This randomized clinical trial tested the effect of targeted temperature management to 33°C for 48 hours vs 24 hours on neurologic outcomes at 6 months in survivors of out-of-hospital cardiac arrest.
Key Points
Question
Does targeted temperature management at 33°C for 48 hours result in better neurologic outcome compared with standard 24-hour targeted temperature management in unconscious patients with out-of-hospital cardiac arrest?
Findings
In this randomized clinical trial enrolling 355 adults with out-of-hospital cardiac arrest, there was no significant difference in favorable neurologic outcome at 6 months for those treated for 48 hours (69%) vs 24 hours (64%) (difference, 5%).
Meaning
Prolonged targeted temperature management at 33°C did not result in better neurologic outcome; however, the study may have had limited power to detect clinically important differences, and further research may be warranted.
Abstract
Importance
International resuscitation guidelines recommend targeted temperature management (TTM) at 33°C to 36°C in unconscious patients with out-of-hospital cardiac arrest for at least 24 hours, but the optimal duration of TTM is uncertain.
Objective
To determine whether TTM at 33°C for 48 hours results in better neurologic outcomes compared with currently recommended, standard, 24-hour TTM.
Design, Setting, and Participants
This was an international, investigator-initiated, blinded-outcome-assessor, parallel, pragmatic, multicenter, randomized clinical superiority trial in 10 intensive care units (ICUs) at 10 university hospitals in 6 European countries. Three hundred fifty-five adult, unconscious patients with out-of-hospital cardiac arrest were enrolled from February 16, 2013, to June 1, 2016, with final follow-up on December 27, 2016.
Interventions
Patients were randomized to TTM (33 ± 1°C) for 48 hours (n = 176) or 24 hours (n = 179), followed by gradual rewarming of 0.5°C per hour until reaching 37°C.
Main Outcomes and Measures
The primary outcome was 6-month neurologic outcome, with a Cerebral Performance Categories (CPC) score of 1 or 2 used to define favorable outcome. Secondary outcomes included 6-month mortality, including time to death, the occurrence of adverse events, and intensive care unit resource use.
Results
In 355 patients who were randomized (mean age, 60 years; 295 [83%] men), 351 (99%) completed the trial. Of these patients, 69% (120/175) in the 48-hour group had a favorable outcome at 6 months compared with 64% (112/176) in the 24-hour group (difference, 4.9%; 95% CI, −5% to 14.8%; relative risk [RR], 1.08; 95% CI, 0.93-1.25; P = .33). Six-month mortality was 27% (48/175) in the 48-hour group and 34% (60/177) in the 24-hour group (difference, −6.5%; 95% CI, −16.1% to 3.1%; RR, 0.81; 95% CI, 0.59-1.11; P = .19). There was no significant difference in the time to mortality between the 48-hour group and the 24-hour group (hazard ratio, 0.79; 95% CI, 0.54-1.15; P = .22). Adverse events were more common in the 48-hour group (97%) than in the 24-hour group (91%) (difference, 5.6%; 95% CI, 0.6%-10.6%; RR, 1.06; 95% CI, 1.01-1.12; P = .04). The median length of intensive care unit stay (151 vs 117 hours; P < .001), but not hospital stay (11 vs 12 days; P = .50), was longer in the 48-hour group than in the 24-hour group.
Results
In 355 patients who were randomized (mean age, 60 years; 295 [83%] men), 351 (99%) completed the trial. More patients in the 48-hour group had a favorable outcome, but this was not statistically significant. Six-month mortality was not different between the groups. Adverse events were more common in the 48-hour group than in the 24-hour group. There was no significant difference in the time to mortality (hazard ratio, 0.79; 95% CI, 0.54-1.15; P = .22). The median length of ICU stay (151 vs 117 hours; P < .001), but not hospital stay (11 vs 12 days; P = .50), was longer in the 48-hour group than in the 24-hour group.
| No. (%) of Patients | Difference, % (95% CI) |
RR (95% CI) | P Value | ||
|---|---|---|---|---|---|
| 48-Hour Group (n = 175) | 24-Hour Group (n = 176) |
||||
| Primary outcome: CPC score of 1 or 2 at 6 mo | 120 (69) | 112 (64) | 4.9 (−5 to 14.8) | 1.08 (0.93 to 1.25) | .33 |
| Secondary outcomes | |||||
| Mortality at 6 mo | 48 (27) | 60 (34) | −6.5 (−16.1 to 3.1) | 0.81 (0.59 to 1.11) | .19 |
| Any adverse event | 169 (97) | 161 (91) | 5.6 (0.6 to 10.6) | 1.06 (1.01 to 1.12) | .03 |
Conclusions and Relevance
In unconscious survivors from out-of-hospital cardiac arrest admitted to the ICU, targeted temperature management at 33°C for 48 hours did not significantly improve 6-month neurologic outcome compared with targeted temperature management at 33°C for 24 hours. However, the study may have had limited power to detect clinically important differences, and further research may be warranted.
Trial Registration
clinicaltrials.gov Identifier: NCT01689077
Introduction
Short- and long-term outcomes in unconscious patients admitted to the intensive care unit (ICU) after out-of-hospital cardiac arrest are characterized by prognostic uncertainty and a high risk of death and neurologic deficit. To improve the chance of survival and neurologic recovery, international guidelines recommend use of targeted temperature management (TTM), together with urgent coronary angiography and percutaneous coronary intervention when appropriate, and delayed multimodal prognostication before withdrawal of care.
The results of the TTM trial (Target Temperature Management 33°C versus 36°C After Out-of-Hospital Cardiac Arrest) showed that TTM to 36°C had benefits similar to those of TTM to 33°C, but the optimal duration of cooling is still under debate. Current recommendations state that the patient’s temperature should be kept at a target of 32°C to 36°C for at least 24 hours. This recommended duration was based on the results of the 2 largest randomized clinical trials on TTM conducted with adult patients with out-of-hospital cardiac arrest, which used 24 hours of cooling. Nevertheless, in some units, longer cooling periods (up to 72 hours) were not uncommon, and for newborns with anoxic-asphyxia brain injury, cooling periods of 72 hours were considered standard practice. Animal studies have also suggested a potential benefit of prolonged cooling, but for adults with out-of-hospital cardiac arrest, this approach is currently supported only by retrospective data from small cohort studies.
Because of this specific knowledge gap, this clinical trial was designed to compare long-term neurologic outcomes in patients managed with 48-hour TTM at 33°C compared with the standard 24-hour duration.
Methods
Study Design
The Time-differentiated Therapeutic Hypothermia trial was an investigator-initiated, blinded-outcome-assessor, parallel, pragmatic, multicenter, randomized clinical trial conducted in 10 European ICUs. The study protocol (Supplement 1) was approved by the ethics committee in each participating center or country (list of ethical approvals available in Supplement 2). The study was conducted according to the requirements of the Declaration of Helsinki; written informed consent was obtained from the next of kin or a legal surrogate before randomization, and from each patient who regained mental capacity, according to local ethical approval. An independent data and safety monitoring committee performed predefined blinded interim analyses when 175 patients had been included, evaluating only safety issues and mortality at 6 months. Accuracy of collected study data was monitored by the principal investigator (see Supplement 2) or a person nominated within the study group.
Patients
All patients admitted to the ICU after an out-of-hospital cardiac arrest of presumed cardiac origin were screened for eligibility, with the inclusion criteria older than 17 years and younger than 80 years, sustained return of spontaneous circulation for more than 20 consecutive minutes, and Glasgow Coma Scale score less than 8. Patients with shockable and nonshockable rhythms were eligible; patients with unwitnessed asystole were excluded. A full description of the exclusion criteria is available in Supplement 2. Randomization had to take place within the first 23 hours after a core temperature less than or equal to 34°C had been reached.
Randomization and Blinding
After screening for eligibility, patients were randomly assigned in a 1:1 ratio to 1 of the 2 study groups, using a web-based central randomization procedure provided by the Department of Clinical Medicine, Aarhus University, Denmark (Figure 1). Randomization was carried out within strata defined by study site, age (<60 vs ≥60 years), and initial rhythm (shockable or not), using blinded randomly permuted block sizes of 6, 4, and 2. Medical personnel caring for the study patients, research staff entering data into the electronic case report form, and relatives were aware of group assignments.
Figure 1. Screened, Excluded, and Included Patients in the Study of Targeted Temperature Management.
OHCA indicates out-of-hospital cardiac arrest; ICU, intensive care unit; ROSC, return of spontaneous circulation.
aReasons for not meeting inclusion (eligibility) criteria were as follows: not cardiac OHCA (n=75), age (n=53), Glasgow Coma Scale score >8 (n=49), and no stable ROSC (n=14).
bExclusion criteria met were as follows: estimated time from collapse to ROSC >60 min (n=40), cardiac arrest with presumed noncardiac cause (eg, trauma, aorta dissection, intracerebral disease, massive bleeding, hanging, or hypoxemia) (n=63), in-hospital cardiac arrest (n=6), terminal disease or do-not-resuscitate order (n=15), severe coagulopathy (anticoagulant therapy, including thrombolysis, was not an exclusion criteria) (n=8), unwitnessed OHCA with asystole as first rhythm (n=21), time from cardiac arrest to initiation of cooling >240 min (n=19), neurologic disease with cognitive impairment (n=8), persistent cardiogenic shock, systolic blood pressure <80 mm Hg despite vasoactive treatment, or aortic balloon pump intervention (n=46), suspected or confirmed acute intracerebral bleeding (n=9), suspected or confirmed acute stroke (n=6), and acute coronary artery bypass surgery (n=1).
cOther reasons for exclusion were as follows: patient died before enrollment (n=10), other interventional study precluding co-enrollment (n=5), patients transferred to other ICU because of bed availability (n=5), and patient not native to country of treatment, rendering follow-up difficult or impossible (n=3).
dThe patient lost to follow-up was known to be alive and was included in the survival analyses, but could not be included in the primary analyses due to lack of primary outcome data.
Neurologic prognostication and decisions about withdrawal of treatment were made by medical personnel independent of the research team. Physicians and health personnel assessing 6-month functional outcomes and the statisticians were unaware of the study group assignments. During the analysis phase, the 2 study groups were identified as group 1 and group 2, and the results were incorporated in a final report by the writing group before data were unblinded.
Intervention
Patients were randomized to TTM at 33°C (±1°C) for 24 or 48 hours. Surface and invasive cooling methods were allowed, including boluses of intravenous cold fluids for induction of TTM (4°C). The aim was to reach target temperature as fast as possible. Temperature was measured in the bladder, rectum, or esophagus, or with intravascular probes according to local practices. Timing of the 24- or 48-hour duration started from the first point at which the core temperature was 34°C or lower. At the end of the 24- or 48-hour period, rewarming was performed at a maximal rate of 0.5°C/h until a core temperature of 37°C was reached. Sedation was used in both groups until rewarming was complete. The trial protocol included recommendations on supportive treatment, including the use of vasopressors, mechanical ventilation, and blood pressure targets.
In patients with severe bleeding, life-threatening arrhythmias, or refractory low cardiac output, cooling could be prematurely stopped and the temperature increased to 36°C to 37°C by the treating physicians, independent of the research team. These patients were analyzed in their assigned treatment group in a modified intention-to-treat analysis, but excluded from the per-protocol analysis.
Neurologic Prognostication
All centers agreed to provide active treatment until 72 hours after normothermia, with the exception of patients with brain death or refractory shock with multiple organ dysfunctions. Patients who remained unconscious despite cessation of sedation were assessed according to trial protocol recommendations (Supplement 1) with a combination of neurologic examination, electroencephalography, somatosensory-evoked potentials, computed tomography, and magnetic resonance imaging of the brain according to the decision of the attending physicians. Decisions to withdraw life-supporting therapy were made by a multidisciplinary team according to protocol recommendations and local practice, and were recorded in the electronic case report form.
Adverse Events and Hospital Stay
Adverse events data were collected throughout the hospital stay, including the occurrence of a new cerebral abnormality (including seizures), hypotension, cardiac arrhythmia, gastrointestinal feeding intolerance, renal failure, infections (in particular, pneumonia), and bleeding (including need for transfusion) (Supplement 1 and 2). Time receiving ventilator assistance (time to extubation) was collected, as well as length of stay in the ICU and in the hospital and the use of medical procedures (ie, tracheostomy, echocardiography, gastroscopy, and computed tomography scan).
Follow-up and Outcome
All surviving patients were followed up until 6 months after enrollment. The primary outcome, neurologic outcome at 6 months after the cardiac arrest, was defined by and based on the Cerebral Performance Categories score: 1, alert, able to work and lead a normal life; 2, moderate cerebral disability and sufficient cerebral function for part-time work; 3, severe cerebral disability, dependent on others, and impaired brain function; 4, coma and vegetative state; and 5, dead or certified brain dead. A Cerebral Performance Categories score of 1 or 2 was considered a favorable neurologic outcome. Assessors blinded to the treatment allocation performed the assessment during a telephone or person-to-person interview. The main secondary outcomes were 6-month mortality and time to death. Cause of death was reported as multiple organ dysfunction, brain death, withdrawal of life-supporting treatment, and other or undetermined reasons.
Statistical Analyses
For 80% power (2-sided P = .05) to show an absolute difference of 15% (50% vs 65%), a sample size of 338 patients was required. The choice of 15% as detectable difference was based on the effect sizes observed in the original TTM studies from 2002 and on observational data. The expected outcome of 50% was estimated according to the percentage of patients with good outcome in the 2 randomized studies from 2002, 49% and 55%, and supported by registry data. To account for potential loss to follow-up, sample size was inflated by 5% to 355 participants. No adjustment was made to the sample size analysis to account for the preplanned interim safety analysis. A modified intention-to-treat analysis (including all randomized patients who initiated the intervention and did not withdraw their consent) was undertaken in accordance with the protocol (Supplement 1) and the previously published analysis plan by an independent statistician blinded to treatment allocation. An additional per-protocol analysis was also conducted, excluding patients in whom cooling was terminated earlier than scheduled.
Categorical data were compared with χ2 tests for equal proportion (or Fisher exact test), t test for normally distributed data, and Wilcoxon test otherwise. Results are presented as numbers (percentages), means (SD), and medians (interquartile range [IQR]), as appropriate. Binomial outcomes are presented as proportions (95% CIs), with group comparisons presented as unadjusted differences (95% CIs) and as both unadjusted and adjusted relative risks (95% CIs), which were derived from Poisson regression with robust error variance.
Unadjusted and adjusted 6-month survival analyses were performed with Cox proportional hazards regression, with results reported as hazard ratios. Survival time is presented with Kaplan-Meier curves and was compared between groups by using the log-rank test, with proportionality assumptions verified with Schoenfeld residuals. Resource usage variables were log transformed, with unadjusted and adjusted analysis performed by using mixed hierarchic linear modeling, with results presented as geometric means, ratios of geometric means, and Hodges-Lehmann differences between medians, each with 95% CIs. Multivariable analyses were performed by adjusting for a priori–defined covariates (trial site, age, sex, initial cardiac arrest rhythm, time to return of spontaneous circulation, and bystander-initiated life support), with patients nested within site and site treated as a random effect. Second subgroup analyses were performed on the following predefined subgroups: age (<60 or ≥60 years), cardiac rhythm (shockable or not shockable), time to return of spontaneous circulation (<25 or ≥25 minutes), bystander-initiated life support (performed or not performed), method of cooling (invasive or noninvasive), time from return of spontaneous circulation to target temperature (<240 or ≥240 minutes), out-of-hospital cardiac arrest score (<20 or ≥20 points), and the site with the highest number of recruited patients compared with the other sites. Subgroup results are presented as a forest plot, with heterogeneity among subgroups determined by fitting an interaction between treatment and subgroup.
Temperatures during the first 24 hours of TTM and from completion of TTM (after 24 or 48 hours) to normothermia (37°C) were analyzed with repeated-measures analysis of variance, with pairwise post hoc comparisons performed by using t tests. Because multiple comparisons were considered, in the absence of any post hoc adjustment of the significance level, secondary outcomes should be considered as hypothesis generating and interpreted as exploratory. Because the number of missing values for primary and secondary outcomes and for variables needed for the adjusted analysis was minimal, no imputation was performed.
All analyses were conducted with SAS version 9.4 and SPSS version 22.0. A 2-sided P < .05 was used to indicate statistical significance.
Results
Patients
Between February 2013 and June 2016, all patients with out-of-hospital cardiac arrest (n = 907) who were admitted to a participating ICU were screened; 716 met the inclusion criteria. Of these patients, 361 had at least 1 exclusion criterion so that 355 patients were randomized, 176 to the 48-hour TTM group and 179 to the 24-hour TTM group. The population in the modified intention-to-treat primary outcome analysis included 175 patients in the 48-hour group and 176 in the 24-hour group (Figure 1). One patient was lost to follow-up but was known to be alive and therefore included in the mortality analysis. Baseline characteristics were comparable between groups (Table 1).
Table 1. Baseline and Prerandomization Characteristics of the Intention-to-Treat Population.
| No. (%) of Patients | ||
|---|---|---|
| 48-Hour Group (n = 175) |
24-Hour Group (n = 176) |
|
| Demographic characteristics | ||
| Age, mean (SD), y | 61 (12) | 60 (12) |
| Male sex | 144 (82.3) | 148 (84.1) |
| Weight, mean (SD), kga | 86 (17) | 86 (16) |
| Neurologic function before arrest | ||
| Normal, CPC score 1 | 172 (98) | 169 (96) |
| Some disability, CPC score 2 | 3 (2) | 7 (4) |
| Medical history | ||
| Previous myocardial infarction | 28 (16) | 26 (15) |
| Previous PCI or CABG | 29 (17) | 26 (15) |
| Previous cardiac arrest | 0 | 3 (2) |
| Chronic heart failure (NYHA class IV) | 5 (3) | 13 (7) |
| Chronic obstructive pulmonary disease | 13 (7) | 11 (6) |
| Liver cirrhosis | 3 (2) | 0 |
| Chronic renal failure with dialysis | 1 (<1) | 1 (<1) |
| Diabetes mellitus | 35 (20) | 28 (16) |
| Immunosuppression | 2 (<1) | 1 (<1) |
| Previous stroke | 11 (6) | 14 (8) |
| Cardiac arrest location | ||
| Home | 89 (51) | 102 (58) |
| Public place | 70 (40) | 66 (38) |
| Other out-of-hospital | 16 (9) | 8 (5) |
| Arrest witnessed | ||
| Bystander | 153 (87) | 147 (84) |
| Emergency medical services | 7 (4) | 15 (9) |
| Unwitnessedb | 15 (9) | 14 (8) |
| Resuscitation factors | ||
| Bystander-initiated CPR | 147 (84) | 144 (82) |
| Shockable rhythm | 160 (91) | 152 (86) |
| AED used | 38 (22) | 43 (24) |
| Time to basic life support, median (IQR), mina | 1 (0-2) | 1 (0-2) |
| Time to advanced life support, median (IQR), minc | 8 (5-11) | 8 (5-11) |
| Time to return of spontaneous circulation, median (IQR)d | 20 (15-30) | 21 (16-27) |
| Mechanical chest compression used | 43 (25) | 47 (27) |
| Out-of-hospital treatment | ||
| Epinephrine | 110 (63) | 109 (62) |
| Amiodarone | 79 (45) | 68 (39) |
| Immediate interventional cardiology | ||
| Coronary angiography | 146 (83) | 144 (82) |
| Percutaneous coronary intervention | 76 (43) | 69 (39) |
| Clinical status on ICU admission | ||
| Receiving mechanical ventilation at ICU admission | 175 (100) | 176 (100) |
| Glasgow Coma Scale score, median (IQR)e | 3 (3-3) | 3 (3-3) |
| Time from ICU admission to randomization, median (IQR), h | 15.2 (5.9-19.9) | 15 (7.5-19.5) |
| Temperature, mean (SD), °Cf | 35 (1.1) | 34.9 (1.0) |
| Mean arterial blood pressure, mean (SD), mm Hgg | 82 (21) | 80 (18) |
| Lactate, median (IQR), mg/dLc | 23.4 (12.6-40.5) | 24.3 (13.5-45.9) |
| Creatinine, mean (SD), mg/dL | 1.2 (0.7) | 1.2 (0.4) |
| pH, mean (SD) | 7.27 (0.10) | 7.27 (0.10) |
| Pao2, median (IQR), mm Hg | 118 (91-183) | 120 (90-176) |
| Paco2, mean (SD), mm Hg | 46 (10) | 45 (10) |
Abbreviations: AED, automated external defibrillator; CABG, coronary artery bypass grafting; CPC, Cerebral Performance Categories (1, alert, able to work and lead a normal life; 2, moderate cerebral disability and sufficient cerebral function for part-time work; 3, severe cerebral disability, dependent on others, and impaired brain function; 4, coma and/or vegetative state; and 5, dead or certified brain dead); CPR, cardiopulmonary resuscitation; ICU, intensive care unit; IQR, interquartile range; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Data missing for 1 patient. In some cases, the weight was estimated and not measured.
When the cardiac arrest was unwitnessed, intervals were calculated from the time of the call to emergency medical services.
Data missing for 2 patients.
Data missing for 3 patients.
Untestable because of sedation in 105 patients.
Data missing for 8 patients.
Data missing for 4 patients.
Intervention
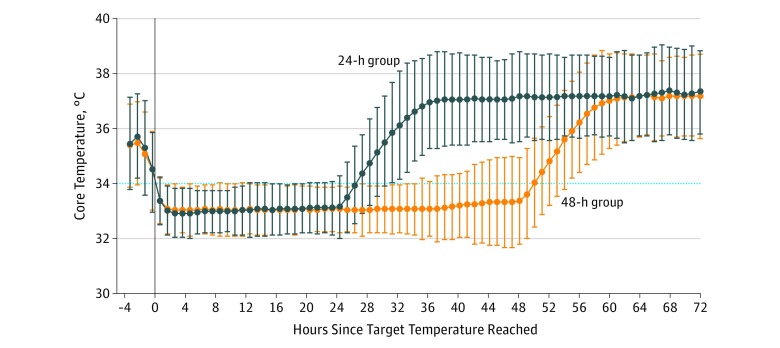
The mean body temperature at ICU admission was 35°C (SD, 1.1°C) in the 48-hour group and 34.9°C (SD, 1.0°C) in the 24-hour group. Invasive cooling with an intravascular catheter was the most common cooling method (n = 218 [62%]), with no significant differences between groups (Table 2). Time from return of spontaneous circulation to target temperature was shorter in the 48-hour group than in the 24-hour group (281 [IQR, 217-360] vs 320 [IQR 241-410] minutes; P = .01). After achievement of the target temperature (≤34°C), there were no significant differences in body temperature between groups until 24 hours (mean, 33.0°C [SD, 0.5°C] in the 24-hour group and 33.1°C [SD, 0.5°C] in the 48-hour group; P = .66) (Figure 2). The intervention was stopped early in 11 patients in the 48-hour group (6%) and 3 patients (2%) in the 24-hour group (Table 2). The rewarming rate did not differ significantly between the 2 groups (0.4°C [SD, 0.2°C] per hour in the 24-hour group and 0.3°C [SD, 0.2°C] per hour in the 48-hour group; P = .07) (Table 2). Temperatures were significantly lower in the 48-hour group at 60 hours but not at 72 hours from when the target temperature was achieved (Table 2). ICU parameters in regard to other aspects of care were similar between groups during the first 24 hours (eTable 2 in Supplement 2). During the intervention, the majority of patients were sedated with propofol (n = 305) and remifentanil (n = 164). Midazolam (n = 46) and fentanyl (n = 89) were also used in a minority of patients. Infusion of neuromuscular-blocking drugs was used in 61 patients.
Table 2. Timing, Methods, and Results of the Cooling Intervention With Included Protocol Violations.
| 48-Hour Group (n = 175) |
24-Hour Group (n = 176) |
P Value | |
|---|---|---|---|
| Time to intervention, median (IQR), min | |||
| Time from ROSC to start of TTMa | 102 (47-174) | 112 (33-185) | .92 |
| Time from ROSC to achievement of target temperatureb | 281 (217-360) | 320 (241-404) | .01 |
| TTM methods used, No. (%) | |||
| Surface coolingc | 75 (43) | 81 (46) | .55 |
| Invasive cooling catheterc | 114 (65) | 104 (59) | .24 |
| Cold fluid bolus | 65 (37) | 58 (33) | .41 |
| Rewarming, mean (SD) | |||
| Rate, °C/h | 0.3 (0.2) | 0.4 (0.2) | .08 |
| Rewarming duration, h | 10 (4) | 10 (3) | .65 |
| Protocol violations | |||
| Rewarmed to 36°C or above before scheduled time (protocol violations), No. (%) | 11 (6.3) | 3 (1.7) | .03 |
| Bradycardia or conduction disturbance | 3 | 0 | |
| Arrhythmia | 3 | 0 | |
| Refractory cardiac arrest | 1 | 0 | |
| Circulatory shock | 2 | 0 | |
| Excessive sedation requirement | 1 | 0 | |
| Brain death | 0 | 2 | |
| Equipment problem | 1 | 0 | |
| Unspecified | 0 | 1 | |
| Temperatures after TTM, mean (SD), °C | |||
| 60 h from target temperature | 37.0 (1.0) | 37.2 (0.6) | .02 |
| 72 h from target temperature | 37.2 (0.8) | 37.4 (0.7) | .16 |
Abbreviations: IQR, interquartile range; ROSC, return of spontaneous circulation; TTM, targeted temperature management.
Data missing for 72 patients. Exact time was impossible to determine because of pre-ICU start of cooling.
Data missing for 5 patients.
Some units used both surface and invasive cooling.
Figure 2. Core Temperature of the Intervention Groups.
Temperatures in the study groups until 72 hours after achieving target temperature (≤34°C [dotted horizontal line]), with T0 defined as the time target temperature was reached. Temperature data were available for 347 of 352 patients and were recorded with variable frequency (median, 188; interquartile range, 61-798) during the depicted period, with no statistically significant difference in frequency between groups (P = .15). Values are presented as mean ±2 SDs.
Outcomes
Six-month neurologic outcome was available for 351 patients (Figure 1); 120 of 175 patients in the 48-hour group (69%; 95% CI, 62%-75%) and 112 of 176 in the 24-hour group (64%; 95% CI, 56%-71%) had a favorable neurologic outcome (absolute difference, 4.9%; 95% CI, −5% to 14.8%; relative risk, 1.08; 95% CI, 0.93-1.25; P = .33) (Table 3). Similar results were obtained in the adjusted and the per-protocol analyses (eTable 2 in Supplement 2). The lack of a significant effect of TTM duration on neurologic outcome was consistent across all predefined subgroups (eFigure 1 in Supplement 2).
Table 3. Primary and Secondary Outcomes After Targeted Temperature Management.
| 48-Hour Group (n = 175) | 24-Hour Group (n = 176) | Difference, % (95% CI) | Relative Risk or Ratio of Geometric Means (95% CI) | P Value | |
|---|---|---|---|---|---|
| Primary outcome, No. (%) | |||||
| CPC score of 1 or 2 at 6 moa | 120 (69) | 112 (64) | 4.9 (−5 to 14.8) | 1.08 (0.93 to 1.25) | .33 |
| Secondary outcomes, No. (%) | |||||
| ICU mortality | 26 (15) | 30 (17) | −2.1 (−9.7 to 5.5) | 0.88 (0.54 to 1.42) | .59 |
| Hospital mortality | 40 (23) | 44 (25) | −2 (−10.9 to 6.9) | 0.92 (0.63 to 1.34) | .66 |
| Mortality at 6 mo | 48 (27) | 60 (34) | −6.5 (−16.1 to 3.1) | 0.81 (0.59 to 1.11) | .19 |
| Adverse events, No. (%) | |||||
| Any adverse event | 169 (97) | 161 (91) | 5.6 (0.6 to 10.6) | 1.06 (1.01 to 1.12) | .03 |
| Pneumonia | 86 (49) | 76 (43) | 6.2 (−4.2 to 16.6) | 1.14 (0.91 to 1.44) | .24 |
| Any bleeding | 17 (10) | 23 (13) | −3.3 (−9.9 to 3.3) | 0.75 (0.41 to 1.35) | .33 |
| Resource use, median (IQR) | |||||
| Time receiving mechanical ventilation, hb,c | 120 (99 to 146) | 87 (72 to 106) | 26 (16 to 36) | 1.37 (1.19 to 1.59) | <.001 |
| Survivors, h | 121 (98 to 149) | 85 (69 to 105) | 28 (18 to 38) | 1.41 (1.22 to 1.64) | <.001 |
| Nonsurvivors, h | 107 (71 to 163) | 151 (89 to 256) | −79 (−205 to 48) | 0.71 (0.37 to 1.37) | .35 |
| ICU length of stay, hc | 151 (127 to 178) | 117 (99 to 138) | 28 (15 to 41) | 1.3 (1.14 to 1.47)c | <.001 |
| Survivors, h | 184 (141 to 240) | 134 (103 to 175) | 30 (17 to 44) | 1.37 (1.22 to 1.54) | <.001 |
| Nonsurvivors, h | 92 (66 to 129) | 88 (64 to 121) | 9 (−25 to 44) | 1.04 (0.66 to 1.64) | .86 |
| Hospital length of stay, dc | 11.1 (9.3 to 13.3) | 11.8 (9.9 to 14.1) | −0.85 (−2.6 to 0.9) | 0.94 (0.79 to 1.11) | .50 |
| Survivors, d | 15.9 (12.6 to 20) | 17.2 (13.7 to 21.6) | −1.15 (−3.1 to 0.8) | 0.93 (0.8 to 1.06) | .28 |
| Nonsurvivors, d | 5.1 (3.6 to 7.1) | 5.3 (3.8 to 7.5) | 0 (−1.9 to 1.9) | 0.95 (0.64 to 1.41) | .79 |
The relative risks are unadjusted. The P values are from the results of χ2 analysis of categorical data from a nonparametric comparison of continuous data. CPC indicates Cerebral Performance Categories; ICU, intensive care unit; IQR, interquartile range.
In the 24-hour group, 100% equals 176 because 1 patient was alive but lost to follow-up. CPC score: 1, alert, able to work and lead a normal life; 2, moderate cerebral disability and sufficient cerebral function for part-time work; 3, severe cerebral disability, dependent on others, and impaired brain function; 4, coma and vegetative state; 5, dead or certified brain dead.
Time calculated as time to extubation.
Comparison of the geometric means between groups. Difference was calculated with the Hodges-Lehmann estimator.
Mortality at 6 months was 27% (95% CI, 21%-34%) in the 48-hour group and 34% (95% CI, 27%-41%) in the 24-hour group (difference, −6.5%; 95% CI, −16.1% to 3.1%; relative risk, 0.81; 95% CI, 0.59-1.11; P = .19). The median follow-up time was 184 days (IQR, 33-196 days) in the 48-hour group and 181 days (IQR, 15-193 days) in the 24-hour group (P = .43). There were no significant differences in time to death overall or in the adjusted and per-protocol analyses (Figure 3 and eTable 2 in Supplement 2). There were no differences in the other prespecified primary and secondary outcomes between groups, ie, Cerebral Performance Categories scores at hospital discharge, 3 months, and 6 months, or in the level of consciousness at 3 days from the arrest (eTable 3 in Supplement 2). Causes of death were comparable in the 2 groups, and there were no significant differences in the number of patients for whom life-supporting treatment was withdrawn for cerebral reasons (eTable 4 in Supplement 2).
Figure 3. Probability of Death With Standard and Prolonged Targeted Temperature Management.
Kaplan-Meier probability of death from randomization to 6 months (200 days) in the study groups. Median follow-up time was 184 days (IQR, 33-196 days) in the 48-hour group and 181 days (IQR, 15-193 days) in the 24-hour group.
Adverse Events
The proportion of patients with 1 or more adverse events was significantly higher in the 48-hour group (97%) than in the 24-hour group (91%) (difference, 5.6%; 95% CI, 0.6%-10.6%; relative risk, 1.06; 95% CI, 1.01-1.12; P = .04) (Table 3). Significantly more patients had hypotension in the 48-hour group than in the 24-hour group (62% vs 49%; P = .013) (eTable 6 in Supplement 2). There were no significant differences in the rates of pneumonia or bleeding between the groups; however, severe bleeding was more common in the 24-hour than in the 48-hour group (4% vs 1%; P = .03).
Resource Use
The median ICU length of stay was longer in the 48-hour than in the 24-hour group (151 hours [IQR, 127-178 hours] vs 117 hours [IQR, 99-138 hours]; P < .001), but there was no significant difference in hospital length of stay (Table 2). There were no significant differences between groups in the use of mechanical assist devices, tracheostomy, echocardiography, gastroscopy, or other operative procedures. Four patients in the 48-hour group had coronary artery bypass grafting compared with none in the 24-hour group (eTable 6 in Supplement 2).
Discussion
In this pragmatic, international, multicenter, randomized clinical trial, 48-hour TTM at 33°C was compared with standard 24-hour TTM in comatose patients admitted to the ICU after an out-of-hospital cardiac arrest of presumed cardiac origin. There were no significant differences between groups in the rates of favorable neurologic outcome or survival at 6-month follow-up. Patients who received TTM for 48 hours had a higher incidence of adverse events and a longer ICU length of stay. These results were consistent across predefined subgroups and after adjustment for prerandomization characteristics. However, the study may have had limited power to detect clinically important differences.
Current resuscitation guidelines recommend TTM at 33°C to 36°C for at least 24 hours. Previous studies have focused on the concept of cooling and on which core temperature should be targeted, but not on duration. The overall survival rate and proportion of patients with good neurologic outcome at 6 months were higher than in previous TTM studies, and higher than anticipated when sample size calculation was performed. Although general improvements in out-of-hospital and in-hospital care over time may partly explain this observation, differences between the current study population and those of previous studies may also be important. For example, the rate of bystander cardiopulmonary resuscitation and use of automated external defibrillators were higher in the current study than in previous randomized clinical trials and observational studies. Furthermore, compared with the TTM trial, patients in the current study were younger and more frequently had a shockable rhythm, as well as slightly shorter times from cardiac arrest to return of spontaneous circulation. A high percentage of patients also had immediate interventional cardiac procedures (coronary angiography or percutaneous coronary intervention), factors known to influence outcome after out-of-hospital cardiac arrest. Nevertheless, the current study included comatose patients after an anoxic injury, which is the population in whom TTM appeared to be effective in previous trials, supporting that findings in the current study are likely to be generalizable.
Duration of TTM in the prolonged-cooling group was set to 48 hours. There is no scientific evidence that specifically supports 48 hours or other durations longer than 24 hours. The 48-hour period was selected to balance a clear augmentation of “cooling dose” against an expected prolonged ICU stay and the risk of more adverse events.
In this study, adverse event rates may appear high but are comparable to those of previous trials on TTM at 33°C. More patients were rewarmed to 36°C before conclusion of the intervention in the 48-hour group compared with the 24-hour group, but overall rewarming rates and causes were similar to those of a previous study. Furthermore, the higher rate of rewarming and adverse effects was expected, given the longer treatment. Although 48-hour TTM was associated with slightly more adverse events than 24-hour TTM, most of them were mild and did not appear to affect neurologic outcome. In neonates with hypoxic-ischemic brain injury, TTM at 33°C for 72 hours is standard practice; prolonged cooling for 120 hours in one trial resulted in significant harm. Unlike the findings of one observational study, the 48-hour group had no increased risk of severe arrhythmias or pneumonia, which are known complications with TTM.
This study has several strengths. It is a multicenter study and has clear recommendations for interventions. Both invasive and noninvasive cooling methods were allowed, which represents current management and thus increases the generalizability of the findings. Because TTM affects neurologic prognostication and some patients regain consciousness long after the initial anoxic injury, a multimodal prognostication approach was used to avoid self-fulfilling outcome with withdrawing of life-sustaining treatment. Finally, the initial intensive care management was similar between groups. The majority of patients underwent electroencephalography, somatosensory-evoked potential, or cerebral computed tomography scan as part of this multimodal approach, with no significant differences between groups.
Limitations
This study also has several limitations. Sample size calculation using a 15% absolute difference in the primary outcome may have increased the risk of a type II error. The protocol was, however, written before the publication of the TTM study. The assumed treatment effect was comparable to that in the original TTM trials and also appeared supported by observational data. Recent randomized clinical trials on the effect of TTM in adults after status epilepticus, in children after cardiac arrest, and in neonates after asphyxia used similar effect sizes, with an assumed improvement of 10% to 15% in the intervention group (at 33°C) compared with controls. Although this study did not find a statistically significant difference between treatment groups, the 95% CIs around the difference (−5% to 14.8%) indicate that a true difference between groups is unlikely to lie outside this range. The 5% higher rate of 6-month favorable neurologic outcome in the prolonged-cooling group could represent a clinically meaningful difference. A trial to confirm or reject such a 5% absolute difference in good neurologic outcome between groups would, according to the unadjusted differences, require a much larger study with approximately 3000 patients. In addition, as in other studies, it was not possible to blind the ICU staff to the treatment group. The 6-month evaluation was, however, carried out by research personnel from outside the ICU. In most of the countries participating in the trial, ethical requirements did not allow allocation to the study before consent from a legal surrogate. This may have introduced bias toward exclusion of some patients otherwise eligible on admission to the ICU. The presence or absence of brain stem reflexes on admission to the ICU was not collected, and therefore imbalances between treatment groups in regard to these important components cannot be excluded. Finally, as in other studies of patients with cardiac arrest, good neurologic outcome was defined as a Cerebral Performance Categories score of 1 or 2, which may be considered a rather crude measure of neurologic outcome. Therefore, this study cannot provide definitive conclusions about the potential effects of the studied intervention on other patient outcomes, such as quality of life or more refined measures of cognitive function.
Conclusions
In unconscious survivors from out-of-hospital cardiac arrest who were admitted to the ICU, TTM for 48 hours did not significantly improve 6-month neurologic outcome compared with TTM for 24 hours. However, the study may have had limited power to detect clinically important differences, and further research may be warranted.
Trial Protocol
eMethods. Supplementary methods
eFigure 1. Forest plot of 8 preplanned sub-group analyses
eTable 1. Laboratory and blood gas parameters during the first three days in the ICU
eTable 2. Adjusted analyses
eTable 3. Neurological outcome scores and discharge destinations in the two groups
eTable 4. Cause and time to death in the intention-to-treat population
eTable 5. Adverse events during hospital stay
eTable 6. Interventions and examinations during the ICU stay
eReferences
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256-1264. [DOI] [PubMed] [Google Scholar]
- 2.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18)(suppl 2):S465-S482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C vs 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197-2206. [DOI] [PubMed] [Google Scholar]
- 4.Donnino MW, Andersen LW, Berg KM, et al. ; ILCOR ALS Task Force . Temperature management after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132(25):2448-2456. [DOI] [PubMed] [Google Scholar]
- 5.Hypothermia After Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556. [DOI] [PubMed] [Google Scholar]
- 6.Nagao K, Kikushima K, Watanabe K, et al. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ J. 2010;74(1):77-85. [DOI] [PubMed] [Google Scholar]
- 7.Hifumi T, Kuroda Y, Kawakita K, et al. ; J-PULSE-Hypo Investigators . Effect of admission Glasgow Coma Scale motor score on neurological outcome in out-of-hospital cardiac arrest patients receiving therapeutic hypothermia. Circ J. 2015;79(10):2201-2208. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran S, Laptook AR, Pappas A, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312(24):2629-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzopardi D, Strohm B, Marlow N, et al. ; TOBY Study Group . Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140-149. [DOI] [PubMed] [Google Scholar]
- 10.Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39(6):1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh GJ, Kwon WY, Kim KS, et al. Prolonged therapeutic hypothermia is more effective in attenuating brain apoptosis in a swine cardiac arrest model. Crit Care Med. 2014;42(2):e132-e142. [DOI] [PubMed] [Google Scholar]
- 12.Bisschops LL, van der Hoeven JG, Mollnes TE, Hoedemaekers CW. Seventy-two hours of mild hypothermia after cardiac arrest is associated with a lowered inflammatory response during rewarming in a prospective observational study. Crit Care. 2014;18(5):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagawa E, Dote K, Kato M, et al. Do lower target temperatures or prolonged cooling provide improved outcomes for comatose survivors of cardiac arrest treated with hypothermia? J Am Heart Assoc. 2015;4(9):e002123. doi: 10.1161/JAHA.115.002123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84. [DOI] [PubMed] [Google Scholar]
- 15.Brain Resuscitation Clinical Trial II Study Group A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med. 1991;324(18):1225-1231. [DOI] [PubMed] [Google Scholar]
- 16.Grossestreuer AV, Abella BS, Sheak KR, et al. Inter-rater reliability of post-arrest Cerebral Performance Category (CPC) scores. Resuscitation. 2016;109:21-24. doi: 10.1016/j.resuscitation.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557-563. [DOI] [PubMed] [Google Scholar]
- 18.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371(9628):1955-1969. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen N, Hovdenes J, Nilsson F, et al. ; Hypothermia Network . Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926-934. [DOI] [PubMed] [Google Scholar]
- 20.Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkegaard H, Pedersen AR, Pettilä V, et al. A statistical analysis protocol for the time-differentiated Target Temperature Management After Out-of-Hospital Cardiac Arrest (TTH48) clinical trial. Scand J Trauma Resusc Emerg Med. 2016;24(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. [DOI] [PubMed] [Google Scholar]
- 23.Adrie C, Cariou A, Mourvillier B, et al. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: the OHCA score. Eur Heart J. 2006;27(23):2840-2845. [DOI] [PubMed] [Google Scholar]
- 24.Becker LB, Aufderheide TP, Graham R. Strategies to improve survival from cardiac arrest: a report from the Institute of Medicine. JAMA. 2015;314(3):223-224. [DOI] [PubMed] [Google Scholar]
- 25.Mourvillier B, Tubach F, van de Beek D, et al. Induced hypothermia in severe bacterial meningitis: a randomized clinical trial. JAMA. 2013;310(20):2174-2183. [DOI] [PubMed] [Google Scholar]
- 26.Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311(1):45-52. [DOI] [PubMed] [Google Scholar]
- 27.Kirkegaard H, Rasmussen BS, de Haas I, et al. Time-differentiated Target Temperature Management After Out-of-Hospital Cardiac Arrest: a multicentre, randomised, parallel-group, assessor-blinded clinical trial (the TTH48 trial): study protocol for a randomised controlled trial. Trials. 2016;17(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legriel S, Lemiale V, Schenck M, et al. ; HYBERNATUS Study Group . Hypothermia for neuroprotection in convulsive status epilepticus. N Engl J Med. 2016;375(25):2457-2467. [DOI] [PubMed] [Google Scholar]
- 29.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators . Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372(20):1898-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Supplementary methods
eFigure 1. Forest plot of 8 preplanned sub-group analyses
eTable 1. Laboratory and blood gas parameters during the first three days in the ICU
eTable 2. Adjusted analyses
eTable 3. Neurological outcome scores and discharge destinations in the two groups
eTable 4. Cause and time to death in the intention-to-treat population
eTable 5. Adverse events during hospital stay
eTable 6. Interventions and examinations during the ICU stay
eReferences