Abstract
Neurons are terminally differentiated cells that use their microtubule arrays not for cell division but rather as architectural elements required for the elaboration of elongated axons and dendrites. In addition to acting as compression-bearing struts that provide for the shape of the neuron, microtubules also act as directional railways for organelle transport. The stability properties of neuronal microtubules are commonly discussed in the biomedical literature as crucial to the development and maintenance of the nervous system, and have recently gained attention as central to the etiology of neurodegenerative diseases. Drugs that affect microtubule stability are currently under investigation as potential therapies for disease and injury of the nervous system. There is often a lack of consistency, however, in how the issue of microtubule stability is discussed in the literature, and this can affect the design and interpretation of experiments as well as potential therapeutic regimens. Neuronal microtubules are considered to be more stable than microtubules in dividing cells. On average, this is true, but in addition to an abundant stable microtubule fraction in neurons, there is also an abundant labile microtubule fraction. Both are functionally important. Individual microtubules consist of domains that differ in their stability properties, and these domains can also differ markedly in their composition as well as how they interact with various microtubule-related proteins in the neuron. Myriad proteins and pathways have been discussed as potential contributors to microtubule stability in neurons.
Keywords: microtubule, neuron, axon, dendrite, stable, labile, microtubule stability, tubulin, polyamination, acetylation, detyrosination, katanin, spastin, fidgetin, taxol, nocodazole, MAP6, tau, Alzheimer’s disease, neurodegeneration, CAMSAP, +tip
Introduction
The neuron is an extraordinary cell. In all of nature, it is the cell whose exaggerated morphology is most intimately related to its unique functions and challenges. A typical vertebrate neuron consists of a small soma (situated in the brain, spinal cord, or peripheral ganglion), several dendrites, and a single elongated axon. The axon is specialized to transmit information, while the dendrites form a receptive field for incoming information. The axon is effectively unlimited in its growth potential and can traverse great distances within the body, reaching, for example, from the spinal cord to the toes. Dendrites, by contrast, are generally short, stout, and highly branched. In addition to these morphological differences, axons and dendrites also differ compositionally. While axons contain a subset of organelles found in the soma, dendrites generally contain all of the organelles found in the soma. Most protein synthesis and organelle biogenesis occur within the soma, thus demanding highly sophisticated mechanisms for actively transporting specific proteins and organelles into each type of process. Retrograde transport of cargoes is also critically important, for example in sending signaling molecules back to the soma and also for protein degradation pathways. During development, there is a great deal of motility involved in the wiring of the nervous system, so that migrating neurons arrive at their proper locations and so that the tips of axons reach their appropriate target tissues. Thus, a number of challenges must be met for neurons to establish and maintain their morphological, functional, and compositional polarity. Meeting these challenges is the work of sophisticated architectural elements within the neuron and tightly regulated mechanisms of transport and motility. Prominent among these architectural/transport elements are microtubules.
Microtubules are abundant in neurons, occupying axons and dendrites as paraxially aligned arrays. These microtubule arrays provide a structural backbone for axons and dendrites that allows them to acquire and maintain their specialized morphologies. In addition to acting as compression-bearing struts, microtubules are the major long-distance railways along which proteins and organelles are actively transported in both directions within axons and dendrites. Microtubules are critically important for early developmental stages of the neuron, such as migration of the soma and the cue-dependent navigation of the growth cone at the tip of the elongating axon. Microtubules are also important throughout life, for the neuron to maintain its proper shape, to support axonal and dendritic transport, and to accommodate shape changes such as alterations in dendritic morphology that may correspond with cognitive plasticity even in old age. Neuronal microtubules have been the topic of many review articles over the past several years (Baas and Ahmad, 2013; Baas and Buster, 2004; Baas and Lin, 2011; Conde and Caceres, 2009; Dubey et al., 2015; Kapitein and Hoogenraad, 2015; Lewis et al., 2013; Prokop, 2013).
Microtubules are hollow polymers of tubulin subunits. Neurons, like other eukaryotic cells, contain a pool of free tubulin subunits and a pool of microtubules. These two pools dynamically exchange with one another such that microtubules undergo bouts of assembly and disassembly. In a purified tubulin preparation, microtubules undergo frequent bouts of assembly and disassembly that can be quite dramatic, with entire microtubules disassembling in events called catastrophes. In living cells, a certain portion of the microtubule mass is stabilized such that it exchanges subunits with the free tubulin pool much more slowly and potentially not at all. The terms labile and stable have been used to respectively describe the microtubule fraction that displays rapid dynamics and the microtubule fraction that does not. A balance between labile and stable microtubule fractions contributes to the morphological stability of cells, with a greater proportion of the microtubule mass being stable in cells with relatively stable morphologies, such as neurons. Both developing and adult neurons contain substantial levels of stable and labile microtubule mass, but a higher proportion of the microtubule mass is stable in adult neurons compared to developing neurons (Ferreira and Caceres, 1989; Lim et al., 1989). In many neurodegenerative diseases, there is a gradual loss of microtubule mass from neurons, and this has been assumed to be due to destabilization of the microtubules leading to their depolymerization. Many proteins and pathways have been implicated in microtubule stability, but with lingering questions as to mechanism. After decades of study, a great deal has been resolved on the issue, but there is still much that remains unknown. Summarizing what is known and not known about microtubule stability in neurons is the purpose of this article.
Microtubule organization in neurons
Before discussing microtubule stability in neurons, some background information on microtubules and how they are organized in neurons is necessary. Tubulin is a heterodimer consisting of an alpha-tubulin subunit and a beta-tubulin subunit, each of which is a different primary gene product with high affinity for one another. In vertebrates, there are generally around 6–7 alpha tubulin genes and about the same number of beta tubulin genes. Because the two subunits are not the same, the microtubule is intrinsically polar. One end, called the plus end, is favored for the addition and subtraction of subunits, while the other end, called the minus end, is less favored for these dynamics. Beta-tubulin is present at the plus-end of the microtubule, while alpha-tubulin is present at the minus-end. The polarity of the microtubule is manifested not only at its two ends, but also along the length of the polymer. This structural polarity is recognized by molecular motor proteins, which are enzymes that use the energy of ATP hydrolysis to walk along the surface of the microtubule. Cytoplasmic dynein walks toward the minus end of the microtubule, while most members of the kinesin superfamily walk toward the plus end of the microtubule. Each molecular motor protein can potentially interact with different types of cargoes, such that the polarity orientation of microtubules in different regions of the neuron is a major determinant of where particular cargoes are transported. In the axon, microtubules are nearly uniformly oriented, with their plus ends directed away from the soma, while in vertebrate dendrites, the microtubules have a non-uniform (i.e., mixed) polarity orientation (Baas et al., 1989; Baas et al., 1988; Baas and Lin, 2011). Microtubules in dendrites of some lower animals are more predominantly minus-end-distal (Stone et al., 2008; Yan et al., 2013), indicating an even more profound difference between axonal and dendritic microtubule organization.
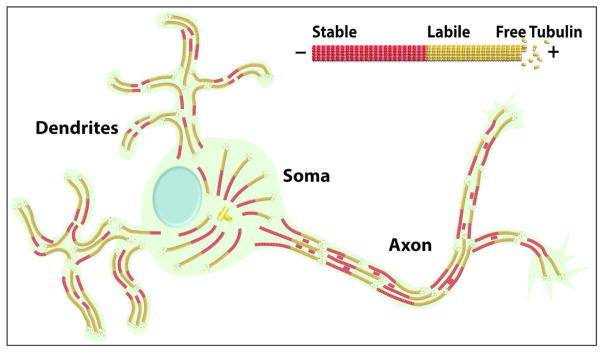
These distinct microtubule polarity patterns, shown in figure 1, are one of the most essential and earliest developmental differences to arise between axons and dendrites, and are undoubtedly of key importance for many of the compositional and morphological differences that distinguish the two types of processes. For example, dendrites contain Golgi outposts and axons do not, which makes sense because Golgi elements are transported toward minus ends of microtubules by cytoplasmic dynein (Black and Baas, 1989). Axons may become longer and more slender than dendrites because vesicular organelles that expand the cell membrane have a unidirectional vector to the tip of the growing axon via their plus-end-distal microtubules, while the same is not true of dendrites (Baas and Lin, 2011). Various other factors beyond polarity orientation contribute to the regulation of these polarity-related events, such as targeting information within the motor proteins themselves, post-translational modifications of the tubulin subunits that comprise microtubules in different neuronal compartments, and the composition of microtubule-associated proteins that decorate microtubules in different regions of the neuron.
Figure 1. Microtubule organization in the vertebrate neuron.
Schematic of typical vertebrate neuron with one axon and multiple dendrites. Microtubules are nearly uniformly oriented in the axon, and non-uniformly oriented in the dendrites. Microtubules in both the axon and the dendrites consist individually of a stable domain (shown in red) and a labile domain (shown in yellow), with the labile domain toward the plus end of the microtubule. Short mobile microtubules in the axon are entirely stable. Microtubules in the axon and dendrites vary in their length, with none of them attached to the centrosome. In the axon, a higher percentage of the total microtubule mass is stable compared to the situation in the dendrite.
In simple non-neuronal cells, microtubules are usually organized by structures such as the centrosome, which act as nucleation sites for the formation of the microtubules. The microtubules assemble with the minus end within the centrosome and the plus end growing away from the centrosome. This generates an orderly array of uniformly oriented microtubules. In developing neurons, microtubules are also nucleated at the centrosome (located in the soma of the neuron), but the microtubules are then released from the centrosome for subsequent transport into axons and dendrites (Ahmad and Baas, 1995; Ahmad et al., 1994; Ahmad et al., 1999; Yu et al., 1993). The microtubules are deployed by molecular motor proteins that move the microtubules specifically with either plus end leading (into axons and dendrites) or with minus end leading (into dendrites only). Thus, in neurons, the organization of microtubules relative to their polarity is determined not by the attachment of the microtubules to their sites of nucleation, but instead by the motor proteins that transport them into each type of neuronal process (Ahmad et al., 1998; Baas and Ahmad, 1993; Lin et al., 2012; Sharp et al., 1997; Yu et al., 1997). Recent studies suggest that local nucleation of microtubules might also occur in axons and/or dendrites, especially as the microtubule-nucleating capability of the centrosome wanes during neuronal maturation (Stiess et al., 2010); this may potentially contribute to the complexity of the mechanisms by which axonal and/or dendritic microtubule arrays are established, at least in some types of neurons in some organisms. In the case of the leading process of a migratory neuron, most of the microtubules remain attached to the centrosome (Falnikar et al., 2011), which is important for the soma to be pulled along with the leading process as it lunges forward during brain development.
Another critically important feature of the neuronal microtubule arrays is that each array consists of individual microtubules that assume a variety of different lengths and degrees of stability. The array of microtubules within an axon or dendrite extends from the soma to the tip of the process, but individual microtubules generally do not traverse the entire length of the process. Some microtubules are only a few micrometers in length (or even less than a micrometer), while others can achieve great lengths, exceeding a hundred micrometers or even hundreds of micrometers in longer axons (Bray and Bunge, 1981; Letourneau, 1982; Tsukita et al., 1982; Yu and Baas, 1994). As noted earlier, the longer microtubules are important for two reasons: they act as architectural struts that oppose the retraction of the axon or the dendrite, and they act as major long-distance railways for organelle transport. The short microtubules are important because they are highly mobile and provide the means by which tubulin is actively transported within the axon and presumably dendrites as well. A short mobile microtubule may act as a nucleation seed to give rise to a long stationary microtubule, or it may entirely or partially depolymerize to yield subunits to be used by other microtubules for their elongation. Short mobile microtubules may also act as information carriers to convey as yet unidentified proteins or signals from one region of the neuron to another (Dent and Baas, 2014).
Microtubule stability in neurons
In the early days of microtubule research, biochemical preparations of tubulin were commonly prepared from adult bovine or porcine brain, purified through cycles of warm and cold conditions. In an appropriate buffer, microtubules assemble in the warm and disassemble in the cold, allowing for progressive cycles of purification. Tubulin prepared in this fashion generally also contains an assortment of microtubule-associated proteins, which can subsequently be removed by phosphocellulose chromatography. Microtubules assembled from pure tubulin were shown to be highly dynamic, undergoing rapid phases of assembly and disassembly. Such dynamics were tempered in the presence of the microtubule-associated proteins. It was shown that in addition to the appropriate warm temperature (37°C), magnesium and GTP are also required for microtubule assembly to occur as well as a tubulin concentration that exceeds a certain critical concentration. The plus end was shown to have a lower critical concentration for assembly than the minus end. As well as cold, calcium was shown to induce disassembly of microtubules, as do drugs such as colchicine or nocodazole that bind to tubulin subunits, effectively lowering the concentration of tubulin subunits relevant to the dynamics of the microtubule.
Subsequent studies demonstrated that microtubule assembly and disassembly are governed by a mechanism known as dynamic instability (Mitchison and Kirschner, 1984). This mechanism depends upon the fact that free tubulin exists with hydrolysable GTP associated with beta tubulin. The hydrolysis of GTP-tubulin to GDP-tubulin occurs only after the tubulin subunits have assembled into the microtubule. Given that GTP hydrolysis takes some time to occur and that dynamics occur principally at the plus end of the microtubule, the older region of the microtubule toward the minus end is richer in GDP-tubulin than the newer region of the microtubule toward the plus end. If GTP hydrolysis catches up to the addition of new subunits such that there is no longer a ring of GTP-tubulins at the plus end, the microtubule undergoes rapid disassembly called catastrophe. As long as there is GTP-tubulin forming a “cap” at the plus end of the microtubule, it can keep assembling. Because of the stochastic nature of this mechanism, different microtubules within a population undergo assembly or disassembly at the same time.
In living cells, the most dynamic microtubules display rapid bouts of assembly and disassembly, but there is another feature of the model at play called selective stabilization. Microtubules can be stabilized either by the capture of their plus ends, for example by proteins and structures in the cell cortex, or by binding along the length of the microtubule of stabilizing proteins. The latter would presumably include the microtubule-associated proteins in the tubulin preparation that cycle with the tubulin, but it remains poorly understood exactly which microtubule-associated proteins are responsible for conferring stability to microtubules in living cells. When a microtubule or a region of a microtubule is stabilized, it may still undergo subunit exchange with the soluble tubulin pool, but such exchange is slow compared to the rapid bouts of assembly and disassembly characteristic of dynamically unstable microtubules.
Microtubules that undergo rapid dynamics, according to these principles, are said to be labile, while microtubules that are stabilized according to these principles [meaning that the microtubules still undergo dynamics, albeit far more slowly than the labile fraction; see (Li and Black, 1996)] are said to be stable. However, the stable fraction defined in this manner is not the only stable fraction present in neurons. During the early steps in the tubulin purification procedure, adult brain matter is chopped, homogenized and centrifuged under cold conditions, and a large pellet is discarded. In theory, all of the tubulin in these preparations should be in the supernatant if all of the microtubules were disassembled by cold, but in fact, the pellet contains a great deal of tubulin, presumably in the form of microtubules that are stable to cold. When the biochemical properties of tubulin in the cold-stable fraction were compared with the properties of the temperature-cycled tubulin, much of the tubulin in the cold-stable fraction was shown to be extremely basic in charge, as assessed by two-dimensional electrophoresis. Not only was this different from the cycling tubulin, but no tubulin isoform produced by any tubulin gene or known post-translational modification of tubulin could explain this behavior (Baas, 2013; Brady et al., 1984).
From the biochemical studies alone, it was impossible to know if the cold insoluble tubulin represented a physiological structure or some artifact of the preparation. The cold-stable fraction was also resistant to solubilization by calcium or anti-microtubule drugs, and hence if this fraction represented microtubules, these microtubules were far more stable than the stable category described above. Such microtubules would presumably be so stable as to be non-dynamic. It was proposed that in the axons of adult neurons, the cold-stable tubulin exists as multiple distinct regions on longer microtubules that are not entirely cold stable (Brady et al., 1984). Subsequent electron microscopic work provided some support for this idea (Sahenk and Brady, 1987), although it was never firmly resolved whether individual MTs contain multiple stable regions or just one stable region. The functional hypothesis was that an especially stable tubulin fraction would serve to preserve the organization of the microtubule array, acting as nucleating elements to ensure that microtubule assembly occurs from pre-existing microtubules rather than haphazardly. An increase in the levels of cold-stable tubulin as neurons mature was posited to contribute to the decline in morphological plasticity that occurs as axons achieve their adult wiring. In cultured neurons from embryos or newborn animals, only about 6% of the microtubule mass is cold stable (Black et al., 1984), while in the adult brain, this fraction has not been quantified but is notably higher (Black et al., 1984).
After almost three decades of uncertainty as to what causes cold stability, recent studies have shown that the unique properties of cold-stable tubulin can be attributed to polyamination (Song et al., 2013). This post-translational modification, which is catalyzed by transglutaminases, is known to make proteins more basic, whereas most modifications make proteins more acidic or are neutral. In addition, polyamination usually causes proteins to become stable, insoluble and resistant to proteolysis, and transglutaminase activity is known to increase as neurons mature [for discussion, see (Song et al., 2013); (Baas and Ahmad, 2013)]. Polyamination contrasts with other known modifications of tubulin associated with microtubule stability, namely acetylation and detyrosination, as these other modifications do not confer stability but rather accumulate on more stable microtubules or microtubule regions because they are long-lived (Garnham and Roll-Mecak, 2012; Song and Brady, 2015).
Thus, there are at least three stability categories of microtubule polymer in neurons: labile, stable, and cold-stable. The labile category is relatively deficient in acetylated, detyrosinated and polyaminated tubulin, while the stable category is rich in acetylated and detyrosinated tubulin but still deficient in polyaminated tubulin. The cold-stable category is rich in polyaminated tubulin and presumably acetylated and detyrosinated tubulin as well. The stable category is stabilized by accessory factors such as microtubule-associated proteins, and still undergoes dynamics, albeit more slowly compared to the labile category. The cold-stable category is stabilized by polyamination of its tubulin subunits, and is so stable as to not undergo dynamics. Any individual microtubule could theoretically consist of all three stability classes in different regions of the microtubule.
There is another level of complication with regard to the issue of microtubule cold-stability. As defined above, the cold-stable fraction is that fraction of the microtubule mass that is resistant to depolymerization by cold in biochemical preparations. However, the same rules about microtubule cold stability do not apply to cells that are intact. When intact cells are exposed to ice-cold temperatures, microtubules can become resistant to cold-induced depolymerization, and this is because a protein now called MAP6 (originally called STOP for stable tubule only peptide) binds to the microtubules in response to the cold temperatures (Delphin et al., 2012). In neurons, this effect is especially profound and can result in a near complete preservation of the microtubules against loss in the presence of cold (Baas et al., 1994; Jones et al., 1980). Such cold stability is lost in cells experimentally depleted of MAP6. In biochemical preparations, MAP6 no longer affords microtubules such protection against cold, so the only microtubule fraction that is cold stable is that stabilized by polyamination of the tubulin itself. The cold-stability afforded to microtubules in vivo by MAP6 is presumably important for animals that are challenged with especially cold temperatures, but the significance of the MAP6 effect on microtubules probably extends beyond that, given that most warm-blooded animals are unlikely to experience notable drops in temperature in their neurons. [MAP6 might play a role in conferring stability on the stable microtubule domains, which is suggested by a preferential enrichment of MAP6 on stable microtubule domains of cultured neurons that have not been exposed to cold; see (Slaughter and Black, 2003)]. Mutations or knockouts of MAP6 result in rodents with schizophrenia-like or neuroleptic behaviors (Andrieux et al., 2002; Volle et al., 2013). Such cold-stability in cells is not afforded by tau or MAP2 or presumably other conventional MAPs but rather is a specialized property of MAP6. Even highly overexpressed tau or MAP2 that stabilizes cellular microtubules against any detectable depolymerization by nocodazole provides no stabilization of the microtubules against cells being exposed to cold (Baas et al., 1994). For the purposes of the discussion here, the term ‘cold stable’ is used to describe the polyaminated fraction, as MAP6-binding appears to be a dynamic process that can temporarily provide cold stability to otherwise labile microtubules. It should also be noted, however, that the cold-stable fraction (i.e., polyaminated fraction) is stable to all factors that are known to cause microtubules to disassemble, so stability to cold, per se, should not be taken as the main distinguishing feature of this fraction. Perhaps in the future, it will be wise to refer to the cold-stable fraction as the polyaminated fraction.
On the flip side, there is some evidence that enrichment of microtubules in one particular isotype of tubulin, namely beta-III tubulin, might make them less stable than microtubules without beta-III-tubulin, all other things being equal. In vertebrate cells (as indicated earlier), there are roughly 6–7 alpha tubulin genes and 6–7 beta tubulin genes, with their tubulin gene products intermingling to build the microtubules of the cells of the body. Beta-III tubulin is neuron-specific, the only exception being Sertoli cells, and hence histologists commonly use beta-III tubulin as a marker to distinguish neurons from other cell types (Katsetos et al., 2003b). There are human diseases that arise from mutations in beta-III tubulin that afflict the nervous system (Tischfield et al., 2010). In vitro studies show that microtubules assembled from beta-III tubulin (together with an alpha tubulin) are more dynamic than microtubules assembled from other beta tubulins together with the same alpha tubulin (Panda et al., 1994). It is unknown if this is true in the presence of the myriad of microtubule-related proteins in cells or of the microtubules containing various dosages of beta-III tubulin together with other tubulin isotypes (as is the case in neurons), but the results suggest that perhaps one reason why neurons express beta-III tubulin is to allow for more microtubule dynamicity than would otherwise be possible in the face of whatever microtubule stabilizers are present in neurons. For example, neuronal progenitors that are already committed to a neuronal fate and already expressing neuronal microtubule-related proteins are still undergoing cell division, and the presence of beta-III tubulin might be important for the microtubules to be sufficiently dynamic in order to participate in mitosis in the face of those neuronal microtubule factors (Haendel et al., 1996). The same might be true, for example, of growth cone guidance, which requires microtubules to be very dynamic at the tip of the growing axon (Challacombe et al., 1997; Lowery and Van Vactor, 2009; Tanaka et al., 2005). An issue with beta-III tubulin is that it tends to make cellular microtubules resistant to taxol treatment for cancer therapy, which is problematic in that tumors of the brain tend to strongly express beta-III tubulin (Katsetos et al., 2003a).
Individual microtubules in the axon consist of domains that differ in stability
While the mystery of the cold-stable tubulin fraction remained on the back burner for years, the general idea of stable microtubules acting as microtubule-nucleating elements in the axon became popular. This thought is attractive because microtubules in axons and dendrites are not attached to any known nucleating structure such as the centrosome, but rather are free at both ends and yet highly organized. The idea that each microtubule had one or more stable regions that would resist depolymerization and hence maintain the polarity pattern of the microtubule array theoretically solved many problems for the neuron. A great deal of progress was made on this issue, especially in the 1980s and 1990s, using cultured fetal or newborn chick or rat neurons as an experimental model, as they are amenable to a range of experimental manipulations and microscopy approaches. It had been known that cultures of primary neurons treated with anti-microtubule drugs displayed far less microtubule loss over similar periods of time than did cultures of simpler cells such as fibroblasts. However, it was unknown whether the microtubule mass that remained after 15–30 minutes in such drugs was any more or less stable than the microtubule mass that had depolymerized, as the entirety of the microtubule mass could theoretically have been undergoing slow gradual depolymerization. To address this matter quantitatively, cultures were treated with nocodazole for various times, ranging from 15 minutes to 6 hours, and then the levels of microtubule mass in axons were assessed by quantitative electron microscopy (Baas and Black, 1990; Baas et al., 1991). Roughly half the microtubule mass in the axon depolymerized within the first 15 minutes, while the remaining microtubule mass depolymerized very slowly, with some remaining even after 6 hours in the drug. On the basis of quantitative analyses using this approach, it was concluded that roughly 58% the microtubule mass in the axons of these cultured neurons is stable while roughly 42% is labile. As indicated earlier, a small portion of the stable fraction in developing neurons (and a larger fraction in adult neurons) is polyaminated and hence virtually non-dynamic.
These studies, it should be noted, were conducted on the middle regions of axons of rat sympathetic neurons that had been grown on rat-tail collagen for several days. Other studies indicate that the microtubule mass in the distal region of the axon, near the growth cone, is especially enriched with the labile microtubule fraction, and that younger axons, grown for just a day in culture, have a higher proportion of the labile fraction, even in their middle regions. Furthermore, the type of neuron may also be a factor, as some studies suggest a higher fraction of the microtubule mass is labile in hippocampal neurons compared to sympathetic neurons. Nevertheless, the overall theme of axons containing stable and labile categories of microtubule mass seems to be broadly applicable. The idea that the stable and labile categories exist as regions on individual microtubules remained a speculation, however, until confirmed by immunoelectron microscopic studies in the early 1990s (Baas and Black, 1990; Baas et al., 1991).
Immunoelectron microscopy with an antibody against tyrosinated tubulin (tubulin that had not yet been post-translationally detyrosinated) revealed a remarkable correlation between antibody labeling and the stability category of the microtubules in the axons of cultured rat sympathetic neurons (Baas and Black, 1990; Baas et al., 1991). In control neurons, roughly half the microtubule profiles labeled densely for tyrosinated tubulin while the other half of the profiles labeled not at all. (The word “profiles” is used to indicate regions of microtubules appearing in thin sections of the neurons prepared for transmission electron microscopy, as portions of any individual microtubule may appear on different thin sections.) Almost no labeled profiles appeared in axons of neurons treated for 15 minutes with nocodazole, indicating that the labeled profiles correspond to the labile category of microtubule mass. Small numbers of labeled profiles remaining after 15 minutes of nocodazole treatment were interpreted as “newly stabilized,” such that they had not yet had sufficient time to become detyrosinated after being stabilized (Baas et al., 1993). Occasionally, in the electron micrographs of axons not treated with the drug, images were obtained of microtubule profiles displaying a sharp transition between a labeled region and an unlabeled region, with the unlabeled region always appearing proximal in the axon to the labeled region. In addition, when the neurons were treated with nocodazole, and then rinsed free of nocodazole for a few minutes, all new microtubule assembly in the axon occurred by elongation of labile regions (labeled for tyrosinated tubulin) from the plus ends of the stable regions (not labeled for tyrosinated tubulin) (Baas and Ahmad, 1992). Collectively, these results indicate that microtubules in the axon consist of a stable domain toward the minus end of the microtubule and a labile domain toward the plus end of the microtubule, with all microtubule assembly in the axon presumably restricted to the elongation of existing stable domains. Later studies using immunofluorescence confirmed the domain structure of axonal microtubules, using a preparation that splayed apart the bundled microtubules in the axonal shaft so that improved resolution could be achieved on individual microtubules (Brown et al., 1993).
It remains unknown how exactly these findings on developing axons relate to the earlier findings on adult axons, wherein microtubules were posited to contain multiple distinct stable regions, presumably with intervening labile regions. Practically, the intervening labile regions would be just as stable, though, because disassembly of the microtubule occurs only from the ends. However, neurons are rich in microtubule-severing proteins that can break the microtubule along its length (see later), the effects of which would be very different in the case of a long continuous stable domain versus a microtubule with intermittent stable regions.
Given the polarity orientation of axonal microtubules, these results provide a satisfactory explanation for why microtubule domains reaching into the distal region of the developing axon contiguous with the growth cone are mainly labile. The question arises as to whether microtubules reaching into the growth cone appear to be especially labile simply because of the coalescence of labile domains from many different microtubules, or if the labile domains in the growth cone are even more labile than the labile domains in the axonal shaft. To resolve this issue, the concentration of the antibody against tyrosinated tubulin was titrated, and it was found that the relative amount of tyrosinated tubulin with microtubules in the growth cone region is greater than the amount in the labile domains of microtubules in the axonal shaft. Relatively higher levels of tyrosinated tubulin were found in the microtubules in the growth cone region, suggesting that all labile domains are not equally labile, with those in the distal region of a growth axon being especially labile (Ahmad et al., 1993). More work needs to be done on this issue because there is still uncertainty as to how drug stability, normal rates of turnover and tyrosination state of the microtubules all relate to one another. For example, the growth cone could simply have a different balance of the enzymes that control the tubulin tyrosination cycle. However, earlier classic studies reported growth cone microtubules to be special in their dynamic properties (Bamburg et al., 1986), lending credence to the view that these observations on tyrosinated tubulin indeed reflect the existence of an especially labile/dynamic class of microtubule polymer extending into the growth cone.
It is not known whether unacetylated tubulin would be as good of a marker for labile domains in the axon as tyrosinated tubulin, as no antibodies exist to unacetylated tubulin. Antibodies to either detyrosinated or acetylated tubulin show enrichment in stable domains, but the immunoreactivity also appears in the labile domains (Baas et al., 1991). Apparently both stable and labile domains have modified subunits whereas the principal difference is that stable domains have demonstrably less unmodified tubulin, such that staining for tyrosinated tubulin is a good marker.
Studies using the same drug-based approach on the dendrites of cultured rat sympathetic neurons revealed that dendrites also have populations of microtubule mass that depolymerize in the presence of nocodazole at the same rates as the labile and stable categories of microtubule mass in the axon (Baas et al., 1991). However, the labile fraction comprises 75% of the total microtubule mass while the stable fraction comprises only 25%. Curiously, labeling for tyrosinated tubulin is not a good marker that distinguishes stable and labile microtubule mass in the dendrite, as even the stable fraction labels fairly strongly. Why the stable fraction in dendrites never achieves such a degree of detyrosination remains a mystery, but might relate to levels or activity of the enzymes involved in the tyrosination/detyrosination cycle. Interestingly, after nocodazole treatment to depolymerize the labile fraction, the proportion of the microtubule mass that is plus-end-distal in the dendrites of cultured rat sympathetic neurons jumps from 56% to 74%, suggesting that, if dendritic microtubules have a domain structure similar to axonal microtubules, the labile domains are relatively longer in the case of the minus-end-distal microtubules compared to the plus-end-distal microtubules (Baas et al., 1991). Dendritic microtubules probably do have a domain structure similar to axonal microtubules, as biotin-tubulin injected into cultured rat sympathetic neurons assembles from microtubules of both orientations in the dendrite with no indication of de novo nucleation in either the axon or the dendrite (Wang et al., 1996; Yu et al., 1996). Figure 1 shows these ideas on the domain structure of axonal and dendritic microtubules.
Microtubule end-targeting proteins
One of the most exciting areas of contemporary microtubule research revolves around a category of proteins called +tips (Akhmanova and Steinmetz, 2015). These proteins associate with the plus end of the microtubule but only during bouts of assembly. Some have their own affinity for the plus end of the microtubule, by recognizing the GTP cap, while others have an affinity for other +tips and become +tips themselves for that reason. It has been quipped that there is a “zoo” of +tips at the microtubule’s plus end, and these proteins (which include EB1, EB3, CLIPs, CLASPs and others) have been the topic of extensive recent reviews (Akhmanova and Hoogenraad, 2005). Fluorescently conjugated +tips have been a boon to live-cell imaging of microtubule assembly and organization in many cell types, so much so, that it is sometimes easy to forget that these proteins have important functions of their own. In neurons, the +tips can influence the assembly dynamics of the labile domains, and integrate their behavior with, for example, other elements of the machinery of growth cone behavior (Bearce et al., 2015). They can also be important players in the “search and capture” mechanism of microtubule-stabilization, wherein a microtubule undergoing bouts of dynamic instability can be selectively stabilized by plus end capture by other cellular structures, usually in the cell cortex. This can be important, for example, in stabilizing microtubules on the side of a cell corresponding to the direction of migration, in response to an environmental cue. In this regard, +tips have been studied in the context of growth cone behaviors (Neukirchen and Bradke, 2011).
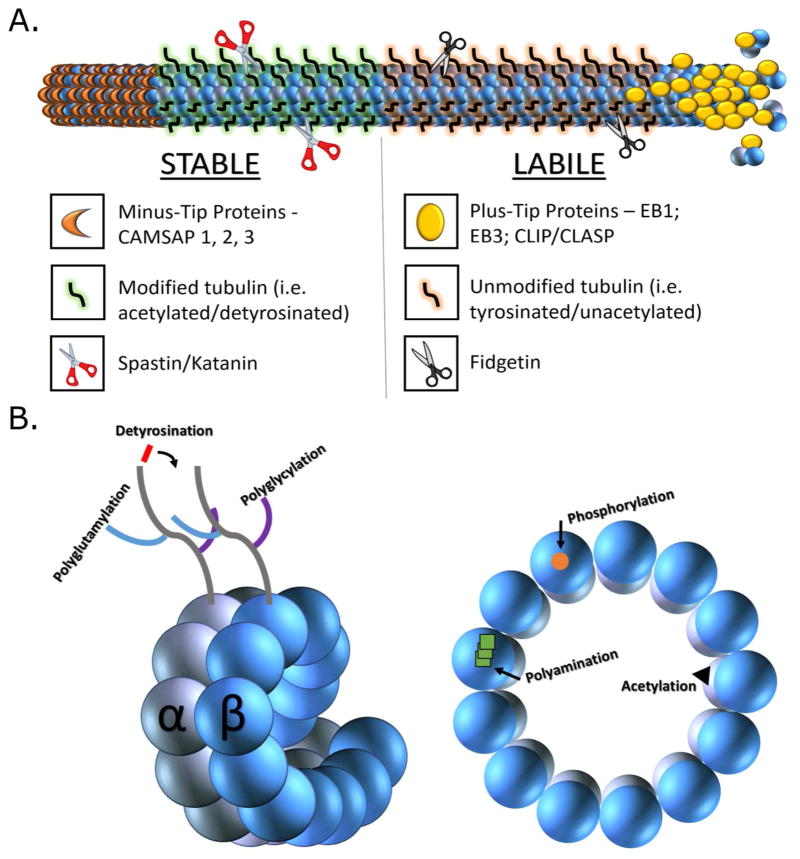
A long-standing mystery is why the minus ends of microtubules in the neuron, free from the centrosome, seem to be almost completely non-dynamic rather than simply being slower in their dynamics, as is the case of minus ends compared to plus ends of microtubules in vitro. This is the case in most cells that have free minus ends of microtubules, not just neurons. The existence of a minus-end cap has long been suspected but identification of such a cap has been elusive. In neurons, it might seem reasonable to attribute the stability of microtubule minus ends to the polyaminated tubulin fraction, but this is questionable, as developing neurons contain so little of this fraction. Recent studies implicate a new category of proteins called calmodulin-regulated spectrin-associated proteins (CAMSAPs) (Akhmanova and Hoogenraad, 2015). CAMSAPs, sometimes referred to as minus-end binding proteins, have been shown to bind to free minus-ends of microtubules in various cell types, and thereby block the addition or subtraction of subunits from that end of the microtubule (Jiang et al., 2014). Such a cap appears to be especially important for inhibiting the capacity of microtubule “depolymerases” such as kinesin-13 to tear apart the microtubule from its minus end, but would presumably also suppress the dynamic addition or loss of subunits from the minus end as occurs in test tube experiments on purified tubulin. Figure 2 shows the domain structure of an axonal microtubule, together with information on tubulin post-translational modifications, as well as microtubule end-targeting proteins at each of its two ends.
Figure 2. Stability domains of axonal microtubules and related molecules and modificiations.
Microtubules in the axon individually consist of a stable domain toward the minus end of the microtubule and a labile domain toward the plus end of the microtubule. Panel A schematically indicates the two domains, which compositionally differ in their levels of post-translationally acetylated and detyrosinated tubulin subunits. Modified subunits (shown with green highlighted tubulin ‘tails’) are enriched in the stable domain while unmodified subunits (shown with orange highlighted tubulin ‘tails’) are enriched in the labile domain. The tip of the labile domain (plus end of the microtubule) is associated during bouts of microtubule assembly with plus-tip proteins such as EB1, EB3, CLIP and CLASP. The tip of the stable domain (minus end of the microtubule) is associated with minus-tip proteins termed CAMSAPs, which often take on a linear structure as they compete for tubulin subunits during the formation of a cap that prevents microtubule disassembly from the minus end. The two domains differ in their interactions with various microtubule-related proteins, such as the severing proteins katanin and spastin which target stable domains and the severing protein fidgetin, which targets labile domains. Panel B shows a more detailed view of tubulin post-translational modifications (detyrosination, acetylation, phosphorylation, polyglycylation and polyglutamylation) and where they occur in the tubulin subunits comprising the microtubule lattice. Note: In dendrites, the stable and labile regions of microtubules do not differ in such a pronounced way in their content of post-translationally modified tubulins, as is the case in the axon.
Three CAMSAPs have been identified in vertebrates, and these are termed CAMSAP-1, CAMSAP-2, and CAMSAP-3. CAMSAP-3 was originally called Nezha, and identified in association with adhesion plaques in non-neuronal cells (Meng et al., 2008). Rather than appearing as a simple dot on the end of the microtubule, immunostained or tagged CAMSAPs, when ectopically expressed, often appear as short stretches along the microtubule toward its minus end. This suggests a mechanism by which CAMSAPs compete with tubulin subunits for the minus end of the microtubule, until enough CAMSAP accumulates to limit any further dynamics. In a recent study, it was shown that CAMSAP2 is the predominant family member in vertebrate neurons, and that depletion of CAMSAPs from neurons results in detrimental effects on microtubule levels and stability as well as stunted neuronal morphologies (Yau et al., 2014).
Microtubule-severing proteins
Microtubule-related enzymes that cut or break microtubules are called microtubule-severing proteins. They are AAA proteins that form hexamers on the surface of the microtubule, and yank on a tubulin subunit to extract it from the microtubule lattice, causing the microtubule to break (Roll-Mecak and McNally, 2010; Roll-Mecak and Vale, 2006). Such breakage or “severing” of microtubules can occur near their minus ends within the centrosome to release the microtubule so it can then be transported into an axon or a dendrite. Severing can also occur at the plus end, in which case, subunits are peeled off the more dynamic end of the microtubule. But what happens if the severing protein breaks the microtubule somewhere along its length? If the severing event occurs in the stable domain, the result would be two new microtubules, one with a stable and labile domain, and the other being exclusively a stable fragment that can then assemble a new labile domain. In this fashion, severing in the stable domain creates new microtubules. This is important as the axon grows longer and the dendritic arbor grows more complex, and especially during branch formation, to supply new microtubules after delivery of new microtubules from the centrosome becomes less practical to suit the needs of the developing or mature neuron. If the severing event occurs in the labile domain, however, the result would be very different, as there would be one microtubule with a stable domain and a shorter labile domain, but the microtubule fragment without a stable domain would presumably vanish as it would completely depolymerize into subunits (Jean and Baas, 2013). Thus, severing in the labile domain would not create new microtubules, but rather would pare away the labile domains, keeping them shorter than they would otherwise be. It has been posited that certain microtubule-severing proteins target the stable domains and other microtubule-severing proteins target the labile domains (Baas and Ahmad, 2013). If this is the case, expression levels and activities of these various severing proteins could be tightly regulated to enable the microtubule array of the axon to expand during axonal development, and then to tamp down the expansion once the axon has reached its target.
The best known of the microtubule-severing proteins are katanin and spastin, both of which have a preference for stable domains. This preference is dictated by the specificity of these two severing proteins for regions of microtubules that are rich in tubulins that have been post-translationally acetylated or polyglutamylated (Lacroix et al., 2010; Sudo and Baas, 2010). Suppressing katanin or spastin has deleterious effects on axonal growth and branching (Ahmad et al., 1999; Karabay et al., 2004; Qiang et al., 2010; Yu et al., 2008), as would be expected, given the important roles these proteins play in generating new microtubules via the severing of existing ones. Whether microtubule-severing proteins exist that favor the labile domain has not been extensively studied, but new evidence suggests that fidgetin has this preference, targeting labile domains through a specificity for tubulins that are not post-translationally acetylated (Baas and Ahmad, 2013). Collectively, these observations suggest a functional relationship between the stability properties of microtubules and the effects of microtubule-severing proteins in the neuron (see figure 2). In adult neurons, the existence of microtubule-severing proteins may be especially relevant to the patches of cold-stable (polyaminated) tubulin that have been posited to exist along the length of an individual microtubule, as such regions might be preserved as fragments in the face of severing events that would otherwise result in complete loss of the microtubule.
Live-cell imaging of cultured neurons has now revealed that microtubules moving in these axons are very short relative to the range of microtubule lengths within the array. Mobile microtubules are generally about 7 micrometers in length (He et al., 2005; Wang and Brown, 2002), and are believed to arise from the severing of longer microtubules by enzymes such as katanin or spastin. They would presumably be the remnants of the stable domains, as explained above, but further yet, they could represent microtubules severed down to their cold-stable regions. Curiously, in bouts of imaging of around 15 minutes, these short mobile microtubules display no obvious length changes, which suggest that they may be unusually stable. Eventually, to serve the function of being a transportable microtubule-organizing center, they would have to begin to elongate again. Whether some would have to shorten again in order to convey tubulin for other microtubules to elongate is debatable, as the short stable microtubules could theoretically convey tubulin as labile domains growing from them, although such a possibility has not yet been appreciated in the live-cell imaging. The stability of the short mobile microtubules may also be important for regulating their transport properties, as the relevant motor protein may recognize factors such as CAMSAPs or tubulin polyamination.
Microtubule stability and the tubulin code
A long-standing mystery of microtubule biology has been why cells so intricately regulate tubulin post-translational modifications that do not confer stability on microtubules but often reflect their stability. Evolutionarily well conserved enzymes exist that confer and also reverse the modification, often with the modification occurring on the microtubule and its reversal occurring on the free tubulin subunit. A great deal of evidence now supports the hypothesis that post-translational modifications of tubulin impose a code on the microtubule lattice called the tubulin code (Garnham and Roll-Mecak, 2012; Yu et al., 2015). The idea is that many (but not all) microtubule-related protein have a preferential affinity for microtubules (or domains of microtubules) that are relatively enriched or deficient in certain modified tubulins. For example, kinesin-1 displays a preference for microtubules rich in detyrosinated and acetylated tubulins, while kinesins 5 and 13 display a preference for microtubules rich in tyrosinated tubulin (Kahn et al., 2015; Peris et al., 2009; Sirajuddin et al., 2014);. Thus, the stability properties of a microtubule (or domain of a microtubule) may make it a more or less favored substrate for various proteins such as molecular motors. Other examples of proteins sensitive to the tubulin code include microtubule-severing proteins, as discussed earlier (Lacroix et al., 2010; Leo et al., 2015; Sudo and Baas, 2010), and certain +tips that have a preference for tyrosinated over detyrosinated tubulin (Peris et al., 2006). Thus, whatever causes a microtubule (or microtubule domain) to be more or less stable may result in an alteration of its “tubulin code” that, in turn, causes the microtubule or (microtubule domain) to preferentially interact with a particular subset of microtubule-related proteins. One reason why this is important is that often when experiments demonstrate a functional role for the stable or labile microtubule fraction, it may not be the stability properties of the microtubule, per se, that is relevant so much as the composition of the microtubule (i.e. its code of tubulin modifications) that accompany its stability properties.
Microtubule stability in nervous system injury
Regeneration of injured adult axons is limited, particularly in the central nervous system (CNS). This is because injured axons tend to retract, because they encounter obstacles such as scar tissue and inhibitory molecules, and because their growth rates do not match that of a juvenile axon. There has been attention in recent years on microtubules as promising targets for coaxing injured adult axons to regenerate. Interestingly, microtubule-stabilizing drugs have been shown to positively impact the regenerative capacity of injured adult CNS axons (Hellal et al., 2011; Ruschel et al., 2015; Sengottuvel et al., 2011). However, these results are somewhat surprising because the dynamic properties of microtubules are important, especially in the distal tip of the axon, for the capacity of the axon to form a viable growth cone, to turn properly in response to external cues, and to grow with the vitality typical of the developing nervous system (Bradke et al., 2012; Challacombe et al., 1997; Lowery and Van Vactor, 2009; Tanaka et al., 1995). Such observations would suggest that the more intuitive approach to coaxing axonal regeneration would be increasing the proportion or the amount of labile microtubule mass in the adult axon, especially in its distal area near the growing tip (Bradke et al., 2012). Other work indicates that post-translational tubulin modifications are relevant to axonal regeneration, as it appears that axons regenerate better in the peripheral nervous system (PNS) because the microtubules in the damaged region of the axon become less acetylated in response to injury (Cho and Cavalli, 2012). Such a reduction in microtubule acetylation does not occur in the CNS, suggesting that tubulin modifications that accompany microtubule stability negatively impact the capacity of the axon to regenerate.
Microtubule-stabilizing drugs increase microtubule acetylation and other tubulin modifications, suggesting that the positive effects of these drugs are not due to recapitulating the mechanisms of axonal growth that occur during development. One possibility is that stabilized microtubules may enable the tip of the axon to push the axon through normally inhibitory environments, but that kind of mechanism is far askew of how the normally dynamic growth cone of the axon functions during development. Recent work has not entirely reproduced the original results with these drugs (Popovich et al., 2014), and suggests that the observed boosts in axonal growth may be due to effects of the drugs on neighboring non-neuronal cells that form the growth-inhibitory glial scar. In order to add more labile microtubule mass to the regenerating axon, proteins that tamp down the expansion of labile domains may be powerful targets for drug development. As noted earlier, fidgetin has been identified as a microtubule-severing protein that pares back labile domains of microtubules in vertebrate neurons (Leo et al., 2015), and hence might be an especially good target for future drug development.
Microtubule stability in nervous system disease
Neurodegenerative diseases are often associated with a gradual loss of microtubule mass from axons and dendrites. Such microtubule loss is best documented in diseases called tauopathies, in which tau is both hyper-phosphorylated and abnormally acetylated. As a result, tau dissociates from microtubules (Duan et al., 2012; Yoshiyama et al., 2013). Pure tauopathies are caused by mutations in tau that render it prone to such effects. Alzheimer’s disease, the most common neurodegenerative disease, does not involve mutations in tau, but rather tau becomes hyper-phosphorylated and acetylated in response to abnormal amyloid-β. Schematic illustrations suggest that microtubules disintegrate as they lose tau, presumably by a loss-of-function mechanism, as tau is classically considered a microtubule-stabilizing protein. Such a mechanism would suggest that stable domains of microtubules become destabilized, hence increasing the proportion of the microtubule mass that is labile, which would then somehow lead to a degradation of the microtubule mass. Given that the loss of microtubule mass occurs gradually over years, it is not unreasonable that the detachment of one of many microtubule stabilizers could explain the gradual microtubule loss that occurs during the disease. Another loss-of-function model for tauopathy posits that detachment of tau from microtubules causes them to degrade not by stable domains becoming labile, but rather because microtubules when not bound to tau become more sensitive to microtubule-severing proteins, mainly katanin (Qiang et al., 2006; Sudo and Baas, 2011). A completely different possibility is a gain-of-function mechanism wherein the abnormal tau, either in the form of soluble protein or in the form of abnormal filaments, produces toxicity that can have myriad ill effects on the axon, including microtubule loss (Kanaan et al., 2012). This could be due, for example, to the hyper-activation of kinases that regulate other microtubule-regulatory proteins, or the abnormal tau filaments could sequester other proteins that normally contribute to microtubule stability (LaPointe et al., 2009). A recent study suggests that when tau abnormally enters dendrites during Alzheimer’s disease, the dendritic microtubules degrade because they become more polyglutamylated and hence more sensitive to spastin (Zempel et al., 2013). Loss-of-function and gain-of-function mechanisms may apply to a number of different neurodegenerative disorders in which loss of microtubule mass or a change in microtubule stability has been documented, including Amyotrophic Lateral Sclerosis, Hereditary Spastic Paraplegia, Parkinson’s disease, and others (Cappelletti et al., 2015; Coyne et al., 2014; Fanara et al., 2007; Solowska et al., 2014).
In recent years, there had been enthusiasm about the idea that neurons afflicted with tauopathies and other neurodegenerative diseases may benefit from a preservation of microtubule mass by treatment with microtubule-stabilizing drugs. This idea is buoyed by work on animal models for neurodegenerative diseases and injuries in which positive effects of microtubule-stabilizing drugs have been documented using methods ranging from histology to behavior (Brunden et al., 2010; Hellal et al., 2011; Sengottuvel et al., 2011; Zhang et al., 2012; Zhang et al., 2005). In human patients, there are potential problems of increasing microtubule stability throughout the neuron and throughout the entire nervous system, if the drugs are taken systematically. A recent study on spartin, a protein that when mutated causes hereditary spastic paraplegia, indicates that in some cases axonal degeneration may be due to too much stabilization of microtubules (Nahm et al., 2013); this serves as a reminder that the labile microtubule fraction is just as important as the stable fraction (Carrillo et al., 2013). Indeed, a broad theme of the present article is that both stable and labile domains of microtubules are functionally important, with each domain having its own roles to play, and interacting preferentially with a different complement of microtubule-related proteins. Proponents of the microtubule-stabilization strategy would argue that the drugs will be introduced at concentrations sufficiently low that they would not have deleterious effects, but at the same time would have sufficiently positive effects to correct for the microtubule abnormalities that accompany the disease. A clinical trial for Epothilone D conducted on patients with mild Alzheimer’s disease ended in 2013 and evaluation of the drug was discontinued (Zempel and Mandelkow, 2015).
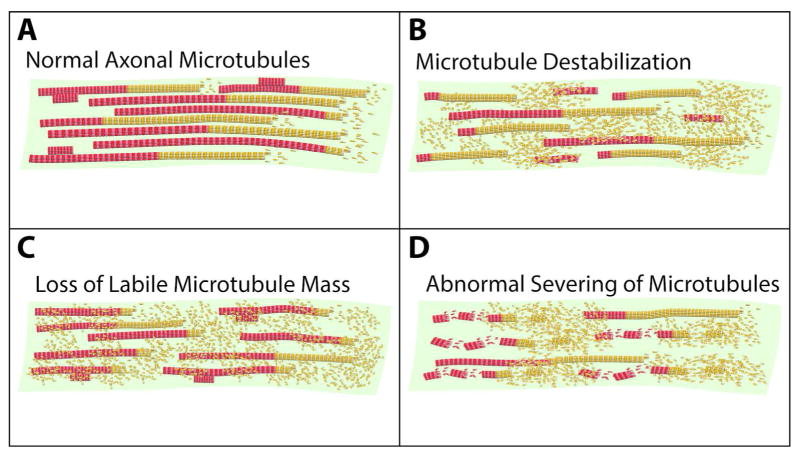
A key issue with regard to the microtubule loss that occurs during neurodegenerative diseases is whether the loss is principally of the stable fraction or the labile fraction. The general assumption has been that the stable fraction becomes labile and as a result is prone to disassembly. However, a different possibility is that the labile fraction is preferentially lost, leaving the neuron with less total microtubule mass. In this case, what microtubule mass that is left would be predominantly stable. If the latter is the case, microtubule-stabilizing drugs may not be an effective strategy because such drugs would further stabilize the microtubule fraction that is already stable, while stabilizing the labile domains so that they may not be able to fulfill their normal functions. In a recent study on the brains of deceased Alzheimer’s patients, it was determined via quantitative Western blotting that the levels of total alpha tubulin were diminished relative to non-diseased brain, and so too were the levels of acetylated tubulin. However, the ratio of acetylated to total tubulin was increased, which would be consistent with the idea that the microtubule loss is of the predominantly labile regions (Tsushima et al., 2015; Zhang et al., 2015). If this is the case, just as with nerve injury discussed above, the more effective strategy may be one that increases labile microtubule mass. Figure 3 shows three potential scenarios of microtubule loss during neurodegeneration, namely microtubule destabilization, preferential loss of labile domains, and abnormal microtubule severing.
Figure 3. Hypothetical mechanisms of microtubule loss from neurons during neurodegenerative diseases.
Panel A shows the normal situation in the axon with individual microtubules displaying stable and labile domains. Panel B shows a scenario for potential loss of microtubule mass during neurodegeneration in which the stable domains are gradually destabilized, thus shifting individual microtubules to shorter stabile domains and longer labile domains that undergo greater depolymerization. Panel C shows enhanced depolymerization of the labile domains, without destabilization of the stable domains. Panel D shows microtubule loss due to increased microtubule severing. Severing in the labile domain results in complete depolymerization of the resulting labile microtubule fragments. Severing in the stable domain produces stable fragments with some accompanying depolymerization during the severing event. These three mechanistic possibilities could also apply to microtubule loss in dendrites.
Methods for assessing microtubule stability in neurons
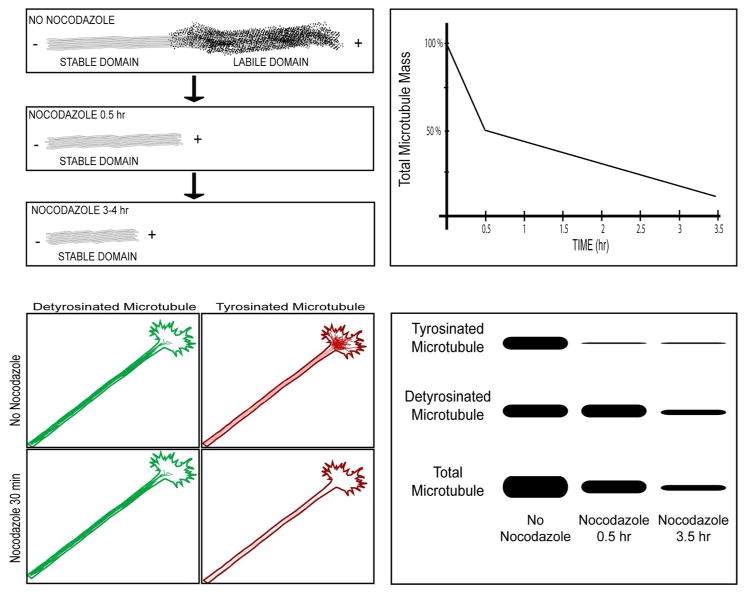
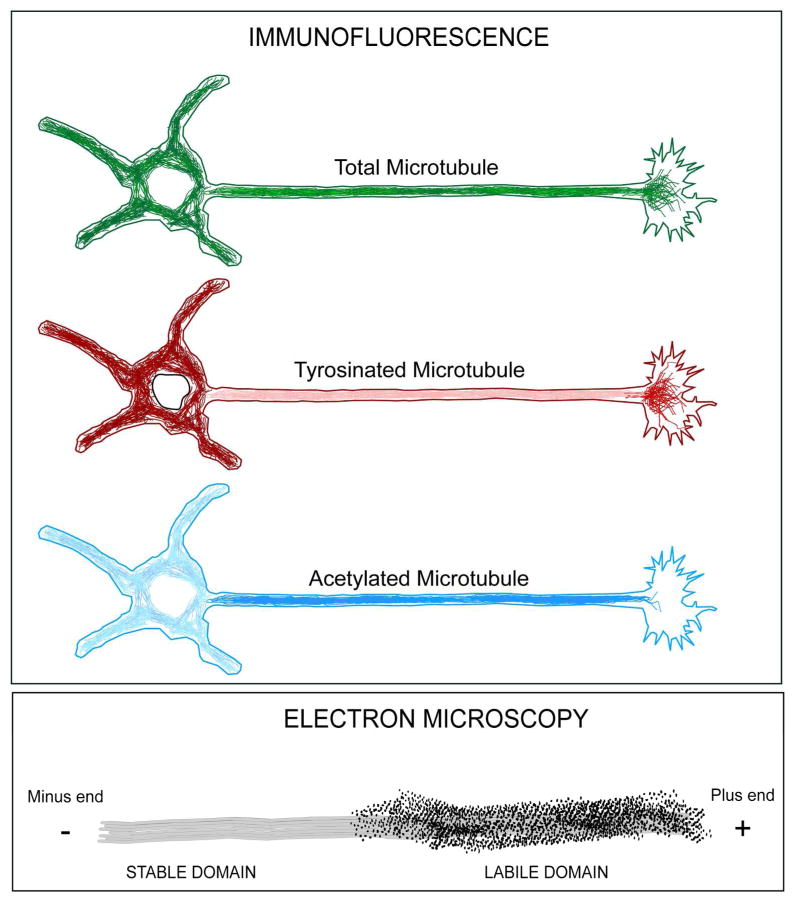
For experimental analyses, the question arises as to how to quantify and visualize the stable and labile microtubule fractions in axons and dendrites. The nocodazole approach from the late 1980s and early 1990s is a good one for studies on cultured neurons (Baas and Black, 1990; Baas et al., 1991), but has not been adapted to studies on intact nerve tissue. In this method, discussed earlier, cultures are treated with nocodazole for various amounts of time and then the microtubule levels are quantified by electron microscopy, quantitative immunofluorescence, or Western blotting, and the data are graphically plotted as total microtubule mass (as a percentage of control levels) versus time in the drug. The drug-induced diminution in microtubule mass is biphasic, with the labile fraction lost during the first 15–30 minutes and the stable fraction decaying much more slowly, over a period of hours (figure 4). Immunostaining for acetylated or detyrosinated tubulin is a relatively simple approach for providing information on microtubule stability at different ages or experimental conditions, or to compare diseased/experimental and healthy/control neurons, and this approach has become routine for use in both cell culture and nerve tissue (figure 5). Both of these methods are useful, but not without their caveats. For example, as indicated earlier, at least in primary neuronal culture, immunostaining for tyrosinated tubulin is a very good distinguisher of stable and labile domains of microtubules in axons, but not dendrites or somata (Baas et al., 1991). In different parts of the neuron, at different developmental stages, in different kinds of neuron, or in disease/injury scenarios, the enzymes that regulate tubulin modifications may vary in concentration and activity, such that immunostaining for acetylated, tyrosinated or detyrosinated tubulin may or may not be a reliable method for quantitatively comparing microtubule stability properties. In addition, levels of any of these stability-relevant isoforms of tubulin should be taken as a ratio against total tubulin in microtubules, or the result may be misinterpreted. For example, as discussed earlier in the context of Alzheimer’s disease, diminution in acetylated tubulin staining in an experimental scenario could mean that the fraction of the total microtubule mass that is acetylated has diminished, or it could be reflective of a diminution of total microtubule mass wherein the proportion of the total that is acetylated might actually be higher than in the control. A potential caveat with the nocodazole approach is that depolymerizing such a large fraction of the microtubule mass may release factors from that microtubule fraction that can then bind to the microtubule fraction that remains, potentially rendering the stable fraction more stable than it normally is. Another approach is to induce depolymerization by dilution, which is to extract the neurons in a detergent that releases free tubulin but does not artificially stabilize the microtubules (Baas and Heidemann, 1986). The microtubules will then depolymerize at a rate reflective of their dynamic properties, with the labile fraction depolymerizing quickly and the stable fraction depolymerizing slowly. The caveat with this approach is that the detergent extraction could result in the loss of factors from the microtubules that influence their stability, although using a mild detergent and/or very low concentration of detergent can minimize this.
Figure 4. Nocodazole-based approach for assaying stable and labile microtubule fractions in cultured neurons.
Top three panels on the right schematically show electron microscopy experiment in which the labile domain of the axonal microtubule is preferentially labeled by colloidal gold secondary antibody and tyrosinated tubulin primary antibody. After 15–30 minutes in nocodazole, the labile domains are entirely depolymerized, leaving behind only the stable domains. After an additional few hours in drug, the stable domain is reduced but much more slowly than the labile domain was lost. The graph to the left of these panels shows a hypothetical quantification of data derived from this kind of experiment in which the microtubule levels are plotted against time in drug. A biphasic loss of microtubule mass is revealed with a rapidly lost labile fraction and a slowly depolymerizing stable fraction. The lower panel to the left shows a parallel immunofluorescence experiment in which the labile domains (rich in tyrosinated tubulin) are depolymerized by 15–30 minutes in the drug, leaving behind the stable domains (rich in detyrosinated tubulin). The lower panel to the right shows the same experiment, as assessed by Western blotting rather than microscopy.
Figure 5. Immuno-microscopy-based approaches for assaying stable and labile microtubule fractions in cultured neurons.
Top panel shows a hypothetical triple-label immunofluorescence experiment in which cultured neurons were pre-extracted in a microtubule-stabilizing buffer (to release free tubulin) prior to fixation, and then labeled with antibodies to total tubulin (with green secondary antibody), tyrosinated tubulin (with red secondary antibody), and acetylated tubulin (with blue secondary antibody). Total tubulin is relatively evenly distributed in dendrites, the axon, and soma, with microtubules also extending into the growth cone at the tip of the axon. Tyrosinated tubulin is relatively enriched in the dendrites, soma and growth cone microtubules. Acetylated tubulin is relatively enriched in the axonal shaft (which would also be true of detyrosinated tubulin, not shown in figure). The lower panel shows electron microscopy of axonal microtubule labeled with primary antibody to tyrosinated tubulin and secondary antibody conjugated to colloidal gold. The labile domain is densely labeled while the stable domain is unlabeled.
A more contemporary approach is to express fluorescently tagged tubulin in the neurons, allow time for it to incorporate through the microtubule array, and then bleach regions of the neuron. The recovery of the fluorescence reflects the rate of exchange of the microtubules with the free tubulin pool, with the labile fraction recovering quickly and the stable fraction recovering more slowly (Edson et al., 1993). This approach, called FRAP (fluorescence recovery after photobleaching) is free of the concerns of the other approaches, but has not yet been sufficiently refined so as to distinguish stability classes of microtubules. Presumably, after a quick influx of diffusible fluorescent tubulin, a bleached zone in the axonal microtubule array would recover in a biphasic fashion, first with the recovery of the labile portion of the microtubules and then with the recovery of the stable portion (figure 6). However, this approach is significantly flawed by the fact that fluorescent microtubules from the regions of the axon flanking the branch can move through the bleached zone (rapid transport of short microtubules and potential sliding of longer microtubules; see (Del Castillo et al., 2015; He et al., 2005), and hence the recovery of fluorescence can be due as much to microtubule movements as microtubule dynamics [see also (Yu et al., 1996)]. A variant of this approach is to microinject tagged tubulin, wait various periods of time, extract to release free tubulin, fix the cells, and visualize the timetable by which the tagged tubulin incorporates into microtubules; the labile fraction incorporates the tagged tubulin quickly while the stable fraction incorporates the tagged tubulin more slowly (Li and Black, 1996). Assaying for microtubule stability in vivo (especially in the case of adult animals) has been more challenging, but is especially important for studies on animal models of diseases and therapeutic approaches for restoring microtubule levels and properties to normal. Some interesting and novel approaches are now being explored (Barten et al., 2012; Kleele et al., 2014).
Figure 6. FRAP-based approach for assaying stable and labile microtubule fractions in cultured neurons.
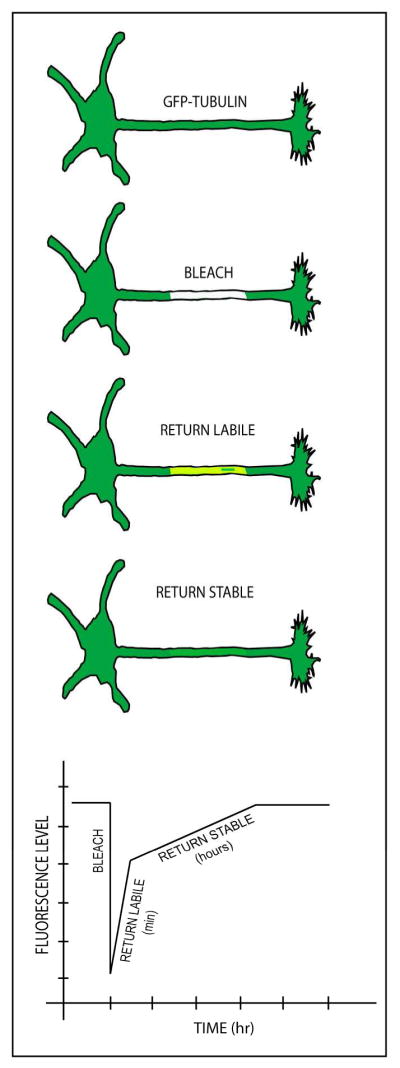
In the FRAP-based approach, cultured neurons are transfected to express fluorescently-tagged tubulin, with sufficient time allowed for the tagged tubulin to incorporate into the stable and labile microtubule fractions. Then, a bleached zone is created in the axon, after which the return of fluorescence is recorded through digital imaging. In theory, after a very rapid initial influx of free fluorescent tubulin (not shown), the fluorescence is recovered biphasically, with the first phase returning in 15–30 minutes, and the rest returning over a matter of hours. These two phases would correspond to the labile and stable microtubule fractions, respectively. FRAP has been used in the past to study microtubules in cultured neurons, but the more sophisticated use of the method to reveal stable and labile fractions remains hypothetical, as is the predicted quantification, shown graphically at the bottom of the schematic illustrations of the recovery phases. A notable shortcoming of this approach is the mobility of fluorescent microtubules into the bleached region from the fluorescent zones flanking the bleached region, as indicated in the third illustration, which could theoretically contribute to the recovery of fluorescence of the bleached zone as much as microtubule dynamics.
Factors that affect microtubule stability in the neuron
It is not uncommon in the biomedical literature for the stability of a microtubule to be confused with its stiffness or mobility or other properties that may be indirectly related to its stability but technically are not stability. As described earlier, stability refers to the rate at which a microtubule undergoes subunit exchange with the soluble tubulin pool, which is reflected in the sensitivity of the microtubule to the reduction in the effective concentration of the tubulin pool by tubulin-sequestering drugs or dilution. Stability also refers to the sensitivity of the microtubule to cold and/or calcium (which is not always the same as sensitivity to dilution or drugs). The mistaken gestalt that sometimes comes to mind is that a stable microtubule is necessarily stiffer or less mobile than a labile microtubule. Some proteins that stabilize microtubules could also make them stiffer or less mobile, but this is not always true. For example, a microtubule could be stable but not as stiff as a microtubule that is labile. As discussed earlier, short mobile microtubules in the axon are stable, while immobile microtubules often have long labile domains. Microtubules that are highly bundled are often thought of as very stable, but this is also not necessarily true, as for example, labile domains extending into the growth cone could be bundled and yet still labile. All of this begs the most perplexing question of all, which is what factors actually determine the stability properties of a microtubule. The literature is rife with talk of proteins that act as microtubule stabilizers or destabilizers, but the issue is not as simple as it is often portrayed. A protein that stabilizes a microtubule would transform a labile domain into a stable domain, or at least make either domain more stable than it would otherwise be. A protein that destabilizes a microtubule would transform a stable domain into a labile domain, or at least make either domain less stable than it would otherwise be.
Myriad proteins are said to stabilize microtubules, and these include traditional fibrous MAPs (microtubule-associated proteins) such as tau, MAP2 and MAP1b, as well as other proteins, such as doublecortin, that bind to the microtubule lattice (Dehmelt and Halpain, 2005; Mandelkow and Mandelkow, 1995; Tucker, 1990). Crosslinking proteins such as plakons have also been implicated in microtubule stability (Fuchs and Yang, 1999). Most proteins that interact with microtubules can stabilize them in vitro, if present at high concentrations, and the same is true under conditions of high overexpression of such proteins in living cells. Tau and its related family members, MAP2 and MAP4, have repeats of microtubule-binding domains on their c-terminal end, and these are thought to bind in a series along the microtubule lattice to hold it together and thereby prevent depolymerization of the microtubule (Kadavath et al., 2015). However, the on/off rate of association and dissociation of these proteins with the microtubule in living cells is only milliseconds (Janning et al., 2014), which seems inconsistent with tau or its family members (which presumably have similar dwell times on the microtubule lattice) transforming highly dynamic labile microtubule domains into stable long-lived domains. Curiously, tau and MAP1b are more enriched on the labile domains extending into the distal region of growing axons than on microtubules in the main shaft of the axon where stable and labile domains intermingle (Black et al., 1994; Black et al., 1996; Kempf et al., 1996). [This contrasts with MAP6, for which there is evidence of enrichment on stable microtubule domains; see (Slaughter and Black, 2003)]. Whether or not MAP1b is phosphorylated may play a role in how it affects microtubule stability (Goold et al., 1999). Doublecortin binds in the groove between the longitudinal protofilaments of the microtubule and would seem ideally located to stabilize the microtubule against depolymerization, and yet most data suggest that doublecortin’s main roles are to regulate microtubule stiffness important for growth cone guidance (Jean et al., 2012), constrain the microtubule’s protofilament number to 13 (Bechstedt and Brouhard, 2012), and regulate the integration of microtubules with the actin cytoskeleton underlying such events as axonal branch formation (Tint et al., 2009). MAP1b also has myriad functions apart from influencing microtubule stability (Villarroel-Campos and Gonzalez-Billault, 2014). One possibility is that microtubule stability in neurons is the result of the combined effects of a large pool of stabilizers, but another possibility is that the microtubule-stabilizing properties of many of these proteins have been a red herring all along, and at least some of them are not really stabilizers of microtubules in the physiological context.
Many proteins termed microtubule destabilizers actually promote microtubule disassembly, and would be more accurately termed microtubule depolymerizers. Examples are stathmin and its neuron-specific variant called SCG10, which shift microtubules toward disassembly by sequestering tubulin subunits and by promoting catastrophe, as well as certain “depolymerizing” kinesins (kinesin-8 and kinesin-13) that use the forces of ATP hydrolysis to tear subunits from the ends of microtubules (Chauvin and Sobel, 2015; Niwa, 2015). By depolymerizing the labile domains of microtubules, such proteins would reduce the total microtubule mass and shift the microtubule array to a greater proportion of the total being stable. Whether proteins actually exist that transform stable domains of microtubules into labile domains remains to be seen.
There are hundreds of published articles on neuronal development, disease and injury that invoke various proteins, pathways and mechanisms that draw authors to converge on changes in microtubule stability as the downstream explanation for their results. Assuming the lion’s share of these studies are interpreted appropriately, the mind boggles at how alterations in so many different proteins and pathways simultaneously could be so crucial to keeping microtubules as stable as they normally are. There may be a complex interplay of factors; for example, regions of microtubules that are rich in tau are generally deficient in doublecortin (Tint et al., 2009), suggesting that one may displace the other. In knockout animals, the absence of one microtubule-associated protein can lead to the overexpression of another (Ke et al., 2012). As indicated earlier, certain proteins that do not directly stabilize microtubules may have that indirect effect by protecting the microtubule from proteins that promote severing or depolymerization, and certain tubulin modifications that do not directly stabilize microtubules may render the microtubule more or less favored for interaction with various microtubule-related proteins, some of which could affect microtubule stability. Work conducted on developing neurons is not easily considered alongside work on adult neurons, as many features of their stability properties can be quite different. Complexities abound such that, despite over 30 years of progress on the issue, the molecular basis of microtubule stability is still shrouded in mystery.
Acknowledgments
The work of the authors is supported by grants to PWB from the Craig H. Neilsen Foundation (Grant 259350), the National Institutes of Health (NIH; NINDS; Grant R01 NS28785), and the Department of Defense (GW120037 and GW140086). ANR is supported by the NIH Ruth L. Kirschstein National Research Service Award (Fellowship F31 11882224). LL is supported by a Drexel Dean’s Fellowship Award for Collaborative Research. This article was inspired by discussions held at the third bi-annual “Emerging Concepts in Neuronal Cytoskeleton” meeting, held in Puerto Varas, Chile in 2015, and improved through helpful comments from Dr. Mark M. Black of Temple University.
References
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. Journal of cell science. 1995;108(Pt 8):2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. The Journal of cell biology. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FJ, Joshi HC, Centonze VE, Baas PW. Inhibition of microtubule nucleation at the neuronal centrosome compromises axon growth. Neuron. 1994;12:271–280. doi: 10.1016/0896-6273(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Pienkowski TP, Baas PW. Regional differences in microtubule dynamics in the axon. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:856–866. doi: 10.1523/JNEUROSCI.13-02-00856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FJ, Yu W, McNally FJ, Baas PW. An essential role for katanin in severing microtubules in the neuron. The Journal of cell biology. 1999;145:305–315. doi: 10.1083/jcb.145.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: mechanisms and functions. Current opinion in cell biology. 2005;17:47–54. doi: 10.1016/j.ceb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Hoogenraad CC. Microtubule minus-end-targeting proteins. Current biology: CB. 2015;25:R162–171. doi: 10.1016/j.cub.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16:711–726. doi: 10.1038/nrm4084. [DOI] [PubMed] [Google Scholar]
- Andrieux A, Salin PA, Vernet M, Kujala P, Baratier J, Gory-Faure S, Bosc C, Pointu H, Proietto D, Schweitzer A, et al. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes & development. 2002;16:2350–2364. doi: 10.1101/gad.223302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW. Microtubule stability in the axon: new answers to an old mystery. Neuron. 2013;78:3–5. doi: 10.1016/j.neuron.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The plus ends of stable microtubules are the exclusive nucleating structures for microtubules in the axon. The Journal of cell biology. 1992;116:1231–1241. doi: 10.1083/jcb.116.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The transport properties of axonal microtubules establish their polarity orientation. The Journal of cell biology. 1993;120:1427–1437. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. Beyond taxol: microtubule-based treatment of disease and injury of the nervous system. Brain: a journal of neurology. 2013;136:2937–2951. doi: 10.1093/brain/awt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ, Pienkowski TP, Brown A, Black MM. Sites of microtubule stabilization for the axon. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:2177–2185. doi: 10.1523/JNEUROSCI.13-05-02177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. The Journal of cell biology. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. The Journal of cell biology. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Buster DW. Slow axonal transport and the genesis of neuronal morphology. J Neurobiol. 2004;58:3–17. doi: 10.1002/neu.10281. [DOI] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Heidemann SR. Microtubule reassembly from nucleating fragments during the regrowth of amputated neurites. The Journal of cell biology. 1986;103:917–927. doi: 10.1083/jcb.103.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Lin S. Hooks and comets: The story of microtubule polarity orientation in the neuron. Developmental neurobiology. 2011;71:403–418. doi: 10.1002/dneu.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Pienkowski TP, Cimbalnik KA, Toyama K, Bakalis S, Ahmad FJ, Kosik KS. Tau confers drug stability but not cold stability to microtubules in living cells. Journal of cell science. 1994;107(Pt 1):135–143. doi: 10.1242/jcs.107.1.135. [DOI] [PubMed] [Google Scholar]
- Baas PW, Slaughter T, Brown A, Black MM. Microtubule dynamics in axons and dendrites. Journal of neuroscience research. 1991;30:134–153. doi: 10.1002/jnr.490300115. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bray D, Chapman K. Assembly of microtubules at the tip of growing axons. Nature. 1986;321:788–790. doi: 10.1038/321788a0. [DOI] [PubMed] [Google Scholar]
- Barten DM, Fanara P, Andorfer C, Hoque N, Wong PY, Husted KH, Cadelina GW, Decarr LB, Yang L, Liu V, et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearce EA, Erdogan B, Lowery LA. TIPsy tour guides: how microtubule plus-end tracking proteins (+TIPs) facilitate axon guidance. Front Cell Neurosci. 2015;9:241. doi: 10.3389/fncel.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechstedt S, Brouhard GJ. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Developmental cell. 2012;23:181–192. doi: 10.1016/j.devcel.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Baas PW. The basis of polarity in neurons. Trends in neurosciences. 1989;12:211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Black MM, Cochran JM, Kurdyla JT. Solubility properties of neuronal tubulin: evidence for labile and stable microtubules. Brain research. 1984;295:255–263. doi: 10.1016/0006-8993(84)90974-0. [DOI] [PubMed] [Google Scholar]
- Black MM, Slaughter T, Fischer I. Microtubule-associated protein 1b (MAP1b) is concentrated in the distal region of growing axons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:857–870. doi: 10.1523/JNEUROSCI.14-02-00857.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Slaughter T, Moshiach S, Obrocka M, Fischer I. Tau is enriched on dynamic microtubules in the distal region of growing axons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nature reviews Neuroscience. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Brady ST, Tytell M, Lasek RJ. Axonal tubulin and axonal microtubules: biochemical evidence for cold stability. The Journal of cell biology. 1984;99:1716–1724. doi: 10.1083/jcb.99.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D, Bunge MB. Serial analysis of microtubules in cultured rat sensory axons. Journal of neurocytology. 1981;10:589–605. doi: 10.1007/BF01262592. [DOI] [PubMed] [Google Scholar]
- Brown A, Li Y, Slaughter T, Black MM. Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. Journal of cell science. 1993;104(Pt 2):339–352. doi: 10.1242/jcs.104.2.339. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, Iba M, James MJ, Xie SX, Ballatore C, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti G, Casagrande F, Calogero A, De Gregorio C, Pezzoli G, Cartelli D. Linking microtubules to Parkinson’s disease: the case of parkin. Biochemical Society transactions. 2015;43:292–296. doi: 10.1042/BST20150007. [DOI] [PubMed] [Google Scholar]
- Carrillo RA, Menon K, Zinn K. Is instability good for the brain? Neuron. 2013;77:599–601. doi: 10.1016/j.neuron.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Challacombe JF, Snow DM, Letourneau PC. Dynamic microtubule ends are required for growth cone turning to avoid an inhibitory guidance cue. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:3085–3095. doi: 10.1523/JNEUROSCI.17-09-03085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin S, Sobel A. Neuronal stathmins: a family of phosphoproteins cooperating for neuronal development, plasticity and regeneration. Progress in neurobiology. 2015;126:1–18. doi: 10.1016/j.pneurobio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. The EMBO journal. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nature reviews Neuroscience. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Coyne AN, Siddegowda BB, Estes PS, Johannesmeyer J, Kovalik T, Daniel SG, Pearson A, Bowser R, Zarnescu DC. Futsch/MAP1B mRNA is a translational target of TDP-43 and is neuroprotective in a Drosophila model of amyotrophic lateral sclerosis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:15962–15974. doi: 10.1523/JNEUROSCI.2526-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome biology. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo U, Winding M, Lu W, Gelfand VI. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in neurons. eLife. 2015;4 doi: 10.7554/eLife.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delphin C, Bouvier D, Seggio M, Couriol E, Saoudi Y, Denarier E, Bosc C, Valiron O, Bisbal M, Arnal I, et al. MAP6-F is a temperature sensor that directly binds to and protects microtubules from cold-induced depolymerization. The Journal of biological chemistry. 2012;287:35127–35138. doi: 10.1074/jbc.M112.398339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Baas PW. Microtubules in neurons as information carriers. J Neurochem. 2014;129:235–239. doi: 10.1111/jnc.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Dong S, Gu F, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer’s disease: focusing on tau-mediated neurodegeneration. Translational neurodegeneration. 2012;1:24. doi: 10.1186/2047-9158-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J, Ratnakaran N, Koushika SP. Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci. 2015;9:343. doi: 10.3389/fncel.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson KJ, Lim SS, Borisy GG, Letourneau PC. FRAP analysis of the stability of the microtubule population along the neurites of chick sensory neurons. Cell motility and the cytoskeleton. 1993;25:59–72. doi: 10.1002/cm.970250108. [DOI] [PubMed] [Google Scholar]
- Falnikar A, Tole S, Baas PW. Kinesin-5, a mitotic microtubule-associated motor protein, modulates neuronal migration. Molecular biology of the cell. 2011;22:1561–1574. doi: 10.1091/mbc.E10-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanara P, Banerjee J, Hueck RV, Harper MR, Awada M, Turner H, Husted KH, Brandt R, Hellerstein MK. Stabilization of hyperdynamic microtubules is neuroprotective in amyotrophic lateral sclerosis. The Journal of biological chemistry. 2007;282:23465–23472. doi: 10.1074/jbc.M703434200. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Caceres A. The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Brain Res Dev Brain Res. 1989;49:205–213. doi: 10.1016/0165-3806(89)90022-9. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Garnham CP, Roll-Mecak A. The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton. 2012;69:442–463. doi: 10.1002/cm.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. Journal of cell science. 1999;112(Pt 19):3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- Haendel MA, Bollinger KE, Baas PW. Cytoskeletal changes during neurogenesis in cultures of avain neural crest cells. Journal of neurocytology. 1996;25:289–301. doi: 10.1007/BF02284803. [DOI] [PubMed] [Google Scholar]
- He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. The Journal of cell biology. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janning D, Igaev M, Sundermann F, Bruhmann J, Beutel O, Heinisch JJ, Bakota L, Piehler J, Junge W, Brandt R. Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons. Molecular biology of the cell. 2014;25:3541–3551. doi: 10.1091/mbc.E14-06-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean DC, Baas PW. It cuts two ways: microtubule loss during Alzheimer disease. The EMBO journal. 2013;32:2900–2902. doi: 10.1038/emboj.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean DC, Baas PW, Black MM. A novel role for doublecortin and doublecortin-like kinase in regulating growth cone microtubules. Human molecular genetics. 2012;21:5511–5527. doi: 10.1093/hmg/dds395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Hua S, Mohan R, Grigoriev I, Yau KW, Liu Q, Katrukha EA, Altelaar AF, Heck AJ, Hoogenraad CC, et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Developmental cell. 2014;28:295–309. doi: 10.1016/j.devcel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Jones DH, Gray EG, Barron J. Cold stable microtubules in brain studied in fractions and slices. Journal of neurocytology. 1980;9:493–504. doi: 10.1007/BF01204838. [DOI] [PubMed] [Google Scholar]
- Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7501–7506. doi: 10.1073/pnas.1504081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn OI, Sharma V, Gonzalez-Billault C, Baas PW. Effects of kinesin-5 inhibition on dendritic architecture and microtubule organization. Molecular biology of the cell. 2015;26:66–77. doi: 10.1091/mbc.E14-08-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, Leitman E, Binder LI, Brady ST. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiology of aging. 2012;33:826e815–830. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Hoogenraad CC. Building the Neuronal Microtubule Cytoskeleton. Neuron. 2015;87:492–506. doi: 10.1016/j.neuron.2015.05.046. [DOI] [PubMed] [Google Scholar]
- Karabay A, Yu W, Solowska JM, Baird DH, Baas PW. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:5778–5788. doi: 10.1523/JNEUROSCI.1382-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsetos CD, Herman MM, Mork SJ. Class III beta-tubulin in human development and cancer. Cell motility and the cytoskeleton. 2003a;55:77–96. doi: 10.1002/cm.10116. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Legido A, Perentes E, Mork SJ. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. Journal of child neurology. 2003b;18:851–866. doi: 10.1177/088307380301801205. discussion 867. [DOI] [PubMed] [Google Scholar]
- Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, Ittner LM. Lessons from tau-deficient mice. International journal of Alzheimer’s disease. 2012;2012:873270. doi: 10.1155/2012/873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleele T, Marinkovic P, Williams PR, Stern S, Weigand EE, Engerer P, Naumann R, Hartmann J, Karl RM, Bradke F, et al. An assay to image neuronal microtubule dynamics in mice. Nature communications. 2014;5:4827. doi: 10.1038/ncomms5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, Gerlich DW, Janke C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. The Journal of cell biology. 2010;189:945–954. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. Journal of neuroscience research. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo L, Yu W, D’Rozario M, Waddell EA, Marenda DR, Baird MA, Davidson MW, Zhou B, Wu B, Baker L, et al. Vertebrate Fidgetin Restrains Axonal Growth by Severing Labile Domains of Microtubules. Cell Rep. 2015;12:1723–1730. doi: 10.1016/j.celrep.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC. Analysis of microtubule number and length in cytoskeletons of cultured chick sensory neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1982;2:806–814. doi: 10.1523/JNEUROSCI.02-06-00806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Jr, Courchet J, Polleux F. Cell biology in neuroscience: Cellular and molecular mechanisms underlying axon formation, growth, and branching. The Journal of cell biology. 2013;202:837–848. doi: 10.1083/jcb.201305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Black MM. Microtubule assembly and turnover in growing axons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:531–544. doi: 10.1523/JNEUROSCI.16-02-00531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Sammak PJ, Borisy GG. Progressive and spatially differentiated stability of microtubules in developing neuronal cells. The Journal of cell biology. 1989;109:253–263. doi: 10.1083/jcb.109.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu M, Mozgova OI, Yu W, Baas PW. Mitotic motors coregulate microtubule patterns in axons and dendrites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:14033–14049. doi: 10.1523/JNEUROSCI.3070-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Current opinion in cell biology. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Nahm M, Lee MJ, Parkinson W, Lee M, Kim H, Kim YJ, Kim S, Cho YS, Min BM, Bae YC, et al. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron. 2013;77:680–695. doi: 10.1016/j.neuron.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukirchen D, Bradke F. Neuronal polarization and the cytoskeleton. Semin Cell Dev Biol. 2011;22:825–833. doi: 10.1016/j.semcdb.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Niwa S. Kinesin superfamily proteins and the regulation of microtubule dynamics in morphogenesis. Anatomical science international. 2015;90:1–6. doi: 10.1007/s12565-014-0259-5. [DOI] [PubMed] [Google Scholar]
- Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11358–11362. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris L, Thery M, Faure J, Saoudi Y, Lafanechere L, Chilton JK, Gordon-Weeks P, Galjart N, Bornens M, Wordeman L, et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. The Journal of cell biology. 2006;174:839–849. doi: 10.1083/jcb.200512058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, Andrieux A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. The Journal of cell biology. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Tovar CA, Lemeshow S, Yin Q, Jakeman LB. Independent evaluation of the anatomical and behavioral effects of Taxol in rat models of spinal cord injury. Exp Neurol. 2014;261:97–108. doi: 10.1016/j.expneurol.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. The intricate relationship between microtubules and their associated motor proteins during axon growth and maintenance. Neural Dev. 2013;8:17. doi: 10.1186/1749-8104-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Yu W, Liu M, Solowska JM, Baas PW. Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Molecular biology of the cell. 2010;21:334–344. doi: 10.1091/mbc.E09-09-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Current opinion in cell biology. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, Vale RD. Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? The Journal of cell biology. 2006;175:849–851. doi: 10.1083/jcb.200611149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahenk Z, Brady ST. Axonal tubulin and microtubules: morphologic evidence for stable regions on axonal microtubules. Cell motility and the cytoskeleton. 1987;8:155–164. doi: 10.1002/cm.970080207. [DOI] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol facilitates axon regeneration in the mature CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Kuriyama R, Essner R, Baas PW. Expression of a minus-end-directed motor protein induces Sf9 cells to form axon-like processes with uniform microtubule polarity orientation. Journal of cell science. 1997;110(Pt 19):2373–2380. doi: 10.1242/jcs.110.19.2373. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter T, Black MM. STOP (stable-tubule-only-polypeptide) is preferentially associated with the stable domain of axonal microtubules. Journal of neurocytology. 2003;32:399–413. doi: 10.1023/B:NEUR.0000011334.70648.87. [DOI] [PubMed] [Google Scholar]
- Solowska JM, D’Rozario M, Jean DC, Davidson MW, Marenda DR, Baas PW. Pathogenic mutation of spastin has gain-of-function effects on microtubule dynamics. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:1856–1867. doi: 10.1523/JNEUROSCI.3309-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25:125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, Johnson GV, Brady ST. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron. 2013;78:109–123. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiess M, Maghelli N, Kapitein LC, Gomis-Ruth S, Wilsch-Brauninger M, Hoogenraad CC, Tolic-Norrelykke IM, Bradke F. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Molecular biology of the cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Human molecular genetics. 2011;20:763–778. doi: 10.1093/hmg/ddq521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. The Journal of cell biology. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- Tint I, Jean D, Baas PW, Black MM. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:10995–11010. doi: 10.1523/JNEUROSCI.3399-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Usukura J, Tsukita S, Ishikawa H. The cytoskeleton in myelinated axons: a freeze-etch replica study. Neuroscience. 1982;7:2135–2147. doi: 10.1016/0306-4522(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Tsushima H, Emanuele M, Polenghi A, Esposito A, Vassalli M, Barberis A, Difato F, Chieregatti E. HDAC6 and RhoA are novel players in Abeta-driven disruption of neuronal polarity. Nature communications. 2015;6:7781. doi: 10.1038/ncomms8781. [DOI] [PubMed] [Google Scholar]
- Tucker RP. The roles of microtubule-associated proteins in brain morphogenesis: a review. Brain research Brain research reviews. 1990;15:101–120. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- Villarroel-Campos D, Gonzalez-Billault C. The MAP1B case: an old MAP that is new again. Developmental neurobiology. 2014;74:953–971. doi: 10.1002/dneu.22178. [DOI] [PubMed] [Google Scholar]
- Volle J, Brocard J, Saoud M, Gory-Faure S, Brunelin J, Andrieux A, Suaud-Chagny MF. Reduced expression of STOP/MAP6 in mice leads to cognitive deficits. Schizophrenia bulletin. 2013;39:969–978. doi: 10.1093/schbul/sbs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu W, Baas PW, Black MM. Microtubule assembly in growing dendrites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:6065–6078. doi: 10.1523/JNEUROSCI.16-19-06065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid movement of microtubules in axons. Current biology: CB. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- Yan J, Chao DL, Toba S, Koyasako K, Yasunaga T, Hirotsune S, Shen K. Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans. eLife. 2013;2:e00133. doi: 10.7554/eLife.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, van Beuningen SF, Cunha-Ferreira I, Cloin BM, van Battum EY, Will L, Schatzle P, Tas RP, van Krugten J, Katrukha EA, et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82:1058–1073. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Lee VM, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. Journal of neurology, neurosurgery, and psychiatry. 2013;84:784–795. doi: 10.1136/jnnp-2012-303144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I, Garnham CP, Roll-Mecak A. Writing and Reading the Tubulin Code. The Journal of biological chemistry. 2015;290:17163–17172. doi: 10.1074/jbc.R115.637447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Baas PW. Changes in microtubule number and length during axon differentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:2818–2829. doi: 10.1523/JNEUROSCI.14-05-02818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Centonze VE, Ahmad FJ, Baas PW. Microtubule nucleation and release from the neuronal centrosome. The Journal of cell biology. 1993;122:349–359. doi: 10.1083/jcb.122.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Molecular biology of the cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Schwei MJ, Baas PW. Microtubule transport and assembly during axon growth. The Journal of cell biology. 1996;133:151–157. doi: 10.1083/jcb.133.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sharp DJ, Kuriyama R, Mallik P, Baas PW. Inhibition of a mitotic motor compromises the formation of dendrite-like processes from neuroblastoma cells. The Journal of cell biology. 1997;136:659–668. doi: 10.1083/jcb.136.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM. Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. The EMBO journal. 2013;32:2920–2937. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Mandelkow EM. Tau missorting and spastin-induced microtubule disruption in neurodegeneration: Alzheimer Disease and Hereditary Spastic Paraplegia. Mol Neurodegener. 2015;10:68. doi: 10.1186/s13024-015-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, 3rd, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Su B, Wang C, Siedlak SL, Mondragon-Rodriguez S, Lee HG, Wang X, Perry G, Zhu X. Posttranslational modifications of alpha-tubulin in alzheimer disease. Translational neurodegeneration. 2015;4:9. doi: 10.1186/s40035-015-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]