Abstract
Objective:
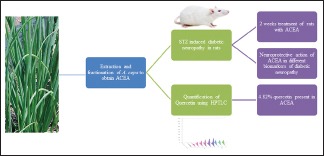
Antioxidant potential has protective effects in diabetic neuropathy (DN); hence, the present study was designed with an objective to quantify quercetin from shade-dried leaves of Allium cepa Lam. and to study its effects on streptozotocin (STZ)-induced chronic DN.
Materials and Methods:
The shade-dried leaves of A. cepa Lam. were extracted with methanol and then fractionated using ethyl acetate (ACEA). The quantification of quercetin in ACEA was evaluated by high-performance thin layer chromatography (HPTLC). The STZ (40 mg/kg) was administered to Sprague-Dawley rats (180–250 g) maintained at normal housing conditions. The STZ was administered once a day for 3 consecutive days. The elevation in blood glucose was monitored for 3 weeks periodically using flavin adenine dinucleotide-glucose dehydrogenase method by Contour TS glucometer. Rats showing blood glucose above 250 mg/dl were selected for the study. Animals were divided into eight groups. ACEA (25, 50, and 100 mg/kg), quercetin (40 mg/kg), metformin (120 mg/kg), and gabapentin (100 mg/kg) were given orally once a day for 2 weeks. The blood glucose level was again measured at the end of treatment to assess DN. Thermal hyperalgesia, cold allodynia, motor incoordination, and neurotoxicity were studied initially and at the end of 2-week treatment. Biochemical parameters were also evaluated after 2-week drug treatment.
Results:
The quercetin present in ACEA was 4.82% by HPTLC. All the ACEA treatment reduces blood glucose level at the end of the 2-week study and shows a significant neuroprotective effect in STZ-induced DN in the above experimental models.
Conclusion:
The quercetin present in ACEA proved protective effect in STZ-induced DN.
SUMMARY
High-performance thin layer chromatography reveals the presence of 4.82% quercetin in Allium cepa ethyl acetate. (ACEA). Its investigation against various diabetic neuropathy biomarkers has proved that ACEA has significant blood glucose reducing action shown neuroprotective action in thermal hyperalgesia, motor incoordination, and biochemical parameters.
Abbreviations Used: HPTLC: High-performance thin layer chromatography, TLC: Thin layer chromatography, UV: Ultraviolet, ACEA: Allium cepa ethyl acetate, STZ: Streptozotocin, LDL: Low-density lipids, HDL: High-density lipids.
Key words: Allium cepa, diabetic neuropathy, high-performance thin layer chromatography, streptozotocin
INTRODUCTION
Diabetic neuropathy (DN) is the most common change and complication that affects more than 50% of patients with diabetes.[1] It is one of the leading causes of death in the world; thus, preventive measures are necessary for patients with diabetes from developing neuropathy.[2] The chances of DN in Indian population are about 26.1%. Peripheral DN is characterized by symptoms such as tingling and numbness, sharp pains or insensitivity to pain, motor incoordination, and loss of sense of vibration. Untreated, it may lead to loss of reflexes and deformities that may progress to gangrene.[3]
There is no definitive treatment for DN at present.[3] Most of the hypoglycemic agents used in allopathic practice to treat diabetes mellitus are reported to have side effects in long-term use. Hence, there is a need to search for effective and safe drugs for which herbal medicine should be investigated as a potential regimen for diabetes and diabetes-related complications such as neuropathy.[4] The antioxidative effects of consumption of Allium cepa bulbs have been associated with a reduced risk of neurodegenerative disorders, many forms of cancer, cataract formation, ulcer development, and prevention of cardiovascular diseases by the inhibition of lipid peroxidation and lowering of low-density lipoprotein (LDL) cholesterol levels.[5] Antioxidant potential has protective effects in DN; hence, in the present study, A. cepa Lam. was selected and its effects on streptozotocin (STZ)-induced chronic DN were studied. It was hypothesized that if A. cepa has a protective effect against DN, it may be due to the flavonoids present in its leaves. Thus, considering quercetin as a standard flavonoid, it was quantified by high-performance thin layer chromatography (HPTLC) method in the current study.
MATERIALS AND METHODS
Drugs and chemicals
STZ was dissolved in cold 0.01 M citrate buffer, pH 4.5, and always prepared freshly for immediate use within 30 min.[6] Metformin (Metmin tablet), Jenburkt Pharmaceuticals Ltd.; gabapentin (Gabapin capsule), Intas Pharmaceuticals; quercetin (Deepa Chemicals); and all other chemicals used in the present study were of analytical grade.
Separation of ethyl acetate fraction from methanolic extract of leaves of Allium cepa, Lam.
Figure 1 explains diagrammatic representation of extraction procedure to obtain ethyl acetate fraction from methanolic extract of leaves of A. cepa, Lam. (ACEA)
Figure 1.
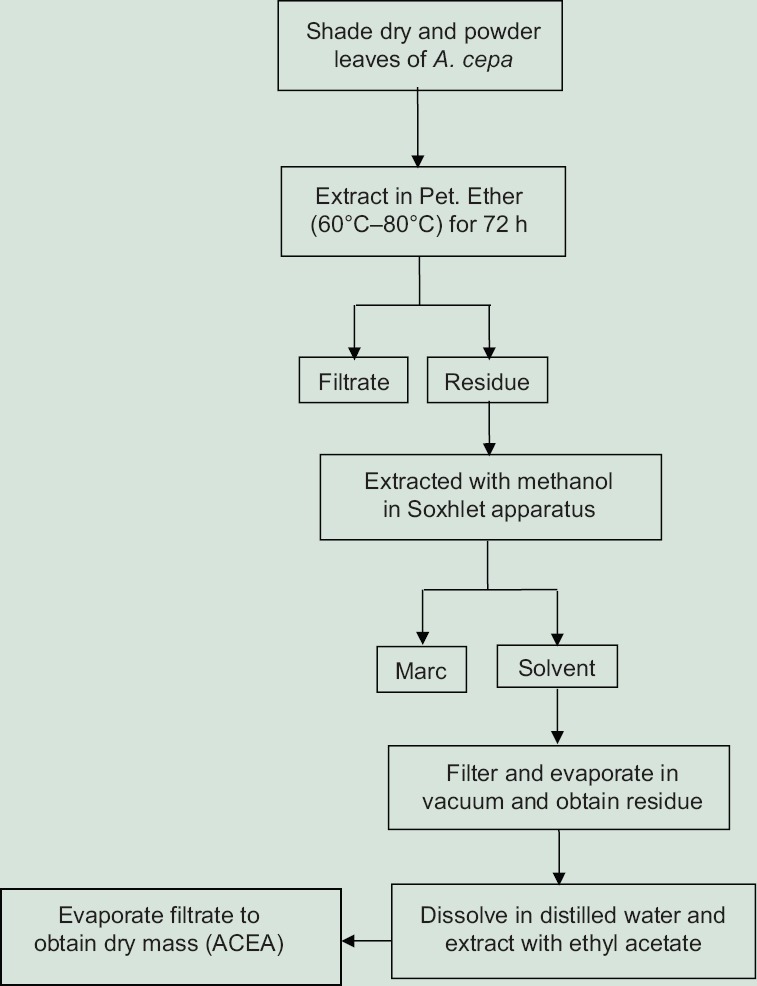
Separation of Ethyl acetate fraction from methanolic extract of leaves of A. cepa
Preparation of test solution
Accurately weighed quantity of ACEA (25, 50, and 100 mg/kg) was dissolved in distilled water to prepare the solution of the extract with appropriate concentration, Tween 80 was used as solubility enhancer.
Phytochemical screening
Phytochemical screening and physical properties of ACEA were carried out employing standard procedures and tests.[6]
Preparation of standard and stock solution
Standard solution of quercetin
A stock solution of quercetin was prepared by dissolving 10 mg of accurately weighed quercetin in methanol and making up the volume to 10 ml with methanol to get final concentration of 1000 μg/ml. It was then sonicated for 10 min.
Preparation of sample solution
Sample solution was prepared by dissolving 100 mg of fraction in ethyl acetate and making up the volume to 10 ml to get the concentration of 10 mg/ml. It was then sonicated for 10 min.
Development of thin layer chromatography technique
Thin layer chromatography (TLC) was performed using standard methods. Small quantity of sample (2 mg/ml) was dissolved in ethyl acetate. Quercetin (1 mg) standard was dissolved in methanol. Different mobile phases with varying concentrations were employed in the screening program and selected the one, in which separation of flavonoid was clear – toluene: ethyl acetate: formic acid (5:4:1).[7,8] All plates were visualized directly after drying and with the help of UV at 254 and 366 nm in UV TLC Visualizer (CAMAG). Later, it was derivatized using anisaldehyde-sulfuric acid reagent.[5] The Rf value of the different spots that were observed was calculated.
Development of high-performance thin layer chromatography technique
The samples were spotted in the form of bands with CAMAG microliter syringe on a precoated silica gel GF254 plates (20 cm × 10 cm with 0.2 mm thickness, E. Merck) using CAMAG Linomat 5, automatic sample spotter of bandwidth 8 mm. The plates were developed in a solvent system in CAMAG glass twin trough chamber previously saturated with the solvent for 30 min. The distance was 7 cm subsequent to the scanning, TLC plates were air dried, and scanning was performed on a CAMAG TLC scanner (CAMAG, Muttenz, Switzerland) in absorbance at 254 nm operated by winCATS software 4.03 version (CAMAG, Muttenz, Switzerland).
Calibration curve for quercetin
The standard solution of quercetin (1000 μg/ml) in different volumes was located on the different TLC plates for preparation of calibration curve (1–2 μl of quercetin) checked for reproducibility. The calibration curve was prepared by plotting the concentration of standard versus average peak area after scanning at 254 nm.
Quantification of quercetin
Stationary phase: Silica gel GF254 plates
Mobile phase: Toluene: ethyl acetate: formic acid (5:4:1)
Standard: Quercetin (1000 μg/ml)
Sample: Ethyl acetate fraction of methanolic extract of A. cepa leaves (10 mg/ml)
Migration distance: 70 mm
Scanning wavelength: 254 nm
Mode of scanning: Absorption (deuterium).
Animals
Sprague-Dawley rats of either sex weighing between 180 and 250 g were used. They were maintained at temperature of 25 ± 2°C and relative humidity of 45%–55% and under standard environmental conditions (12 h light/12 h dark cycles). The animals had free access to rat food (Pranav Agro Industries Ltd., Sangli, India) and water. All the experiments were carried out between 9 and 18 h. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Y B Chavan College of Pharmacy, Aurangabad (Approval number – CPCSEA/IAEC/P'col-52/2015-16/115).
Acute toxicity study
Acute toxicity study was performed under OECD guideline 425.
Induction of experimental diabetic neuropathy
Experimental rats were fasted overnight and injected with STZ at a multiple dose of 40 mg/kg body weight for 3 consecutive days. The solution was injected intraperitoneally within 5 min after dissolving in citrate buffer pH 4.5.[9] The rats in Group NC were injected with distilled water as a vehicle control. The animals were allowed to drink 5% glucose solution overnight to overcome the drug-induced hypoglycemia. Random blood glucose was estimated at the time of induction of diabetes and was checked regularly until stable hyperglycemia was achieved. After a week time for the development of diabetes, the mice with moderate diabetes having glycosuria and hyperglycemia (blood glucose levels of 250 mg/dl) were included in the study as stable hyperglycemic animals.
Experimental design
Group NC: Normal control treated with vehicle
Group DC: STZ-induced diabetic control treated with vehicle
Group DM: Diabetic rats treated with an oral dose of metformin 120 mg/kg
Group ACEA25: Diabetic rats treated with an oral dose of ACEA 25 mg/kg
Group ACEA50: Diabetic rats treated with an oral dose of ACEA 50 mg/kg
Group ACEA100: Diabetic rats treated with an oral dose of ACEA 100 mg/kg
Group DG: Diabetic rats treated with an oral dose of quercetin 40 mg/kg.
Collection of blood and determination of blood glucose
Blood samples from the experimental groups were collected from tail vein. The samples so collected were analyzed for glucose estimation using flavin adenine dinucleotide-glucose dehydrogenase method by Contour TS glucometer.
Diabetic neuropathy
Rats may develop hyperglycemia and other clinical diabetic symptoms within 3 days of STZ injection. After 1 week, rats showing blood glucose above 250 mg/dl were selected for the study. After 3 weeks of stable diabetes, drug treatment was performed, and pretreatment (pre-T/T) studies of parameters of neuropathy were performed. On completion of dosing period, rats were again tested for different parameters of neuropathy.
Neurotoxicity study
In this test, a Rotarod apparatus was rotated at a speed of 15 rpm. All animals were trained to remain on the rotating rod for 5 min. In a drug-treated mouse, the neurological deficit was indicated by the inability of the mouse to maintain equilibrium for 3 min in each of three trials. ACEA was administered, and the animals were tested for neurological deficit and diazepam was kept standard for this.[10]
Thermal hyperalgesia
Tail flick test
The nociceptive response was evaluated by recording the latency to tail withdrawal in response to noxious radiant heating. Tail flick analgesiometer was used, and the latency of withdrawal is calculated manually by stopwatch. Cutoff time is 25 s to prevent tissue damage.[11]
Hot plate method
Animals were individually placed on Eddy's hot plate with the temperature adjusted to 55 ± 1°C. The latency to the first sign of paw licking or jump response was taken as an index of the pain threshold; the cutoff time was 10 s to avoid damage to the paw.[12]
Cold allodynia
Cold allodynia was measured as the number of foot withdrawal responses after the application of acetone to the plantar surface of the paw. A drop of acetone was gently applied to the heel of the rat with a syringe connected to a thin polyethylene tube. A brisk foot withdrawal response, after the spread of acetone over the plantar surface of the paw, was considered as a sign of cold allodynia.[12]
Motor incoordination (beam-walk test)
In this method, the number of hind paw slips as rats crossed the beam-walk apparatus was used to access the sensorimotor ability. The number of time foot loses contact during walking on the beam-walk apparatus is considered as slips.[13]
Biochemical determination
After the completion of treatment, blood was collected from the orbital plexus of overnight fasted rats. The serum was separated; triglycerides, LDL, high-density lipoprotein (HDL), and cholesterol level were determined using respective methods.[14,15]
Statistical analysis
All observations are given in mean ± standard error of mean (n = 6) and data were analyzed using one-way ANOVA or two-way ANOVA as applicable, followed by Tukey's test or Bonferroni posttest using GraphPad Prism (California corporation).
RESULTS
Phytochemical screening
The preliminary phytochemical screening of ACEA revealed the presence of alkaloids, tannins, steroids, triterpenoids, saponins, and flavonoids.
Optimization of sample preparation
Ethyl acetate fraction of methanolic extract leaves of A. cepa was subjected to preliminary phytochemical investigation that revealed the presence of flavonoids. Hence, the extract was selected for further HPTLC analysis. Different solvent systems were tried to achieve a good resolution.[7,8] Finally, the solvent system toluene: ethyl acetate: formic acid (9:1:0.5) was selected.
Thin layer chromatography and high-performance thin layer chromatography
Fingerprint of TLC was studied at both 254 and 366 nm. The band at Rf 0.44 was observed for quercetin and that for the herbal fraction was 0.45 after spray with anisaldehyde-sulfuric acid reagent. The HPTLC chromatogram of standard quercetin and test sample are shown in Figures 2 and 3, respectively. The calibration curve [Figure 4] was linear in the range of 0.1–0.7 μg for quercetin. Further, a correlation coefficient of 0.999 indicates good linearity between concentration and area. ACEA has 4.822% quercetin.
Figure 2.
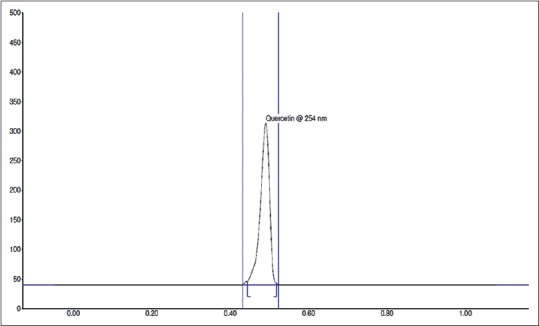
High-performance thin layer chromatography chromatogram of quercetin
Figure 3.
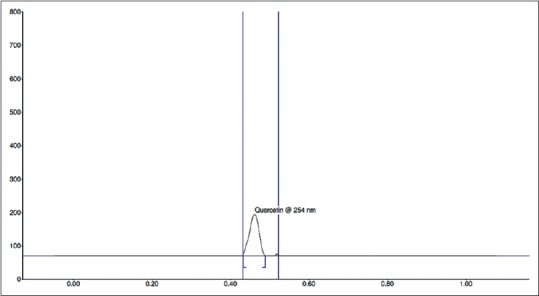
High-performance thin layer chromatography chromatogram of Allium cepa ethyl acetate
Figure 4.
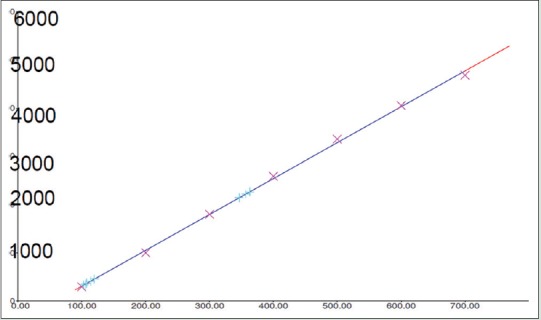
Calibration plot obtained by chromatography of marker compound quercetin. Regression through area, regression mode-linear
Acute toxicity
Animals treated with ACEA were free of any toxicity as per acceptable range given by the OECD guidelines No. 425, and no mortality was found up to 2000 mg/kg.
Effect of Allium cepa ethyl acetate on blood glucose of diabetic mice
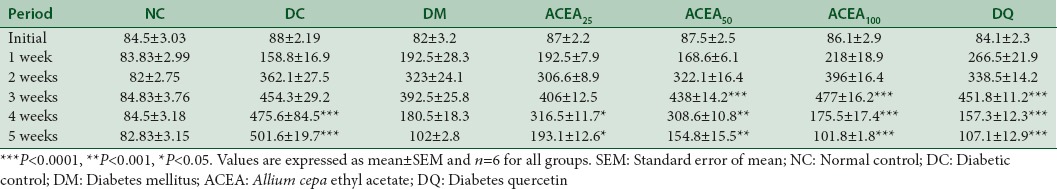
After 3 consecutive days of administration of STZ on 7th day (1st week), no stable hyperglycemia was observed. However, after 2 weeks, stable hyperglycemia was observed. Diabetic rats in all groups showed very significant (P < 0.001) increase in serum glucose level as compared to control animals. Vehicle-treated diabetic control group showed a significant (P < 0.001) increase in serum glucose level on treatment as compared to normal control animals. Metformin, ACEA 25, 50, and 100 mg/kg, and quercetin-treated animals showed a significant (P < 0.001) reduction in serum blood glucose level after treatment as compared to control. Dose- and time-dependent decrease in serum glucose level was observed till 4 weeks of treatment [Table 1].
Table 1.
Effect of Allium cepa ethyl acetate on blood glucose level (mg/dl) in streptozotocin-induced diabetic rats statistical analysis was by a two-way ANOVA with Bonferroni posttest
Diabetic neuropathy
After 3 weeks of stable diabetic condition, diabetic control animals have developed neuropathy after 4 weeks. DN was studied under thermal hyperalgesia, cold allodynia, and motor incoordination.
Neurotoxicity study
Rats treated with ACEA were able to maintain equilibrium on the Rotarod apparatus for complete duration of 5 min even when given in combination of diazepam (3 mg/kg), which is known to induce neurotoxic effects in rodents while diabetic rats and diazepam-treated rats shown neurotoxicity.
Thermal hyperalgesia
Thermal hyperalgesia was evident in STZ-induced diabetic rats as the diabetic control group shows significantly (P < 0.0001) decreased tail and paw withdrawal latency in the animal models of behavioral biomarkers of neuropathy.
Tail flick test
ACEA has significantly (P < 0.0001) increased the tail withdrawal latency of STZ-induced diabetic rats dose dependently, as compared to normal control and diabetic control in tail flick test [Figure 5].
Figure 5.
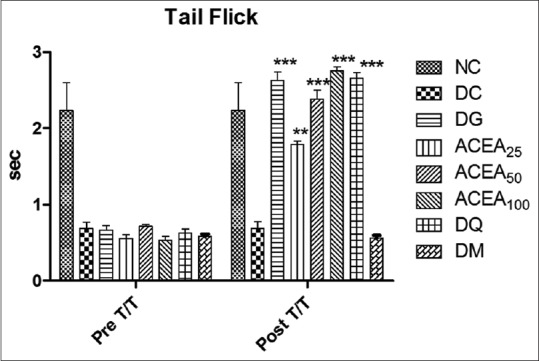
Effect of Allium cepa ethyl acetate, metformin, quercetin, and gabapentin groups both before and after treatment were studied by tail flick test. Statistical analysis two-way ANOVA with Bonferroni posttest was performed. Values are expressed as a mean ± standard error of mean and n = 6 for all groups. ***P < 0.0001, **P < 0.001
Hot plate test
Again ACEA has significantly (P < 0.0001) increased the paw withdrawal latency of STZ-induced diabetic rats dose dependently, as compared to normal control and diabetic control in hot plate test [Figure 6].
Figure 6.
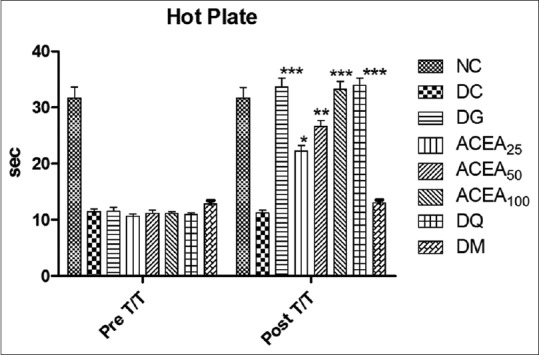
Effect of Allium cepa ethyl acetate, metformin, quercetin, and gabapentin groups both before and after treatment were studied by hot plate test. Statistical analysis two-way ANOVA with Bonferroni posttest was performed. Values are expressed as a mean ± standard error of mean and n = 6 for all groups. ***P < 0.0001, **P < 0.001
Cold allodynia
ACEA has significantly (P < 0.0001) increased the paw withdrawal latency of STZ-induced diabetic rats dose dependently, as compared to normal control and diabetic control in cold allodynia [Figure 7].
Figure 7.
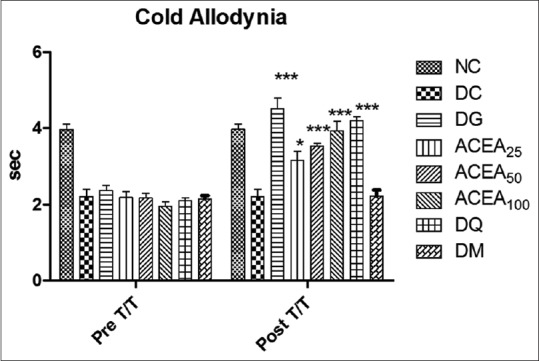
Effect of Allium cepa ethyl acetate, metformin, quercetin, and gabapentin groups both before and after treatment were studied by cold allodynia. Statistical analysis two-way ANOVA with Bonferroni posttest was performed. Values are expressed as a mean ± standard error of mean and n = 6 for all groups. ***P < 0.0001, **P < 0.001
Motor incoordination (beam-walk test)
ACEA has significantly (P < 0.0001) decreased the number of foot slips of STZ-induced diabetic rats dose dependently, as compared to normal control and diabetic control in beam-walk test [Figure 8].
Figure 8.
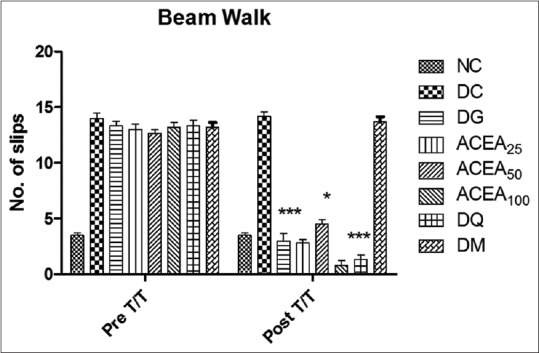
Effect of Allium cepa ethyl acetate, metformin, quercetin, and gabapentin groups both before and after treatment were studied by beam-walk. Statistical analysis two-way ANOVA with Bonferroni posttest was performed. Values are expressed as a mean ± standard error of mean and n = 6 for all groups. ***P < 0.0001, **P < 0.001, *P < 0.005
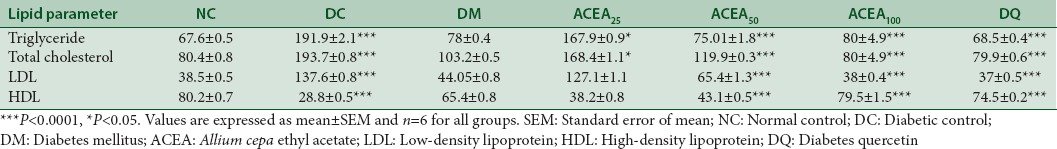
Biochemical parameters
ACEA has significantly (P < 0.0001) decreased triglyceride, cholesterol, and LDL levels while increased HDL level as compared to that of diabetic control [Table 2].
Table 2.
Effect of Allium cepa ethyl acetate on blood lipid parameters (mg/dl) in streptozotocin-induced diabetic rats statistical analysis was by a one-way ANOVA with Tukey test
DISCUSSION
STZ-induced diabetes is a reliable model of experimental diabetes. Previous research indicates that multiple subdiabetogenic doses of STZ partially damage islets and trigger an inflammatory process which is followed by the onset of insulin deficiency.[10] We have followed the multiple dosing type of induction of diabetes by administration of STZ (40 mg/kg) for 3 consecutive days. Observations indicate that diabetes was successfully induced in rats under study.
Phytochemical evaluation of ACEA revealed the presence of alkaloids, tannins, steroids, triterpenoids, saponins, and flavonoids. TLC and HPTLC analysis so far has helped in a variety of research work to get confirm results about active phytoconstituents present in medicinal traditional herbs. In this study, we have targeted to achieve the presence of flavonoids and quantify the amount of active metabolite present by considering quercetin as the marker molecule.[16,17]
The method followed allows reliable quantification of quercetin and provides good resolution and separation of quercetin in A. cepa. The peak purity of quercetin was assessed by comparing the spectra at peak start, peak apex, and peak end positions of the spot [Figures 2, 3 and 9]. In system suitability, the relative standard deviations of the quercetin peak areas and retention factors were 2.47% and 100%, respectively. The results obtained by the adoption of outlined methodologies clearly support that these may help in qualitative-quantitative analyses of quercetin from any unknown plant source within the range of the designed standardized curve for quercetin [Tables 3 and 4]. Methods that are so far in use elute several other compounds along with the flavonoids while the currently investigated technique is found to be optimum for identifying and separating flavonoids. The linear relationship between the peak areas and the concentrations prove the dependability of the present method. Applying the result that ACEA has 4.822% quercetin proves that this fraction is valuable in the fields of medicine and therapeutics.
Figure 9.
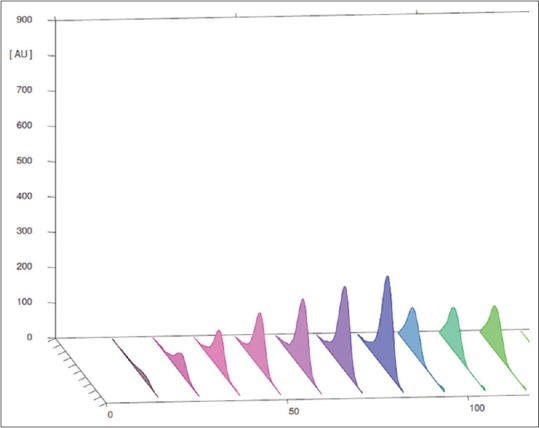
UV spectra of calibration curve for Allium cepa with quercetin as marker
Table 3.
Details of thin layer chromatography and high-performance thin layer chromatography chromatogram of quercetin

Table 4.
Details of thin layer chromatography and high-performance thin layer chromatography chromatogram of Allium cepa ethyl acetate

Results show that ACEA has potential antidiabetic activity. All forms of diabetes are characterized by chronic hyperglycemia and lead to several acute and chronic complications among which neuropathy is the most common complication.[12] There exist various behavioral, functional, structural, and molecular biomarkers which are widely used as models to investigate the etiology of DN and also used to screen the efficacy of the potential therapeutic interventions.[5] Behavioral studies in diabetic rats focus on the response to a painful as well as nonpainful sensory stimulus, thereby measuring hyperalgesia and allodynia, respectively. We have studied DN using animal models such as thermal hyperalgesia (tail flick test and hot plate test), cold allodynia, and motor incoordination (beam-walk test). These models are supposed to be behavioral biomarkers of DN.[14] After the 4th week, diabetic control group has shown signs of neuropathy while treated group has shown improvement in their condition. ACEA has shown dose-dependent protective action in thermal hyperalgesia, cold allodynia, and motor incoordination as compared to that of metformin-treated group and diabetic control because it is found that metformin has negligible effect against DN while ACEA has reduced DN.
Earlier studies reported that various plants that possess a wide variety of natural antioxidant constituents such as tannins, saponins, alkaloids, flavonoids, phenolic acids, and polyphenols and enhance free radical scavenging activities and are also responsible to ameliorate change in antioxidant enzymes and may prove helpful for treatment of diabetic-related complications.[18] ACEA proved the presence of these phytochemicals and more specifically flavonoids namely quercetin. The present study indicates that STZ-induced diabetic rats show elevated blood glucose level and reduced pain threshold in thermal hyperalgesia and cold allodynia and also reduced motor incoordination.
In chronic diabetes, there always occurs some common lipid abnormalities such as hypertriglyceridemia and hypercholesterolemia.[9] This study has shown similar effects; diabetic control animals have elevated levels of triglycerides, total cholesterol, and LDL while ACEA has shown effects similar to quercetin-treated groups that is decreased levels of these lipids. HDL being an antiatherogenic lipoprotein acts as a protective factor,[9] the levels of HDL were found to be decreased in diabetic control group while increased in ACEA-treated group.
As hypothesized about the presence of quercetin, quantification was done and to link the action of ACEA with its quercetin content, quercetin-treated group was also added in the protocol which opens a gateway for its future prospective toward the pathway or mechanism with which ACEA might be acting.
CONCLUSION
A rapid and simple HPTLC method for quantitative estimation of quercetin present in ACEA has been developed. The method used in this work resulted in good peak shape and enabled good resolution of quercetin from other constituents of the plant material. Since recovery was 100%, there was no interference with the quercetin peak from other constituents present in the plant. As it was found that ACEA contains quercetin, this fraction justifies its use for many medicinal studies including DN. The present investigation has shown that ACEA has protective action against DN in STZ-induced diabetic rats and has the presence of quercetin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Netaji TN, Dipak GP, Rahul SS, Rajkumari SS. Effect of rutin on early diabetic neuropathy in experimental animals. J Nat Prod Plant Resour. 2014;4:1–9. [Google Scholar]
- 2.Subhasree N, Kamella A, Kaliappan I, Agrawal A, Dubey GP. Antidiabetic and antihyperlipidemic activities of a novel polyherbal formulation in high fat diet/streptozotocin induced diabetic rat model. Indian J Pharmacol. 2015;47:509–13. doi: 10.4103/0253-7613.165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadig PD, Revankar RR, Dethe SM, Narayanswamy SB, Aliyar MA. Effect of Tinospora cordifolia on experimental diabetic neuropathy. Indian J Pharmacol. 2012;44:580–3. doi: 10.4103/0253-7613.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammed M, Khan D, Hemant DU, Syed SM. Effects of ethyl acetate fraction of Z. mauritiana, Lam. Leaves on STZ induced diabetes and diabetic neuropathy in mice. Int J Exp Pharmacol. 2014;4:109–16. [Google Scholar]
- 5.Vamshi KS, Rao KN, David B, Sandhya S, Sudhakar K, Saikumar P, et al. A comprehensive review on Allium cepa. J Adv Pharm Res. 2010;1:94–100. [Google Scholar]
- 6.Khandelwal KR. Practical Pharmacognosy Techniques and Experiments. 20th ed. Pune: Nirali Prakashan; 2010. [Google Scholar]
- 7.Gupta AK. Quality Standards of Indian Medicinal Plants. Vol. 3. New Delhi: Indian Council of Medicinal Research; 2005. [Google Scholar]
- 8.Sharma V, Janmeda P. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arabian J Chem. 2014:1–6. [Google Scholar]
- 9.Sivaprasad P, Mainak C, Poulami M, Suchandra M, Sudipta D, Pallab KH. Antidiabetic, antioxidant and anti-hyperlipidaemic activity of Cucumis callosus in streptozotocin-induced diabetic rats. Int J Pharm Sci Res. 2016;7:1978–84. [Google Scholar]
- 10.Arora S, Ojha SK, Vohora D. Characterisation of streptozotocin induced diabetes mellitus in Swiss albino mice. Glob J Pharm. 2009;3:81–4. [Google Scholar]
- 11.Calcutt NA. Experimental models of painful diabetic neuropathy. J Neurol Sci. 2004;220:137–9. doi: 10.1016/j.jns.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Une HD, Sarveiya VP, Pal SC, Kasture VS, Kasture SB. Nootropic and anxiolytic activity of saponins of Albizzia lebbeck leaves. Pharmacol Biochem Behav. 2001;69:439–44. doi: 10.1016/s0091-3057(01)00516-0. [DOI] [PubMed] [Google Scholar]
- 13.Kuhad A, Sharma S, Chopra K. Lycopene attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pain. 2008;12:624–32. doi: 10.1016/j.ejpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Talbot S, Chahmi E, Dias JP, Couture R. Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J Neuroinflammation. 2010;7:36. doi: 10.1186/1742-2094-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee KL. Medical Laboratory Technology. Vol. II, III. New Delhi: Tata McGraw Hill Publishing Company Limited; 2005. [Google Scholar]
- 16.Doshi GM, Une HD. Quantification of quercetin and rutin from Benincasa hispida seeds and Carissa congesta roots by high-performance thin layer chromatography and high-performance liquid chromatography. Pharmacognosy Res. 2016;8:37–42. doi: 10.4103/0974-8490.171098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doshi GM, Une HD. High performance thin layer chromatography and high performance liquid chromatography determination of quercetin from Polyalthia longifolia leaves. Free Radic Antioxid. 2015;5:60–4. [Google Scholar]
- 18.Lanjhiyana S, Garabadu D, Ahirwar D, Bigoniya P, Rana AC, Patra KC, et al. Antidiabetic activity of methanolic extract of stem bark of Elaeodendron glaucum Pers. in alloxanized rat model. Adv Appl Sci Res. 2011;2:47–62. [Google Scholar]


