Abstract
Background:
Chlorhexidine belongs to a group of medicines called antiseptic antibacterial agents. Chlorhexidine is commonly used for the care and clean off the skin, hands, and wounds. In recent years, medicinal and aromatic plants have been used for prevention of disease, maintaining health, and improving disease in traditional and modern medicine as a medicament. According to recent research, cineole is the isolated active agent of eucalyptus oil and possesses antimicrobial activity. It was demonstrated that cineole could enhance the antimicrobial effects of the other antiseptics.
Objective:
The aim of this study was to investigate the efficacy of 1,8-cineole on the antimicrobial effect of chlorhexidine against some microorganisms.
Materials and Methods:
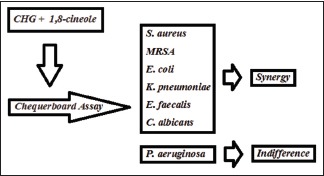
The effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate (CHG) was tested using seven different microorganisms. In this study, CHG (128–0.125 mg/l) and cineole (512–2 g/l) were analyzed together and separately using checkerboard assay. Interactions between CHG and 1,8-cineole have been identified as synergistic, indifferent, or antagonistic.
Results:
Synergistic activity was demonstrated between CHG and 1,8-cineole against Staphylococcus aureus, methicillin-resistant S. aureus, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, and Candida albicans. Indifferent interactions for these compounds were demonstrated against Pseudomonas aeruginosa.
Conclusion:
CHG antiseptic properties were found to be increased when CHG was used in combination with 1,8-cineole. In this way, CHG will reveal stronger effect against microorganisms.
SUMMARY
Cineole has increased the antimicrobial activity of chlorhexidine gluconate against all microorganisms except Pseudomonas aeruginosa
In topical application, using cineole in combination with chlorhexidine may be easier, eradicate certain resistant bacteria by increasing the antimicrobial efficacy.
Abbreviation Used: CHG: Chlorhexidine gluconate, MRSA: Methicillin-resistant S. aureus, MHB: Mueller Hinton broth, SDB: Sabouraud dextrose broth, CFU: Colonyforming unit, FIC: Fractional inhibitory concentration, FICI: FIC index, EO: Eucalyptus oil.
Key words: 1,8-cineole; chequerboard assay; chlorhexidine gluconate
INTRODUCTION
Effective disinfection and antisepsis are essential in preventing infections within the health-care setting. For this purpose, a variety of disinfectant and antiseptic agents have been used for the killing of microorganisms and the inhibition of microbial growth so far. Inappropriate usage of antisepsis and disinfection substances accelerates the process of resistance to these agents. Chlorhexidine gluconate (CHG) is a disinfectant/antiseptic agent possessing broad-spectrum antimicrobial activity. CHG (2%) in isopropyl alcohol (70%) is used as a skin antiseptic.[1] CHG is widely used for skin disinfection, in intensive care patient bathrooms, in prevention of colonization of methicillin-resistant Staphylococcus aureus (MRSA), in catheter applications as impregnated into the catheter zones cover in the wound care, in the mouthwash to prevent ventilator-associated pneumonia, and in antiseptic solution for oral hygiene and in dental practices. In addition, 0.5%–4% concentrations of CHG are used as hand antiseptics for hand hygiene. CHG is not effective against mycobacteria and bacterial spores. Although CHG has stronger efficacy against Gram-positive bacteria, it is less effective against Gram-negative bacteria and especially Pseudomonas aeruginosa.[2,3] In short, CHG has broad bactericidal activity against many Gram-positive and Gram-negative bacteria and also against Candida albicans which is a yeast.[4] In spite of the proven antimicrobial activity of CHG, recent research has demonstrated that both aqueous and alcoholic preparations of CHG poorly penetrate the human skin.[5,6] The antimicrobial efficacy of many essential oils has been known for many years. Some studies had revealed a synergistic effect between natural ingredients and disinfectants and some antimicrobial agents.[7,8] In the recent studies, many essential oils not only have antimicrobial activity against some microorganisms grown in planktonic and biofilm but also have synergistic antimicrobial activity when combined with CHG against biofilms of some microorganisms.[6,9] Based on this idea, in this study, we investigated the antimicrobial activity of 1,8-cineole alone and in combination with CHG against various microorganisms. Eucalyptus essential oils rich in 1,8-cineole (70%) have been used by inhalation in pulmonary infections.[10,11,12,13,14] Cineole is a cyclic ether and monoterpenoid and no toxic effect on tissue.[15,16] Therefore, its antimicrobial effect has been studied in various investigations. Intensive studies were performed to reveal some herbal antibacterial products. They have the potential to be an effective solution for the problem of antibiotic resistance development.[7,17,18,19]
The aim of this investigation was to compare the antimicrobial efficacy of the 1,8-cineole, the major constituent of the eucalyptus essential oils, alone and in combination with CHG against some microorganisms.
MATERIALS AND METHODS
Chemicals
1,8-cineole (99%, v/v) (Lot BCBN3332V), aqueous CHG (2%, v/v), and dimethyl sulfoxide (DMSO) (5%, v/v) were purchased from Sigma-Aldrich.
Test microorganisms
S. aureus (ATCC 25923), MRSA (clinical isolate), P. aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 51299), Klebsiella pneumoniae (ATCC 700603) and C. albicans (ATCC 90028) were obtained from Anadolu University, Faculty of Pharmacy, Department of Pharmaceutical Microbiology in Turkey. These microorganisms were stored at −80°C on MicroBank beads (Pro-Lab Diagnostics, UK) until used.
Preparation of antimicrobial agents and microbial suspensions
1,8-cineole was dissolved in DMSO. Aqueous CHG was diluted in Mueller Hinton broth (MHB) or Sabouraud dextrose broth (SDB) to produce a stock solution of 512 mg/l, and 1,8-cineole was diluted in either MHB or SDB to obtain stock suspensions of 512 g/l. Suspensions of test microorganisms which were grown in medium (Mueller-Hinton agar or Sabouraud Dextrose agar) were prepared by inoculating in either MHB or SDB and incubated overnight. The suspensions were then diluted in MHB or SDB and adjusted to a final concentration of 1 × 107 colony forming units/ml.
Chequerboard assay to assess the antimicrobial activity of chlorhexidine gluconate in combination with 1,8-cineole
To evaluate the interaction of 1,8-cineole and CHG, the antimicrobial activity of 1,8-cineole in combination with CHG was assessed against the test microorganisms by the use of chequerboard assays.[20] The tests were performed in duplicate. In this method, combinations of varying concentrations of two different active substances were examined on the same microorganisms. Further, the effect on the test microorganisms of the each antimicrobial agent was analyzed separately in microplates, and the minimum inhibitory concentration (MIC) of the each agent alone and in combination was determined in stages. First of all, preparing serial double dilutions of each antimicrobial (CHG 128–0.125 mg/l) 100 μl of CHG was added to the wells of the columns of the 96-well microtiter plate in decreasing concentrations, and 100 μl of 1,8-cineole (1,8-cineole 512–2 g/l) was added in reducing concentrations to the rows. Each well was then inoculated with 10 μl (105 microorganisms per well) of the test microorganisms. Control wells containing each agent individually in MHB or SDB were incorporated and inoculated as described previously. Following, 24 h of incubation in at 37°C for bacteria or 30°C for C. albicans, the MICs of the each agent alone and in combination were determined as described previously.[21,22] Eventually, after the MIC values of each agent alone and in combination were determined, fractional inhibitory concentration (FIC) for each agent and FIC index (FICI) were calculated using the following formulas for each microorganism. At the end of this process, CHG/1,8-cineole combinations were classified as synergistic (FICI [≤0.5]), indifferent (FICI [>0.5–≤4.0]), or antagonistic (FICI [>4.0]).[21,22]
FICA = MIC of antimicrobial in combination (CHG-1,8-cineole)/MIC of CHG alone
FICB = MIC of antimicrobial in combination (1,8-cineole-CHG)/MIC of 1,8-cineole alone
FICI = FICA + FICB.
RESULTS
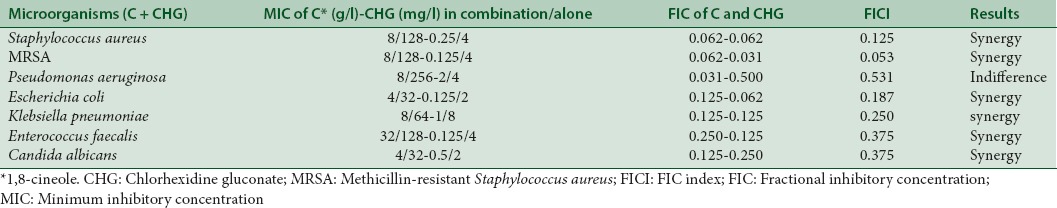
In this study, CHG and 1,8-cineole showed significant levels of synergistic interaction against some microorganisms whereas CHG interacted indifferently with 1,8-cineole against P. aeruginosa [Table 1]. The MIC values of CHG alone and in combination with 1,8-cineole were found as 4 mg/l and 0.25 mg/l for S. aureus, 4 mg/l and 0.125 mg/l for MRSA, 4 mg/l and 2 mg/l for P. aeruginosa, 2 mg/ml and 0.125 mg/l for E. coli, 8 mg/l and 1 mg/l for K. pneumoniae, 5 mg/l and 0.125 mg/l for E. faecalis, and 2 mg/l and 0.5 mg/l for C. albicans, respectively. The MIC values of 1,8-cineole alone and in combination with CHG were found as 128 g/l and 8 g/l for S. aureus, 128 g/l and 8 g/l for MRSA, 256 g/l and 8 g/l for P. aeruginosa, 32 g/l and 4 g/l for E. coli, 64 g/l and 8 g/l for K. pneumoniae, 128 g/l and 32 g/l for E. faecalis, and 32 g/l and 4 g/l for C. albicans, respectively [Table 1].
Table 1.
Antimicrobial activities of chlorhexidine gluconate alone and in combination with 1,8-cineole against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis and Candida albicans
DISCUSSION
CHG is a reliable antiseptic and has been widely used. However, despite the use of CHG and other wound antiseptics, infections caused by some resistant bacteria, and fungi can develop. The emergence of such hospital infections could also lead to great problems. The principle target of chlorhexidine is the bacterial cytoplasmic membrane, which results in the loss of structural organization and integrity, and coagulation and precipitation of cytoplasmic constituents usually occur.[23] Eucalyptus oils (EO) and its main component 1,8-cineole are thought to act on the plasma membranes, the same target of CHG. Therefore, the interaction of EO and CHG requires further studies to establish the mode of action of the potential synergism.[24] Previous research that has investigated the synergistic activity of an essential oil, and an antimicrobial agent has suggested that the synergism may be due to their action on both different or similar targets on the bacterial cells.[25,26]
In this study, we investigated the antimicrobial activity of CHG in combination with 1,8-cineole and determined the synergistic, indifferent, and antagonistic interactions between CHG and 1,8-cineole against S. aureus, MRSA, E. coli, K. pneumoniae, E. faecalis, P. aeruginosa, and C. albicans using checkerboard analysis. The calculated FIC values showed that synergistic activity was observed for CHG in combination with 1,8-cineole against all test microorganisms grown in suspension with the exception of P. aeruginosa where indifference effect was seen [Table 1].
The aim of this current study was to investigate the antimicrobial properties of 1,8-cineole, the main component of eucalyptol alone and in combination with aqueous CHG. The results showed that CHG combined with 1,8-cineole demonstrated synergistic antimicrobial activity in some of the tested microorganisms. The results of this study are consistent with previous research, verifying that 1,8-cineole, possess antimicrobial activity, such properties were also confirmed for CHG.[27] Hendry et al. were showed the same synergistic effect for cineole and CHG combination.[6]
This synergy between CHG and 1,8-cineole was found much more effective against S. aureus and MRSA. However, synergistic interaction between CHG and 1,8-cineole did not reach the strong level against E. faecalis despite the fact that E. faecalis is a Gram-positive bacterium. The synergy was also unable to reach the strong level for K. pneumoniae, E. coli, and C. albicans similar to the results of E. faecalis. MIC values of CHG and 1,8-cineole greatly diminished against P. aeruginosa for CHG in combination with 1,8-cineole, but this level is not sufficient for the definition of synergy. In conclusion, the use of such antiseptics in combination with very low or nontoxic substances such as 1,8 cineole can lead to beneficial results. Therefore, the formulations of CHG combined with such antiseptics should be developed for future usage.
CONCLUSION
The synergistic activity between CHG and 1,8-cineole may be of benefit in improved skin antisepsis and other antiseptic applications for the elimination of microcolonies which are likely to exhibit increased resistance to CHG alone. Further researches for the toxic effects of 1,8-cineole and similar plant-derived antibacterial agents and investigation of such agents in combinations with other antiseptics using a greater number of microorganisms will provide a better understanding of the subject.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are very grateful to Zerrin Cantürk (PhD) at Pharmaceutical Microbiology, Anadolu University, for supplying the clinical strains for this study.
REFERENCES
- 1.Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2007;65(Suppl 1):S1–64. doi: 10.1016/S0195-6701(07)60002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 3.Macias JH, Arreguin V, Munoz JM, Alvarez JA, Mosqueda JL, Macias AE. Chlorhexidine is a better antiseptic than povidone iodine and sodium hypochlorite because of its substantive effect. Am J Infect Control. 2013;41:634–7. doi: 10.1016/j.ajic.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Hope CK, Wilson M. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob Agents Chemother. 2004;48:1461–8. doi: 10.1128/AAC.48.5.1461-1468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karpanen TJ, Worthington T, Conway BR, Hilton AC, Elliott TS, Lambert PA. Penetration of chlorhexidine into human skin. Antimicrob Agents Chemother. 2008;52:3633–6. doi: 10.1128/AAC.00637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendry ER, Worthington T, Conway BR, Lambert PA. Antimicrobial efficacy of Eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J Antimicrob Chemother. 2009;64:1219–25. doi: 10.1093/jac/dkp362. [DOI] [PubMed] [Google Scholar]
- 7.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–90. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 8.Ben Marzoug HN, Romdhane M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, et al. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits) Molecules. 2011;16:1695–709. doi: 10.3390/molecules16021695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpanen TJ, Worthington T, Conway BR, Hilton AC, Elliott TS, Lambert PA. Permeation of chlorhexidine from alcoholic and aqueous solutions within excised human skin. Antimicrob Agents Chemother. 2009;53:1717–9. doi: 10.1128/AAC.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juergens UR, Stöber M, Vetter H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur J Med Res. 1998;3:508–10. [PubMed] [Google Scholar]
- 11.Kehrl W, Sonnemann U, Dethlefsen U. Therapy for acute nonpurulent rhinosinusitis with cineole: Results of a double-blind, randomized, placebo-controlled trial. Laryngoscope. 2004;114:738–42. doi: 10.1097/00005537-200404000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Su YC, Ho CL, Wang EI, Chang ST. Antifungal activities and chemical compositions of essential oils from leaves of four Eucalyptus. Taiwan J For Sci. 2006;21:49–61. [Google Scholar]
- 13.Safaei-Ghomi J, Ahd AA. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn Mag. 2010;6:172–5. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taur DJ, Kulkarni VB, Patil RY. Chromatographic evaluation and anthelmintic activity of Eucalyptus globulus oil. Pharmacognosy Res. 2010;2:125–7. doi: 10.4103/0974-8490.65504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boland DJ, Brophy JJ, House AP, editors. Eucalyptus leaf oils: Use, chemistry, distillation, and marketing. Melbourne: Incata Press; 1991. [Google Scholar]
- 16.Baek N, Kennelly EJ, Kardono LB, Tsauri S, Padmaniwata K, Doajarto DD, et al. Flavonoids and a proanthocyanidin from rhizomes of Selliguea feei. Phytochemistry. 1994;36:513–8. [Google Scholar]
- 17.Tabassum N, Hamdani M. Plants used to treat skin diseases. Pharmacogn Rev. 2014;8:52–60. doi: 10.4103/0973-7847.125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saraswathy A, Shakila R, Lavanya SM, Arunmozhidevi A. Essential oil constituents of Illicium griffithii and its antimicrobial activity. Pharmacogn Mag. 2010;6:208–11. doi: 10.4103/0973-1296.66938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdian-rizi MR. Chemical composition and antimicrobial activity of the essential oil of Artemisia annua L. from Iran. Pharmacogn Res. 2009;1:21–4. [Google Scholar]
- 20.Shin S, Lim S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J Appl Microbiol. 2004;97:1289–96. doi: 10.1111/j.1365-2672.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 21.Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42:872–4. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White RL, Burgess DS, Manduru M, Bosso JA. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob Agents Chemother. 1996;40:1914–8. doi: 10.1128/aac.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpanen TJ, Worthington T, Hendry ER, Conway BR, Lambert PA. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with Eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J Antimicrob Chemother. 2008;62:1031–6. doi: 10.1093/jac/dkn325. [DOI] [PubMed] [Google Scholar]
- 25.Fyfe L, Armstrong F, Stewart J. Inhibition of Listeria monocytogenes and Salmonella enteriditis by combinations of plant oils and derivatives of benzoic acid: The development of synergistic antimicrobial combinations. Int J Antimicrob Agents. 1997;9:195–9. doi: 10.1016/s0924-8579(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 26.Filoche SK, Soma K, Sissons CH. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol. 2005;20:221–5. doi: 10.1111/j.1399-302X.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 27.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]


