Abstract
Female sex workers (FSW) living with HIV in sub-Saharan Africa have poor engagement to HIV care and treatment. Understanding the HIV care and treatment engagement experiences of FSW has important implications for interventions to enhance care and treatment outcomes. We conducted a systematic review to examine the HIV care experiences and determinants of linkage and retention in care, antiretroviral therapy (ART) initiation, and ART adherence and viral suppression among FSW living with HIV in sub-Saharan Africa. The databases PubMed, Embase, Web of Science, SCOPUS, CINAHL, Global Health, Psycinfo, Sociological Abstracts, and Popline were searched for variations of search terms related to sex work and HIV care and treatment among sub-Saharan African populations. Ten peer-reviewed articles published between January 2000 and August 2015 met inclusion criteria and were included in this review. Despite expanded ART access, FSW in sub-Saharan Africa have sub-optimal HIV care and treatment engagement outcomes. Stigma, discrimination, poor nutrition, food insecurity, and substance use were commonly reported and associated with poor linkage to care, retention in care, and ART initiation. Included studies suggest that interventions with FSW should focus on multilevel barriers to engagement in HIV care and treatment and explore the involvement of social support from intimate male partners. Our results emphasise several critical points of intervention for FSW living with HIV, which are urgently needed to enhance linkage to HIV care, retention in care, and treatment initiation, particularly where the HIV prevalence among FSW is greatest.
Keywords: literature review, sex work, HIV care and treatment experiences, Africa
Introduction
Globally, female sex workers (FSW) remain disproportionately burdened by HIV and are a key population for engaging in HIV care and treatment to both improve these women’s health and stem ongoing HIV transmission. The HIV prevalence among FSW worldwide is 12% (Baral et al., 2012). FSW have more than 13.5-times increased odds of HIV infection than women in the general population of reproductive age in low and middle income countries (Baral et al., 2012). When compared to other regions, sub-Saharan Africa holds the highest HIV prevalence among FSW, with nearly 40% of FSW living with HIV (Baral et al., 2012).
Expanded antiretroviral therapy (ART) access among the general population has led to substantial improvements to the overall health and well-being of those living with HIV. ART adherence can significantly maintain or restore immune function while also reducing viral load and the likelihood for onward transmission (Cohen et al., 2011; Grinsztejn et al., 2014; Group et al., 2015). The numerous benefits of ART are, however, reliant on successful engagement in HIV care and treatment, including linkage to care shortly after HIV diagnosis, retention in pre-ART care, timely initiation onto ART, and optimal ART adherence for viral suppression (Gardner, McLees, Steiner, Del Rio, & Burman, 2011; McNairy & El-Sadr, 2012; Mountain et al., 2014b). FSW living with HIV must be linked to care and initiate treatment to receive the individual immunological and clinical benefits of ART, such as viral suppression. Also, given the evidence supporting ART for treatment as prevention (Granich et al., 2010; Smith, Powers, Kashuba, & Cohen, 2011), FSW who are virally suppressed decrease the likelihood for ongoing transmission to their sexual partners (Cohen et al., 2011; Gardner et al., 2011).
As countries in sub-Saharan Africa begin to implement universal testing and treatment strategies to provide immediate ART to all those testing HIV-positive, there has been recent attention to estimating the proportion of key populations, such as FSW, at each step of the HIV treatment cascade from HIV diagnosis to viral suppression on ART. Currently, prevalence data is limited on linkage and retention in HIV care prior to ART initiation (Mountain et al., 2014b). A recent meta-analysis of HIV care continuum estimates that among FSW living with HIV, ART initiation ranges from 19% in Kenya to 48% in Rwanda and current ART use ranging from 23% in Kenya to 70% in Burkina Faso (Mountain et al., 2014a; Mountain et al., 2014b). Strategies that enhance linkage and retention to HIV care, initiation of ART, and viral suppression through ART adherence are urgently needed to maximise the benefits of ART among this key population.
While estimates of HIV care and treatment engagement outcomes among FSW are improving, our understanding of barriers and facilitators for linkage to HIV care and treatment for FSW living with HIV is limited. Among general populations of people living with HIV in low-income countries, reasons found for not being linked to care or initiating ART have included poor health provider communication and barriers to accessing services (e.g. transportation, cost) (Fehringer et al., 2006; Harris et al., 2011; Layer et al., 2014a; Layer et al., 2014b; Tuller et al., 2010; U.S. Agency for International Development [USAID], 2013). Among people living with HIV in high-income countries, barriers have included depression, social instability, substance use, and literacy levels (Harris et al., 2011; Winter, Halpern, Brozovich, & Neu, 2014). FSW frequently experience stigma, discrimination, and violence, which likely exacerbates these known barriers to HIV care and treatment (Baral et al., 2012; Chersich et al., 2013; Scambler & Paoli, 2008; Scheibe, Drame, & Shannon, 2012). Despite the implementation of lifelong ART for all women who are pregnant or breastfeeding for the prevention of mother-to-child transmission (Option B+) in many countries within sub-Saharan Africa, FSW may face additional stigma and discrimination when attending antenatal care visits without a male partner (Beckham et al., 2015; Beckham et al., 2016). To improve HIV care and treatment outcomes for FSW living with HIV in sub-Saharan Africa, it is imperative to systematically review the existing evidence on FSW’s experiences with linkage and retention to care and treatment initiation and adherence in this region.
For this systematic review, our objective was to examine and synthesise the findings in the quantitative and qualitative literature regarding the care experiences and factors associated with linkage to and retention in HIV care, treatment initiation, and ART adherence and viral suppression among FSW living with HIV in sub-Saharan Africa.
Methods
Search strategy
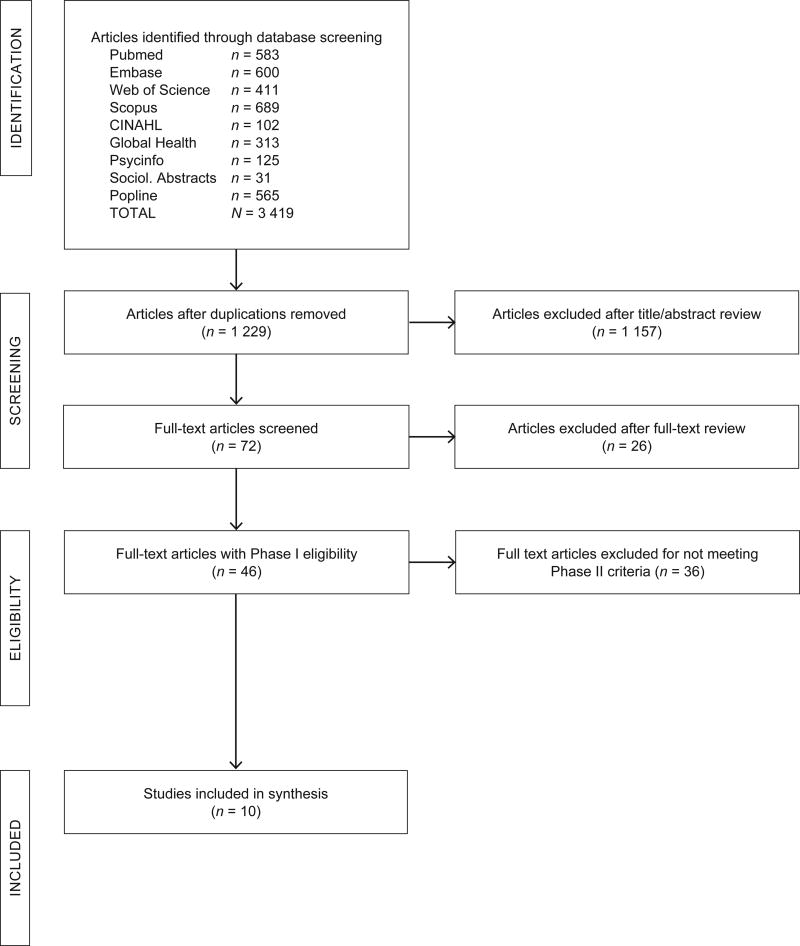
To identify articles on the HIV care and treatment experiences and determinants of FSWs living with HIV, we used established criteria for systematic reviews, as defined by The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement (Moher, Liberati, Tetzlaff, & Altman, 2010). We also consulted guidelines and strategies for conducting systematic reviews with findings from both qualitative and quantitative data (Petticrew & Roberts, 2008; Harden, 2010; Atkins, Launiala, Kagaha, & Smith, 2012). Details of the article selection process are shown in Figure 1.
Figure 1.
Flow chart of study selection for inclusion in systematic review
We conducted our search in two phases. The first phase sought to identify all articles published in English peer-reviewed journals published between 2000 and 2015 that described the experiences of FSW living with HIV with engagement in HIV care and treatment. Articles were included if they provided results that specifically described the social, behavioural and/or health system experiences of FSW living with HIV. This included articles such as FSW living with HIV as a sub-sample within a research study on FSW, key populations, or people living with HIV. Articles were excluded if they did not disaggregate the results of FSWs living with HIV. Articles that focused solely on biological or clinical measurements of disease progression or transmission among this population were also excluded.
We conducted our initial search on 22 November 2013 and updated the search on 30 July 2015. We used the databases of PubMed, Embase, Web of Science, SCOPUS, CINAHL, Global Health, Psycinfo, Sociological Abstracts, and Popline. Our search terms were:
-
((‘HIV-positive’ OR ‘HIV positive’ OR ‘HIV seropositive’ OR ‘living with HIV’ OR ‘living with AIDS’ OR PLWH OR PLWA OR PLWHA OR PLHIV)
AND
(‘sex work’ OR ‘sex worker’ OR ‘sex workers’ OR prostitute*)
AND
(female* OR women)).
Selection criteria and data extraction
Phase 1 had three review steps: 1. Title review, 2. Abstract review, 3. Full-text review. Each review step included two independent reviewers who evaluated whether or not the article should be included, based on the following a priori inclusion criteria:
-
(a)
Included female sex workers living with HIV. We excluded articles that were solely about transgender-identified female sex workers because their experiences were likely to differ from the population of cisgender female sex workers. We included articles that specifically mentioned sex worker or prostitute. We excluded articles that included only mentions of transactional sex, but no indication that the women self-identified as FSW.
-
(b)
Included original empirical quantitative or qualitative data. We excluded articles that were commentaries, letters-to-the-editor, systematic reviews, or meta-analyses and did not present any original data. We also excluded articles where we could not disaggregate which results were from FSW living with HIV.
-
(c)
Included data collected after 2000. We limited our search to data that is more reflective of the current era of HIV care and treatment.
-
(d)
Article published in English.
After the two primary reviewers finalised their decisions, any discrepancies were discussed among all four reviewers (DC, RZ, PF, KL) until a final decision was agreed upon. The initial title and abstract review resulted in a total of 72 articles that met the inclusion criteria and went through final paper review. For full-text review, two reviewers separately extracted key data about each paper using a standardised data abstraction sheet and made a final recommendation for inclusion. Reviewers used the same a priori inclusion criteria for each step of Phase 1. At the conclusion of Phase 1, we had 46 articles published in English-language peer-reviewed journals since 2000 that were focused on the experiences of FSW living with HIV.
In Phase 2, we aimed to identify a sub-sample of the 46 articles from Phase 1 that addressed determinants related to linkage to HIV care, retention to HIV care, ART initiation, and ART adherence and viral suppression among FSW in sub-Saharan Africa. To do so, we had two reviewers independently review the extractions of each of the 46 articles to identify whether or not an article used data collected in sub-Saharan Africa and had data on this population’s HIV care and treatment experiences. Any discrepancies were discussed by all reviewers until a final decision was made. Of the 46 articles from Phase 1, we identified ten articles from sub-Saharan that fit the inclusion criteria and are the focus of the results presented below.
To synthesise the findings from the ten articles, we first organised results by the care and treatment steps of the HIV care cascade: linkage and retention to care, ART initiation, and ART adherence and viral suppression. Given the focus on the care experiences and determinants of care among FSW living with HIV, the HIV testing step of the HIV care cascade was not included as part of our results. We also developed a conceptual framework to organise results using a multilevel framework that includes individual, interpersonal, and structural levels. For quantitative articles, we assessed how each outcome was measured and compared reported determinants of HIV care and treatment. For qualitative studies, we identified themes highlighted by each article and findings related to HIV care and treatment engagement outcomes. Because qualitative articles tended to provide more in-depth information on these women’s lives, we used the articles to provide illustrative quotes and gain a deeper understanding of these women’s HIV care and treatment experiences.
Results
The final sample of ten articles were all published after 2011 (Table 1). The sample size of FSW ranged from 20 to 870. They came from Rwanda (n = 1), Zimbabwe (n = 2), Benin (n = 2), Burkina Faso (n = 1), Nigeria (n = 1), Swaziland (n = 1), Kenya (n = 1), and Uganda (n = 1).
Table 1.
Characteristics of studies including determinants and care experiences of HIV care and treatment of female sex workers living with HIV
| Study | Location | Design | Period | Sample size | Objective | Key findings related to FSWs living with HIV |
|---|---|---|---|---|---|---|
| Quantitative | ||||||
| Braunstein et al (2011) | Kigali, Rwanda | Longitudinal cohort survey | 2006–2007 | 141 FSW living with HIV | To evaluate linkage-to-care, sexual behaviour change, and psychosocial experiences among newly diagnosed FSWs in Rwanda. | 85% of women had enrolled in HIV-related medical care by the follow up visit. Main barriers to treatment were perceived good health and high CD4 count. About 30% on ART reported missing pills. Common reasons for non-adherence were forgetting, lack of food, interruptions due to hospitalisation or imprisonment. Participants on ART generally had positive attitudes toward ART. |
| Cowan et al. (2013) | Victoria Falls, Hwange and Mutare, Zimbabwe | Cross-sectional survey | 2011 | 870 FSW | To determine the HIV prevalence and the extent of HIV prevention and care engagement among FSWs in Zimbabwe. | Approximately half of FSW living with HIV knew their status prior to the survey and of those 51–74% were enrolled in HIV care. Only 26–38% of all FSW who tested positive were accessing ART. Most taking ART received treatment at the hospital. Many reported gender-based violence and police harassment. |
| Diabaté et al (2013) | Cotonou, Benin | Longitudinal cohort study | 2008–2011 | 747 FSW of whom 120 were FSW living with HIV | To determine the effect of taking ART on sexual behaviour among FSWs and if this differs from non-FSW people living with HIV. | Sexual behaviour was not altered in the general population or among FSWs following ART initiation. After ART initiation, unsafe sex was mainly due to whether there are related risk reduction strategies. |
| Diabaté et al (2011) | Cotonou, Benin | Longitudinal cohort study | 2008–2010 | 53 FSW living with HIV 318 non-FSW people living with HIV | To determine if there was a difference in ART adherence, mortality rate and virologic response between FSWs living with HIV and non-FSW people living with HIV | FSW living with HIV had significantly worse adherence, higher mortality and lower CD4 gains during the first year on ART than non-FSW people living with HIV. Main reasons for missed treatment was being out of the home and running out of pills. FSWs have greater challenges for treatment adherence due to mobility due to avoiding harassment from police, brother owners or boyfriends and looking for new clients. |
| Konate et al. (2011) | Burkina Faso | Longitudinal cohort study | 2003–2005 | 658 high risk women including 169 FSWs living with HIV at baseline | To determine the effectiveness of a peer-led intervention that combined prevention and care among high-risk women. | FSW received ART and treatment adherence support from clinical psychologists and group education sessions. Condom use at last sex with regular partners, regular clients and new clients significantly improved from baseline to last visit. |
| Lawan et al. (2012) | Kano, Nigeria | Cross-sectional study | 2011 | 124 FSW, of whom 32 were FSW living with HIV | To investigate the perceptions about the risk and prevention of STIs and HIV/AIDS and to determine prevalence of STIs and HIV/AIDS in Kano among FSWs. | All FSW living with HIV continued unprotected sex with clients and unhealthy sexual behaviour and treatment-seeking behaviour, despite a good knowledge and perception of HIV/AIDS/STI prevention. The majority were receiving care at a hospital. |
| Qualitative | ||||||
| Fielding-Miller et al. (2014) | Swaziland | In-depth interviews (2 per participant) | 2010 | 20 FSW living with HIV | To examine the HIV prevention, care, and treatment needs of FSW who are living with HIV | FSW discussed difficulty in taking ART on an empty stomach |
| Mbonye et al. (2014) | Kampala Uganda | In-depth interviews (3 per participant) | 2010–2011 | 40 FSW | To explore the drivers of alcohol consumption and associated risky sexual behaviour among female sex workers in Kampala. | FSW living with HIV reported alcohol use affected medication adherence as they would forget to take medicine. |
| Mtetwa et al. (2013) | Harare, Zimbabwe | Focus group discussions | 2011 | 38 FSW living with HIV | To explore reasons for non-attendance at health services and experiences of barriers and facilitators to engagement in care for FSWs living with HIV. | FSW experienced stigma and discrimination by health workers and also experienced barriers to health care, such as time commitment and cost of transport and treatment. |
| Mixed methods | ||||||
| Benoit et al. (2013) | Kibera, Kenya | Cross-sectional mixed methods | 2011 | 30 FSW living with HIV | To understand the benefits of intimate partner relationships among FSW living with HIV | FSW with intimate partners had lower income, but received more financial support from partners and experienced lower intimate partner violence. FSW also described higher partner emotional support and more assistance with taking HIV medications. This included provision of money for treatment and treatment reminders. More FSW had disclosed HIV status than FSW status. |
FSW: female sex worker
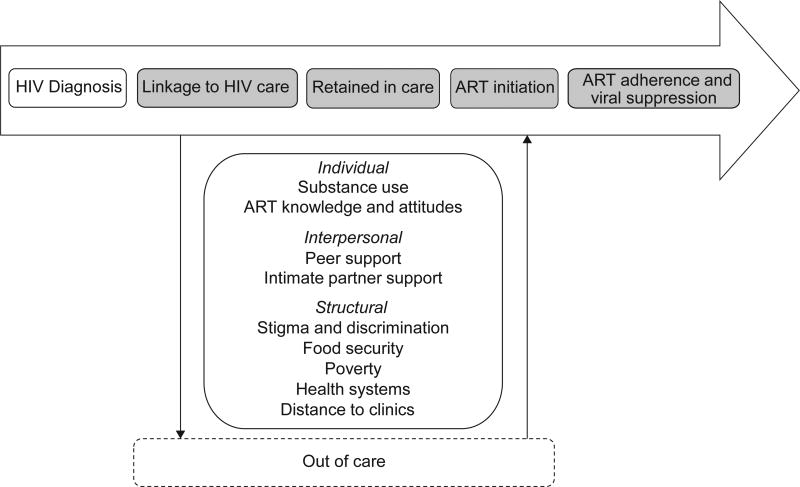
Overall, critical barriers and facilitators were noted at the individual, interpersonal, and systems levels for engagement in HIV care and treatment among FSW (Figure 2). At the individual level, the articles describe that substance use and ART knowledge and attitudes influences linkage and retention in care, ART initiation, and ART adherence. At the interpersonal level, peer and intimate partner support were important determinants for engagement in care and treatment. At the structural level, articles within our review emphasised experiences of stigma and discrimination from healthcare workers and poor health systems, such as long waiting lines and distance to clinics, as barriers to linkage and retention in care and ART initiation. Food security and underlying poverty experienced by FSW also played an important role for engagement in care and treatment.
Figure 2.
Key determinants and HIV care and treatment experiences among female sex workers living with HIV at the individual, interpersonal, and structural levels throughout the HIV care continuum, as adapted from Zulliger, et al (under review). HIV care continuum steps shaded in grey were the focus on the present systematic review.
Linkage and retention to HIV care
The evidence on linkage and retention in care was limited as only two articles quantitatively described linkage to HIV care or retention in HIV care among FSWs living with HIV in sub-Saharan Africa. Both the Rwanda and Nigeria articles assessed linkage to care as receiving any HIV-related medical care (Braunstein et al., 2011; Lawan, Abubakar, & Ahmed, 2012). Although the majority FSWs in these study populations were receiving HIV care, FSW’s positive experiences with healthcare providers and ART knowledge positively influenced HIV care engagement and retention specifically among FSW in Rwanda (Braunstein et al., 2011).
Overall, these two studies showed that most FSW living with HIV had reported linking to HIV care. The article from Kano, Nigeria found brothel-based FSW were most likely to receive HIV care within ART clinics in public hospitals rather than receiving care at medicine stores, faith-based health centres or traditional healers (Lawan et al., 2012). Among FSW not in care in Rwanda, many women believed that HIV care was not necessary until they were symptomatic or had worsened immunological health (Braunstein et al., 2011). In multivariable analyses, factors associated with being out-of-care in Rwanda included breastfeeding, having a known HIV-infected sexual partner, and reported condom use at last sex. Among FSW in care, structured interviews showed that FSW generally had positive attitudes towards ART as well as knowing the purpose and benefits of ART (Braunstein et al., 2011).
ART initiation
Overall, our search showed that six articles examined ART initiation (Braunstein et al., 2011; Diabaté et al., 2011; Konate et al., 2011; Cowan et al., 2013; Diabaté et al., 2013; Mtetwa, Busza, Chidiya, Mungofa, & Cowan, 2013). Five of the articles used quantitative methods to assess determinants of ART initiation, while one article used qualitative methods to provide a more in-depth understanding of care experiences related to ART initiation among FSW in Zimbabwe (Mtetwa et al., 2013). These articles highlighted important barriers to ART initiation, including stigma and poor nutrition.
One article reported on a research project that provided ART as part of a tailored intervention package for FSW in Burkina Faso (Konate et al., 2011). Both professional and non-professional (bar waitresses, fruit sellers, etc.) FSW who were part of the open-cohort in Burkina Faso received ART, in addition to treatment adherence support from clinical psychologists and group education sessions (Konate et al., 2011). With that support, approximately 30% of FSW living with HIV initiated ART.
Within three urban and rural areas of Zimbabwe, 56% of FSW on ART reported receiving treatment within a primary care ART clinic and 41% reported receiving treatment at a hospital (Cowan et al., 2013). FSW living with HIV in Zimbabwe discussed discrimination and hostility from hospital staff and reported financial and logistical barriers to treatment (Mtetwa et al., 2013). This included the negative attitudes they would receive during examinations and counselling, as described by one Zimbabwean participant:
She opened my file and I saw her face just changed instantly, and she actually frowned and looked at me like I was disgusting her. Her first words to me were, ‘so you are a prostitute and you actually have the guts to come here to waste our time and drugs on you, why do you do such things anyway? Why can’t you find a man of your own and get married’? (Mtetwa et al., 2013).
These types of harsh judgements by healthcare providers were a significant barrier to women’s motivation to initiate ART. Other types of public humiliation from hospital staff that women in Zimbabwe described included public announcements in the waiting room which stated that all sex workers should move to the back of the waiting line or stand in a separate line (Mtetwa et al., 2013).
Besides poor treatment by healthcare providers, FSW in Zimbabwe described other barriers related to the financial burden of initiating ART. They said that initiating ART treatment had a prohibitive financial burden due to the costs of regular testing and doctors’ visits. Nutrition was also revealed as a barrier to treatment as FSW were worried that being on ART would require more nutritious diets than their current diets, and therefore the food would become more of a financial burden. FSW also perceived travel time for receiving treatment as burdensome and that it encroached on their available time to earn money (Mtetwa et al., 2013).
ART adherence and viral suppression
ART adherence and viral suppression was assessed in six articles (Braunstein et al., 2011; Konate et al., 2011; Benoit et al., 2013; Fielding-Miller, Mnisi, Adams, Baral, & Kennedy, 2014; Mbonye, Rutakumwa, Weiss, & Seeley, 2014; Goldenberg et al., 2016). Lack of adequate food was often linked to the difficulty of adhering to ART (Braunstein et al., 2011; Fielding-Miller et al., 2014). Additionally, substance use was strongly associated with gaps in ART treatment and likelihood of a detectable viral load (Mbonye et al., 2014). One article highlighted the importance of intimate partner support for ART adherence (Benoit et al., 2013).
Reported adherence was relatively high among FSW who participated in an intervention where ART was provided in Burkina Faso (Konate et al., 2011). Within the first six months post ART initiation, over 80% of FSW achieved adherence levels of 95% or higher, measured by pill counts. Nearly all FSW who initiated ART had reached viral suppression within six months. ART adherence continued to increase to 92% at 12 months post ART initiation.
FSW in both Rwanda and Swaziland described hunger or not having adequate food to take pills as reasons for non-adherence (Braunstein et al., 2011; Fielding-Miller et al., 2014). Approximately 30% of FSW in Rwanda reported ever missing a pill since initiating ART, while 14% reported missing pills in the prior three days (Braunstein et al., 2011). Qualitative interviews among FSW in Swaziland revealed that FSW were counselled at the clinics to consume ‘healthy foods’ in order to manage their HIV infection and disease progression (Fielding-Miller et al., 2014). FSW specifically expressed anxiety of being unable to take their HIV medication on an empty stomach. One FSW described the importance of eating prior to taking ART to facilitate drug absorption:
You don’t necessarily have to eat tasty food to take the pills. Just any food that will settle in the stomach and allow for digestion of the pills because you cannot take the pills on an empty stomach (Fielding-Miller et al., 2014, p. 5).
FSW living with HIV in Swaziland felt they could not regularly afford or access food, particularly healthy foods such as fruits and vegetables, therefore food insecurity presented a potential barrier to their adherence to their medication.
Substance use was found to be associated with gaps in ART among FSW living with HIV in Uganda. They described their concern for the effect of alcohol on their health and adherence (Mbonye et al., 2014). Some of these FSW expressed their desire to stop using alcohol and felt it was necessary to leave sex work to abstain from alcohol. Also, FSW openly discussed that drinking inhibited their ability to remain adherent to ART because it limited their ability to remember taking their pills (Mbonye et al., 2014).
A study in Kenya identified that FSW who were on ART reported receiving support from intimate partners—including monetary, financial, and emotional support—that enabled them to better adhere to ART (Benoit et al., 2013). Several FSW on ART reported that their intimate partners would buy their medications when needed. One FSW described that her and her intimate partner would share the responsibility of clinic travel for ART. Some FSW also stated that they received reminders from their partners to take medications. One FSW explicitly shared that her intimate partner would send reminders by cell phone to take her pills when they were not together. Additionally, intimate partners encouraged their FSW partners to maintain a healthy lifestyle, such as reducing alcohol use, exercising, and eating healthy foods (Benoit et al., 2013).
Discussion
Our findings from this systematic review characterised determinants and care experiences of HIV care and treatment among FSW living with HIV in sub-Saharan Africa. FSW living with HIV are first and foremost women; therefore known barriers and facilitators to HIV care and treatment for women in sub-Saharan Africa are also applicable for FSW. However, FSW face additional challenges to HIV care and treatment at multiple levels.
Our findings complement previous research that documented large gaps globally along these first crucial steps of the HIV care continuum among FSW (Mountain et al., 2014a; Risher, Mayer, & Beyrer, 2015). Though the evidence base was limited to only ten articles, we found several key factors that influence FSW linkage to HIV care, retention, ART initiation, and treatment adherence at multiple levels, and which merit future research in a broader range of settings and populations. At the individual level, we found that substance use can negatively impede engagement in HIV care and treatment, while accurate ART knowledge and positive attitudes of treatment improves engagement throughout the HIV care continuum. At the interpersonal level, social support from peers or intimate partners can lead to optimal ART adherence and ultimately viral suppression. At the structural level, stigma and discrimination from healthcare workers and poor health systems adversely affects linkage and retention to care and ART initiation. Furthermore, food security and poverty were found to be substantial factors affecting ART initiation and adherence. Although more research is needed to address the broad range of FSW populations and settings, these results emphasise critical entry points for interventions to enhance HIV care and treatment for FSW living with HIV in sub-Saharan Africa.
Stigma and discrimination occurred during linkage to HIV care and ART initiation for FSW living with HIV. FSW often face multiple levels of stigma and discrimination related to the social and structural context of sex work (Scambler & Paoli, 2008; Logie, James, Tharao, & Loutfy, 2011; Baral et al., 2012; Scheibe et al., 2012; Chersich et al., 2013). Therefore, it is not surprising that this stigma and discrimination continue to occur and are perhaps exacerbated among FSW living with HIV. In our review, FSW highlighted that stigma and discrimination specifically within the healthcare setting were significant barriers to their engagement in care and treatment, which is likely due to their sex work practices and HIV status combined (Logie et al., 2011; USAID, 2013). Interventions focused on healthcare service providers to reduce stigma and discrimination, such as sex work sensitisation training, are urgently needed to improve HIV care and treatment outcomes for FSW, a finding that has been highlighted in other studies (Zulliger et al., 2015).
Social support, especially from peers or intimate male partners, could help overcome some of the barriers related to stigma and discrimination. Peer support and health navigation holds strong promise for improving engagement throughout the HIV care continuum. Peer support, in addition to social environment cohesion among FSW, has been associated with FSW’s willingness to engage in HIV testing and treatment initiation (Hong, Fang, Li, Liu, & Li, 2008; Deering et al., 2009). HIV care and treatment interventions should also build on the intimate partner dynamics among FSW. Strategies that enhance trust and communications between partners and partner engagement in care could improve emotional quality support for ART initiation (Fleming, Barrington, Perez, Donastorg, & Kerrigan, 2015; Syvertsen et al., 2015).
Nutrition plays an important role for ART initiation and adherence among FSW living with HIV in sub-Saharan Africa. Food insecurity has been intrinsically linked with sex work (Oyefara, 2007; Weiser et al., 2007; Anema, Vogenthaler, Frongillo, Kadiyala, & Weiser, 2009) and lack of adequate food and poverty often motivates women to engage in sex work. While engagement in sex work can be income generating, FSW may continue to struggle with food insecurity. Our findings indicate that FSW—particularly within Rwanda and Swaziland—understood the importance of eating healthy foods or any food at all in order to prevent negative ART side effects (Braunstein et al., 2011; Fielding-Miller et al., 2014). While healthful diets are important, a frequent barrier to treatment initiation or continuation among FSWs was a lack of food. Food supplements and context-specific nutritional counselling are valuable interventions to improve food security and to promote ART initiation (Mamlin et al., 2009). It is important that this messaging, however, be realistic to FSWs’ available resources. Failure to do so can introduce additional barriers to ART among FSW who are food-insecure.
The synergistic relationship between substance use and sex work is well-known. Often substance use is associated with women entering into sex work (Wechsberg, Luseno, Lam, Parry, & Morojele, 2006; Strathdee et al., 2015). Others may use substances to facilitate soliciting clients and to cope with the challenges from engaging in sex work (de Graaf, Vanwesenbeeck, van Zessen, Straver, & Visser, 1995; El-Bassel, Witte, Wada, Gilbert, & Wallace, 2001; Chersich et al., 2007; Gupta, Raj, Decker, Reed, & Silverman, 2009; Li, Li, & Stanton, 2010). Findings from our review demonstrate that substance use, particularly alcohol use, is also a barrier to engaging in HIV care and treatment among FSW. To date, there are few interventions focused on reducing substance use while improving ART uptake (Deering et al., 2009; Donastorg, Barrington, Perez, & Kerrigan, 2014), and none with FSW in sub-Saharan Africa. Substance use can impair cognitive functions, which in turn may adversely affect health seeking behaviour such as receiving and initiating HIV care and treatment (Chitwood, McBride, French, & Comerford, 1999; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003; Sohler et al., 2007; Simmonds & Coomber, 2009; Lancaster et al., 2016). Integrating substance use treatment with HIV care and treatment programmes, as resources allow, may reach FSW and improve HIV care and treatment outcomes.
FSW could be an ideal population to benefit from investigational treatment and prevention modalities. Preliminary trial results have suggested the potential effectiveness of long-acting injectable antiretrovirals for viral suppression (Spreen, Margolis, & Pottage, 2013; Kerrigan, Mantsios, Margolis, & Murray, 2016; Margolis et al., 2016a; Margolis et al., 2016b). If effective, long-acting injectables hold great promise for improving adherence by alleviating the burden of a daily pill for HIV treatment.
This review identified important gaps within the current literature that could enhance our understanding of HIV care and treatment experiences for FSW living with HIV in sub-Saharan Africa. ART initiation and ultimate adherence with the goal of viral suppression is critical for improving not only health outcomes but also onward transmission (Cohen et al., 2011; Gardner et al., 2011). Our systematic review reveals the limited literature among FSW living with HIV in sub-Saharan Africa, where the HIV prevalence is highest globally. Also, the lack of evidence on linkage and retention in HIV care among FSW is concerning as countries move towards providing universal treatment. Universal treatment strategies may continue to widen current disparities in linkage and retention to care among FSW. Furthermore, our review emphasises the large variation of engagement in HIV care and treatment among diverse FSW populations and settings in the region. The majority of articles within our review provide insights from cross-sectional quantitative or qualitative data. Further research, including longitudinal research, is imperative in order to provide more clarity on the temporality of determinants significantly affecting engagement and disengagement in HIV care and treatment for FSW living with HIV in sub-Saharan Africa.
There are limitations to this systematic review. First, our search criteria of sex work may have affected our final set of articles presented within this review. Women who engaged in transactional sex but did not self-identify as sex workers were not included within the population of our final set of articles. Second, our search was restricted to peer-reviewed published literature. Our findings do not include the grey literature and non-peer reviewed journals that may have additional insights on the HIV care experiences among FSW living with HIV. Third, the articles included in our review had varying typologies of FSW. Therefore, interpretations from our findings must be further explored within various specific FSW populations. Finally, there may be other important factors that are influencing these women’s HIV care and treatment experiences that have yet to be researched or published. Thus, this review should be considered a synthesis of the current published literature and not a definitive review of all determinants of the care and treatment experiences of FSW living with HIV. Nonetheless, our findings highlight several future lines of research and potential interventions to improve HIV care and treatment initiation experiences for FSW living with HIV in sub-Saharan Africa.
Conclusions
This systematic review revealed important barriers and facilitators to engagement in HIV care and treatment among FSW in sub-Saharan Africa. The evidence showed that stigma, discrimination, poor nutrition and food insecurity, and substance use impeded FSW’s engagement in these critical steps of the HIV care continuum. Developing tailored interventions that address these known barriers for FSW living with HIV in sub-Saharan Africa is crucial to prevent ongoing transmission and improve health outcomes among this population.
Acknowledgments
This work was supported by the NIAID T32 training grant (T32 AI0700).
References
- Anema A, Vogenthaler N, Frongillo EA, Kadiyala S, Weiser SD. Food insecurity and HIV/AIDS: Current knowledge, gaps, and research priorities. Current HIV/AIDS Reports. 2009;6(4):224–231. doi: 10.1007/s11904-009-0030-z. http://dx.doi.org/10.1007/s11904-009-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins S, Launiala A, Kagaha A, Smith H. Including mixed methods research in systematic reviews: Examples from qualitative syntheses in TB and malaria control. BMC Medical Research Methodology. 2012;12(1):62–68. doi: 10.1186/1471-2288-12-62. http://dx.doi.org/10.1186/1471-2288-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, Kerrigan D. Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. The Lancet Infectious Diseases. 2012;12(7):538–549. doi: 10.1016/S1473-3099(12)70066-X. http://dx.doi.org/10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- Beckham SW, Shembilu CR, Brahmbhatt H, Winch PJ, Beyrer C, Kerrigan DL. Female sex workers’ experiences with intended pregnancy and antenatal care services in southern Tanzania. Studies in Family Planning. 2015;46(1):55–71. doi: 10.1111/j.1728-4465.2015.00015.x. http://dx.doi.org/10.1111/j.1728-4465.2015.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham SW, Likindikoki S, Galai N, Mwampashi A, Shembilu C, Mantsios A, Kerrigan D. Pregnancy, HIV and denial of care among female sex workers in southern Tanzania: implications for elimination of vertical transmission; Paper presented at the Conference of the International AIDS Society; Durban, South Africa. 2016. [Google Scholar]
- Benoit C, Roth E, Hallgrimsdottir H, Jansson M, Ngugi E, Sharpe K. Benefits and constraints of intimate partnerships for HIV positive sex workers in Kibera, Kenya. International Journal for Equity in Health. 2013;12(1):76–87. doi: 10.1186/1475-9276-12-76. http://dx.doi.org/10.1186/1475-9276-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein SL, Umulisa MM, Veldhuijzen NJ, Kestelyn E, Ingabire CM, Nyinawabega J, Nash D. HIV diagnosis, linkage to HIV care, and HIV risk behaviors among newly diagnosed HIV-positive female sex workers in Kigali, Rwanda. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;57(4):e70–e76. doi: 10.1097/QAI.0b013e3182170fd3. http://dx.doi.org/10.1097/QAI.0b013e3182170fd3. [DOI] [PubMed] [Google Scholar]
- Chersich MF, Luchters SM, Malonza IM, Mwarogo P, King’ola N, Temmerman M. Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. International Journal of STD & AIDS. 2007;18(11):764–769. doi: 10.1258/095646207782212342. http://dx.doi.org/10.1258/095646207782212342. [DOI] [PubMed] [Google Scholar]
- Chersich MF, Luchters S, Ntaganira I, Gerbase A, Lo YR, Scorgie F, Steen R. Priority interventions to reduce HIV transmission in sex work settings in sub-Saharan Africa and delivery of these services. Journal of the International AIDS Society. 2013;16(1):17980–17987. doi: 10.7448/IAS.16.1.17980. http://dx.doi.org/10.7448/IAS.16.1.17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DD, McBride DC, French MT, Comerford M. Health care need and utilization: A preliminary comparison of injection drug users, other illicit drug users, and nonusers. Substance Use & Misuse. 1999;34(4–5):727–746. doi: 10.3109/10826089909037240. http://dx.doi.org/10.3109/10826089909037240. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. The New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. http://dx.doi.org/10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan FM, Mtetwa S, Davey C, Fearon E, Dirawo J, Wong-Gruenwald R, Hargreaves JR. Engagement with HIV prevention treatment and care among female sex workers in Zimbabwe: A respondent driven sampling survey. PLoS One. 2013;8(10):e77080. doi: 10.1371/journal.pone.0077080. http://dx.doi.org/10.1371/journal.pone.0077080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R, Vanwesenbeeck I, van Zessen G, Straver CJ, Visser JH. Alcohol and drug use in heterosexual and homosexual prostitution, and its relation to protection behaviour. AIDS Care. 1995;7(1):35–47. doi: 10.1080/09540129550126948. http://dx.doi.org/10.1080/09540129550126948. [DOI] [PubMed] [Google Scholar]
- Deering KN, Shannon K, Sinclair H, Parsad D, Gilbert E, Tyndall MW. Piloting a peer-driven intervention model to increase access and adherence to antiretroviral therapy and HIV care among street-entrenched HIV-positive women in Vancouver. AIDS Patient Care and STDs. 2009;23(8):603–609. doi: 10.1089/apc.2009.0022. http://dx.doi.org/10.1089/apc.2009.0022. [DOI] [PubMed] [Google Scholar]
- Diabaté S, Chamberland A, Zannou DM, Geraldo N, Azon-Kouanou A, Massinga-Loembe M, Alary M. Sexual behaviour after antiretroviral therapy initiation in female sex workers and HIV-positive patients from the general population, Cotonou, Benin. AIDS Care. 2013;25(11):1426–1432. doi: 10.1080/09540121.2013.772279. http://dx.doi.org/10.1080/09540121.2013.772279. [DOI] [PubMed] [Google Scholar]
- Diabaté S, Zannou DM, Geraldo N, Chamberland A, Akakpo J, Ahouada C, Alary M. Antiretroviral therapy among HIV-1 infected female sex workers in Benin: A comparative study with patients from the general population. World Journal of AIDS. 2011;1(03):94–99. http://dx.doi.org/10.4236/wja.2011.13014. [Google Scholar]
- Donastorg Y, Barrington C, Perez M, Kerrigan D. Abriendo Puertas: Baseline findings from an integrated intervention to promote prevention, treatment and care among FSW living with HIV in the Dominican Republic. PLoS One. 2014;9(2):e88157. doi: 10.1371/journal.pone.0088157. http://dx.doi.org/10.1371/journal.pone.0088157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bassel N, Witte SS, Wada T, Gilbert L, Wallace J. Correlates of partner violence among female street-based sex workers: Substance abuse, history of childhood abuse, and HIV risks. AIDS Patient Care and STDs. 2001;15(1):41–51. doi: 10.1089/108729101460092. http://dx.doi.org/10.1089/108729101460092. [DOI] [PubMed] [Google Scholar]
- Fehringer J, Bastos FI, Massard E, Maia L, Pilotto JH, Kerrigan D. Supporting adherence to highly active antiretroviral therapy and protected sex among people living with HIV/AIDS: The role of patient-provider communication in Rio de Janeiro, Brazil. AIDS Patient Care and STDs. 2006;20(9):637–648. doi: 10.1089/apc.2006.20.637. http://dx.doi.org/10.1089/apc.2006.20.637. [DOI] [PubMed] [Google Scholar]
- Fielding-Miller R, Mnisi Z, Adams D, Baral S, Kennedy C. ‘There is hunger in my community’: A qualitative study of food security as a cyclical force in sex work in Swaziland. BMC Public Health. 2014;14(1):79–89. doi: 10.1186/1471-2458-14-79. http://dx.doi.org/10.1186/1471-2458-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming PJ, Barrington C, Perez M, Donastorg Y, Kerrigan D. Strategies for recruiting steady male partners of female sex workers for HIV research. AIDS and Behavior. 2015;19(2):362–368. doi: 10.1007/s10461-014-0894-9. http://dx.doi.org/10.1007/s10461-014-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. http://dx.doi.org/10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg SM, Montaner J, Duff P, Nguyen P, Dobrer S, Guillemi S, Shannon K. Structural barriers to antiretroviral therapy among sex workers living with HIV: Findings of a longitudinal study in Vancouver, Canada. AIDS and Behavior. 2016;20(5):977–986. doi: 10.1007/s10461-015-1102-2. http://dx.doi.org/10.1007/s10461-015-1102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granich R, Crowley S, Vitoria M, Smyth C, Kahn JG, Bennett R, Williams B. Highly active antiretroviral treatment as prevention of HIV transmission: Review of scientific evidence and update. Current Opinion in HIV and AIDS. 2010;5(4):298–304. doi: 10.1097/COH.0b013e32833a6c32. http://dx.doi.org/10.1097/COH.0b013e32833a6c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, Cohen MS. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: Results from the phase 3 HPTN 052 randomised controlled trial. The Lancet Infectious Diseases. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. http://dx.doi.org/10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Neaton JD. Initiation of antiretroviral therapy in early asymptomatic HIV infection. The New England Journal of Medicine. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. http://dx.doi.org/10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J, Raj A, Decker MR, Reed E, Silverman JG. HIV vulnerabilities of sex-trafficked Indian women and girls. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics. 2009;107(1):30–34. doi: 10.1016/j.ijgo.2009.06.009. http://dx.doi.org/10.1016/j.ijgo.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden A. Mixed-methods systematic reviews: Integrating quantitative and qualitative findings. Focus: A Publication of the National centre for the Dissemination of Disability Research (NCDDR) Technical Brief. 2010;(25) [Google Scholar]
- Harris J, Pillinger M, Fromstein D, Gomez B, Garris I, Kanetsky PA, Gross R. Risk factors for medication non-adherence in an HIV infected population in the Dominican Republic. AIDS and Behavior. 2011;15(7):1410–1415. doi: 10.1007/s10461-010-9781-1. http://dx.doi.org/10.1007/s10461-010-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Fang X, Li X, Liu Y, Li M. Environmental support and HIV prevention behaviors among female sex workers in China. Sexually Transmitted Diseases. 2008;35(7):662–667. doi: 10.1097/OLQ.0b013e31816b322c. http://dx.doi.org/10.1097/OLQ.0b013e31816b322c. [DOI] [PubMed] [Google Scholar]
- Kerrigan D, Mantsios A, Margolis D, Murray M. Experiences with long-acting injectable ART: A qualitative study among people living with HIV participating in a phase II study of cabotegravir rilpivirine (LATTE-2) in the United States and Spain; Paper presented at the Conference of the International AIDS Society; Durban, South Africa. 2016. [Google Scholar]
- Konate I, Traore L, Ouedraogo A, Sanon A, Diallo R, Ouedraogo JL, Nagot N. Linking HIV prevention and care for community interventions among high-risk women in Burkina Faso—the ARNS 1222 ‘Yerelon’ cohort. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;57(Suppl 1):S50–S54. doi: 10.1097/QAI.0b013e3182207a3f. http://dx.doi.org/10.1097/QAI.0b013e3182207a3f. [DOI] [PubMed] [Google Scholar]
- Lancaster KE, Go VF, Lungu T, Mmodzi P, Hosseinipour MC, Chadwick K, Miller WC. Substance use and HIV infection awareness among HIV-infected female sex workers in Lilongwe, Malawi. The International Journal on Drug Policy. 2016;30:124–131. doi: 10.1016/j.drugpo.2016.02.020. http://dx.doi.org/10.1016/j.drugpo.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawan UM, Abubakar S, Ahmed A. Risk perceptions, prevention and treatment seeking for sexually transmitted infections and HIV/AIDS among female sex workers in Kano, Nigeria. African Journal of Reproductive Health. 2012;16(1):61–67. [PubMed] [Google Scholar]
- Layer EH, Brahmbhatt H, Beckham SW, Ntogwisangu J, Mwampashi A, Davis WW, Kennedy CE. ‘I pray that they accept me without scolding’: Experiences with disengagement and re-engagement in HIV care and treatment services in Tanzania. AIDS Patient Care and STDs. 2014a;28(9):483–488. doi: 10.1089/apc.2014.0077. http://dx.doi.org/10.1089/apc.2014.0077. [DOI] [PubMed] [Google Scholar]
- Layer EH, Kennedy CE, Beckham SW, Mbwambo JK, Likindikoki S, Davis WW, Brahmbhatt H. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One. 2014b;9(8):e104961. doi: 10.1371/journal.pone.0104961. http://dx.doi.org/10.1371/journal.pone.0104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li X, Stanton B. Alcohol use among female sex workers and male clients: An integrative review of global literature. Alcohol and Alcoholism (Oxford, Oxfordshire) 2010;45(2):188–199. doi: 10.1093/alcalc/agp095. http://dx.doi.org/10.1093/alcalc/agp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie CH, James L, Tharao W, Loutfy MR. HIV, gender, race, sexual orientation, and sex work: A qualitative study of intersectional stigma experienced by HIV-positive women in Ontario, Canada. PLoS Medicine. 2011;8(11):e1001124. doi: 10.1371/journal.pmed.1001124. http://dx.doi.org/10.1371/journal.pmed.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamlin J, Kimaiyo S, Lewis S, Tadayo H, Jerop FK, Gichunge C, Einterz R. Integrating nutrition support for food-insecure patients and their dependents into an HIV care and treatment program in Western Kenya. American Journal of Public Health. 2009;99(2):215–221. doi: 10.2105/AJPH.2008.137174. http://dx.doi.org/10.2105/AJPH.2008.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, Eron J, Yazdanpanah Y, Griffith SK, Spreen WR. Cabotegravir rilpivirine as long-acting maintenance therapy: LATTE-2 week 32 results; Paper presented at the 23rd Conference on Retroviruses and Opportunistic Infections (CROI).2016a. [Google Scholar]
- Margolis DA, Podzamczer D, Stellbrink H-J, Lutz T, Angel J, Richmond G, Spreen W. Cabotegravir rilpivirine as long-acting maintenance therapy: LATTE-2 week 48 results; Paper presented at the Conference of the International AIDS Society; Durban, South Africa. 2016b. [Google Scholar]
- Mbonye M, Rutakumwa R, Weiss H, Seeley J. Alcohol consumption and high risk sexual behaviour among female sex workers in Uganda. African Journal of AIDS Research. 2014;13(2):145–151. doi: 10.2989/16085906.2014.927779. http://dx.doi.org/10.2989/16085906.2014.927779. [DOI] [PubMed] [Google Scholar]
- McNairy ML, El-Sadr WM. The HIV care continuum: No partial credit given. AIDS (London, England) 2012;26(14):1735–1738. doi: 10.1097/QAD.0b013e328355d67b. http://dx.doi.org/10.1097/QAD.0b013e328355d67b. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. http://dx.doi.org/10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Mountain E, Mishra S, Vickerman P, Pickles M, Gilks C, Boily MC. Antiretroviral therapy uptake, attrition, adherence and outcomes among HIV-infected female sex workers: A systematic review and meta-analysis. PLoS One. 2014a;9(9):e105645. doi: 10.1371/journal.pone.0105645. http://dx.doi.org/10.1371/journal.pone.0105645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain E, Pickles M, Mishra S, Vickerman P, Alary M, Boily MC. The HIV care cascade and antiretroviral therapy in female sex workers: Implications for HIV prevention. Expert Review of Anti-Infective Therapy. 2014b;12(10):1203–1219. doi: 10.1586/14787210.2014.948422. http://dx.doi.org/10.1586/14787210.2014.948422. [DOI] [PubMed] [Google Scholar]
- Mtetwa S, Busza J, Chidiya S, Mungofa S, Cowan F. ‘You are wasting our drugs’: Health service barriers to HIV treatment for sex workers in Zimbabwe. BMC Public Health. 2013;13(1):698–704. doi: 10.1186/1471-2458-13-698. http://dx.doi.org/10.1186/1471-2458-13-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyefara JL. Food insecurity, HIV/AIDS pandemic and sexual behaviour of female commercial sex workers in Lagos metropolis, Nigeria. Journal of Social Aspects of HIV/AIDS Research Alliance. 2007;4(2):626–635. doi: 10.1080/17290376.2007.9724884. http://dx.doi.org/10.1080/17290376.2007.9724884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petticrew M, Roberts H. Systematic reviews in the social sciences: A practical guide. Malden: John Wiley & Sons; 2008. [Google Scholar]
- Risher K, Mayer KH, Beyrer C. HIV treatment cascade in MSM, people who inject drugs, and sex workers. Current Opinion in HIV and AIDS. 2015;10(6):420–429. doi: 10.1097/COH.0000000000000200. http://dx.doi.org/10.1097/COH.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler G, Paoli F. Health work, female sex workers and HIV/AIDS: Global and local dimensions of stigma and deviance as barriers to effective interventions. Social Science & Medicine. 2008;66(8):1848–1862. doi: 10.1016/j.socscimed.2008.01.002. http://dx.doi.org/10.1016/j.socscimed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Scheibe A, Drame FM, Shannon K. HIV prevention among female sex workers in Africa. Journal of Social Aspects of HIV/AIDS Research Alliance. 2012;9(3):167–172. doi: 10.1080/17290376.2012.743809. http://dx.doi.org/10.1080/17290376.2012.743809. [DOI] [PubMed] [Google Scholar]
- Simmonds L, Coomber R. Injecting drug users: A stigmatised and stigmatising population. The International Journal on Drug Policy. 2009;20(2):121–130. doi: 10.1016/j.drugpo.2007.09.002. http://dx.doi.org/10.1016/j.drugpo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Smith K, Powers KA, Kashuba ADM, Cohen MS. HIV-1 treatment as prevention: The good, the bad, and the challenges. Current Opinion in HIV and AIDS. 2011;6(4):315–325. doi: 10.1097/COH.0b013e32834788e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni M-L, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care and STDs. 2007;21 doi: 10.1089/apc.2007.9985. (s1, S1), S-68–S-76. http://dx.doi.org/10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Current Opinion in HIV and AIDS. 2013;8(6):565–571. doi: 10.1097/COH.0000000000000002. http://dx.doi.org/10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, West BS, Reed E, Moazan B, Azim T, Dolan K. Substance use and HIV among female sex workers and female prisoners: Risk environments and implications for prevention, treatment, and policies. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;69(Suppl 2):S110–S117. doi: 10.1097/QAI.0000000000000624. http://dx.doi.org/10.1097/QAI.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvertsen JL, Bazzi AR, Martinez G, Rangel MG, Ulibarri MD, Fergus KB, Strathdee SA. Love, trust, and HIV risk among female sex workers and their intimate male partners. American Journal of Public Health. 2015;105(8):1667–1674. doi: 10.2105/AJPH.2015.302620. http://dx.doi.org/10.2105/AJPH.2015.302620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of non-adherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. The American Journal of Medicine. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. http://dx.doi.org/10.1016/S0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in south-western Uganda: A qualitative study. AIDS and Behavior. 2010;14(4):778–784. doi: 10.1007/s10461-009-9533-2. http://dx.doi.org/10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Agency for International Development (USAID) Strategic assessment to define a comprehensive response to HIV in Iringa. Tanzania: Research brief, Linkages to care; 2013. http://www.jhsph.edu/research/centers-and-institutes/research-to-prevention/publications/iringa/ltc-brief-final.pdf. [Google Scholar]
- Wechsberg WM, Luseno WK, Lam WK, Parry CD, Morojele NK. Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in Pretoria. AIDS and Behavior. 2006;10(2):131–137. doi: 10.1007/s10461-005-9036-8. http://dx.doi.org/10.1007/s10461-005-9036-8. [DOI] [PubMed] [Google Scholar]
- Weiser SD, Leiter K, Bangsberg DR, Butler LM, Percy-de Korte F, Hlanze Z, Heisler M. Food insufficiency is associated with high-risk sexual behavior among women in Botswana and Swaziland. PLoS Medicine. 2007;4(10):1589–1597. doi: 10.1371/journal.pmed.0040260. http://dx.doi.org/10.1371/journal.pmed.0040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MC, Halpern M, Brozovich A, Neu N. Evaluation of an HIV adherence counseling program in La Romana, Dominican Republic. Journal of the International Association of Providers of AIDS Care. 2014;13(4):361–365. doi: 10.1177/2325957413514630. http://dx.doi.org/10.1177/2325957413514630. [DOI] [PubMed] [Google Scholar]
- Zulliger R, Maulsby C, Barrington C, Holtgrave D, Donastorg Y, Perez M, Kerrigan D. Retention in HIV care among female sex workers in the Dominican Republic: Implications for research, policy and programming. AIDS and Behavior. 2015;19(4):715–722. doi: 10.1007/s10461-014-0979-5. http://dx.doi.org/10.1007/s10461-014-0979-5. [DOI] [PubMed] [Google Scholar]