Abstract
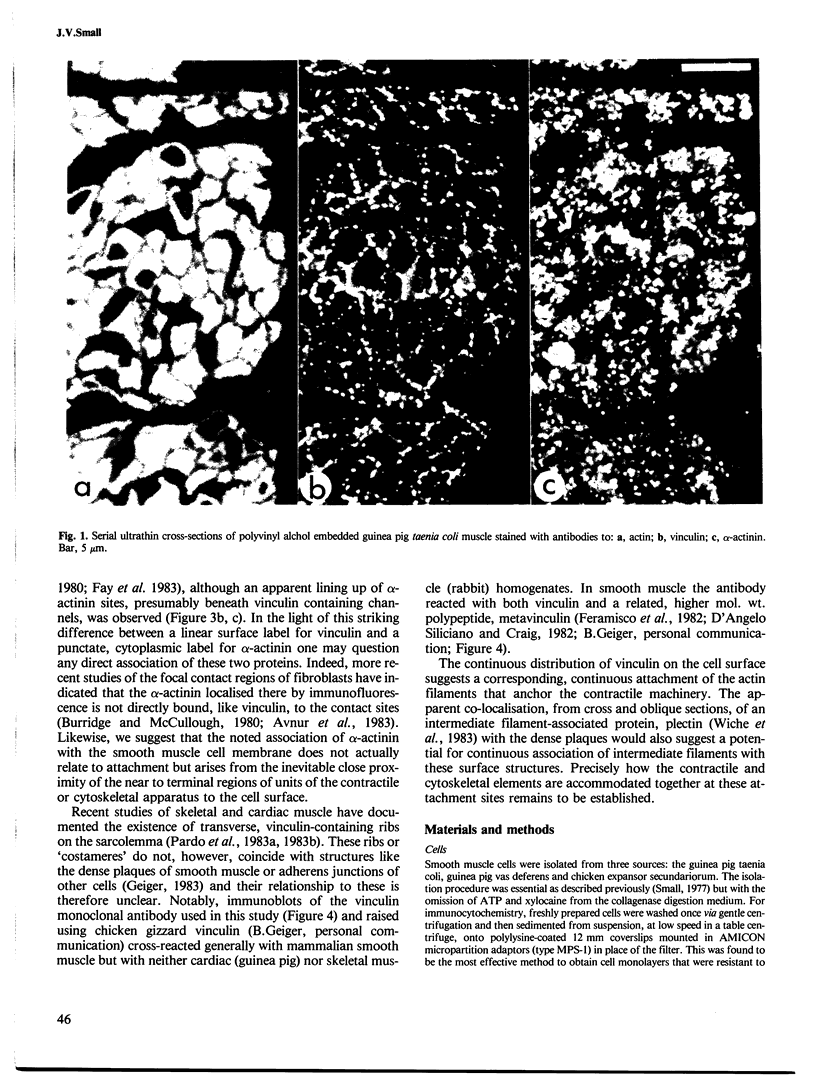
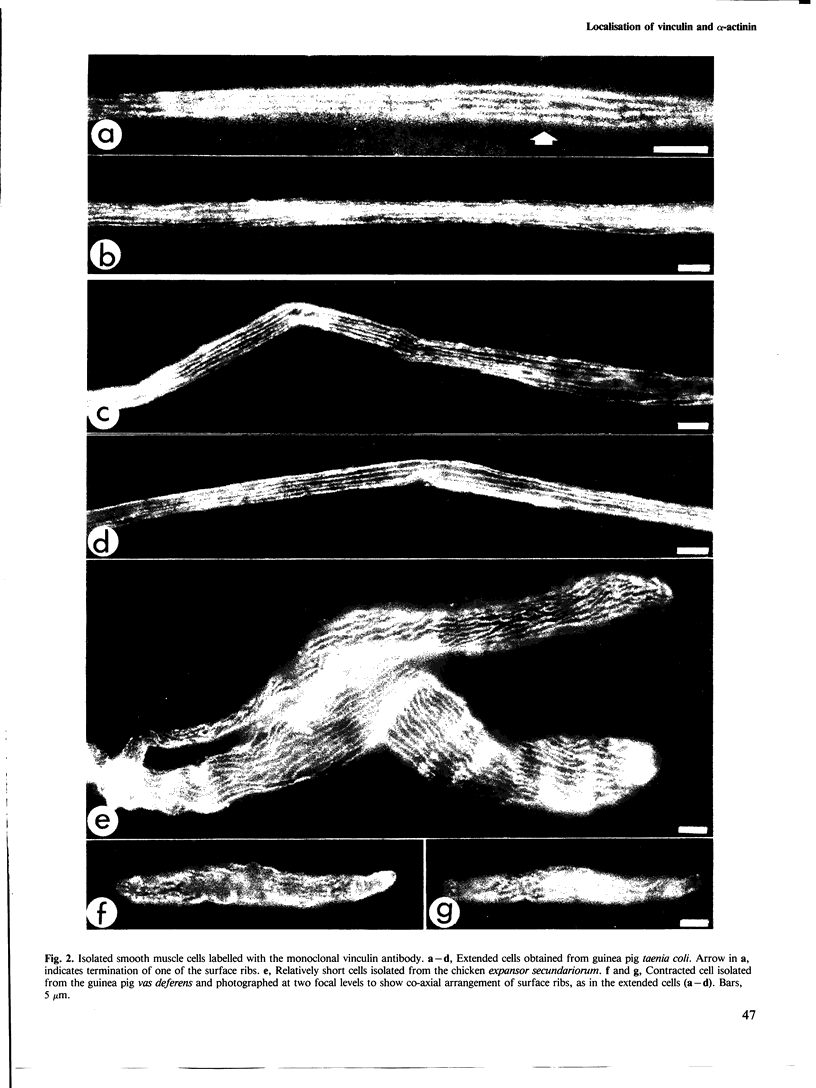
Antibodies to vinculin, a component of actin-membrane attachment sites, revealed by immunofluorescence microscopy a parallel co-axial array of continuous rib-like bands on the surface of isolated vertebrate smooth muscle cells. Images of extended and shortened cells showed that these ribs remain co-axially organised on contraction. Reference to earlier studies and labelling of thin sections indicates that the ribs correspond in position to the adhesion plaques previously described in electron microscope studies. Alpha-actinin showed a punctate distribution consistent with its presence in the cytoplasmic dense bodies, but did not show a constant association with the vinculin-containing ribs. It is suggested that alpha-actinin is an intracellular actin linker and not membrane associated, as earlier supposed, and that vinculin is, as deduced by others, a mediator of actin membrane attachment. The apparent co-association of these two proteins, noted previously, is concluded to arise from the inevitable geometrical apposition of peripheral and pre-terminal parts of the contractile machinery with the cell membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton F. T., Somlyo A. V., Somlyo A. P. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975 Oct 15;98(1):17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- Avnur Z., Small J. V., Geiger B. Actin-independent association of vinculin with the cytoplasmic aspect of the plasma membrane in cell-contact areas. J Cell Biol. 1983 Jun;96(6):1622–1630. doi: 10.1083/jcb.96.6.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby R. M. Double-immunofluorescent staining of isolated smooth muscle cells. I. preparation of anti-chicken gizzard alpha-actinin and its use with anti-chicken gizzard myosin for co-localization of alpha-actinin and myosin in chicken gizzard cells. Histochemistry. 1980;69(2):113–130. doi: 10.1007/BF00533128. [DOI] [PubMed] [Google Scholar]
- Bond M., Somlyo A. V. Dense bodies and actin polarity in vertebrate smooth muscle. J Cell Biol. 1982 Nov;95(2 Pt 1):403–413. doi: 10.1083/jcb.95.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., McCullough L. The association of alpha-actinin with the plasma membrane. J Supramol Struct. 1980;13(1):53–65. doi: 10.1002/jss.400130106. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Simpson F. O., Bertaud W. S. Surface features of smooth muscle cells from the mesenteric artery and vas deferens. J Cell Sci. 1971 Mar;8(2):427–443. doi: 10.1242/jcs.8.2.427. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Mannherz H. G. Distribution of actin and the actin-associated proteins myosin, tropomyosin, alpha-actinin, vinculin, and villin in rat and bovine exocrine glands. Eur J Cell Biol. 1983 May;30(2):167–176. [PubMed] [Google Scholar]
- Fay F. S., Delise C. M. Contraction of isolated smooth-muscle cells--structural changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Fujiwara K., Rees D. D., Fogarty K. E. Distribution of alpha-actinin in single isolated smooth muscle cells. J Cell Biol. 1983 Mar;96(3):783–795. doi: 10.1083/jcb.96.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Fisher B. A., Bagby R. M. Reorientation of myofilaments during contraction of a vertebrate smooth muscle. Am J Physiol. 1977 Jan;232(1):C5–14. doi: 10.1152/ajpcell.1977.232.1.C5. [DOI] [PubMed] [Google Scholar]
- Gabella G., Blundell D. Effect of stretch and contraction on caveolae of smooth muscle cells. Cell Tissue Res. 1978 Jul 5;190(2):255–271. doi: 10.1007/BF00218174. [DOI] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983 Aug 11;737(3-4):305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Geiger B., Schmid E., Franke W. W. Spatial distribution of proteins specific for desmosomes and adhaerens junctions in epithelial cells demonstrated by double immunofluorescence microscopy. Differentiation. 1983;23(3):189–205. doi: 10.1111/j.1432-0436.1982.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Lowy J., Vibert P. J., Haselgrove J. C., Poulsen F. R. The structure of the myosin elements in vertebrate smooth muscles. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):191–196. doi: 10.1098/rstb.1973.0022. [DOI] [PubMed] [Google Scholar]
- Pardo J. V., Siliciano J. D., Craig S. W. Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983 Oct;97(4):1081–1088. doi: 10.1083/jcb.97.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenberg C. F., Haselgrove J. C. Filaments and ribbons in vertebrate smooth muscle. Nature. 1974 May 10;249(453):152–154. doi: 10.1038/249152a0. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Meta-vinculin--a vinculin-related protein with solubility properties of a membrane protein. Nature. 1982 Dec 9;300(5892):533–535. doi: 10.1038/300533a0. [DOI] [PubMed] [Google Scholar]
- Small J. V. Contractile units in vertebrate smooth muscle cells. Nature. 1974 May 24;249(455):324–327. doi: 10.1038/249324a0. [DOI] [PubMed] [Google Scholar]
- Small J. V. Studies on isolated smooth muscle cells: The contractile apparatus. J Cell Sci. 1977 Apr;24:327–349. doi: 10.1242/jcs.24.1.327. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Butler T. M., Bond M., Somlyo A. P. Myosin filaments have non-phosphorylated light chains in relaxed smooth muscle. Nature. 1981 Dec 10;294(5841):567–569. doi: 10.1038/294567a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Usukura J., Ishikawa H. Myosin filaments in smooth muscle cells of the guinea pig taenia coli: a freeze-substitution study. Eur J Cell Biol. 1982 Oct;28(2):195–201. [PubMed] [Google Scholar]
- Wiche G., Krepler R., Artlieb U., Pytela R., Denk H. Occurrence and immunolocalization of plectin in tissues. J Cell Biol. 1983 Sep;97(3):887–901. doi: 10.1083/jcb.97.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]