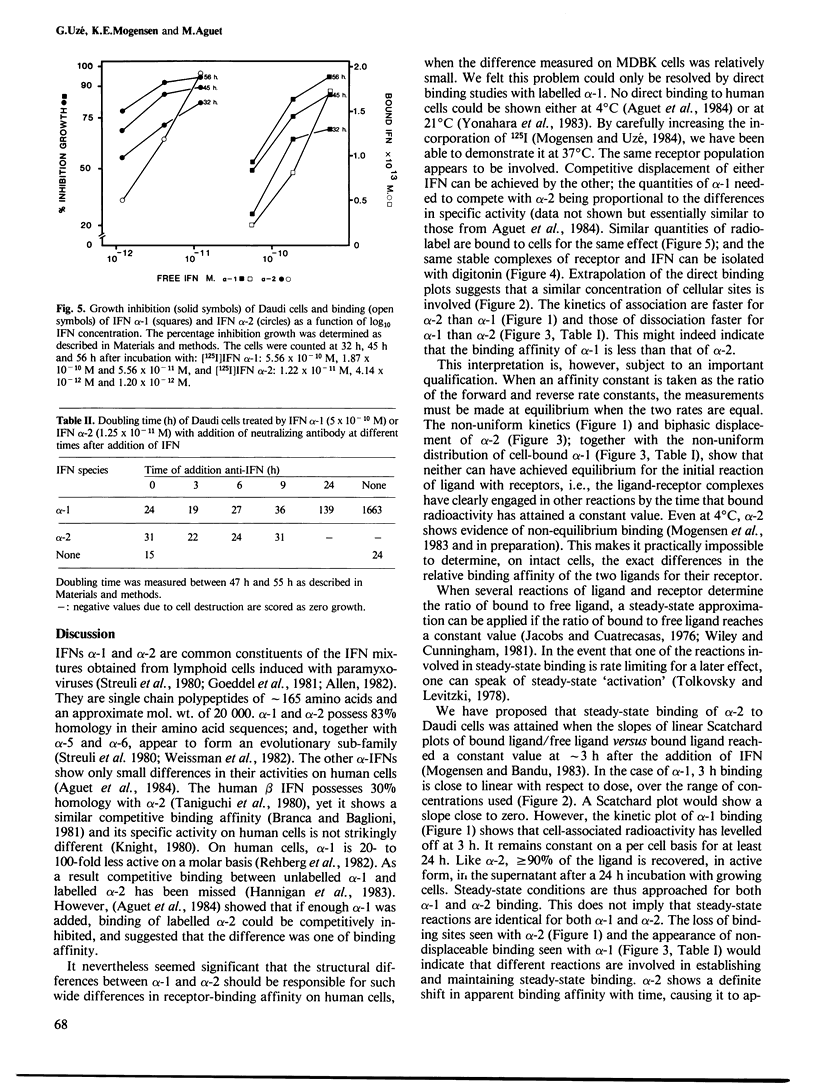
Abstract
Two related human alpha interferons with 83% homology in their primary sequences show a similar specific activity on nonhuman cells, but a striking difference on human cells, on which alpha-1 shows 1-5% of the specific molar activity displayed by alpha-2. Both interferons were labelled with 125I, and their binding kinetics followed on growing cultures of the human Burkitt line Daudi. Binding of alpha-1 showed slower rates of association and faster rates of dissociation implying that differences in apparent binding affinity were responsible for the differences in specific molar activity. However, binding was shown to reach steady-state rather than an equilibrium, so differences in the dynamics of the ligand-receptor complexes may represent amplification of differences in the initial binding constant. alpha-2, but not alpha-1, induces a marked loss of binding sites leading to a high affinity steady-state binding. Inhibition of cell multiplication by both interferons depends on a continued stimulation by free ligands at steady-state. It is proposed that the differences in specific molar activity are, in the main, kinetic and cause alpha-1 and alpha-2 to behave respectively as "slow' and "fast' interferons.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Aguet M., Gröbke M., Dreiding P. Various human interferon alpha subclasses cross-react with common receptors: their binding affinities correlate with their specific biological activities. Virology. 1984 Jan 15;132(1):211–216. doi: 10.1016/0042-6822(84)90105-3. [DOI] [PubMed] [Google Scholar]
- Aguet M., Mogensen K. E. Interferon receptors. Interferon. 1983;5:1–22. [PubMed] [Google Scholar]
- Allen G. Structure and properties of human interferon-alpha from Namalwa lymphoblastoid cells. Biochem J. 1982 Dec 1;207(3):397–408. doi: 10.1042/bj2070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V., Mogensen K. E., Muscettola M., Pacini A., Paulesu L., Pessina G. P., Skiftas S. Degradation of human 125I-interferon alpha by isolated perfused rabbit kidney and liver. J Lab Clin Med. 1983 Jun;101(6):857–863. [PubMed] [Google Scholar]
- Borden E. C. Interferons: in pursuit of the promise. Prog Clin Biol Res. 1983;132E:287–296. [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Branca A. A., Faltynek C. R., D'Alessandro S. B., Baglioni C. Interaction of interferon with cellular receptors. Internalization and degradation of cell-bound interferon. J Biol Chem. 1982 Nov 25;257(22):13291–13296. [PubMed] [Google Scholar]
- Daubas P., Mogensen E. A radioimmunoassay of human leukocyte interferon using protein A-containing Staphylococcus aureus. J Immunol Methods. 1982;48(1):1–12. doi: 10.1016/0022-1759(82)90204-6. [DOI] [PubMed] [Google Scholar]
- Eid P., Mogensen K. E. Isolated interferon alpha-receptor complexes stabilized in vitro. FEBS Lett. 1983 May 30;156(1):157–160. doi: 10.1016/0014-5793(83)80268-3. [DOI] [PubMed] [Google Scholar]
- GIFFORD G. E., TOY S. T., LINDENMANN J. Studies on vaccinia virus plaque formation and its inhibition by interferon. II. Dynamics of plaque formation by vaccinia virus in the presence of interferon. Virology. 1963 Mar;19:294–301. doi: 10.1016/0042-6822(63)90067-9. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Vigneri R., Cohen D., Pliam N. B., Kahn C. R. Intracellular binding sites for insulin are immunologically distinct from those on the plasma membrane. Nature. 1977 Oct 20;269(5630):698–700. doi: 10.1038/269698a0. [DOI] [PubMed] [Google Scholar]
- Gresser I. On the varied biologic effects of interferon. Cell Immunol. 1977 Dec;34(2):406–415. doi: 10.1016/0008-8749(77)90262-3. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Hannigan G. E., Gewert D. R., Fish E. N., Read S. E., Williams B. R. Differential binding of human interferon-alpha subtypes to receptors on lymphoblastoid cells. Biochem Biophys Res Commun. 1983 Jan 27;110(2):537–544. doi: 10.1016/0006-291x(83)91183-x. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Inglot A. D. The hormonal concept of interferon. Brief review. Arch Virol. 1983;76(1):1–13. doi: 10.1007/BF01315699. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. The mobile receptor hypothesis and "cooperativity" of hormone binding. Application to insulin. Biochim Biophys Acta. 1976 May 21;433(3):482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Johnson L. K., Vlodavsky I., Baxter J. D., Gospodarowicz D. Nuclear accumulation of epidermal growth factor in cultured rat pituitary cells. Nature. 1980 Sep 25;287(5780):340–343. doi: 10.1038/287340a0. [DOI] [PubMed] [Google Scholar]
- Jonak G. J., Knight E., Jr Selective reduction of c-myc mRNA in Daudi cells by human beta interferon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1747–1750. doi: 10.1073/pnas.81.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Peptide hormone-induced receptor mobility, aggregation, and internalization. N Engl J Med. 1981 Jul 9;305(2):77–88. doi: 10.1056/NEJM198107093050206. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Szigeti R., Klein E., Klein G., Gruest J., Montagnier L., Taira H., Hall A., Nagata S., Weissmann C. Effect of interferon-alpha 1 from E. coli on some cell functions. Science. 1980 Sep 19;209(4463):1431–1435. doi: 10.1126/science.6158096. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T. Kinetic evidence for an activation step following binding of human interferon alpha 2 to the membrane receptors of Daudi cells. Eur J Biochem. 1983 Aug 1;134(2):355–364. doi: 10.1111/j.1432-1033.1983.tb07575.x. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T., Vignaux F., Aguet M., Gressner I. Binding of 125I-labelled human alpha interferon to human lymphoid cells. Int J Cancer. 1981 Nov 15;28(5):575–582. doi: 10.1002/ijc.2910280508. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T., Vignaux F., Uze G., Eid P. Receptor mediated pathways for interferon action: in vivo implications. Med Oncol Tumor Pharmacother. 1984;1(2):77–85. doi: 10.1007/BF02934978. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Pyhälä L., Cantell K. Raising antibodies to human leukocyte interferon. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):443–450. doi: 10.1111/j.1699-0463.1975.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Overall J. C., Jr, Spruance S. L., Green J. A. Viral-induced leukocyte interferon in vesicle fluid from lesions of recurrent herpes labialis. J Infect Dis. 1981 Apr;143(4):543–547. doi: 10.1093/infdis/143.4.543. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The principles of drug action. Proc R Soc Med. 1960 Oct;53:815–820. doi: 10.1177/003591576005301002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg E., Kelder B., Hoal E. G., Pestka S. Specific molecular activities of recombinant and hybrid leukocyte interferons. J Biol Chem. 1982 Oct 10;257(19):11497–11502. [PubMed] [Google Scholar]
- Schreiber A. B., Lax I., Yarden Y., Eshhar Z., Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Shechter Y., Chang K. J., Jacobs S., Cuatrecasas P. Modulation of binding and bioactivity of insulin by anti-insulin antibody: relation to possible role of receptor self-aggregation in hormone action. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2720–2724. doi: 10.1073/pnas.76.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Hall A., Boll W., Stewart W. E., 2nd, Nagata S., Weissmann C. Target cell specificity of two species of human interferon-alpha produced in Escherichia coli and of hybrid molecules derived from them. Proc Natl Acad Sci U S A. 1981 May;78(5):2848–2852. doi: 10.1073/pnas.78.5.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980 Sep 19;209(4463):1343–1347. doi: 10.1126/science.6158094. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Mantei N., Schwarzstein M., Nagata S., Muramatsu M., Weissmann C. Human leukocyte and fibroblast interferons are structurally related. Nature. 1980 Jun 19;285(5766):547–549. doi: 10.1038/285547a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Klotz I. M. Macromolecule--small molecule interactions: analytical and graphical reexamination. Arch Biochem Biophys. 1971 Nov;147(1):178–185. doi: 10.1016/0003-9861(71)90325-0. [DOI] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Nagata S., Boll W., Fountoulakis M., Fujisawa A., Fujisawa J. I., Haynes J., Henco K., Mantei N., Ragg H. Structure and expression of human IFN-alpha genes. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 24;299(1094):7–28. doi: 10.1098/rstb.1982.0102. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. A steady state model for analyzing the cellular binding, internalization and degradation of polypeptide ligands. Cell. 1981 Aug;25(2):433–440. doi: 10.1016/0092-8674(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]
- Yonehara S., Yonehara-Takahashi M., Ishii A., Nagata S. Different binding of human interferon alpha 1 and alpha 2 to common receptors on human and bovine cells. Studies with recombination interferons produced in Escherichia coli. J Biol Chem. 1983 Aug 10;258(15):9046–9049. [PubMed] [Google Scholar]



