Bruce and colleagues used bone marrow chimeras to show that bone marrow-derived cells need not express PrP for normal scrapie infection and neuroinvasion, whereas radioresistant cells do need to express PrP for infection to proceed (8). They concluded from these results that the FDC was the B cell-dependent, PrP-expressing cell, although in fact they did not exclude roles for other cells, including radioresistant macrophages. Indeed, the original studies that showed typical scrapie in tumor necrosis factor receptor-deficient mice that lack FDCs (5) argue against a requirement for FDCs, as do our results with CJD-infected, lymphotoxin β (LTβ)-deficient mice. LTβ− mice also lack FDCs but show only a minor delay in the onset of spongiform encephalopathy (9). Studies using a soluble form of the LTβ receptor to inhibit LT signaling in scrapie were interpreted differently (10), but effects could not be extended long-term to find whether FDCs were essential for disease progression. Moreover, most scrapie infectivity (>98%) in the spleen is present in the lymphoid compartment, even though most PrP staining is associated with FDCs, which are stromal cell components (11). Curiously, high levels of infectivity were found in splenic B cells but not in B cells isolated from the blood of these scrapie-infected mice, even though splenic B cells recirculate through blood every 2–3 days and white blood cell infectivity has been documented in different CJD models, including natural human disease (12, 13). Overall, a confusing picture has emerged, and it is still not certain which cells harbor infectivity, whether there are different cell subsets for agent replication and transit, how the agent enters the brain, or the precise relationship of either PrP expression or pathology to infectivity. In many instances pathologic PrP and infectivity correlate poorly in tissues (e.g., refs. 14 and 15), in transgenic mice with PrP overexpression (16), and during agent purification (e.g., refs. 17 and 18).
To further investigate these questions, we used a series of mouse strains that harbor a deletion of the Ig JH locus (JhD) (19) and either do or do not carry partially reconstituting Ig transgenes (Tgs; ref. 20). These mice are partially or completely deficient in B cell functions (see Table 1). One strain, membrane IgM construct (mIg Tg), has B cells that cannot secrete Ig; a second, membrane plus secreted IgM construct [(m+s)Ig Tg], has B cells that can secrete only IgM; and a third lacks B cells (and FDCs) altogether. We infected these mice with a “fast” strain of CJD (FU). All of the B cell-impaired mouse strains proved equally susceptible to this agent, although they all lagged control wild-type (wt) mice by ≈50 days to the onset of clinical disease. Thus, complete B cell function allows for optimal neuroinvasion but is not required for it. Nor is there a requirement for FDCs, which were absent in the B cell-deficient mice but present in the other two mutant strains. These data, taken in context of prior work, lead to a revised view of the role of immune system cells in the propagation of infection and expression of disease.
Table 1.
Summary of mutant mice used in this study
| Mouse strain | Transgene | B cells | Serum IgM |
|---|---|---|---|
| JhD | None | None | None |
| mIgTg | Vh186.2-Cμ-m | Yes | None |
| (m+s)Ig Tg | Vh186.2-Cμ-m+s | Yes | Yes |
Methods
Mice.
Mice used in this study have been described (20). Briefly they include: (i) wt Balb/CByJ mice (The Jackson Laboratory); (ii) mice without B cells (JhD); (iii) JhD mice expressing a Tg containing an IgH V region (Vh186.2) linked to a mutated IgM constant region in which the exon encoding the secreted form along with the polyadenylation site for that mRNA were deleted, yielding mice with surface IgM-positive B cells that cannot secrete Ig (mIg Tg); and (iv) JhD mice expressing a similar Tg, except that the IgM constant region is unmutated and thus can direct synthesis of both membrane and secreted IgM [(m+s)Ig Tg]. The latter mice have markedly reduced levels of IgG, but normal levels of IgM, because the Tg construct does not contain the genes for IgG expression and the JH regions of the endogenous IgH loci are deleted. The Tg mice were bred and typed for the Tg by PCR as described (20). Tg-negative littermates are homozygous for the JhD mutation and thus lack B cells; these mice were used throughout this study as the “JhD” or B cell-deficient mice, thus ensuring identical genetic backgrounds for all of the manipulated mice.
CJD Agent Infection and Tissue Analyses.
The FU agent strain of CJD (21) was passaged in CD-1 mice. Intraperitoneal inoculations were performed on the left side with 100 μl per mouse. For oral infection, mice were starved for 24 h and then fed individually with a Cheerio (General Mills, Minneapolis) freshly soaked with 25 μl of diluted brain homogenate containing ≈2 × 105 infectious doses, repeated three times weekly for 3 weeks. After clinical signs developed, mice were killed and one half of the brain was fixed for histopathology and the other half was frozen for protein analyses as described (22). Stock FU brain homogenates were titered to determine standard intracerebral infectious units (IU) as reported (21, 23) within ≈4 weeks of inoculating transgenic and control mice peripherally. Verification of intracerebral IU was necessary because we found that FU stock homogenates can lose infectivity with prolonged storage at −70°C (unpublished data). To minimize variation between experimental groups, mice were all bred to be 4–6 weeks old on the day of inoculation; the groups of mice compared here were all inoculated by the same route on the same day from the identical FU stock vial. Confirmation of clinical CJD was done by using a series of antibodies for immunocytochemistry as previously detailed (9, 22), whereas chemiluminescence was used for Western blot detection (15, 24)
Results
In the first experiment we used very low to limiting dilutions of the FU agent to accentuate potential differences between peripherally inoculated groups (9). Because the i.p. route is at least 103 less efficient than the intracerebral route with this agent (unpublished observations), ≈5 × 103 IU were inoculated i.p. into wt background and Tg mice [10 wt, 17 JhD, 9 mIg, and 9 (m+s)Ig]. In parallel, CD-1, wt, and the three transgenic groups (9–10 mice per group) were fed the same FU stock for a cumulative dose of 2 × 106 IU per mouse. As expected, wt mice inoculated i.p. became sick only after a very prolonged time, and only 9 of these 10 inoculated wt mice showed clinical signs before 450 days (mean ± SEM 404 ± 2 days). These 9 positive wt mice all went on to develop terminal disease within the next 2 weeks (419 ± 3 days), and on histologic examination exhibited marked spongiform changes in the brain with pathologic PrP deposition. Western blotting of the contralateral hemisphere further confirmed that PrP was resistant to limited protease digestion (PrP-res), an indication of its amyloid structure. In contrast, the negative wt mouse as well as all the other inoculated Tg mice killed at the same or at slightly later times showed no evidence of clinical CJD. Because 4 of these 35 Tg mice began to die or show clinical changes unrelated to CJD such as lymphomas, the rest of the Tg mice were killed between 440 and 450 days for disease analyses. None of these mice exhibited spongiform lesions or pathologic PrP in the brain either by histology or by Western blotting. In summary, there was a clear difference in the onset of neurologic disease between wt and all three Tg genotypes at limiting doses, but we were unable to determine the extent of this difference because other natural diseases began to kill the mice. Notably, Tg mice without pathology included groups that developed mature B cells and FDC (ref. 20 and see below). Additionally, although the cumulative oral dose was higher, none of five groups of mice (n = 9 each), including CD-1, wt, and the three different Tg models developed any stigmata of CJD by 450 days. Thus the oral route is even less efficient than the i.p. route with the FU agent in mice.
Previous experiments with LTβ knockout mice lacking FDC but with a longer natural lifespan than the Tg mice above showed that mice eventually displayed disease at ≥550 days with limiting i.p. doses of FU; i.e., they are not resistant to infection, but only develop brain pathology more slowly (9). The more prolonged incubation in LTβ knockout mice became prominent only at very low doses, when incubations are variable and deviate significantly from the linear dilution curve (23). To determine whether any of these new complementary Tg models were completely resistant to infection, as would be predicted for the JhD mice but not for either of the Tg mice by the prevailing model (25), we challenged wt and Tg mice i.p. with 100-fold higher doses of agent than above (≈5 × 105 IU).
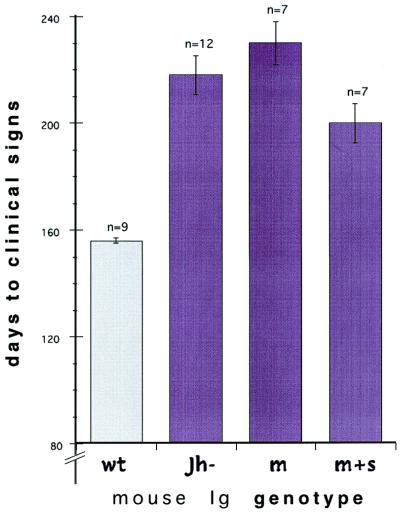
Fig. 1 shows the 44 to 74-day difference in clinical incubation time between the wt and each of the three mutant mouse groups inoculated i.p. (P ≤ 0.0001 for each mutant–wt comparison). These differences were comparable when scored for more terminal signs of disease, whereas incubation differences between transgenic JhD and mIg or (m+s)Ig groups were not significant (P = 0.22 and 0.07, respectively, ANOVA). The prolongation of incubation time in the mutant compared with wt mice was not entirely explained by B cell or FDC changes; development of B cells producing both membrane and soluble IgM, along with resultant FDC, did not shorten the incubation time compared with B cell-deficient mice. Moreover, replacement of only membrane IgM, a feature that allowed B cell development as well as FDC differentiation (see below), yielded an incubation time marginally longer than that in JhD mice bearing no FDCs (230 ± 8 versus 218 ± 7.5 days). Thus, the presence of FDCs as well as B cells did not appreciably accelerate disease, indicating that these cells have a relatively small role in the delivery of FU to the brain.
Figure 1.
Incubation in days to signs of clinical disease with higher i.p. doses of FU-CJD in wt BALB/c mice, JhD mice, JhD mice with replaced surface IgM (mIg), and JhD mice with replacement of both surface and secreted IgM (m+s)Ig. Error bars show SEM and n gives the number of mice in each group. Clinical signs in wt mice were less developed when scored versus the Tg mice, and thus the differences between wt and the other groups are probably somewhat less than graphed.
We also compared JhD- and wt-background mice inoculated intracerebrally by using two doses of the same stock homogenate. Brain inoculation of ≈500 IU showed a similar incubation in wt and JhD mice (175 ± 3 versus 188 ± 1 days, respectively, to clinical disease). Similarly, with a 100-fold dilution, wt mice developed clinical signs at 189 ± 2 versus 197 ± 1 days for JhD mice. The small increase in incubation time in JhD mice (8–13 days) was significant in both intracerebral comparisons (P < 0.01). This difference could be meaningless biologically, or alternatively might indicate a minor difference in the susceptibility of the JhD mice based on a role of B cell functions in brain pathogenesis.
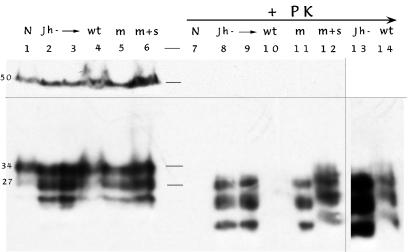
Despite differences in incubation time after i.p. inoculation between wt and Tg mice, biochemical analyses of brains revealed comparable PrP-res band patterns (Fig. 2). Samples on the left were not treated with proteinase K (lanes 1–6), whereas parallel samples 7–12 were digested. The PrP-res bands in the wt lane 10 are faint, probably because these mice were killed early, but bands were obvious with longer exposures (lane 14). Similar homogenate loads, as well as effective digestions, were routinely determined by probing the same blot with an α-tubulin antibody as shown (≈50-kDa bands in lanes 1–6 that disappear after digestion). Histologic evaluation further verified extensive disease in all of the Tg mice as shown in representative samples of i.p. inoculated animals (Fig. 3 A–C). The typical low-power distribution of pathologic PrP (red) is depicted in Fig. 3A from a mIg Tg mouse. Higher-power examination revealed spongiform lesions with abnormal PrP deposition, as shown in the hippocampus from a mIg Tg mouse and a JhD mouse (Fig. 3 B and C, respectively). Astrocytic hypertrophy and microglial changes were also comparable to those caused by the FU agent in terminally ill CD-1 mice (ref. 24 and data not shown).
Figure 2.
Western blot of brain homogenates from representative mice injected i.p. with higher doses. The band at ≈50 kDa in lanes 1–6 is α-tubulin and indicates equivalent loads in each lane. It is absent in the replicate samples treated with proteinase K (+PK, lanes 7–12). The lower portion of this same blot shows host PrP bands before digestion, with the two most prominent bands at 34 and 27 kDa. After treatment with proteinase K for PrP-res detection, these bands are reduced in size (markers indicated in lane between samples). Uninoculated normal mice (N) show no PrP-res, whereas JhD (lanes 8 and 9), mIg (lane 11), and (m+s)Ig (lane 12) samples show abundant PrP-res after short exposure. Faint bands in wt mice (as in lane 10) are seen in longer exposures of lanes 9 and 10 (shown in lanes 13 and 14). This indicated premature killing of wt mice, possibly by as much as 15–20 days. This incomplete pathology was also verified histologically (data not shown).
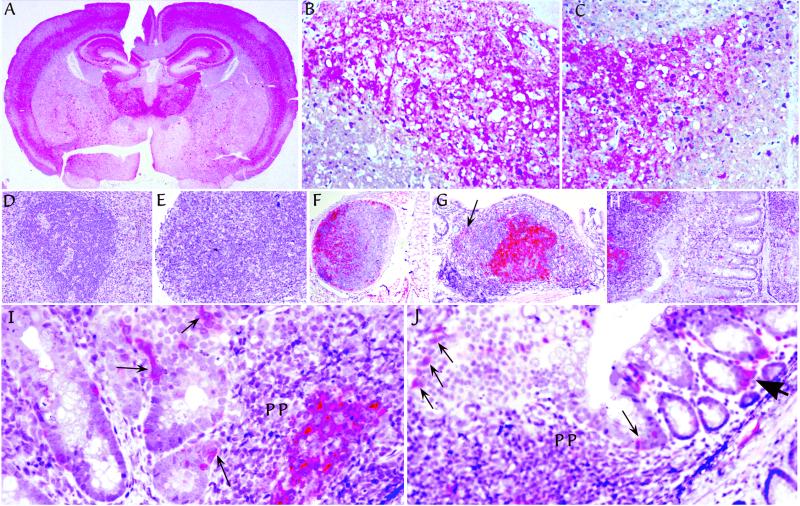
Figure 3.
Pathologic PrP (red) in tissues from representative Tg mice after i.p. infection. (A) Typical distribution after FU-CJD infection of mice. This example is from a mIg Tg mouse killed at 223 days. Comparable pathology was found in intracerebral infections with no abnormal PrP found in age-matched uninoculated controls (ref. 24 and data not shown). Higher-power examinations of comparable hippocampal regions, from a mIg Tg mouse at 236 days (B), and a JhD mouse at 212 days (C), show spongiform change with fine and coarse deposits of pathologic PrP. Typical spleen (D) and mesenteric lymph node (E) in a JhD B cell deficient mouse at 212 days show lymphoid disorganization and absence of FDCs by PrP staining. Normal FDCs were also not detectable by S-100 protein staining in JhD mice (data not shown). In contrast, FDCs have abundant pathologic PrP in lymph nodes of (m+s)Ig Tg mice, including a distant lymph node in lung at 199 days (F), and in a Peyer's patch of the ileum at 177 days (G). Arrow in G points to one of many PrP-positive small cells dispersed outside the germinal center with a dendritic-to-macrophage morphology. Pathologic PrP was equally abundant on FDCs of mIg Tg mice without secreted Ig, as shown at 223 days in Peyer's patch at the left in H. mIg Tg mice (but not JhD or uninfected mice), also showed PrP-positive cells in epithelium in the region of Peyer's patches as in I (arrows). Some of these interspersed cells are consistent with M cells. Similar M cells are seen in a row just above lymphoid cells of a Peyer's patch at low-power (thin arrows in J). J also shows labeled single cells with a dendritic morphology in the lamina propria adjacent to glands at the lumen, as well as clusters of dendritic cells (large arrowhead).
Whereas Tg mice showed no differences in brain pathology, there was a clear difference in the accumulation of pathologic PrP in lymphoid organs. As expected, mice lacking B cells had disorganized lymphoid architecture (Fig. 3 D and E). We have previously shown that FDCs can be identified by S-100 (9) and, as expected, none were detected in JhD mice stained with S-100 antibodies. FDCs were, however, obvious in all mIg and (m+s)Ig mice (ref. 20 and data not shown). Moreover, abnormal PrP that accumulates on the surface of FDCs of infected mice (9) was entirely absent in JhD mice. Fig. 3 D and E show the lack of germinal centers and the complete absence of PrP-positive FDCs in the spleen and lymph node of i.p. inoculated JhD mice. In contrast, abundant pathologic PrP accumulated on FDCs of the spleen and lymph nodes of both mIg and (m+s)Ig mice. It is likely that some of these lymph nodes had become infected by way of the bloodstream because they do not drain the peritoneum. Fig. 3F shows a lymph node in distant lung tissue with abundant pathologic PrP.
Peyer's patches in both (m+s)Ig (Fig. 3G) and mIg-only mice (Fig. 3F) were positive for abnormal PrP in contrast to parallel uninoculated controls. The FDCs in germinal centers were particularly positive, as were additional scattered myeloid cells outside the germinal centers (e.g., at arrow in Fig. 3G). These dispersed cells had a dendritic-to-macrophage morphology. In addition, scattered cells in the epithelial region overlying Peyer's patches also displayed pathologic PrP in IgM-reconstituted mice, but not in JhD mice. Some of these more superficial cells were morphologically consistent with the M cell (Fig. 3 I and J, thin arrows), a cell type dependent for its development on the presence of B cells (26). Such PrP-positive cells were not seen in uninfected controls (data not shown). Other migratory cells in the lamina propria that were also positive for abnormal PrP collected under some glands in the lamina propria (Fig. 3J, large arrowhead) and had a morphology consistent with dendritic cells. The finding of PrP positive cells in the glandular region again strongly suggested agent spread by migratory white blood cells.
Discussion
We further dissected the roles of B cells in CJD by studying mutant mice that have B cells and FDCs but do not secrete Ig at all, or that secrete only IgM. Despite B cell and FDC differences among mutants, all these groups showed essentially the same incubation time. These similar incubations establish, in contrast to previous reports on scrapie, that FDCs and B cells make only a small contribution to the peripheral spread of FU-CJD. Because our results are positive ones with obvious clinical signs, spongiform encephalopathy, and comparable pathologic PrP accumulations documented for all these mice, we believe they are definitive. This conclusion is in complete accord with previous results in LTβ-deficient mice that also lack FDCs. Only a small delay in the onset of CJD was observed in LTβ−/− mice as compared with their wt counterparts (9). LTβ−/− mice, moreover, had a genetic background (C57/Bl6) different from the BALB/c background of the currently studied mice. This further rules out other genetic differences that could have obscured an FDC effect on incubation time. Similarly, mice lacking tumor necrosis factor receptor 1, which do not have FDCs, were also susceptible to scrapie (5).
In apparent contradiction to our results, μMT mice, which also lack B cells and FDCs, were initially reported to be resistant to scrapie (5). Further studies, however, demonstrated scrapie pathology with little overt clinical disease (27), indicating that resistance was incomplete. Additionally, mice treated for 7 weeks with soluble LTβ-receptor, a treatment that blocks signaling necessary for the maintenance of FDC, showed reduced scrapie agent in the spleen during treatment (10). Nevertheless, the conclusion that low spleen or lymphoid tissue infectivity levels simply prevented agent neuroinvasion could not be documented because treatment effects were transient. Moreover, manipulations of tumor necrosis factor-family molecules that affect FDCs can have many other effects because of the wide expression of these molecules. For example, high-affinity IgG Ab responses are attenuated in LTβ-receptor treated mice. Limiting infectious doses can also magnify minor differences attributable to very extended, variable incubation times as well as to biologic inconsistencies of agent clearance (15, 23). Finally, different agent strains may preferentially use particular immune pathways or cells. Although strain differences are poorly explained by the concept of the prion, strains are well documented and different CJD agents can induce markedly different disease patterns (21, 24). Nevertheless, we clearly demonstrate progression of a peripheral CJD agent in the absence of B cells and FDCs, leading to the conclusion that the roles of these cells in promoting spongiform encephalopathy have probably been overestimated (25).
Of interest was the fact that the mIg Tg mice, with surface but not secreted IgM, accumulated pathologic PrP on FDCs in their lymphoid organs. Such accumulations were not seen in the mice lacking B cells and FDCs. These two experimental situations unlink the accumulation of PrP on FDCs to incubation time for spongiform encephalopathy.
We have recently shown by confocal microscopy that most or all of the pathologic PrP resides on the surface of FDCs (9), and data here demonstrate that antigen–antibody complexes are not required for this aggregation. FDCs are specialized for trapping antigens (Ags), a process that normally occurs after opsonization by IgG, complement fragments, or both. This allows FDCs to capture complexed Ag by means of Fc receptors and/or complement receptors. This type of capture could also enhance CJD agent-trapping within PrP–amyloid complexes at the surface of FDCs. Both mechanisms of normal Ag-trapping on FDCs typically depend on Abs; either IgG to allow binding to Fc receptors or to promote complement fixation by the classical pathway, thereby enabling C3-derivatized Ags to bind complement receptors. It is notable that ample amounts of FDC-associated PrP were seen in our mice that lacked Abs because various model protein or hapten–protein conjugate Ags are not trapped by FDCs in mIg Tg mice, even after primary or secondary immunization (20). Thus an Ab-independent mechanism must account for PrP accumulation on FDCs. One possibility is that PrP and/or its amyloid aggregates have an intrinsic ability to fix complement or to activate the alternative complement pathway, as is the case for certain bacterial and viral proteins. In this instance, trapping would be dependent, at least in part, on the complement receptor.
We also observed pathologic PrP in certain gut epithelial cells and myeloid cells in the region of Peyer's patches. Some of these PrP-stained cells in the epithelial layer are probably M cells because staining was not seen in B cell-deficient mice, which lack M cells (26). At the very least, these epithelial cells with abnormal PrP implicate a centripetal flow of infection, i.e., in the opposite direction from normal luminal invasion. Again, it is likely in this instance that macrophages and dendritic cells migrated from the bloodstream to carry the FU agent because such cells were seen in the adjacent lamina propria, which is isolated from obvious contact with nerve endings. Similarly, blood-borne infection explains most parsimoniously the involvement of lung lymph nodes after i.p. inoculation, as well as previously documented perivascular microglia with abnormal PrP (9).
The whole notion of whether specific immune system cells are required absolutely for agent replication and transit from the periphery into the brain is called into question by the partial, variable, or even inconsistent effects of different immune cell manipulations on disease progression. These inconsistencies have been explained away under the model of a specific requirement for FDCs (25). Our data argue strongly against that interpretation. An attractive alternative is that general immunocompetence is more permissive for agent replication and spread, and that the degree of immunological compromise overall will generally correspond to the degree that agent susceptibility is impaired. Many mechanisms may be involved. Indeed, complex immune functions are compromised in each of the mutant mice we studied. All lack IgG and therefore have very reduced opsonization, Fc receptor signaling, and complement fixation. Impaired Ag-presentation due to the lack of B cells in JhD mice, as well as the restricted repertoire imposed by the exclusive expression of the same VH region in every B cell of the Ig Tg mice, may also underlie the reduced efficiency of FU infection that was evident by the ≈50-day longer incubations in Tg as compared with wt mice. The concept that general immunocompetence is permissive for optimal CJD infection is further supported by both immunostimulatory and antiinflammatory studies (e.g., refs. 22, 28 and 29). Together such studies suggest that inflammation itself, inflammatory cells, and inflammatory mediators tend to promote these infections in an additive or incremental fashion.
In such a context IgG Abs may substantially propel some agent strains. IgG Abs were absent or markedly diminished in all three of the mutant strains we studied. Interestingly, IgG Abs were also reduced in all other mutants reported to delay progressive scrapie infection. It is known, moreover, that IgG binding to Ags and pathogens can markedly enhance the ability of macrophages and dendritic cells to take up Ags and pathogens by means of Fc receptors, as shown for example with Leishmania mexicana (30). Thus, IgG Abs could enhance agent uptake into myeloid cells, which may in turn heighten agent replication (22, 31). Chemoprophylaxis studies on scrapie similarly implicate a crucial role for myeloid cells in spreading peripheral infection (32).
In summary, our data help exclude the concept that B cells or FDCs are absolutely required for infection and spread to the brain. Instead, they suggest that general immune competence is required for optimal disease and that other cell types such as macrophages or dendritic cells may have a greater role in this process than is generally appreciated. The demonstration of FU infectivity in such myeloid cells (9) lends support to this idea, and we propose that IgG interactions may enhance infection of myeloid and perhaps other peripheral cells. An important role for myeloid cells in spreading infection has not been excluded by any studies to date, and further experiments targeting these cells can be used to test this hypothesis.
Acknowledgments
We thank Zhi Yun Lu and Mark Chernyak for help with blots and histochemistry, and Ann Haberman for characterizing (m+s)Ig Tg mice. This work was supported by National Institutes of Health Grants NS12674 and NS34569 (to L.M.) and AI43603 (to M.J.S.).
Abbreviations
- CJD
Creutzfeldt–Jacob disease
- FDC
follicular dendritic cell
- PrP
host prion protein
- LT
lymphotoxin
- JhD
Ig JH locus deleted
- Tg
transgene/transgenic
- mIg
membrane IgM construct
- (m+s)Ig
membrane plus secreted IgM construct
- FU
“fast” strain of CJD
- wt
wild type
- IU
infectious units
- PrP-res
PrP resistant to limited protease digestion
- Ag
antigen
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Eklund C M, Kennedy R C, Hadlow W J. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Manuelidis E E, Gorgacz E J, Manuelidis L. Science. 1978;200:1069–1071. doi: 10.1126/science.349691. [DOI] [PubMed] [Google Scholar]
- 3.Diringer H. Arch Virol. 1984;82:105–109. doi: 10.1007/BF01309373. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda Y, Gibbs C, Amyx H, Gajdusek D. Infect Immun. 1983;41:154–161. doi: 10.1128/iai.41.1.154-161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein M A, Frigg R, Flechsig E, Raeber A J, Kalinke U, Bluethmanns H, Bootz F, Suter M, Zinkernagel R M, Aguzzi A. Nature (London) 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 6.Klein M A, Frigg R, Raeber A J, Flechsig E, Hegyi I, Zinkernagel R M, Weissmann C, Aguzzi A. Nat Med. 1998;4:1429–1433. doi: 10.1038/4022. [DOI] [PubMed] [Google Scholar]
- 7.Kitamoto T, Muramoto T, Mohri S, Doh-Ura K, Tateishi J. J Virol. 1991;65:6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown K L, Stewart K, Ritchie D L, Mabbott N A, Williams A, Fraser H, Morrison W I, Bruce M E. Nat Med. 1999;5:1308–1312. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 9.Manuelidis L, Zaitsev I, Koni P, Lu Z-Y, Flavell R, Fritch W. J Virol. 2000;74:8614–8622. doi: 10.1128/jvi.74.18.8614-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montrasio F, Frigg R, Glatzel M, Klein M, Mackay F, Aguzzi A, Weissmann C. Science. 2000;288:1257–1259. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- 11.Raeber A, Klein M, Frigg R, Flechsig E, Aguzzi A, Weissmann C. EMBO J. 1999;18:2702–2706. doi: 10.1093/emboj/18.10.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuelidis E E, Kim J H, Mericangas J R, Manuelidis L. Lancet. 1985;ii:896–897. doi: 10.1016/s0140-6736(85)90165-5. [DOI] [PubMed] [Google Scholar]
- 13.Tateishi J. Lancet. 1985;ii:1074. doi: 10.1016/s0140-6736(85)90949-3. [DOI] [PubMed] [Google Scholar]
- 14.Xi Y G, Ingrosso A, Ladogana A, Masullo C, Pocchiari M. Nature (London) 1992;356:598–601. doi: 10.1038/356598a0. [DOI] [PubMed] [Google Scholar]
- 15.Manuelidis L, Fritch W. Virology. 1996;215:46–59. doi: 10.1006/viro.1996.0033. [DOI] [PubMed] [Google Scholar]
- 16.Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 17.Manuelidis L, Sklaviadis T, Akowitz A, Fritch W. Proc Natl Acad Sci USA. 1995;92:5124–5128. doi: 10.1073/pnas.92.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riesner D, Kellings K, Post K, Wille H, Serban H, Groth D, Baldwin M A, Prusiner S B. J Virol. 1996;70:1714–1722. doi: 10.1128/jvi.70.3.1714-1722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Trounstine M, Alt F W, Young F, Kurahara C, Loring J F, Huszar D. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 20.Hannum L G, Haberman A M, Anderson S E, Shlomchik M J. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuelidis L. Proc Natl Acad Sci USA. 1998;95:2520–2525. doi: 10.1073/pnas.95.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuelidis L, Fritch W, Xi Y G. Science. 1997;277:94–98. doi: 10.1126/science.277.5322.94. [DOI] [PubMed] [Google Scholar]
- 23.Manuelidis L, Sklaviadis T, Manuelidis E E. EMBO J. 1987;6:341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manuelidis L, Lu Z Y. Neurosci Lett. 2000;293:163–166. doi: 10.1016/s0304-3940(00)01514-7. [DOI] [PubMed] [Google Scholar]
- 25.Bruce M E, Brown K L, Mabbott N A, Farquhar C F, Jeffrey M. Immunol Today. 2000;21:442–446. doi: 10.1016/s0167-5699(00)01696-0. [DOI] [PubMed] [Google Scholar]
- 26.Golovkina T V, Shlomchik M, Hannum L, Chervonsky A. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 27.Frigg R, Klein M A, Hegyi I, Zinkernagel R M, Aguzzi A. J Virol. 1999;73:9584–9588. doi: 10.1128/jvi.73.11.9584-9588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Outram G. In: Slow Virus Diseases of Animals and Man. Kimberlin R, editor. Amsterdam: North–Holland; 1976. pp. 325–355. [Google Scholar]
- 29.Manuelidis L, Fritch W, Zaitsev I. Lancet. 1998;352:456. doi: 10.1016/S0140-6736(05)79191-1. [DOI] [PubMed] [Google Scholar]
- 30.Kima P E, Constant S L, Hannum L, Colmenares M, Lee K S, Haberman A M, Shlomchik M J, McMahon-Pratt D. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker C, Lu Z Y, Zaitsev I, Manuelidis L. J Virol. 1999;73:5089–5097. doi: 10.1128/jvi.73.6.5089-5097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diringer H, Ehlers B. J Gen Virol. 1991;72:457–460. doi: 10.1099/0022-1317-72-2-457. [DOI] [PubMed] [Google Scholar]
- 33.Chan O T, Hannum L G, Haberman A M, Madaio M P, Shlomchik M J. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]