Abstract
Objective
The prognostic value of cardiac troponins in apparently healthy populations is not well established. The aim of this study was to investigate the prognostic properties of high-sensitivity cardiac troponin T (hs-cTnT) for long-term adverse outcomes.
Setting
A community-dwelling prospective survey of residents from two communities in Beijing.
Participants
From September 2007 to January 2009, 1680 participants were initially enrolled. Of these, 1499 (870 females, mean age: 61.4 years) participants completed the survey and were followed up for a median of 4.8 years (IQR: 4.5–5.2).
Outcome measures
The primary outcome was the occurrence of all-cause mortality and major cardiovascular events.
Results
Overall, 820 individuals (54.7%) had detectable hs-cTnT levels. During the follow-up, 52 participants (3.5%) died, 154 (10.3%) had major cardiovascular events and 99 (6.6%) experienced new-onset coronary events. Compared with those with undetectable hs-cTnT levels, participants with hs-cTnT levels in the highest category (≥14 ng/L) had a significantly increased risk for all-cause mortality (adjusted HR (aHR): 2.07, 95% CI 1.05 to 3.01), major cardiovascular events (aHR: 3.27, 95% CI 1.88 to 5.70) and coronary events (aHR: 4.50, 95% CI 2.26 to 9.02) in covariate-adjusted analyses. No differences in stroke incidence were found (aHR: 1.27, 95% CI 0.69 to 2.62). Also, significant associations were presented when hs-cTnT levels were modelled as a continuous variable and when analysing changes in hs-cTnT levels over time with adverse outcomes. The addition of troponin T levels to clinical variables led to significant increases in risk prediction with a marked improvement in the C-statistics (p=0.003 or lower).
Conclusions
In this cohort of individuals from a community-based population, cTnT levels measured with a highly sensitive assay were associated with increases in the subsequent risk for all-cause mortality and major cardiovascular events. These results might support screening for at-risk individuals.
Keywords: biomarkers, high-sensitivity cardiac troponin T, cardiovascular diseases, prognosis, risk assessment.
Strengths and limitations of this study.
Evaluating the prognostic properties of both baseline high-sensitivity cardiac troponin T (hs-cTnT) and change in hs-cTnT for long-term adverse outcomes in a large community-dwelling study.
Reliably detecting minimal subclinical myocardial injury in an apparently healthy population over a wide age range.
Due to the lack of echocardiographic and coronary artery imaging data, no causal explanations on the associations between hs-cTnT and outcomes could be provided.
Introduction
Cardiovascular disease (CVD) is still the primary cause of morbidity and mortality worldwide. There is concordant evidence that prevention is the key to lessening the CVD burden, but predicting the risk of adverse cardiovascular events in low-risk to intermediate-risk individuals is a considerable challenge.1 This subpopulation is at risk for developing CVD but is not identified during the early stages by current risk screening methods.2 3 Circulating biomarkers can be an important supplemental screening tool, and troponins are one of the most interesting biomarkers in this context. Cardiac troponin T (cTnT) is a regulatory protein that is expressed by cardiac myocytes and is released in the setting of myocardial injury.4 5 As a sensitive and specific marker of cardiomyocyte damage, cTnT is typically used to exclude or confirm the diagnosis of acute myocardial infarction in the clinical setting.
However, cTnT levels can also be elevated and clinically meaningful in the absence of myocardial ischaemia. Detectable levels of cTnT can also be useful for detecting subclinical CVD and assessing CVD risk in the general population. However, the use of cTnT for clinical applications is limited, as it is only detectable in a small percentage of the general population using standard assays.6 7 Recently, a high-sensitivity cTnT (hs-cTnT) assay has been developed, which can detect concentrations that are 10-fold lower than those detectable with the standard fourth-generation assay. The introduction of this new assay has enabled the detection of very low levels of circulating cTnT, even in an asymptomatic general population.8
Levels of cTnT, below the detection limit of standard assays, have been shown to be independently associated with adverse cardiovascular events in patients with heart failure (HF)9 or stable coronary heart disease (CHD).10 However, the clinical significance of detectable cTnT with the use of the new assay in the apparently healthy population has not been well established. The main objective of this study was to investigate the prognostic value of minimally detectable hs-cTnT levels in predicting adverse clinical events in a population of community-based subjects.
Methods
Study population
This was a prospective observational study of people living in the Pingguoyuan area of the Shijingshan district, which is a metropolitan area of Beijing, China. Between September 2007 and January 2009, all permanent residents of the Han origin, aged 45 years or older (range 45–91 years), from two communities, were invited to participate in a health survey that focused on identifying CVD risk factors. Thirty-one subjects with bedridden status, mental illness and severe systemic diseases were excluded from enrolment. Then, 1832 participants were included and asked to complete questionnaires. Of these, 152 participants with overt CVD (defined as a composite of CHD (myocardial infarction, angina pectoris or coronary insufficiency), cerebrovascular disease (stroke or transient ischaemic attack), congestive HF or peripheral vascular disease) were excluded. Adequate baseline measurements and cardiac biomarkers were obtained in 1680 participants. After the initial evaluation, the recruited subjects were contacted every 2 years for follow-up, and the last follow-up visits were conducted through 30 September 2013. The median follow-up was 4.8 (IQR: 4.5–5.2) years. In our investigation, 181 participants were lost to follow-up (see online supplementary table 1) and were excluded from the study. Complete data were acquired from 1499 participants (return rate: 89.2%). All participants provided written informed consent at the time of enrolment, and the study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital.
bmjopen-2016-013431supp001.pdf (59.3KB, pdf)
Data collection
Baseline characteristics were collected using self-reported standardised questionnaires that included demographics, lifestyle information, medical history and medication use. Anthropometrical measurements were obtained by trained medical doctors, and the body mass index was derived from heights and weights that were measured in participants wearing light clothing and no shoes. Blood pressures were measured twice on the right arm after 5 min of rest in a sitting position, and the mean of two readings was used for further analysis. Participants were followed up for clinical outcomes by interview. Outcome measures were the occurrences of all-cause mortality and major adverse cardiovascular events (MACE). New events were validated by obtaining medical records and adjudicated by two independent and blinded reviewers. In the analyses, survival time was defined as the period from the date of baseline blood sample collection to the date of the first adverse event or end of follow-up.
Biomarker assays
At the time of enrolment, baseline blood samples were collected from participants between 08:00 and 10:00 after at least 12 hours of overnight fasting. Follow-up measurements were performed on blood samples, which were collected 4–5 years later. Serum aliquots were frozen at −80°C until further analyses in a central laboratory. Concentrations of blood glucose (fasting and 2 hours after an oral glucose tolerance), total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, uric acid and serum creatinine were measured by routine laboratory analysis.
Hs-cTnT levels were measured by an electrochemiluminescence immunoassay method using an Elecsys Troponin T highly sensitive assay (Roche Diagnostics, Mannheim, Germany) on the Modular Analytics E170 autoanalyser (Roche Diagnostics). The lower detection limit of the novel assay was 3 ng/L, and the 99th percentile value in apparently healthy individuals has been reported to be 14 ng/L.11 N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations were determined using an electrochemiluminescence immunoassay (Roche Diagnostics) on the Roche analyser. Levels of high-sensitivity C reactive protein (hs-CRP) were measured with the use of an immunoturbidimetric assay (Siemens Healthcare Diagnostics, Plainfield, Indiana, USA) on a Dimension RxL Max analyser (Siemens Healthcare Diagnostics).
Estimated glomerular filtration rate (eGFR) was calculated using the Chinese Modification of Diet in Renal Disease equation. Details of the computing equation have been published elsewhere.12
Definition of endpoints
For outcomes, we used the incidence of mortality and CVD morbidity after the baseline screening. All-cause mortality was determined by review of death certificates. The definition of MACE comprised non-fatal myocardial infarction, newly diagnosed CHD (identified by coronary artery imaging or receiving coronary revascularisation), stroke (ischaemic or haemorrhagic) and cardiovascular mortality. Major coronary events were defined as non-fatal myocardial infarction, newly diagnosed CHD and CHD death. Stroke was characterised as a neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause, including cerebral infarction, intracerebral haemorrhage (ICH) and subarachnoid haemorrhage.13 Cardiovascular mortality was defined as mortality related to atherosclerotic heart disease (fatal myocardial infarction and definite fatal CHD), mortality following cerebrovascular disease or mortality from HF and other CVDs. Non-fatal myocardial infarction was defined by the American Heart Association diagnostic criteria.14
Statistical analysis
Continuous variables are reported as mean±SD or median (IQR), and categorical variables are presented as counts and percentages. Hs-cTnT was modelled as both a categorical and a continuous variable. For the analyses of hs-cTnT as a categorical variable, participants were divided into four categories. Participants with undetectable hs-cTnT were placed in the first category as the reference group. Those with hs-cTnT levels greater than or equal to the previously reported 99th percentile value (14 ng/L)11 were placed in the fourth category, and those with levels between 3 and 14 ng/L were divided into two groups for categories 2 and 3, respectively, based on the median value of hs-cTnT. Demographic and clinical variables were compared across hs-cTnT categories using the one-way analysis of variance tests for continuous measures, Cuzick’s non-parametric trend test for non-normally distributed variables and χ2 tests for categorical variables.
The cumulative incidence of MACE and all-cause mortality for each category of hs-cTnT concentration was evaluated using the Kaplan-Meier method and compared with the log-rank test. Multivariable Cox proportional hazards regression models were used to analyse associations of hs-cTnT categories with outcomes, and category 1 was used as the reference. The models were incrementally adjusted for demographics, traditional cardiovascular risk factors, renal function and other biomarkers (hs-CRP and NT-proBNP). We also carried out an exploratory analysis, in which cTnT was regarded as a continuous variable, and the relationship between natural logarithm-transformed hs-cTnT values and endpoints was assessed. In this analysis, values of cTnT that were below the lower limit of detection were assigned to 1.5 ng/L (ie, one-half of the lower limit of detection).
In view of the markedly skewed distribution of cTnT, changes in concentrations over time were calculated as the difference between the natural logarithm of the concentrations at follow-up and baseline. The association between changes in hs-cTnT levels and outcomes were also evaluated in Cox proportional hazards models. The change in hs-cTnT levels was entered as a continuous variable and was adjusted for the baseline concentration of hs-cTnT and other relevant variables.
The discriminative capacity of a model with and without cTnT was estimated as C-statistics. The area under the receiver operating characteristic curve summarised the diagnostic discrimination. All data were analysed using the SPSS V.17.0 statistical package software. A two-sided value of p<0.05 was considered statistically significant.
Results
Participant characteristics
For the present analyses, 1499 participants were included. The mean (±SD) age of participants at enrolment was 61.4±11.4 years and 58.0% were women. Detectable baseline cTnT levels were found in 820 individuals (54.7%; range 3–176.4 ng/L) and were equal to or higher than 14 ng/L in 172 participants (11.5%). Table 1 shows the baseline characteristics by hs-cTnT categories. Compared with those with lower cTnT levels, individuals with higher levels were older, more likely to be male, more frequently hypertensive and diabetic, had lower eGFR and HDL-C levels, and had higher uric acid and NT-proBNP levels. The use of antihypertensive drugs, antidiabetic drugs and aspirin varied across levels of hs-cTnT.
Table 1.
Characteristics of the study population by baseline high-sensitivity troponin T levels
| Characteristics | Hs-cTnT group (ng/L) | p Value for trend | |||
| Group 1 <3.00 (n=679) |
Group 2 3.00–6.21 (n=324) |
Group 3 6.22–<14 (n=324) |
Group 4 ≥14 (n=172) |
||
| Demographics | |||||
| Age (years) | 58.6±10.3 | 63.1±10.7 | 64.2±12.4 | 61.8±11.5 | <0.001 |
| Male sex, n (%) | 202 (29.7) | 163 (50.3) | 170 (52.5) | 94 (54.7) | <0.001 |
| BMI (kg/m2) | 25.5±3.5 | 25.7±3.2 | 25.6±3.3 | 25.8±3.5 | 0.498 |
| Medical history | |||||
| Hypertension, n (%) | 258 (38.0) | 159 (49.1) | 176 (54.3) | 97 (56.4) | <0.001 |
| Diabetes mellitus, n (%) | 99 (14.6) | 72 (22.2) | 76 (23.5) | 45 (26.2) | <0.001 |
| Current smoking, n (%) | 85 (12.5) | 69 (21.3) | 62 (19.1) | 31 (18.0) | 0.003 |
| Systolic BP (mm Hg) | 131.5±16.5 | 132.7±17.3 | 132.8±17.2 | 133.2±17.1 | 0.041 |
| Diastolic BP (mm Hg) | 77.1±9.7 | 77.3±10.0 | 76.4±10.5 | 76.1±11.1 | 0.372 |
| Laboratory values | |||||
| FBG (mmol/L) | 5.3±1.4 | 5.3±1.5 | 5.5±1.7 | 5.8±1.9 | 0.076 |
| PBG (mmol/L) | 7.5±3.7 | 7.1±3.2 | 8.1±4.3 | 8.9±4.4 | <0.001 |
| Total cholesterol (mmol/L) | 5.1±0.9 | 5.1±0.9 | 5.1±0.9 | 5.0±0.9 | 0.464 |
| Triglycerides (mmol/L) | 1.8±1.1 | 1.7±1.1 | 1.7±1.0 | 2.0±1.4 | 0.04 |
| LDL cholesterol (mmol/L) | 3.0±0.7 | 2.9±0.7 | 3.0±0.7 | 3.0±0.7 | 0.136 |
| HDL cholesterol (mmol/L) | 1.4±0.4 | 1.4±0.3 | 1.3±0.4 | 1.3±0.4 | 0.001 |
| Uric acid (μmol/L) | 279.0±68.2 | 295.9±73.3 | 301.2±75.4 | 309.5±76.2 | <0.001 |
| eGFR (mL/min/1.73 m2) | 90.1±14.5 | 88.7±13.4 | 87.5±15.2 | 84.7±16.5 | 0.001 |
| NT-proBNP (pg/mL) | 37.2 (17.9, 75.3) | 38.9 (17.9, 74.4) | 46.6 (22.3, 90.1) | 49.3 (17.1, 112) | 0.003 |
| Hs-CRP (mg/L) | 2.4 (1.3, 3.4) | 2.3 (1.4, 3.4) | 2.2 (1.5, 3.5) | 2.4 (1.6, 3.6) | 0.383 |
| Medication use | |||||
| Antihypertensives, n (%) | 187 (27.5) | 122 (37.7) | 139 (42.9) | 80 (46.5) | <0.001 |
| Antidiabetics, n (%) | 64 (9.4) | 44 (13.6) | 52 (16.0) | 29 (16.9) | 0.0028 |
| Lipid-lowering drugs, n (%) | 109 (16.1) | 61 (18.8) | 58 (17.9) | 34 (19.8) | 0.149 |
| Aspirin, n (%) | 137 (20.2) | 79 (24.4) | 89 (27.5) | 55 (31.9) | <0.001 |
Values are reported as n (%), mean±SD or median (IQR).
BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C reactive protein; hs-cTnT, highly sensitive cardiac troponin T; LDL, low-density lipoprotein; PBG, postprandial blood glucose; NT-proBNP, N-terminal pro-type-B natriuretic peptide.
Outcomes by baseline hs-cTnT
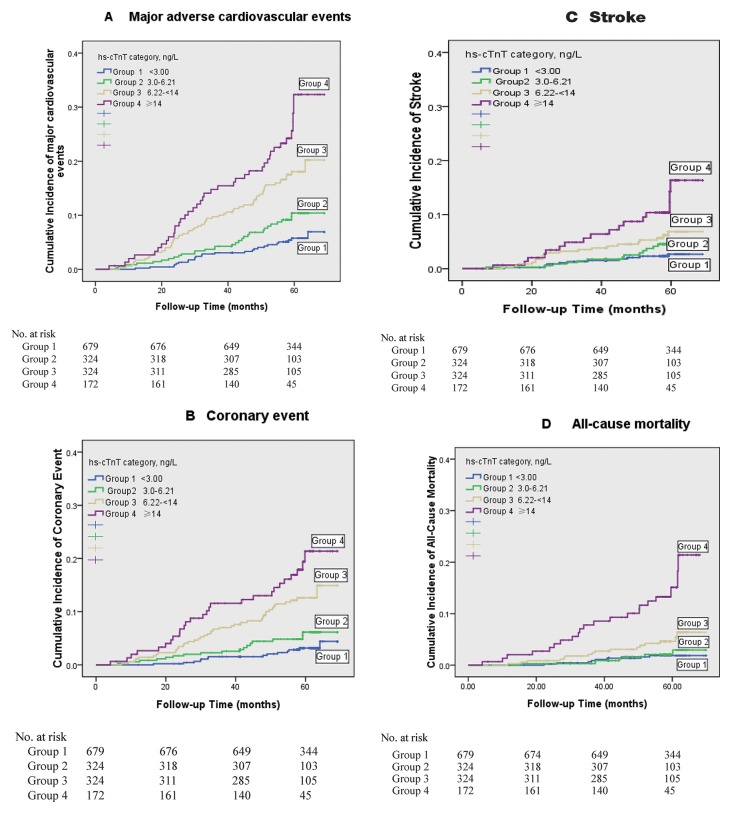
Over a median follow-up period of 4.8 years (IQR: 4.5–5.2), 154 participants experienced new-onset MACE (including 99 coronary events and 61 strokes, and some participants had more than one event). Fifty-two deaths occurred from any cause. The cumulative incidences of MACE and all-cause mortality by hs-cTnT categories are shown in figure 1A–D. As demonstrated by the Kaplan-Meier survival analysis, there was a graded increase in the probability of adverse clinical events across categories. The unadjusted incidence rate for MACE ranged from 3.7% in participants with undetectable hs-cTnT levels to 23.3% in participants in the highest category (p<0.001, log-rank test). Similar trends were discovered for coronary events, stroke and all-cause mortality, and a particularly high risk was found for participants in the highest category.
Figure 1.
Risk for cardiovascular events and all-cause mortality by baseline hs-cTnT level. Kaplan-Meier survival curves indicating cumulative incidence of major adverse cardiovascular events (A), coronary event (B), stroke (C) and all-cause mortality (D) across baseline hs-cTnT categories. Groups are indicated by colours; p<0.001 for all between-group comparisons by the log-rank test. Hs-cTnT, highly sensitive cardiac troponin T.
Associations of baseline hs-cTnT levels with MACE and all-cause mortality
In a series of Cox proportional hazards models adjusting for age and gender (model 1), and further adjusting for traditional cardiovascular risk factors (model 2), higher hs-cTnT categories demonstrated a graded association with MACE. Only a modest attenuation of the hazards was found with further adjustment for renal function (model 3). Although a significant attenuation was discovered with additional adjustments for hs-CRP and NT-proBNP levels (model 4), hs-cTnT levels in the third category (HR: 2.31, 95% CI 1.39 to 3.84) and fourth category (HR: 3.27, 95% CI 1.88 to 5.70) remained independently associated with MACE in the fully adjusted models. Participants in hs-cTnT categories 3 and 4 also had a significantly higher risk of coronary events compared with participants with undetectable levels (HR: 2.76 (95% CI 1.44 to 5.31) and 4.50 (95% CI 2.26 to 9.02), respectively, in model 4; table 2). However, associations of hs-cTnT levels with stroke events were markedly attenuated and no longer significant after adjusting for NT-proBNP (model 4; table 2). Membership in the highest hs-cTnT category was independently associated with all-cause mortality (HR: 2.07, 95% CI 1.05 to 3.01) in both univariate and multivariate analyses.
Table 2.
Cox proportional hazards models analysis for associations between baseline hs-cTnT levels and outcomes
| HR (95% CI) | ||||
| Group 1 <3.00 ng/L |
Group 2 3.0–6.21 ng/L |
Group 3 6.22–<14 ng/L |
Group 4 ≥14 ng/L |
|
| No | 679 | 324 | 324 | 172 |
| MACE | n=25 (3.7%) | n=30 (9.3%) | n=59 (18.2%) | n=40 (23.3%) |
| Model 1 | 1 (Reference) | 1.65 (0.97 to 2.81) | 2.65 (1.64 to 4.27) | 4.20 (2.51 to 7.02) |
| Model 2 | 1 (Reference) | 1.52 (0.89 to 2.59) | 2.41 (1.48 to 3.91) | 3.82 (2.27 to 6.43) |
| Model 3 | 1 (Reference) | 1.52 (0.89 to 2.61) | 2.38 (1.47 to 3.88) | 3.79 (2.26 to 6.39) |
| Model 4 | 1 (Reference) | 1.68 (0.97 to 2.92) | 2.31 (1.39 to 3.84) | 3.27 (1.88 to 5.70) |
| Coronary event | n=14 (2.1%) | n=17 (5.3%) | n=41 (12.7%) | n=27 (15.7%) |
| Model 1 | 1 (Reference) | 1.69 (0.83 to 3.44) | 3.39 (1.82 to 6.29) | 5.23 (2.7 to 10.14) |
| Model 2 | 1 (Reference) | 1.61 (0.78 to 3.29) | 3.25 (1.74 to 6.07) | 5.5 (2.84 to 10.64) |
| Model 3 | 1 (Reference) | 1.57 (0.76 to 3.22) | 3.09 (1.65 to 5.79) | 5.14 (2.65 to 9.98) |
| Model 4 | 1 (Reference) | 1.42 (0.71 to 2.89) | 2.76 (1.44 to 5.31) | 4.50 (2.26 to 9.02) |
| Stroke event | n=11 (1.6%) | n=13 (4.0%) | n=20 (6.2%) | n=17 (9.9%) |
| Model 1 | 1 (Reference) | 1.59 (0.71 to 3.57) | 1.94 (0.91 to 4.14) | 3.84 (1.75 to 8.43) |
| Model 2 | 1 (Reference) | 1.47 (0.65 to 3.29) | 1.77 (0.83 to 3.79) | 3.19 (1.42 to 7.16) |
| Model 3 | 1 (Reference) | 1.47 (0.65 to 3.31) | 1.70 (0.79 to 3.66) | 3.16 (1.41 to 7.10) |
| Model 4 | 1 (Reference) | 1.03 (0.50 to 2.09) | 1.13 (0.57 to 2.14) | 1.27 (0.69 to 2.62) |
| All-cause mortality | n=8 (1.2%) | n=7 (2.2%) | n=16 (4.9%) | n=21 (12.2%) |
| Model 1 | 1 (Reference) | 1.13 (0.41 to 3.14) | 1.99 (0.83 to 4.76) | 6.14 (2.61 to 14.46) |
| Model 2 | 1 (Reference) | 1.13 (0.41 to 3.14) | 1.78 (0.73 to 4.35) | 4.87 (1.99 to 11.88) |
| Model 3 | 1 (Reference) | 1.14 (0.41 to 3.16) | 1.79 (0.73 to 4.38) | 4.87 (1.99 to 11.88) |
| Model 4 | 1 (Reference) | 1.01 (0.40 to 1.15) | 1.09 (0.43 to 2.76) | 2.07 (1.05 to 3.01) |
Models are defined as follows: model 1=adjusted for age and gender; model 2=adjusted for model 1 + presence of hypertension or diabetes mellitus, current smoking status, systolic blood pressure, postprandial blood glucose, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medication use and antidiabetic medication use; model 3=adjusted for model 2 + estimated glomerular filtration rate; model 4=adjusted for model 3 + high-sensitivity C reactive protein and N-terminal pro-B-type natriuretic peptide (both after logarithmic transformation).
hs-cTnT, highly sensitive cardiac troponin T; MACE, major adverse cardiovascular event.
In an exploratory research of hs-cTnT as a continuous variable after natural logarithmic transformation, we found a continuous association with MACE incidence (per one-logarithmic unit increment; HR: 1.67, 95% CI 1.04 to 2.68, p<0.001), coronary events (HR: 1.68, 95% CI 1.35 to 2.08, p<0.001) or all-cause mortality (HR: 1.50, 95% CI 1.26 to 1.79, p=0.003) in fully multivariate models encompassing the variables described in table 2. No association between stroke risk and cTnT as a continuous variable was found in the final adjusted models (HR: 1.06, 95% CI 0.87 to 1.32, p=0.17).
Changes in hs-cTnT concentration during follow-ups and subsequent events
Table 3 presents the results of Cox regression analysis for subsequent events in relation to changes in hs-cTnT concentration as a continuous variable. Changes in hs-cTnT concentration were strongly associated with subsequent events except for stroke, even in multivariable analyses that additionally adjusted for baseline hs-cTnT levels. In the fully adjusted models, the HRs for MACE and coronary events for every unit increase in hs-cTnT concentration were 1.35 (95% CI 1.08 to 1.68, p=0.008) and 1.44 (95% CI 1.17 to 1.77, p=0.001), respectively. However, an increase in hs-cTnT concentration was not significantly associated with stroke events (HR: 1.02, 95% CI 0.64 to 2.19, p=0.242).
Table 3.
Association of changes in high-sensitivity cardiac troponin T (hs-cTnT) concentrations with subsequent events
| Multivariable-adjusted HR | 95% CI | p Value | |
| Major adverse cardiovascular event | 1.35 | 1.08 to 1.68 | 0.008 |
| Coronary event | 1.44 | 1.17 to 1.77 | 0.001 |
| Stroke event | 1.02 | 0.64 to 2.19 | 0.242 |
Data are presented as HRs and 95% CIs for a 1-unit increase in the change in hs-cTnT on a log scale. Multivariable model was adjusted for the covariates listed for model 4 in table 2, plus the baseline hs-cTnT level.
Hs-cTnT and risk prediction
Hs-cTnT provided incremental prognostic information regarding the three endpoints when added to a model based on established risk indicators (table 4). The addition of hs-cTnT increased the C-statistics from 0.671 to 0.698 (p=0.003) for the prediction of all-cause mortality. The prediction of MACE also improved from 0.702 to 0.734 (p<0.001).
Table 4.
Discrimination of adverse outcomes with the addition of high-sensitivity cardiac troponin T (hs-cTnT) to the clinical risk factor model
| Endpoint | C-statistics (95% CI) | ||
| Clinical model | Clinical model + hs-cTnT | p Value | |
| All-cause mortality | 0.671 (0.646 to 0.704) | 0.698 (0.680 to 0.725) | 0.003 |
| Major adverse cardiovascular event | 0.702 (0.680 to 0.726) | 0.734 (0.714 to 0.758) | <0.001 |
| Coronary event | 0.713 (0.701 to 0.735) | 0.749 (0.729 to 0.768) | <0.001 |
| Stroke event | 0.683 (0.665 to 0.709) | 0.697 (0.674 to 0.729) | 0.09 |
The clinical risk factor model included age, gender, body mass index, current smoking status, diabetes mellitus, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol and estimated glomerular filtration rate.
Discussion
The main purpose of the current study was to evaluate the usefulness of measuring hs-cTnT for predicting major cardiovascular events and all-cause mortality in a community-dwelling study of general population. There were several valuable findings. First, the up-to-date generation of cTnT assay allowed for minimal subclinical myocardial injury to be reliably detected in some individuals of a general population covering a wide age range. There was no need to restrict the study to morbid or high-risk individuals. Second, baseline and kinetic changes in hs-cTnT concentration were powerful and independent predictors of long-term MACE and all-cause mortality. Finally, these risk predictions were persistent, even after adjusting for traditional cardiovascular risk factors and biomarkers of inflammation and cardiac wall strain.
Cardiac troponins are generally the preferred biomarkers of myocardial necrosis and are typically used in the diagnosis of acute coronary syndromes. But the recently available hs-cTnT assay has allowed for the detection of much lower concentrations of circulating cTnT. More recent observations using this new assay have shown that very low levels of circulating cTnT can be detectable, these levels of circulating cTnT can provide information about coronary plaque characteristics and mortality in patients with stable CHD,15 16 and very low levels of circulating cTnT can even be detected in the general population.17 18 The exact mechanisms of troponin release in apparently healthy individuals are not well clarified. One possibility is the existence of asymptomatic cardiac ischaemia with minimal myocardial injury, which results in the asymptomatic release of troponins.19 Other mechanisms include cardiomyocyte apoptosis,20 physiological cell turnover,21 or subclinical cardiac structural or functional abnormalities.22 23
The prognostic implications of hs-cTnT assays among apparently healthy individuals have recently been explored in three large cohort studies.18 24 25 However, these studies focused on middle-aged and elderly subjects from Western countries. Our study extends the findings to the Asian population and demonstrates the significance of the hs-cTnT assay in risk assessment. To the best of our knowledge, this is the first study to evaluate the potential utility of the hs-cTnT assay in a Chinese population. One important finding is the observation that the presence of an elevated hs-cTnT level (in the highest category) was associated with adverse outcomes regarding all-cause mortality and MACE. However, there were no associations between minimally detectable hs-cTnT levels, especially those below 6.2 ng/L, and death or MACE. It has been proposed that such levels may be physiological, reflecting normal myocardial cell turnover and apoptosis within the senescent heart tissue.26 The levels may also be a result of the cortisol response to mental stress in healthy adults.27 Further research is required to determine which hs-cTnT threshold value may best be used in risk prediction.
Although other biomarkers, such as hs-CRP and NT-proBNP, have been used to identify apparently healthy individuals who are at increased CVD risk,28 29 our investigation indicates that hs-cTnT was also independent of these biomarkers. In this cohort, although associations with death and cardiovascular events were significantly attenuated after additional adjustments for levels of NT-proBNP, hs-cTnT remained independently predictive of endpoints in the final model, suggesting that the two biomarkers convey slightly different information for cardiac structural and functional abnormalities. The HRs remained statistically significant after adjusting for traditional cardiovascular risk factors and renal function, and were consistent with results from other studies.8 30 These data indicate that very low levels of cTnT may be used to identify subclinical myocardial injury and estimate the risk of cardiovascular events, which are currently not fully estimated by established methods. Also, associations of hs-cTnT concentration with all-cause mortality and MACE were consistent in the stratification analysis defined by sex.
Our data also suggest that even minimal changes in low levels of cTnT have prognostic characteristics and can help to identify individuals at long-term risk for adverse events. There seemed to be a dose-dependent relationship between increased hs-cTnT and increased risk. Similarly, deFilippi et al 24 have reported that changes in cTnT concentrations, which were determined with a highly sensitive assay, are significantly associated with incident HF events and cardiovascular death in community-based older adults. Furthermore, in a prospective cohort of ambulatory older adults, a strong increase in the risk of sudden cardiac death31 and incident atrial fibrillation32 was associated with changes in hs-cTnT concentrations over time. Although these changes may reflect normal physiological variation, their relation to future adverse events, regardless of baseline hs-cTnT levels, indicates that they may really represent dynamic changes in risk stratification.
In contrast to the strong associations with mortality and MACE, hs-cTnT concentration was not associated with stroke occurrence after adjusting for multiple variables. There are several potential explanations for this finding. First, cardiac troponin specifically reflects myocardial necrosis or subclinical myocardial injury. Some studies have indicated that in comparison with coronary atherosclerosis, structural heart abnormalities are more powerful determinants of myocardial injury in the general population.33 34 Second, the Atherosclerosis Risk in Communities Study35 showed that elevated plasma hs-cTnT levels are associated with an increased risk of non-lacunar ischaemic strokes, and especially cardioembolic stroke, but not with haemorrhagic stroke in the general population. Epidemiological data have demonstrated that there are differences in incidence rates of stroke subtypes between Chinese and Western populations. ICH accounts for only 10%–15% of strokes in most Western populations,36 whereas up to 55% of strokes in the Chinese population are due to ICH.37 Although an analysis for ischaemic stroke as an endpoint was conducted (data not shown), less than half of all strokes were due to ischaemia, and it was impossible to achieve statistical significance.
Our study has several limitations. First, cardiovascular treatment has changed over time, and it is possible that a higher frequency of the use of medications, such as statins and antiplatelet drugs, could have lowered the predictive value of hs-cTnT. Second, no echocardiographic and coronary artery imaging data were obtained. Thus, no causal explanations for the associations among hs-cTnT levels, cardiac abnormalities and outcomes could be provided. Third, the incidence rate of adverse events was relatively low in this cohort, and hs-cTnT levels were detected in a considerable proportion. This could limit its potential for predicting long-term events. Finally, the study population was restricted to Chinese residents of Han origin. Extrapolation of the results to other demographic groups should be done with caution. The predictive value of hs-cTnT needs to be further validated in other observational studies.
Conclusion
In this prospective cohort of a community-dwelling population, we found that both baseline cTnT and changes in cTnT, as detected by a highly sensitive assay, were independently associated with adverse outcomes. The results suggest that cTnT may be an important biomarker in the prediction of mortality and cardiovascular events in apparently healthy individuals. Further investigations are warranted to elucidate the mechanisms for cTnT release in these individuals and to test whether active interventions can reduce the associated risk contributed by elevated troponin levels.
Supplementary Material
Acknowledgments
We thank our colleagues at the Department of Laboratory Medicine of the PLA General Hospital for their help with biochemical measurements. We are also grateful to all study participants for their rigorous work.
Footnotes
Contributors: The manuscript has been read and approved by all of the authors. All authors contributed to the intellectual development of this paper.
Conceived and designed the experiments: PY. Performed the experiments: WX, YL.
Analysed the data: WX and YB. Wrote the manuscript: WX.
Supervised data collection: RC, HW.
Funding: This study was supported by grants from the Key National Basic Research Program of China (2012CB517503, 2013CB530804) and the Key Science and Technology Foundation of China (2012ZX09303004-002) to PY.
Competing interests: None declared.
Ethics approval: The study was approved by the Ethics Committee of the Chinese Peoples Liberation Army (PLA) General Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi:10.5061/dryad.bq0rm.
References
- 1. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290:898–904. 10.1001/jama.290.7.898 [DOI] [PubMed] [Google Scholar]
- 2. Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA 2009;302:49–57. 10.1001/jama.2009.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blankenberg S, Zeller T, Saarela O, et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 2010;121:2388–97. 10.1161/CIRCULATIONAHA.109.901413 [DOI] [PubMed] [Google Scholar]
- 4. Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010;31:2197–204. 10.1093/eurheartj/ehq251 [DOI] [PubMed] [Google Scholar]
- 5. Bandstein N, Ljung R, Johansson M, et al. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol 2014;63:2569–78. 10.1016/j.jacc.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 6. Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358:2107–16. 10.1056/NEJMoa0707064 [DOI] [PubMed] [Google Scholar]
- 7. Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol 2008;52:450–9. 10.1016/j.jacc.2008.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eggers KM, Al-Shakarchi J, Berglund L, et al. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J 2013;166:541–8. 10.1016/j.ahj.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 9. Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin t using a highly sensitive assay in patients with chronic heart failure. Circulation 2012;125:280–8. [DOI] [PubMed] [Google Scholar]
- 10. Beatty AL, Ku IA, Christenson RH, et al. High-sensitivity cardiac troponin T levels and secondary events in outpatients with coronary heart disease from the Heart and Soul Study. JAMA Intern Med 2013;173:763–9. 10.1001/jamainternmed.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high–sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–61. 10.1373/clinchem.2009.132654 [DOI] [PubMed] [Google Scholar]
- 12. Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937–44. 10.1681/ASN.2006040368 [DOI] [PubMed] [Google Scholar]
- 13. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies. Circulation 2003;108:2543–9. [DOI] [PubMed] [Google Scholar]
- 15. Oemrawsingh RM, Cheng JM, García-García HM, et al. High-sensitivity Troponin T in relation to coronary plaque characteristics in patients with stable coronary artery disease; results of the ATHEROREMO-IVUS study. Atherosclerosis 2016;247:135–41. 10.1016/j.atherosclerosis.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 16. Giannitsis E, Spanuth E, Horsch A, et al. High-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide predict mortality in stable coronary artery disease: results from the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med 2013;51:2019–28. 10.1515/cclm-2012-0786 [DOI] [PubMed] [Google Scholar]
- 17. Rubin J, Matsushita K, Lazo M, et al. Determinants of minimal elevation in high-sensitivity cardiac troponin T in the general population. Clin Biochem 2016;49:657–62. 10.1016/j.clinbiochem.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367–76. 10.1161/CIRCULATIONAHA.110.005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011;57:2398–405. 10.1016/j.jacc.2010.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011;57:2406–8. 10.1016/j.jacc.2011.01.029 [DOI] [PubMed] [Google Scholar]
- 21. Røsjø H, Andreassen J, Edvardsen T, et al. Prognostic usefulness of circulating high-sensitivity troponin T in aortic stenosis and relation to echocardiographic indexes of cardiac function and anatomy. Am J Cardiol 2011;108:88–91. 10.1016/j.amjcard.2011.02.346 [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki T, Sakai C, Harimoto K, et al. Usefulness of high-sensitivity cardiac troponin T and brain natriuretic peptide as biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Am J Cardiol 2013;112:867–72. 10.1016/j.amjcard.2013.04.060 [DOI] [PubMed] [Google Scholar]
- 23. Ravassa S, Kuznetsova T, Varo N, et al. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: A population-based study. Int J Cardiol 2015;185:177–85. 10.1016/j.ijcard.2015.03.046 [DOI] [PubMed] [Google Scholar]
- 24. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–502. 10.1001/jama.2010.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–12. 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sze J, Mooney J, Barzi F, et al. Cardiac troponin and its relationship to cardiovascular outcomes in community populations - a systematic review and meta-analysis. Heart Lung Circ 2016;25:217–28. 10.1016/j.hlc.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 27. Lazzarino AI, Hamer M, Gaze D, et al. The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults. J Am Coll Cardiol 2013;62:1694–701. 10.1016/j.jacc.2013.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schnell-Inderst P, Schwarzer R, Göhler A, et al. Prognostic value, clinical effectiveness, and cost-effectiveness of high-sensitivity C-reactive protein as a marker for major cardiac events in asymptomatic individuals: a health technology assessment report. Int J Technol Assess Health Care 2010;26:30–9. 10.1017/S0266462309990870 [DOI] [PubMed] [Google Scholar]
- 29. Daniels LB, Clopton P, deFilippi CR, et al. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2015;170:1170–83. 10.1016/j.ahj.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheven L, de Jong PE, Hillege HL, et al. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J 2012;33:2272–81. 10.1093/eurheartj/ehs163 [DOI] [PubMed] [Google Scholar]
- 31. Hussein AA, Gottdiener JS, Bartz TM, et al. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: the Cardiovascular Health Study. J Am Coll Cardiol 2013;62:2112–20. 10.1016/j.jacc.2013.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussein AA, Bartz TM, Gottdiener JS, et al. Serial measures of cardiac troponin T levels by a highly sensitive assay and incident atrial fibrillation in a prospective cohort of ambulatory older adults. Heart Rhythm 2015;12:879–85. 10.1016/j.hrthm.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masson S, Latini R, Mureddu GF, et al. High-sensitivity cardiac troponin T for detection of subtle abnormalities of cardiac phenotype in a general population of elderly individuals. J Intern Med 2013;273:306–17. 10.1111/joim.12023 [DOI] [PubMed] [Google Scholar]
- 34. de Lemos JA, deFilippi CR. Prevalence and significance of detectable troponins as measured by highly sensitive assays in the general population. Coron Artery Dis 2013;24:705–9. 10.1097/MCA.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 35. Folsom AR, Nambi V, Bell EJ, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke 2013;44:961–7. 10.1161/STROKEAHA.111.000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480–6. 10.1161/CIRCULATIONAHA.108.191259 [DOI] [PubMed] [Google Scholar]
- 37. Yang QD, Niu Q, Zhou YH, et al. Incidence of cerebral hemorrhage in the Changsha community. A prospective study from 1986 to 2000. Cerebrovasc Dis 2004;17:303–13. 10.1159/000077341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013431supp001.pdf (59.3KB, pdf)