Abstract
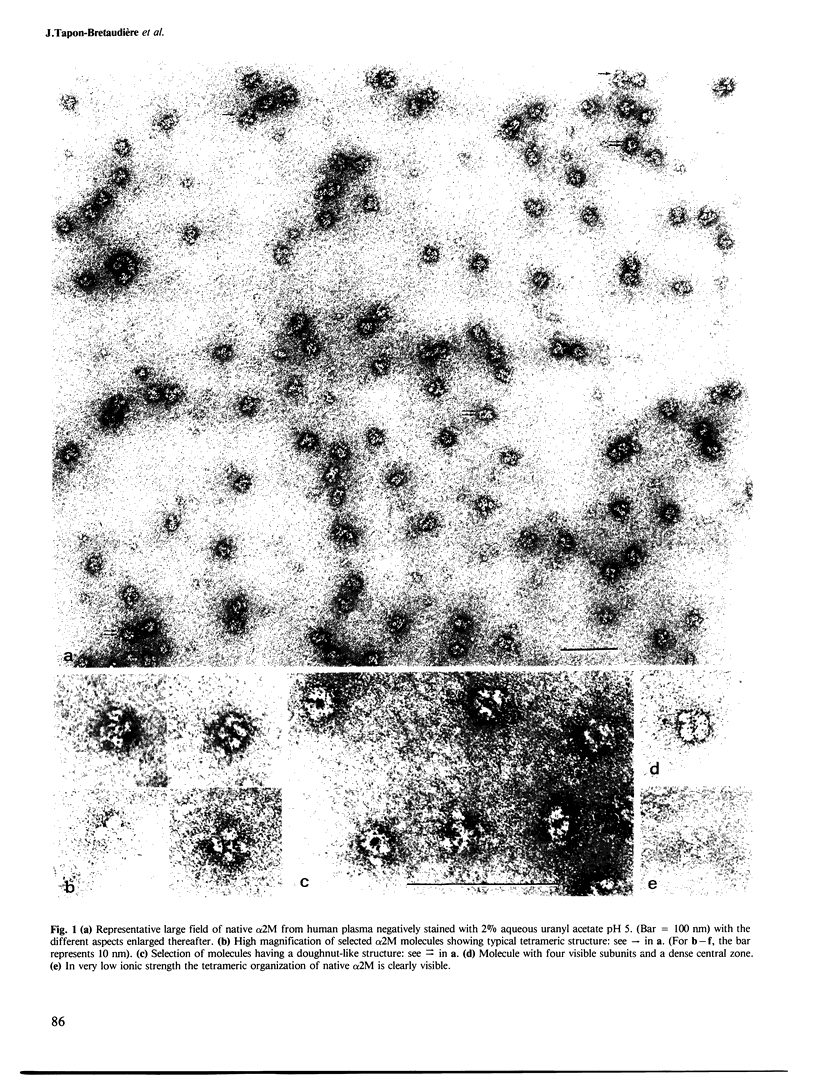
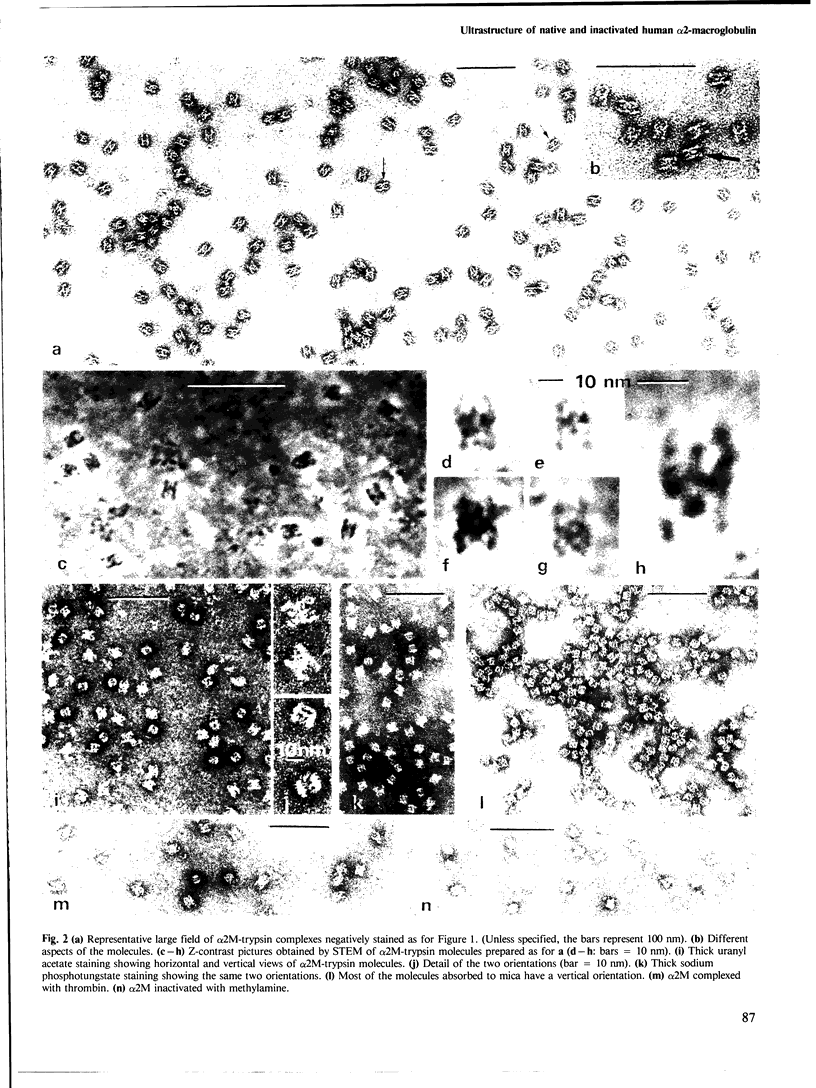
High resolution electron microscopy reveals that fully active alpha 2-macroglobulin (α2M) from fresh human plasma presents a very characteristic tetrameric structure. This native conformation of the α2M molecule is described here for the first time, along with its various orientations in negatively stained preparations. Although the native form is sensitive to inactivation, glutaraldehyde fixation is not necessary for its observation except when ammonium salts are used. The tetrameric structure of α2M undergoes a drastic conformational change when the protein is treated either with trypsin, thrombin or methylamine, as evidenced by the appearance of the typical)+(structure already described in the literature. The various aspects of this second conformation correspond to different orientations of the molecules in the stain film, and depend upon the nature of the support.
Keywords: α2-macroglobulin, conventional and scanning transmission electron microscopy, native form, negative staining, protease inactivated form
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Alpha 2-macroglobulin. Methods Enzymol. 1981;80(Pt 100):737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Fish W. W. Evidence for similar conformational changes in alpha 2-macroglobulin on reaction with primary amines or proteolytic enzymes. Biochem J. 1982 Nov 1;207(2):347–356. doi: 10.1042/bj2070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloth B., Chesebro B., Svehag S. E. Ultrastructural studies of human and rabbit alpha-M-globulins. J Exp Med. 1968 Apr 1;127(4):749–756. doi: 10.1084/jem.127.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branegård B., Osterberg R., Sjöberg B. Small-angle X-ray scattering study of the interaction between human alpha 2-macroglobulin and trypsin. Eur J Biochem. 1982 Mar 1;122(3):663–666. doi: 10.1111/j.1432-1033.1982.tb06489.x. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Curgy J. J., Iftode F., Perasso R., André J., Mory C., Colliex C. A STEM approach to the study of the proteinaceous architecture of a ribosome. Biol Cell. 1984;50(3):247–254. doi: 10.1111/j.1768-322x.1984.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Gauthier F., Leng M., Mouray H. Isolement, propriétés physiques et morphologie en microscopie électronique de l'alpha-1-macroglobuline du sérum de rat. C R Acad Sci Hebd Seances Acad Sci D. 1974 Oct 14;279(16):1409–1412. [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Horne R. W., Ronchetti I. P. A negative staining-carbon film technique for studying viruses in the electron microscope. I. Preparative procedures for examining icosahedral and filamentous viruses. J Ultrastruct Res. 1974 Jun;47(3):361–383. doi: 10.1016/s0022-5320(74)90015-x. [DOI] [PubMed] [Google Scholar]
- Höglund S., Levin O. Electron microscopic studies of some proteins from normal human serum. J Mol Biol. 1965 Jul;12(3):866–871. doi: 10.1016/s0022-2836(65)80333-3. [DOI] [PubMed] [Google Scholar]
- Ikai A., Kitamoto T., Nishigai M. Alpha-2-macroglobulin-like protease inhibitor from the egg white of cuban crocodile (Crocodylus rhombifer). J Biochem. 1983 Jan;93(1):121–127. doi: 10.1093/oxfordjournals.jbchem.a134145. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebreton de Vonne T., Mouray H. Les alpha-macroglobulines. Ann Biol Clin (Paris) 1974;32(3):185–196. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Morelis P., Ambrosioni J. C., Got R., Fontanges R. Observation au microscope électronique du complexe formé par l'alpha-1-macroglobuline de sérum de lapin avec la trypsine. C R Acad Sci Hebd Seances Acad Sci D. 1969 Oct 13;269(15):1453–1454. [PubMed] [Google Scholar]
- Pochon F., Amand B., Lavalette D., Bieth J. Rotational relaxation of free and protease-bound alpha2-macroglobulin. J Biol Chem. 1978 Oct 25;253(20):7496–7499. [PubMed] [Google Scholar]
- Schramm H. J., Schramm W. Computer averaging of single molecules of alpha 2-macroglobulin and the alpha 2-macroglobulin/trypsin complex. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):803–812. doi: 10.1515/bchm2.1982.363.2.803. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Blatrix C. Préparation de l'alpha 2-macroglobuline comme sous-produit du fractionnement. Rev Fr Transfus. 1970 Jun;13(2):141–151. doi: 10.1016/s0035-2977(70)80022-0. [DOI] [PubMed] [Google Scholar]
- Straight D. L., McKee P. A. Effect of protease binding by alpha 2-macroglobulin on intrinsic fluorescence. Biochemistry. 1982 Sep 14;21(19):4550–4556. doi: 10.1021/bi00262a006. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. Kinetics of inactivation of alpha 2-macroglobulin by methylamine, and formation of anomalous complexes with trypsin. Biochem J. 1982 Jan 1;201(1):119–128. doi: 10.1042/bj2010119. [DOI] [PMC free article] [PubMed] [Google Scholar]