Abstract
Improving the specific capacity and electronic conductivity of TiO2 can boost its practical application as a promising anode material for lithium ion batteries. In this work, a three-dimensional networking oxygen-deficient nano TiO2-x/carbon fibre membrane was achieved by combining the electrospinning process with a hot-press sintering method and directly used as a self-standing anode. With the synergistic effects of three-dimensional conductive networks, surface oxygen deficiency, high specific surface area and high porosity, binder-free and self-standing structure, etc., the nano TiO2-x/carbon fibre membrane electrode displays a high electrochemical reaction kinetics and a high specific capacity. The reversible capacity could be jointly generated from porous carbon, full-lithiation of TiO2 and interfacial lithium storage. At a current density of 100 mA g−1, the reversible discharge capacity can reach 464 mA h g−1. Even at 500 mA g−1, the discharge capacity still remains at 312 mA h g−1. Compared with pure carbon fibre and TiO2 powder, the TiO2-x/C fibre membrane electrode also exhibits an excellent cycle performance with a discharge capacity of 209 mA h g−1 after 700 cycles at the current density of 300 mA g−1, and the coulombic efficiency always remains at approximately 100%.
Keywords: fibre membrane, three-dimensional conductive network, oxygen deficiency, electrospinning, hot-press sintering
1. Introduction
TiO2 has been regarded as one of the most promising anodes due to its merits including high redox potential, excellent capacity retention, low self-discharge and less than 4% volume change during Li ion insertion/extraction processes, which endows TiO2 with a good structural stability and long cycle life [1,2]. However, some intrinsic drawbacks, such as low electronic conductivity and Li ion diffusivity, especially low theoretical capacity of 168–335 mA h g−1, and poor rate capability, are still hindering its application in Li-ion batteries [3]. Various strategies have been developed for enhancing the electrochemical performance of TiO2 anodes, e.g., tailoring the morphology as nanorod [4], nanofibre [5], nanotube [6], nanowire [7] or microcone [8], carbon coating [9], and combining with CNTs [10], graphene [11] or mesoporous carbon [12] etc.; although much progress has been achieved to improve the rate capability of TiO2 anodes, there is still a challenge to its specific capacity.
Many works have proved that anatase TiO2 has a tetragonal unit cell that can theoretically accommodate one lithium for every TiO2, corresponding to a theoretical capacity of 335 mA h g−1. However, full lithiation can, up to date, only be achieved with particles smaller than 10 nm in diameter, because for lithiation to Li0.5TiO2, anatase has to undergo a phase transition from tetragonal to orthorhombic, which has been regarded as the maximum electrochemical insertion limit of Li into bulk anatase [13,14], leading to the most reported capacity values with xLi < 1. For this reason, most research on this material is focused on nano-sized or nano-textured forms.
Recently, two new methods were proposed to improve the electrochemical properties of anodes. One is by forming oxygen deficiency on the surface of active materials [15,16]. For example, Brumbarov et al. [15] synthesized oxygen-deficient, carbon-coated TiO2 nanotubes as anode material with remarkably high Li storage capacity reaching the theoretical capacity of x = 1.0, which was interpreted as a result of enhanced electronic conductivity of the TiO2 nanotubes and then enhanced charge-transfer kinetics due to the formation of oxygen vacancies. In addition, many researchers have also focused on the three-dimensional network structures to improve the kinetics of the electrode material, which provide high three-dimensional conductivity, high porosity and large surface area for fast Li+ diffusion and excellent charge transport, and large surface area also for fast interfacial charge collection. Meanwhile, the high porosity can alleviate the volume variation during the Li+ insertion/extraction processes, resulting in a relatively high reversible capacity and cycling stability [17,18]. Zhang et al. [18] reported on one-dimensional TiO2–graphene composite nanofibres (TiO2-G nanofibres) as an anode, which exhibited an initial discharge capacity of 260 mA h g−1 at a current density of 33 mA g−1, and retained a reversible capacity up to 84% after 300 cycles at 150 mA g−1. The improved reversible capacity and cycling performance of the TiO2 nanofibres are attributed to the large surface area of the nanofibres, small nanocrystalline size, large Li nonstoichiometric parameters, and increased electronic conductivity [18]. However, up to date, few studies have been reported on a three-dimensional nano-TiO2 fibre membrane with oxygen deficiency as anode material for Li-ion batteries.
In this work, we prepared a three-dimensional oxygen-deficient nano-TiO2-x/carbon fibre membrane by facile electrospinning method as a self-standing anode for Li-ion batteries. By combining oxygen-deficient merit and three-dimensional network structure, this fibre membrane electrode shows high capacity and rate performance simultaneously, and will be a promising anode for Li-ion batteries.
2. Experimental
The precursor of the TiO2/C nanofibre membrane was prepared by conventional electrospinning method. Firstly, 5 g tetrabutyl titanate (Ti(OC4H9)4, 99.0%) and 4.0 g polyacrylonitrile (PAN, M = 150 000) were dissolved in 5 g and 30 g N,N-dimethylformamide (DMF) solutions respectively to obtain homogeneous solutions A and B. Next, 0.5 ml HNO3 was added to solution A to avoid hydrolysis of Ti4+ ions. Then solution A was added dropwise into solution B and continuously stirred for 12 h to yield a viscous solution for electrospinning. The obtained solution was subsequently electrospun by an electronspinner (Yfolw, 2.2.S-500) at a feeding rate of 0.5 ml h−1 and a high voltage of 23 kV. The distance between the injecting nozzle and the roll receiver was 15 cm. Finally, the electrospun TiO2/C composite fibre membrane precursor was pre-sintered at 280°C in air for 2 h. Then, the pre-sintered fibre membrane was sandwiched between two graphite plates and annealed at 750°C for 1 h under a nitrogen atmosphere. When the furnace was cooled to room temperature, a uniform anatase TiO2/C porous fibre membrane was obtained with a thickness of approximately 200 µm.
A field-emission scanning electron microscope (SEM, JSM-5600LV) was used to observe the morphology and microstructure of the fibres. A Rigaku 2500 X-ray diffractometer equipped with Cu Kα radiation was used to examine the crystalline phases of the membrane between 10° and 80°. The carbon content and graphitized degree of the composite nanofibre were analysed by a MltiEA2000 carbon–sulfur analyser and a BOEN 265964 Raman microscope. X-ray photoelectron spectroscopy (XPS analysis for the fibre membrane was carried out using a Thermo Scientific ESCALAB 250Xi spectrophotometer; an AlK X-ray source was used for the excitation of electrons. Microstructure and composition of the synthesized composites were measured using transmission electron microscopy (TEM; JEOL JEM2010) with an energy dispersive X-ray spectrometer (EDS) attachment and selected area electron diffraction (SAED). The surface area and pore size distribution were determined by nitrogen adsorption/desorption using the Brunauer–Emmett–Teller (BET; NOVA2000e) technique.
All the electrochemical measurements were conducted in 2025 type coin-cells. The self-standing electrode round pieces of 13 mm diameter were directly cut from the larger piece of TiO2/C fibre membrane. The whole weight of a piece of electrode is about 3.6–4.2 mg, and the carbon content is about 32.8%. The electrodes were dried at 110°C overnight and then assembled in an Ar-filled glovebox, using metallic lithium foil as counter and reference electrode, and 1 M LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC) (EC/DEC = 50/50 (v/v)) mixture as the electrolyte solution. The cathode/anode were separated by a Celgard 2400 polypropylene film. The electrode reaction kinetics was analysed by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements using a DHS VMP2 electrochemical workstation and 1287/SI-1260/SI impedance/gain-phase analyser. The rate and cycle performances were evaluated by galvanostatic charge/discharge measurements using a Land 2000 battery tester at various current densities between 0 and 3 V at 25°C. The specific capacity was calculated according to the total quality of the electrode. For comparison, the bare carbon fibre membrane electrode was also prepared by the same process as TiO2/C fibre membrane, and the pure TiO2 powders electrode was fabricated by conventional tape-casting method using commercial Degussa P25 powders as active material. All the electrochemical tests were at the same conditions as those of the TiO2/C fibre membrane.
3. Results and discussion
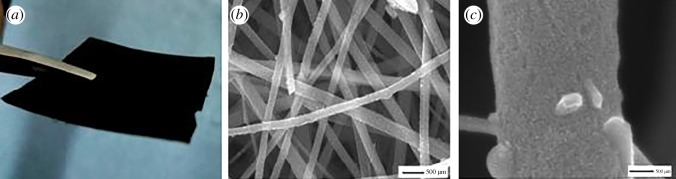
In this work, the three-dimensional nano-TiO2 fibre membrane was prepared by general electrospinning technique and followed by an improved hot-press sintering process as described in our previous work [19,20]. By means of the hot-press sintering step at 750°C for 1 h under N2 atmosphere, the prepared fibre membrane presents a homogeneous surface and good flexibility as shown in figure 1a, which can be directly cut into self-standing electrodes without using binder and metal current. Figure 1b,c shows the macro- and micro-morphologies of the fibre membrane; it can be seen that the nanofibre membrane after calcination clearly indicates the formation of highly interconnected networks, and the higher magnification of the TiO2/C nanofibre image in figure 1c reveals that a highly porous surface of fibre can be evidently observed.
Figure 1.
Optical and SEM images of nano-TiO2/C fibre membrane under different magnifications.
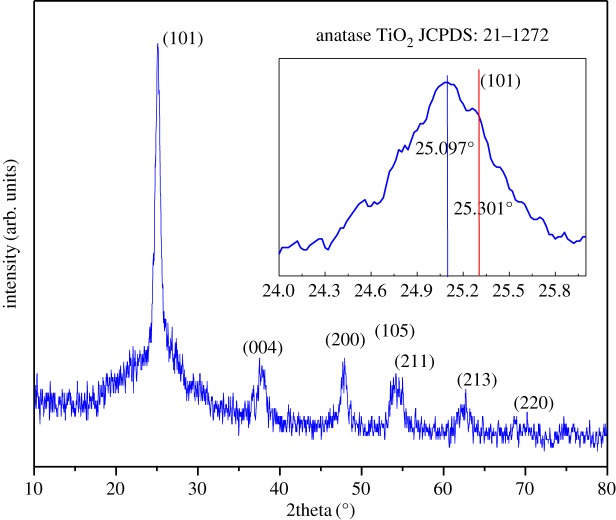
The crystallinity and crystal structure of nano-TiO2/C fibre membrane sintered at 750°C for 1 h were examined by XRD as shown in figure 2. The diffraction peaks show a good match with the standard anatase TiO2 (PDF # 21–1272), except that the main peak (101) presents a small shift to a low angle (as shown in the inset curve), which means the fibre membrane contains pure anatase TiO2, but the cell structure of TiO2 has some lattice dilatation. The crystalline size was calculated using Scherrer formula to be about 9.28 nm on the basis of the (101) peak. Meanwhile, a clearly coarse background can be seen, which is due to the amorphous carbon in the fibre. According to the result of the C-S analyser, the content of C is about 32.8 wt%.
Figure 2.
XRD patterns of the nano-TiO2/C fibre membrane. The inset is the enlargement of the (101) peak.
Raman spectra were also recorded to analyse the surface nature and degree of crystallinity of carbon as shown in figure 3. The broad characteristic peaks at approximately 1351 and approximately 1600 cm−1 are due to the D band (disordered carbon) and G band (graphitic carbon), respectively, and the intensity ratio of D and G bands (ID/IG) is about 1.12, which indicates the porous fibres have been partially graphitized. Compared with pure carbon fibre, the Raman spectra of TiO2/C fibre also shows clear characteristic peaks at 151, 200, 411, 512 and 622 cm−1, which are assigned to the Eg, B1g and A1g vibrational modes of anatase TiO2, respectively. To further investigate the surface structure nature of TiO2, the electronic properties were investigated by using XPS measurement. Figure 4 shows the high-resolution XPS spectra of Ti 2p. The Ti 2p peaks with Ti4+ characteristics (Ti 2p3/2 peak at 459.0 eV and Ti 2p1/2 at 464.9 eV) indicate the presence of Ti4+ at the surface, while comparing with XPS of pure TiO2 powder (electronic supplementary material, figure 1S), the XPS spectra also exhibits a shoulder peak at 457.6 eV, which is a characteristic of Ti3+ [16]. That is to say, the obtained TiO2 should be written as TiO2-x for maintaining chemical valence equilibrium. The ratio of Ti3+ to Ti4+ was calculated to be 7.6/92.4, assumed to be TiO1.962. According to some literature [15,21], defects can be generated in TiO2 structure when sintered in inert atmosphere, in which partial oxygen would be generated and released from the TiO2 particles, leading to Ti4+ reduction and oxygen vacancy generation, and the reduction of Ti4+ to Ti3+ would influence the density of the state distribution. Meanwhile, it is worth noting that this result just explains the phenomenon of the slight shift of the diffraction peaks to the low angles and lattice dilatation, which might be related to the presence of Ti3+ in the crystalline core of TiO2-x, because Ti3+ has a larger ionic radius than Ti4+ (Ti4+: 60.5pm, Ti3+: 67pm).
Figure 3.
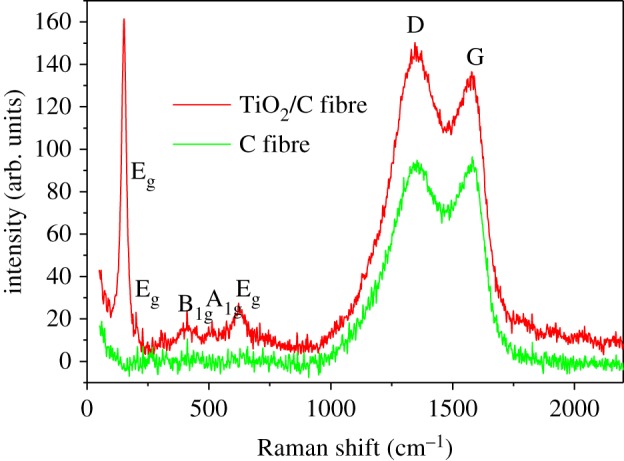
Raman spectra of nano-TiO2/C fibre membrane compared with pure carbon fibre membrane.
Figure 4.
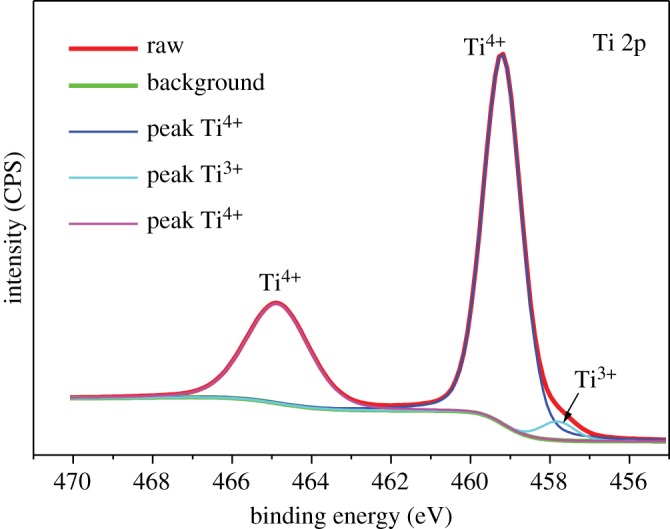
High resolution XPS spectra of Ti 2p for nano TiO2-x/C fibre membrane.
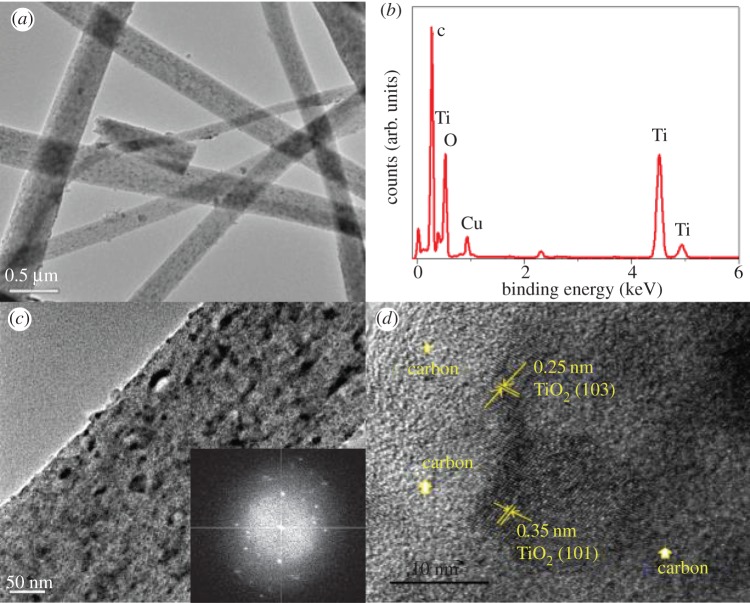
The microstructure of TiO2-x/C fibres was further examined by TEM, EDS and SAED, as shown in figure 5. Figure 5a presents a low magnification image of network TiO2-x/C fibres and figure 5b displays the corresponding EDS spectrum, which presents all the elements of C, Ti, and O of nano-TiO2-x/C fibres. Figure 5c shows a single TiO2-x/C fibre with high porosity. This three-dimensional network and porous structure of the nano-TiO2-x/C fibres can provide high electronic conductivity and open channels or higher contact area for the transport of Li ions and electrons, improving the Li ion storage performance [17,18,22].
Figure 5.
(a,c) TEM images of nano-TiO2-x/C fibre, (b) corresponding EDS spectra and (d) HR-TEM image of nano-TiO2-x/C fibre. The inset image in (c) is the corresponding SAED pattern.
The SAED pattern of nano-TiO2-x/C fibre shown in figure 5c (inset) confirmed the presence of polycrystalline TiO2 and amorphous carbon in the TiO2-x/C fibres. Furthermore, the high resolution TEM image in figure 5d also displays the remarkable lattice fringes with 0.35 nm and 0.25 nm interplanar spacing, corresponding to the typical (101) and (103) planes of anatase TiO2, respectively.
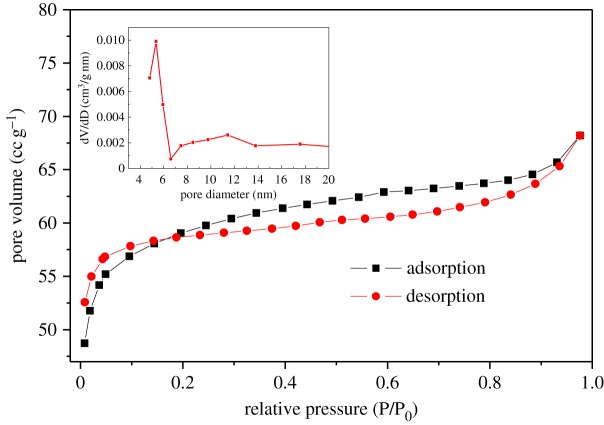
Figure 6 shows the N2 adsorption–desorption isotherms of TiO2-x/C fibre membrane, which exhibits a type IV isotherm and indicates characteristics of porous materials. The BET surface area was measured to be 199.65 m2 g−1, and the pore diameter distribution calculated on the basis of the Barrett–Joyner–Halenda (BJH) method mainly includes mesopores larger than 4 nm. Despite the pure carbon fibres without TiO2 having higher specific surface area (357.7 m2 g−1), the pore diameter distribution is mainly 3–4 nm. This mesoporous structure with larger pore size is useful to access the electrolyte solution and accommodate the volume expansion during the charge/discharge process [23,24].
Figure 6.
N2 adsorption/desorption isotherms of TiO2-x/C fibre membrane and pore size distribution in the inset.
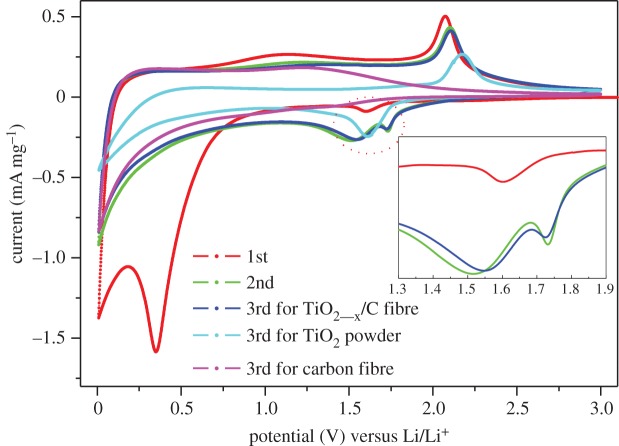
The electrochemical properties of the TiO2-x/C fibre membrane were firstly evaluated by CV and EIS. Figure 7 shows the CV curves of TiO2-x/C fibre membrane (first to third cycles), carbon fibre membrane (third cycle) and TiO2 powders (third cycle), respectively at a scanning speed of 0.1 mV s−1 at the potential range of 0–3 V. Compared with pure carbon fibre and TiO2 powders, it can be clearly confirmed that the CV curve of TiO2-x/C fibre membrane is a combination of the CV curves of carbon fibre and TiO2 powders. That is to say, the carbon in the TiO2-x/C composite fibres can also contribute some capacity to the electrode. For the TiO2-x/C fibre membrane, there are two cathodic peaks appearing at approximately 0.35 and 1.59 V in the first discharge process, where the peak at 0.35 V disappears from the second cycle, indicating the formation of amorphous Li2O and the irreversible solid electrolyte interphase (SEI) layer [25]. After the second cycle, the CV curves tend to overlap, suggesting that the TiO2-x/C fibre membrane electrode exhibits a good cycle stability for the insertion and extraction of Li ions. Based on the literature [26–28], the couple of 1.59/2.1 V is assigned to the Li+ insertion and delithiation reaction for anatase TiO2, which corresponds to the reversible biphasic transition between the tetragonal anatase and orthorhombic LixTiO2. It is noteworthy that for TiO2-x/C fibre membrane, the cathodic peak at 1.59 V was split into two peaks of 1.53 and 1.72 V from the second cycle. According to the analysis of Brumbarov et al. [15], during the two phase transition process of Li-ion insertion into anatase TiO2, the first transition step is from TiO2 to Li-rich Li0.55TiO2, and the second transition step is from Li0.55TiO2 to fully lithiated LiTiO2; these two transitions should take place at 1.72 V and 1.53 V respectively [15]. Therefore, we can conclude that this oxygen-deficient nano-TiO2-x/C fibre membrane is helpful for full lithiation of TiO2.
Figure 7.
CV curves of TiO2-x/C fibre membrane, bare carbon fibre membrane and TiO2 powders. The inset is the partial enlargement of the cathodic peaks for TiO2-x/C fibre membrane at the first to third cycles.
The electrode reaction kinetics is inversely proportional to its electrochemical impedance. Figure 8 displays a typical Nyquist plot of TiO2-x/C fibre membrane electrode, compared with the plots of pure carbon fibres and TiO2 powders. It can be seen that the EIS spectra of TiO2-x/C fibre membrane, carbon fibres and TiO2 powders are all composed of an arc in the high- and medium-frequency region, and an inclined line in the low frequency region. The Nyquist plots are well fit to the equivalent circuit model as shown in figure 8 inset. The high- and medium-frequency semicircle is composed of the electrolyte solution resistance (Re), solid electrolyte interphase resistance (Rsei) and charge transfer resistance (Rct). The inclined line represents the Warburg impedance (ZW). Besides, double layer capacitance (CSei) and a phase element (CPE) are also added to fit the Nyquist plots. According to the model, the values of Rct for the TiO2-x/C fibre membrane electrode, pure carbon fibres and TiO2 powders are calculated to be 69.32, 82.31 and 220.1 Ω respectively, although the three electrodes have a similar ohmic resistance. This result reveals that the TiO2-x/C fibre membrane electrode possesses the lowest charge transfer resistance, which may explain why the hysteresis between lithiation and delithiation peaks of TiO2-x/C fibre membrane electrode is smaller than that of pure TiO2 powder. The enhanced reaction kinetics for TiO2-x/C fibre membrane electrode could be mainly attributed to the high conductive three-dimensional networks, large surface area and high porosity.
Figure 8.
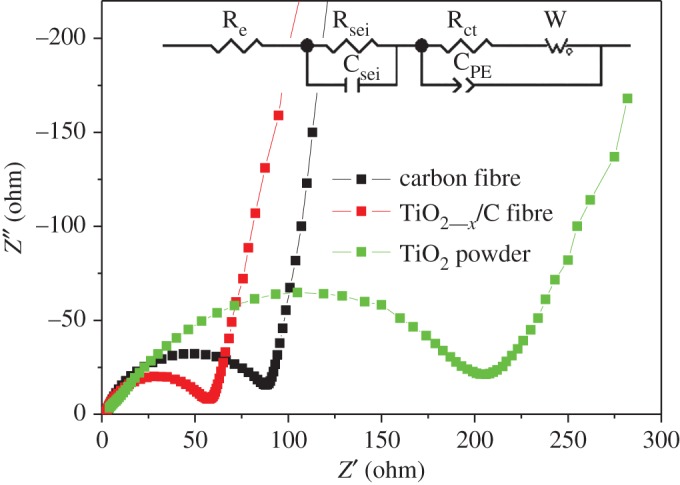
EIS curves of TiO2-x/C fibre membrane, bare carbon fibre and TiO2 powders.
In order to further evaluate the electrochemical performances of the TiO2-x/C fibre membrane electrode, the galvanostatic discharge/charge measurements were implemented at the potential range of 0–3 V. Figure 9 shows the first, second and tenth discharge/charge curves for the TiO2-x/C fibre membrane electrode at a current density of 100 mA g−1. It can be seen that the first discharge (lithiation) and charge (delithiation) capacities are 773 and 514 mA h g−1 respectively; the initial coulombic efficiency (CE) is only 66.5%. The specific capacity was calculated according to the total quality of the electrode. Although the second discharge capacity is decreased to 464 mA h g−1, the CE increases to 94.2%. This phenomenon is consistent with the CV curves for the first and second cycles. The large irreversible capacity is due to the formation of amorphous Li2O and the irreversible SEI layer. While it is noteworthy that the high lithiation capacity enormously exceeds the theoretical capacity of anatase TiO2 and graphite, which may be explained from the CV and discharge/charge curves on the one hand, the capacity is jointly contributed by fully lithiated TiO2 and porous carbon fibre when discharge/charge is at 0–3 V. In addition, according to the ‘job-sharing mechanism’ proposed by Fu et al. [29], an additional Li storage at the interface between two electrode materials in a hybrid electrode could be generated [29–31]; therefore, for the three-dimensional porous TiO2-x/C fibre membrane electrode, the interfacial lithium storage may take place besides insertion, just as the reported graphene (more than 600 mA h g−1) [32] or core/shell TiO2/Li4Ti5O12 (larger than both of them) [33], etc. Besides, the tenth charge/discharge curves show a good overlap with the second charge/discharge curves, suggesting the TiO2-x/C fibre membrane electrode has a good reversibility after the second charge/discharge process.
Figure 9.
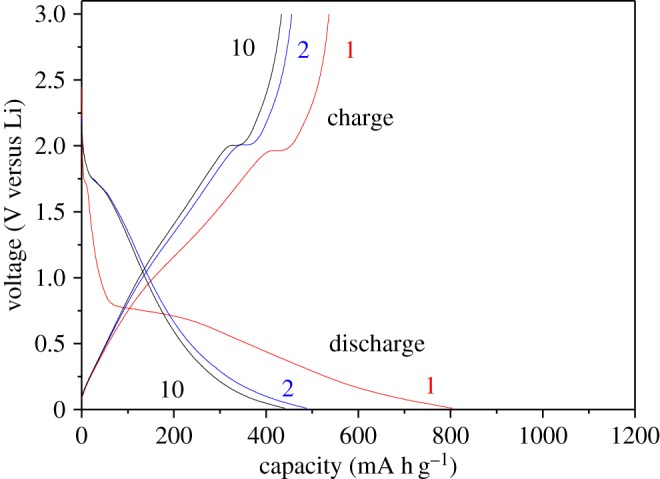
The first, second and tenth charge/discharge curves of TiO2-x/C fibre membrane electrode.
Figure 10 shows the rate performance of the TiO2-x/C fibre membrane electrode. The specific capacity was calculated according to the total quality of the electrode. Compared with the pure carbon fibre membrane and TiO2 powders, the TiO2-x/C composite fibre membrane electrode exhibits higher discharge capacity and stability at the current density irrespective of 100, 200, 300, 400 or 500 mA g−1. Even at 500 mA g−1, the discharge capacity still remains 312 mA h g−1. This high rate capability further determines the possibility that TiO2-x could be fully lithiated and the interfacial lithium storage could happen. Meanwhile, the TiO2-x/C fibre membrane electrode also displays an excellent cycle performance at the current density of 300 mA g−1 as shown in figure 11. Compared with carbon fibre and TiO2 powder, the TiO2-x/C fibre membrane possesses higher specific capacity with cycles, even after 700 cycles the discharge capacity still remains at 209 mA h g−1, and the coulombic efficiency always remains at approximately 100% throughout the cycling test, except for the first cycle.
Figure 10.
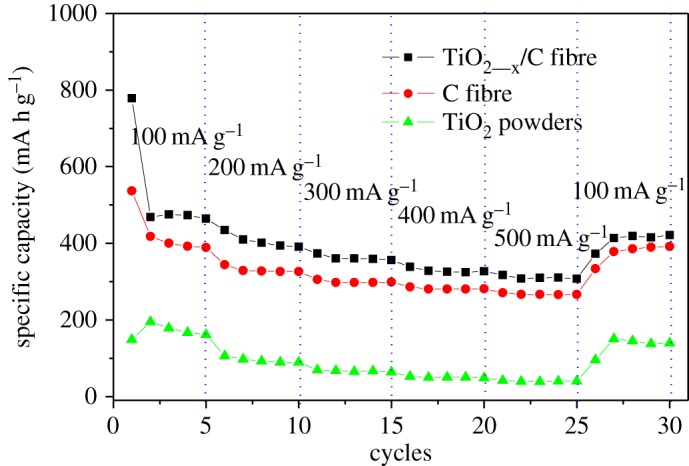
Rate performances of TiO2-x/C fibre membrane electrode, bare carbon fibre membrane and TiO2 powders at different currents under a potential range of 0–3 V.
Figure 11.
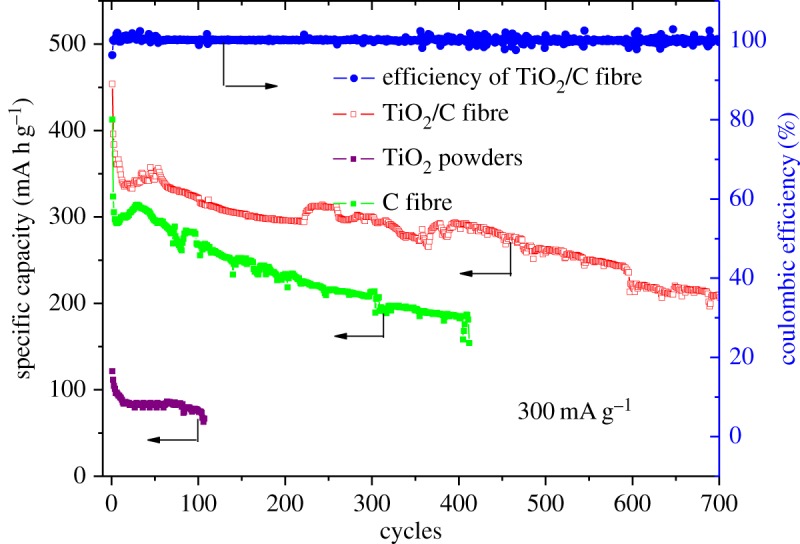
Cycle performances of TiO2-x/C fibre membrane electrode, bare carbon fibre membrane and TiO2 powders at a current of 300 mA g−1 under a potential range of 0–3 V.
The high specific capacity and excellent rate and cycle performances for the TiO2-x/C fibre membrane could be attributed to five aspects. (i) The three-dimensional long-range conductive network greatly improves the conductivity of the TiO2 electrode [18]. (ii) The high specific surface area and high porosity of the fibre membrane electrode can shorten the migration pathway for Li+ insertion and extraction and reduce charge transfer resistance, thus greatly enhancing the electrode reaction kinetics [22,34]. (iii) The conductive carbon wrapping and oxygen deficiency make TiO2 easily fully lithiated to LiTiO2 [15]. (iv) Combing TiO2 with porous carbon fibres could generate abundant interface, leading to interfacial lithium storage. (v) The binder-free, self-standing characteristics improve the structural stability during the charge/discharge process, which enhances the cycle performance [35]. The synergistic effects of all these merits make this three-dimensional self-standing TiO2-x/C fibre membrane an acceptable substitute for graphite, with promise for use as an anode for high capacity and power Li-ion batteries.
4. Conclusion
In summary, the three-dimentional networking oxygen-deficient nano TiO2-x/carbon fibre membrane was successfully prepared by the electrospinning process and hot-press sintering method. This nano TiO2-x/carbon fibre membrane could be directly used as a self-standing anode, and possessed high electrochemical reaction kinetics. The reversible discharge capacity of this electrode can reach 464 mA h g−1 at a current density of 100 mA g−1. Even at 500 mA g−1, the discharge capacity still remained at 312 mA h g−1. Compared with pure carbon fibre and TiO2 powder, the TiO2-x/C fibre membrane electrode also exhibited an excellent cycle performance with a discharge capacity of 209 mA h g−1 after 700 cycles at the current density of 300 mA g−1, and the coulombic efficiency always remained at approximately 100%. The high specific capacity could be jointly generated from porous carbon, full-lithiation of TiO2 and interfacial lithium storage. The high rate capability and cycle performance were attributed to the synergistic effects of three-dimensional conductive networks, surface oxygen deficiency, high specific surface area and high porosity, binder-free and self-standing structure, etc., which make this three-dimensional self-standing TiO2-x/C fibre membrane promising anode for high capacity and power Li-ion batteries.
Supplementary Material
Acknowledgements
We thank the sponsorship of Jiangsu Overseas Research & Training Program for University Young & Middle-aged Teachers and Presidents.
Data accessibility
Our data have been deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h4rs2 [36].
Authors' contributions
L.J.Q., H.C. and J.M.X. carried out the material laboratory work, participated in data analysis, participated in the design of the study and drafted the manuscript; Y.S.S., Z.J., Z.H.A. and C.L.L. carried out the microstructure characterization and analysis; S.X.Q. and X.K.S. coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
Financial support comes from the National Natural Science Foundation of China (grant nos 51474113, 51274106, 51504101) and the Natural Science Foundation of Jiangsu Province (grant no. BK20150514).
References
- 1.Ge MZ, Cao CY, Huang JY, Li SH, Chen Z, Zhang KQ, Al-Deyab SS, Lai YK. 2016. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 4, 6772–6801. (doi:10.1039/C5TA09323F) [Google Scholar]
- 2.Liu Y, Yang YF. 2016. Recent progress of TiO2-based anodes for Li ion batteries. J. Nanomater. 2016, 8123652 (doi:10.1155/2016/8123652) [Google Scholar]
- 3.Deng D, Kim MG, Lee JY, Cho J. 2009. Green energy storage materials: nanostructured TiO2 and Sn-based anodes for lithium-ion batteries. Energy Environ. Sci. 2, 818–837. (doi:10.1039/b823474d) [Google Scholar]
- 4.Qiao H, Luo QH, Wei QF, Cai YB, Huang FL. 2012. Electrochemical properties of rutile TiO2 nanorods as anode material for lithium-ion batteries. Ionics 18, 667–672. (doi:10.1007/s11581-012-0672-5) [Google Scholar]
- 5.Dharani S, Mulmudi HK, Yantara N, Thu Trang PTT, Park NG, Graetzel M, Mhaisalkar S, Mathews N, Boix PP. 2014. High efficiency electrospun TiO2 nanofiber based hybrid organic–inorganic perovskite solar cell. Nanoscale 6, 1675–1679. (doi:10.1039/C3NR04857H) [DOI] [PubMed] [Google Scholar]
- 6.Lee K, Mazare A, Schmuki P. 2014. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem. Rev. 114, 9385–9454. (doi:10.1021/cr500061m) [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yang HX, Lu L. 2014. Topotactically synthesized TiO2 nanowires as promising anode materials for high-performance lithium-ion batteries. Energy Procedia 61, 2562–2566. (doi:10.1016/j.egypro.2014.12.046) [Google Scholar]
- 8.Rhee O, Lee G, Choi J. 2016. Highly ordered TiO2 microcones with high rate performance for enhanced lithium-ion storage. ACS Appl. Mater. Interfaces 8, 14 558–14 563. (doi:10.1021/acsami.6b03099) [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Li W, Shen DK, Zhao DY, Wang GX. 2015. Graphitic carbon conformal coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 137, 13 161–13 166. (doi:10.1021/jacs.5b08743) [DOI] [PubMed] [Google Scholar]
- 10.Wen ZH, Ci SQ, Mao S, Cui SM, He Z, Chen JH. 2013. CNT@TiO2 nanohybrids for high-performance anode of lithium-ion batteries. Nanoscale Res. Lett. 8, 499 (doi:10.1186/1556-276X-8-499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren YM, Zhang J, Liu YY, Li HB, Wei HJ, Li BJ, Wang XY. 2012. Synthesis and superior anode performances of TiO2–carbon–rGO composites in lithium-ion batteries. ACS Appl. Mater. Interfaces 4, 4776−4780. (doi:10.1021/am301131h) [DOI] [PubMed] [Google Scholar]
- 12.Kim JI, Lee JW. 2005. Nanocomposite of TiO2 and mesoporous carbon for high power anode of lithium rechargeable batteries. J. Nanosci. Nanotechnol. 5, 9145–9150. [DOI] [PubMed] [Google Scholar]
- 13.Belak AA, Wang Y, Van der Ven A. 2012. Kinetics of anatase electrodes: the role of ordering, anisotropy and shape memory effects. Chem. Mater. 24, 2894–2898. (doi:10.1021/cm300881t) [Google Scholar]
- 14.Van der Ven A, Bhattacharya J, Belak AA. 2013. Understanding Li diffusion in Li-intercalation compounds. Accounts Chem. Res. 46, 1216–1225. (doi:10.1021/ar200329r) [DOI] [PubMed] [Google Scholar]
- 15.Brumbarov J, Vivek JP, Leonardi S, Valero-Vidal C, Portenkirchner E, Kunze-Liebhauser J. 2015. Oxygen deficient, carbon coated self-organized TiO2 nanotubes as anode material for Li-ion intercalation. J. Mater. Chem. A 3, 16 469–16 477. (doi:10.1039/C5TA03621F) [Google Scholar]
- 16.Jeong G, Kim JG, Park MS, Seo M, Hwang SM, Kim YU, Kim YJ, Kim JH, Dou SX. 2014. Core-shell structured silicon nanoparticles@TiO2-x/carbon mesoporous microfiber composite as a safe and high-performance lithium-ion battery anode. ACS Nano 8, 2977–2985. (doi:10.1021/nn500278q) [DOI] [PubMed] [Google Scholar]
- 17.Wang DL, He H, Han LL, Lin RQ, Wang J, Wu Z, Liu H, Xin HL. 2016. Three-dimensional hollow-structured binary oxide particles as an advanced anode material for high-rate and long cycle life lithium- ion batteries. Nano Energy 20, 212–220. (doi:10.1016/j.nanoen.2015.12.019) [Google Scholar]
- 18.Zhang X, Suresh Kumar P, Aravindan V, Liu HH, Sundaramurthy J, Mhaisalkar SG, Duong HM, Ramakrishna S, Madhavi S. 2012. Electrospun TiO2–graphene composite nanofibers as a highly durable insertion anode for lithium ion batteries. J. Phys. Chem. C 116, 14 780−14 788. (doi:10.1021/jp302574g) [Google Scholar]
- 19.Jing MX, et al. 2016. Electrospinning fabrication and enhanced performance of 3D Li3V2(PO4)3/C fiber membrane as self-standing cathodes for Li-ion battery. Electrochim. Acta 212, 898–904. (doi:10.1016/j.electacta.2016.07.087) [Google Scholar]
- 20.Jing MX, Pi ZC, Zhai HA, Li JQ, Chen LL, Shen XQ, Xi XM, Xiao KS. 2016. Three-dimensional Li3V2(PO4)3/C nanowire and nanofiber hybrid membrane as a self-standing, binder-free cathode for lithium ion batteries. RSC Adv. 6, 71 574–71 580. (doi:10.1039/C6RA13686A) [Google Scholar]
- 21.Padilha ACM, Raebiger H, Rocha AR, Dalpian GM. 2016. Charge storage in oxygen deficient phases of TiO2: defect physics without defects. Sci. Rep. 6, 28871 (doi:10.1038/srep28871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L, Hu H, Li G, Zhu Q, Yu Y. 2014. Hierarchical 3D TiO2@Fe2O3 nanoframework arrays as high-performance anode materials. Nanoscale 6, 6463–6467. (doi:10.1039/c4nr00387j) [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Chen LB, Xu Z, Lu BA. 2015. Electrospinning preparation of ultra-long aligned nanofibers thin films for high performance fully flexible lithium-ion batteries. Nano Energy 12, 339–346. (doi:10.1016/j.nanoen.2014.10.026) [Google Scholar]
- 24.Dong ZX, Kennedy SJ, Wu YQ. 2011. Electrospinning materials for energy-related applications and devices. J. Power Sources 196, 4886–4904. (doi:10.1016/j.jpowsour.2011.01.090) [Google Scholar]
- 25.Guo SM, Liua JR, Qiu S, Wang YR, Yan XR, Wu NN, Wang SY, Guo ZH. 2016. Enhancing electrochemical performances of TiO2 porous microspheres through hybridizing with FeTiO3 and nanocarbon. Electrochim. Acta 190, 556–565. (doi:10.1016/j.electacta.2015.12.135) [Google Scholar]
- 26.Luo W, Hu X, Sun Y, Huang Y. 2012. Surface modification of electrospun TiO2 nanofibers via layer-by-layer self-assembly for high-performance lithium-ion batteries. J. Mater. Chem. 22, 4910–4915. (doi:10.1039/c2jm15197a) [Google Scholar]
- 27.Jiang S, Wang R, Pang M, Wang H, Zeng S, Yue X, Ni L, Qiu S, Zhang Z. 2015. Hierarchical composites of ultrathin carbon self-coated TiO2 nanosheets on reduced graphene oxide with enhanced lithium storage capability. Chem. Eng. J. 280, 614–622. (doi:10.1016/j.cej.2015.06.054) [Google Scholar]
- 28.Wang X, Wang Y, Yang L, Wang K, Lou X, Cai B. 2014. Template-free synthesis of homogeneous yolk–shell TiO2 hierarchical microspheres for high performance lithium ion batteries. J. Power Sources 262, 72–78. (doi:10.1016/j.jpowsour.2014.03.081) [Google Scholar]
- 29.Fu LJ, et al. 2015. ‘Job-Sharing’ storage of hydrogen in Ru/Li2O nanocomposites. Nano Lett. 15, 4170−4175. (doi:10.1021/acs.nanolett.5b01320) [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Xu J, Yang X, Lu F, He S, Yang J, Fan HJ, Wu M.. 2015. Ultrathin anatase TiO2 nanosheets embedded with TiO2-B nanodomains for lithium-ion storage: Capacity enhancement by phase boundaries. Adv. Energy Mater. 5, 1 401 756−1 401 764. (doi:10.1002/aenm.201401756)26190957 [Google Scholar]
- 31.Chen C, Huang Y, An C, Zhang H, Wang Y, Jiao L, Yuan H.. 2015. Copper-Doped Dual Phase Li4Ti5O12−TiO2 nanosheets as high-rate and long cycle life anodes for high-power lithium-ion batteries. ChemSusChem 8, 114−122. (doi:10.1002/cssc.201402886) [DOI] [PubMed] [Google Scholar]
- 32.Sun HY, et al. 2016. Binder-free graphene as an advanced anode for lithium batteries. J. Mater. Chem. A 4, 6886–6895. (doi:10.1039/C5TA08553E) [Google Scholar]
- 33.Balogun MS, Zhu YK, Qiu WT, Luo Y, Huang YC, Liang CL, Lu XH, Tong YX. 2015. Chemically lithiated TiO2 heterostructured nanosheet anode with excellent rate capability and long cycle life for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 7, 25 991−26 003. (doi:10.1021/acsami.5b09610) [DOI] [PubMed] [Google Scholar]
- 34.Naoi K, Kisu K, Iwama E, Sato Y, Shinoda M, Okita N, Naoi W. 2015. Ultrafast cathode characteristics of nanocrystalline-Li3V2(PO4)3/carbon nanofiber composites. J. Electrochem. Soc. 162, A827–A833. (doi:10.1149/2.0021506jes) [Google Scholar]
- 35.Arthur TS, et al. 2011. Three-dimensional electrodes and battery architectures. MRS Bull. 36, 523–531. (doi:10.1557/mrs.2011.156) [Google Scholar]
- 36.Jing M-x, Li J-q, Han C, Yao S-s, Zhang J, Zhai H-a, Chen L-I, Shen X-q, Xiao K-s. 2017. Data from: Electrospinning preparation of oxygen-deficient nano TiO2-x/carbon fibre membrane as a self-standing high performance anode for Li-ion batteries. Dryad Digital Repository. (doi:10.5061/dryad.h4rs2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jing M-x, Li J-q, Han C, Yao S-s, Zhang J, Zhai H-a, Chen L-I, Shen X-q, Xiao K-s. 2017. Data from: Electrospinning preparation of oxygen-deficient nano TiO2-x/carbon fibre membrane as a self-standing high performance anode for Li-ion batteries. Dryad Digital Repository. (doi:10.5061/dryad.h4rs2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Our data have been deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h4rs2 [36].