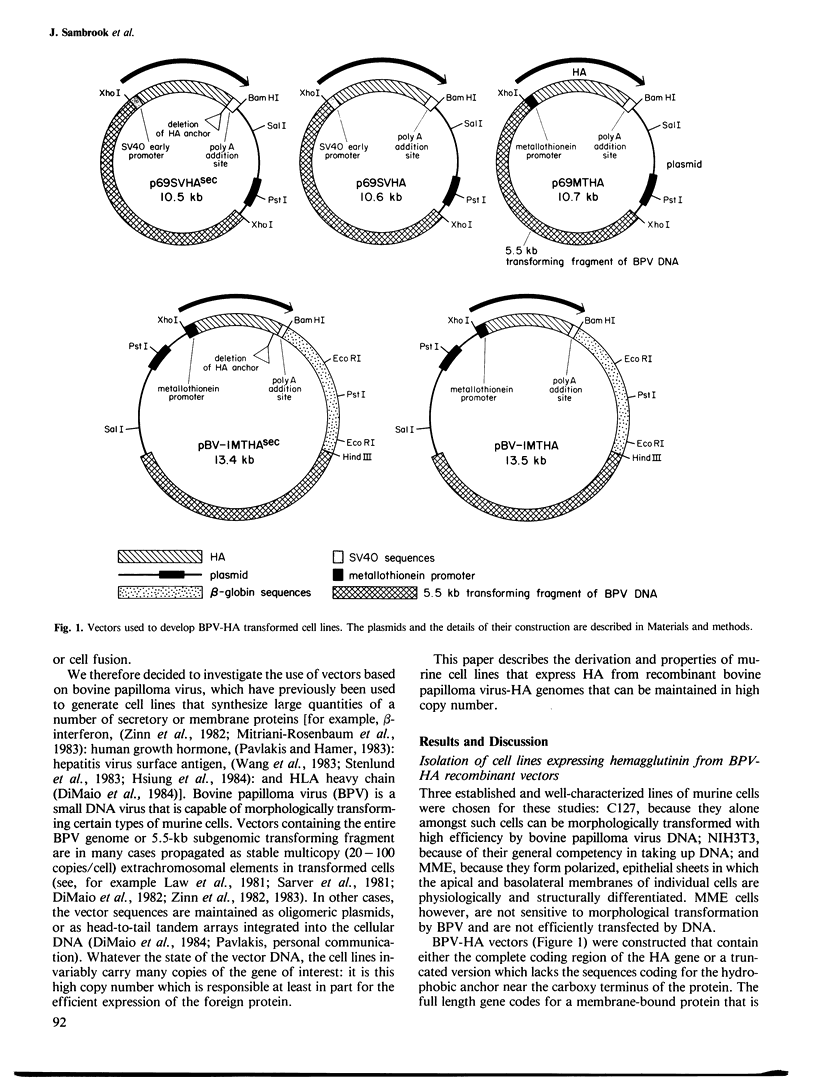
Abstract
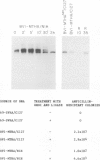
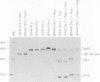
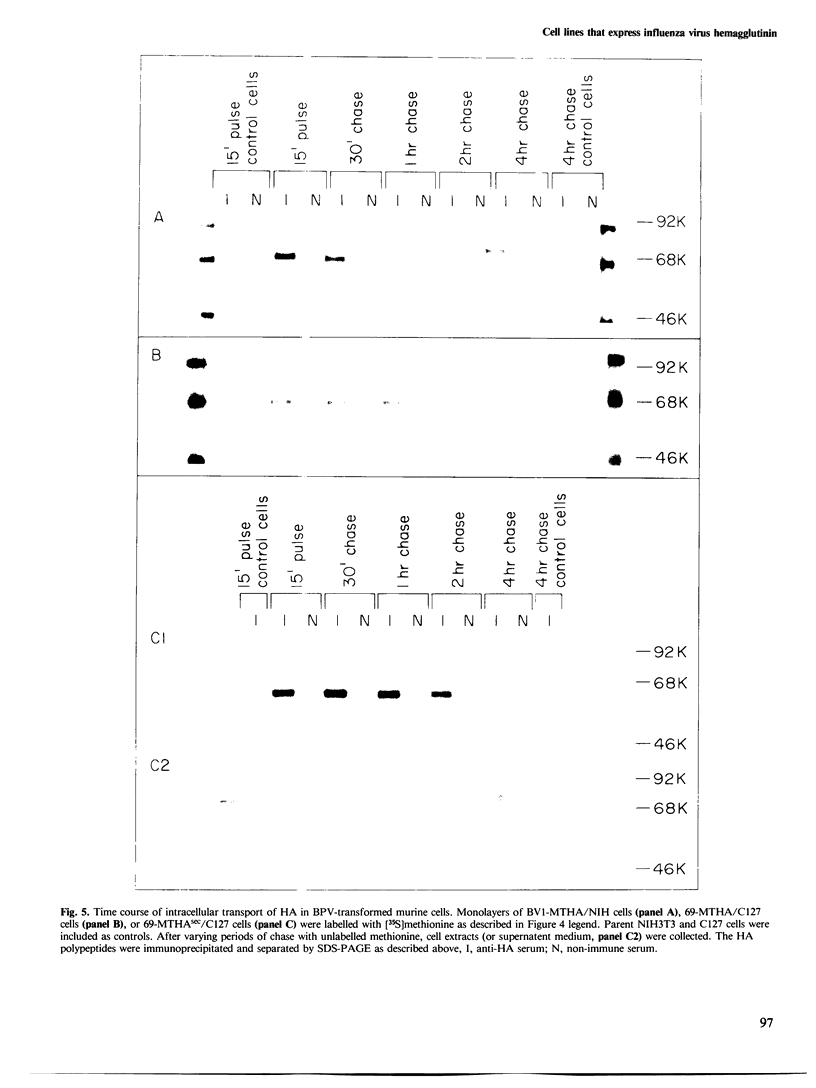
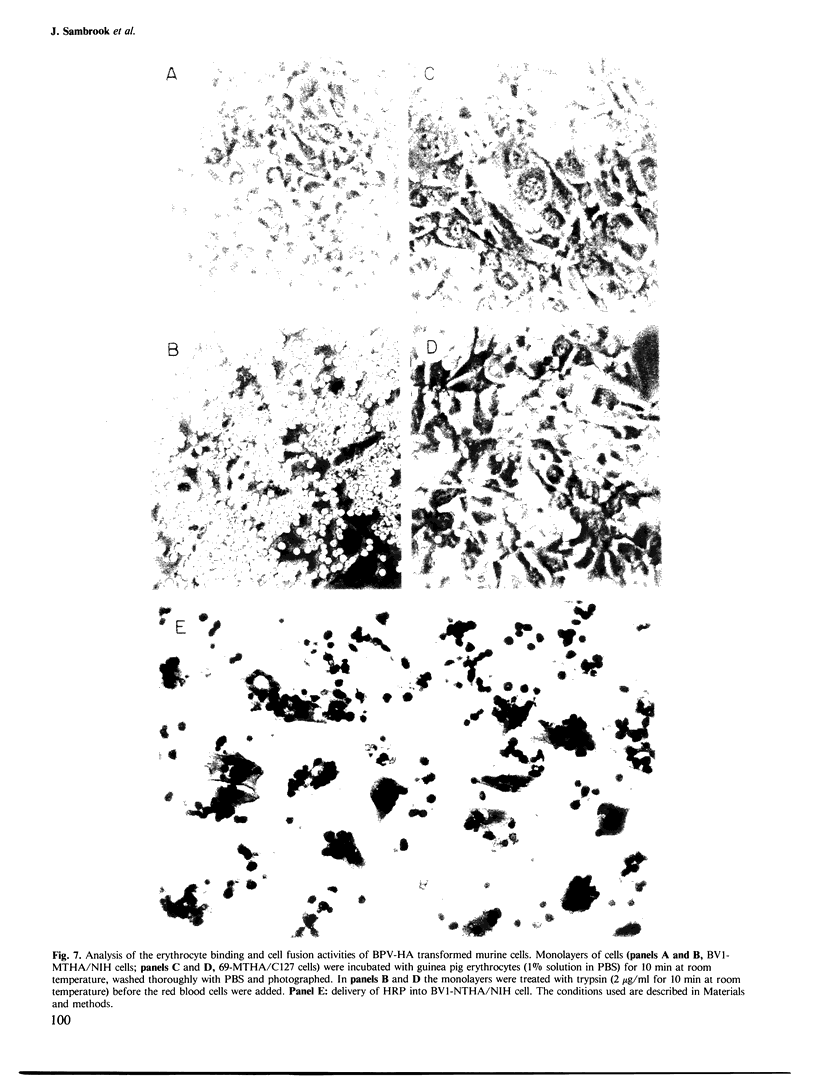
We have developed and characterized several murine cell lines that constitutively express either the full-length, membrane-bound form of influenza virus hemagglutinin (HA) or a truncated version of the protein (HAsec) that lacks the carboxyterminal anchoring sequences and is secreted from cells. cDNAs encoding HA or HAsec were linked to the murine metallothionein-I promoter or the SV40 early promoter, and inserted into plasmids containing the transforming DNA fragment of bovine papilloma virus (BPV). The resulting vectors were introduced into three cultured lines of murine cells--C127, NIH3T3 and MME--either alone or in the presence of a plasmid that carries the aminoglycoside transferase gene of Tn5. The resulting lines of MME cells contained 1-5 copies of the vector in an integrated state and expressed low levels of HA (approximately 10(4) molecules/cell). In contrast, lines of C127 and NIH3T3 cells were obtained that express up to 5 X 10(6) molecules of HA per cell or secrete approximately 10(7) molecules of HAsec per cell per 24 h. Some of these cell lines carry multiple (30-200) copies of the vector in an integrated state; in others, the vector is propagated as unit-length episomes or as oligomers. Both the membrane-bound and secreted forms of HA expressed in these cell lines display a more extensive pattern of glycosylation than HA or HAsec synthesized in simian cells and they are transported to the cell surface more slowly. Pulse-chase experiments suggest that the step which limits the rate at which HA and HAsec travel down the secretory pathway occurs in the rough endoplasmic reticulum before the molecules are transferred to the Golgi apparatus. Using indirect immunofluorescence in combination with a cell sorter, we have shown that the level of expression of HA within cloned populations of producing cells can be variable. However, greater than 90% of the cells in certain cell lines display considerable quantities of HA on their surface, as judged by their ability to bind red blood cells in large numbers. We have taken advantage of the membrane fusion activity of HA to effect the fusion of erythrocytes to these cells and to deliver the contents of red cell ghosts into the cells' cytoplasm.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards R., Little P. F., Annison G., Williamson R., Flavell R. A. Structure of the human G gamma-A gamma-delta-beta-globin gene locus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4827–4831. doi: 10.1073/pnas.76.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Braciale V. L., Henkel T. J., Sambrook J., Gething M. J. Cytotoxic T lymphocyte recognition of the influenza hemagglutinin gene product expressed by DNA-mediated gene transfer. J Exp Med. 1984 Feb 1;159(2):341–354. doi: 10.1084/jem.159.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Dorio R. J., Buck C. A. Manipulation of cell-cell and cell-substratum interactions in mouse mammary tumor epithelial cells using broad spectrum antisera. J Cell Biol. 1981 May;89(2):173–184. doi: 10.1083/jcb.89.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Corbin V., Sibley E., Maniatis T. High-level expression of a cloned HLA heavy chain gene introduced into mouse cells on a bovine papillomavirus vector. Mol Cell Biol. 1984 Feb;4(2):340–350. doi: 10.1128/mcb.4.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Smith A., Bergmann J. E., Rose J. K. Isolation of stable mouse cell lines that express cell surface and secreted forms of the vesicular stomatitis virus glycoprotein. J Cell Biol. 1983 Nov;97(5 Pt 1):1381–1388. doi: 10.1083/jcb.97.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Walling M. Regulation in vivo of a cloned mammalian gene: cadmium induces the transcription of a mouse metallothionein gene in SV40 vectors. J Mol Appl Genet. 1982;1(4):273–288. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsiung N., Fitts R., Wilson S., Milne A., Hamer D. Efficient production of hepatitis B surface antigen using a bovine papilloma virus-metallothionein vector. J Mol Appl Genet. 1984;2(5):497–506. [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A., Davies J. Expression of a transposable antibiotic resistance element in Saccharomyces. Nature. 1980 Oct 30;287(5785):869–871. doi: 10.1038/287869a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Cathala G., Nguyen-Huu M. C. Expression and regulation of a human metallothionein gene carried on an autonomously replicating shuttle vector. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4040–4044. doi: 10.1073/pnas.80.13.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Slater E. P., Herschman H. R. Regulation of metallothionein synthesis in HeLa cells by heavy metals and glucocorticoids. J Cell Physiol. 1981 Jan;106(1):63–74. doi: 10.1002/jcp.1041060108. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Mitrani-Rosenbaum S., Maroteaux L., Mory Y., Revel M., Howley P. M. Inducible expression of the human interferon beta 1 gene linked to a bovine papilloma virus DNA vector and maintained extrachromosomally in mouse cells. Mol Cell Biol. 1983 Feb;3(2):233–240. doi: 10.1128/mcb.3.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979 May;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Hamer D. H. Regulation of a metallothionein-growth hormone hybrid gene in bovine papilloma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(2):397–401. doi: 10.1073/pnas.80.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stenlund A., Lamy D., Moreno-Lopez J., Ahola H., Pettersson U., Tiollais P. Secretion of the hepatitis B virus surface antigen from mouse cells using an extra-chromosomal eucaryotic vector. EMBO J. 1983;2(5):669–673. doi: 10.1002/j.1460-2075.1983.tb01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Wang Y., Stratowa C., Schaefer-Ridder M., Doehmer J., Hofschneider P. H. Enhanced production of hepatitis B surface antigen in NIH 3T3 mouse fibroblasts by using extrachromosomally replicating bovine papillomavirus vector. Mol Cell Biol. 1983 Jun;3(6):1032–1039. doi: 10.1128/mcb.3.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Helenius A., Gething M. J. Haemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature. 1982 Dec 16;300(5893):658–659. doi: 10.1038/300658a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Mellon P., Ptashne M., Maniatis T. Regulated expression of an extrachromosomal human beta-interferon gene in mouse cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4897–4901. doi: 10.1073/pnas.79.16.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]