Abstract
The spectral composition of ambient light varies across both space and time. Many species of jawed vertebrates adapt to this variation by tuning the sensitivity of their photoreceptors via the expression of CYP27C1, an enzyme that converts vitamin A1 into vitamin A2, thereby shifting the ratio of vitamin A1-based rhodopsin to red-shifted vitamin A2-based porphyropsin in the eye. Here, we show that the sea lamprey (Petromyzon marinus), a jawless vertebrate that diverged from jawed vertebrates during the Cambrian period (approx. 500 Ma), dynamically shifts its photoreceptor spectral sensitivity via vitamin A1-to-A2 chromophore exchange as it transitions between photically divergent aquatic habitats. We further show that this shift correlates with high-level expression of the lamprey orthologue of CYP27C1, specifically in the retinal pigment epithelium as in jawed vertebrates. Our results suggest that the CYP27C1-mediated vitamin A1-to-A2 switch is an evolutionarily ancient mechanism of sensory plasticity that appeared not long after the origin of vertebrates.
Keywords: visual ecology, photoreceptor, Petromyzon marinus
1. Introduction
Variability in the intensity and spectral composition of light in the natural world presents fundamental challenges for vision, and many features of the vertebrate eye are adaptations to meet these challenges. In some aquatic environments such as ponds, streams and rivers, specific wavelengths of light are scattered or absorbed by sediment and dissolved organic matter, resulting in a marked shift in the spectral composition of light towards longer wavelengths [1,2]. A wide variety of aquatic organisms optimize visual sensitivity and discrimination by matching the sensitivities of their photoreceptors to the available light spectrum [2–5]. To maintain this match, as light environments change over space and time, many species have evolved mechanisms of visual system plasticity: that allow them to shift photoreceptor sensitivity dynamically. For example, some fish species switch the expression of opsin genes to match their visual sensitivity to ambient wavelengths (e.g. [6,7]). In this study, we focus on another widespread mechanism of visual system plasticity: the ‘rhodopsin–porphyropsin’ switch [8].
Anadromous species such as Pacific salmon (Oncorhynchus sp.) spend most of their adult lives in the ocean where short-wavelength (blue–green) light predominates, but migrate to spawn in inland waterways enriched for long-wavelength (yellow–red) light [9]. To accommodate this shift in the light spectrum, salmon red-shift the sensitivity of their photoreceptors by switching their visual pigment chromophore from 11-cis retinal (a derivative of vitamin A1) to 11-cis 3,4-Didehydroretinal (a derivative of vitamin A2; figure 1a) [8,9]. This shift in the visual system is sometimes referred to as the ‘rhodopsin–porphyropsin’ switch, because vitamin A1-based photopigments have traditionally been called ‘rhodopsins’, whereas the red-shifted vitamin A2-based photopigments are collectively referred to as ‘porphyropsins’ [8]. Chromophore switching allows for adaptation of the visual system to changing spectral conditions and is used by a diversity of fishes, amphibians and reptiles [8,10–12]. Recently, the cytochrome P450 family member CYP27C1 was identified as the enzyme that mediates the conversion of vitamin A1 to A2 in both fish and amphibians [13]. This discovery raises the question of when this mechanism of sensory plasticity first arose during the course of vertebrate evolution.
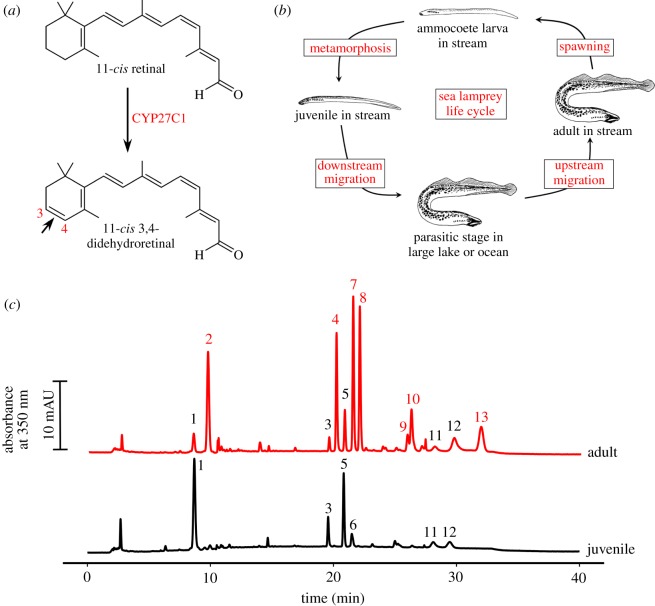
Figure 1.
The eyes of adult upstream migrant lamprey are enriched with vitamin A2 chromophore. (a) In teleost fish and amphibians, CYP27C1 mediates the conversion of 11-cis retinal (a derivative vitamin A1) to 11-cis 3,4-didehydroretinal (a derivative of vitamin A2) to red-shift photoreceptor spectral sensitivities. (b) The sea lamprey moves between streams and large lakes/ocean through the course of its life cycle. (c) Representative HPLC chromatograms of retinoid extracts from juvenile and adult lamprey eyes. 3,4-Didehydroretinoids, unique to the adult eye, are numbered in red, and other retinoids, common to both adult and juvenile, are numbered in black. These peaks were identified based on comparisons to pure standards, published accounts, and spectral characteristics: 1, retinal ester; 2, 3,4-dehydroretinal ester; 3, syn-11-cis-retinal oxime; 4, syn-11-cis-3,4-dehydroretinal oxime; 5, syn-all-trans-retinal oxime; 6, syn-9-cis-retinal oxime; 7, syn-all-trans-3,4-dehydroretinal oxime; 8, syn-9-cis-3,4-dehydroretinal oxime; 9, anti-11-cis-3,4-dehydroretinal oxime; 10, 11-cis-3,4-dehydroretinol; 11, anti-all-trans-retinal oxime; 12, all-trans-retinol; 13, all-trans-3,4-dehydroretinol (peak spectra are given in electronic supplementary material, figure S1, and further details about the peaks are presented in electronic supplementary material, table S1).
Lampreys are jawless (agnathan) vertebrates which, together with hagfish, constitute the cyclostomata, the only surviving group from the earliest branch in the vertebrate subphylum. The lineage giving rise to cyclostomes diverged from the one giving rise to jawed vertebrates during the late Cambrian period about 500 Ma [14]. Like salmon, many lamprey species are anadromous: they begin life in freshwater streams as ammocoete larvae, and after several years undergo metamorphosis to become juveniles. The juveniles subsequently migrate either to large lakes or to the sea before returning to streams or rivers to spawn [15] (figure 1b). Earlier experiments showed that sea lamprey (Petromyzon marinus) can shift the retinoid content of their photoreceptors from vitamin A1 to A2 upon migration to their breeding grounds as adults [16,17]. This finding raises the intriguing possibility that a CYP27C1-mediated vitamin A1-to-A2 switch may have already been present in the last common ancestor of lampreys and jawed vertebrates. To investigate this possibility, we examined the physiology and spectral sensitivity of the photoreceptors of downstream migrating juvenile and upstream migrating adult lamprey and examined how these measures correlated with the vitamin A1 and A2 composition of the eye and the expression of CYP27C1.
2. Results
2.1. The eyes of upstream migrating adult lamprey contain vitamin A2
We sampled lamprey from a recently established land-locked population that spends the parasitic stage of its life cycle in Lake Huron instead of the ocean. To confirm that this population maintains the A1-to-A2 switch, we analysed the retinoid content of the eyes of juvenile and adult lamprey with high-performance liquid chromatography (HPLC). Consistent with coastal populations [16,17], the eyes of downstream migrating juveniles contained only vitamin A1 derivatives, while the eyes of adult lampreys contained both vitamin A1 and A2 derivatives, with the various forms of vitamin A2 making up approximately 82% of the total retinoid content (figure 1c; electronic supplementary material, table S1).
2.2. The physiological responses of juvenile lamprey photoreceptors are similar to those of adults
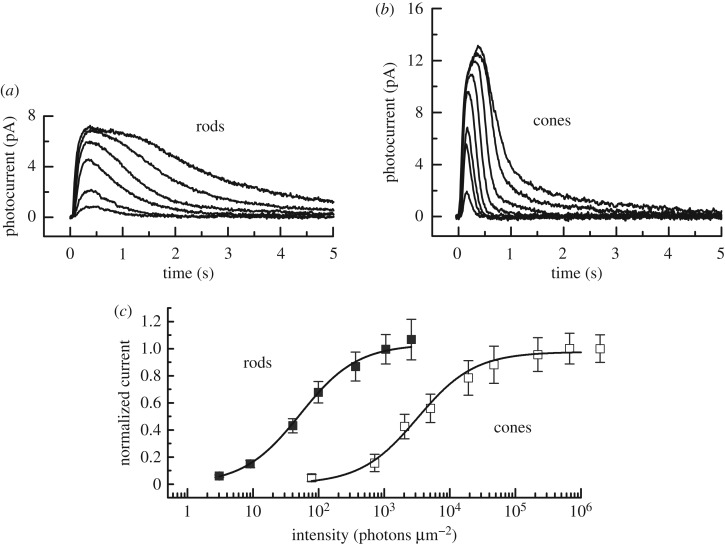
Juvenile lampreys are much smaller than adults, with eyes only about 20% as large in diameter, and the physiology of their photoreceptors has not been previously investigated. To investigate the physiological responses of the photoreceptors of juvenile lamprey, we used suction-electrode recording to measure the responses of single photoreceptors. Responses of juvenile photoreceptors were very similar to those of adults [18] (figure 2a,b). When we plotted the peak amplitude of the light response for the two photoreceptor types as a function of stimulus intensity (figure 2c), juvenile rods were approximately 85-fold more sensitive than cones, comparable to the value we obtained for adults [18]. Juvenile rod responses decay more slowly than cone responses, and single photons produce a response of about 0.4 pA, again very similar to the behaviour of adults photoreceptors [18,19] (electronic supplementary material, figure S2). Thus, the physiological properties of juvenile rods and cones showed no significant differences from those of their adult counterparts.
Figure 2.
Current responses and sensitivity of juvenile lamprey rod and cone photoreceptors to brief light stimuli. (a) Mean responses of 13 rods to 20 ms 500 nm flashes given at t = 0 at the following intensities (in photons μm−2): 9, 40, 98, 366, 1055 and 2591. (b) Mean responses of six cones to 20 ms 600 nm flashes given at t = 0 at the following intensities (in photons µm−2): 78, 711, 2051, 5036, 1.91 × 104, 2.20 × 105, 6.71 × 105 and 6.96 × 105. (c) Normalized mean current response amplitudes (±s.e.) as a function of flash intensity for rods (closed squares) and cones (open squares). The same cells as in (a) and (b). Data for both cell types were fitted with the Michaelis–Menton equation, r/rmax = I/(I + I½), where r/rmax is the normalized current amplitude, I the flash intensity and I½ the flash intensity producing a half-maximal response. Best-fitting values of I½ were 52 photons μm−2 for rods and 3210 photons μm−2 for cones.
2.3. The spectral sensitivity of the rods and cones of adult migrant lamprey are red-shifted relative to those of the juvenile
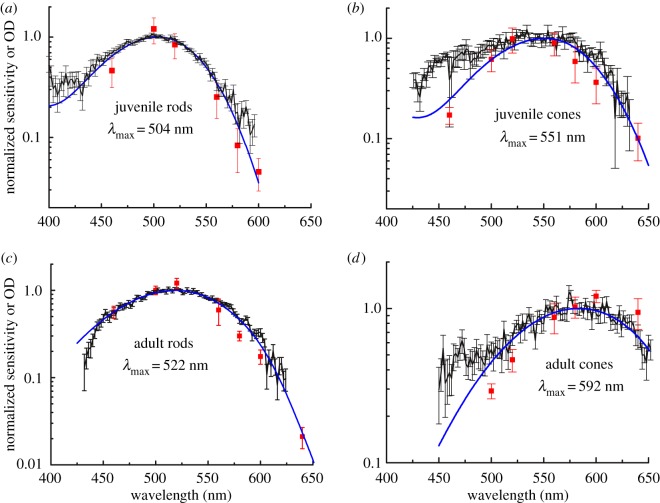
To determine whether the differences in retinoid content between juvenile and adult lampreys are reflected in the spectral sensitivities of individual rods and cones, we recorded the responses of these cells to 460, 500, 520, 560, 580, 600 and 620 nm light. The results of these measurements are presented for juvenile rods and cones (red squares in figure 3a,b) and for adult rods and cones (red squares in figure 3c,d). In separate experiments, we used microspectrophotometry (MSP) to measure the mean normalized pigment absorbance of both juvenile and adult rods and cones, and we superimposed these data on top of the spectral sensitivities (black points in figure 3a,d). We found that juvenile rods have peak sensitivity and absorbance (λmax) at 504 nm, while adult rods have their λmax at 522 nm (cf. figure 3a,c). By contrast, juvenile cones have λmax = 551 nm, whereas adult cones have λmax = 592 nm (cf. figure 3b,d). Thus, the spectral sensitivities of adult rods and cones are red-shifted relative to those of the juvenile.
Figure 3.
The photoreceptor spectral sensitivities of adult upstream migrant lamprey are long-wavelength shifted compared with those of juvenile downstream migrants. Normalized absorption spectra and spectral sensitivities for each condition are superimposed in a single graph. Black data points and black connecting lines represent microspectrophotometric determinations of mean pigment absorbance (±s.e.) normalized to absorbance at λmax, taken from 58 juvenile rods (a), nine juvenile cones (b), 70 adult rods (c) and 23 adult cones (d). Red data points represent the normalized mean spectral sensitivities (± s.e.) from suction-electrode recordings of nine juvenile rods (a), seven juvenile cones (b), 13 adult rods (c) and 14 adult cones (d). Blue curves for (a) and (b) are A1 visual-pigment nomograms [20] for best-fitting λmax's of 504 nm for rods and 551 for cones; or weighted averages of A1 and A2 nomograms for adult photoreceptors (c,d) with best-fitting values of 18% A1 and 82% A2 for rods (c) and 14% A1 and 86% A2 for cones (d). The A1/A2 mixture modelling approach was adapted from Kefalov et al. [21].
To investigate the potential role of chromophore exchange in mediating the shift of rod and cone sensitivities to longer wavelengths in the adult, we fitted the MSP data with curves representing contributions from vitamin A1-based pigment only in juvenile lampreys, and a mixture model that incorporates contributions from pigments based on both vitamin A1 and vitamin A2 in the adult [20] (blue lines, figure 3). The data from juvenile rods and cones show a good fit to a pure vitamin A1-based template (figure 3a,b). By contrast, the visual pigments of adults are best fit by a weighted sum of pigment curves with 82% (rod) and 86% (cone) vitamin A2-based pigment, with the remaining fractions attributable to vitamin A1-based pigment (figure 3c,d). Additionally, the differing magnitudes of the spectral sensitivity shifts in the rods (Δ18 nm) versus cones (Δ41 nm) are consistent with the known dependence of the A1-to-A2 spectral shift on the λmax of the opsin [22,23]. Thus, single-photoreceptor electrophysiological measurements and HPLC analyses both indicate the long-wavelength shift in sensitivity between juvenile and adult lamprey is the result of switching from a vitamin A1-based chromophore to a predominantly vitamin A2-based chromophore [17].
2.4. The accumulation of vitamin A2 in the adult retina is correlated with CYP27C1 expression
Next, we asked if this A1-to-A2 chromophore shift in lamprey is mediated by a homologue of the enzyme CYP27C1, which was recently shown to catalyse the conversion of vitamin A1 into vitamin A2 in teleost fish and amphibians [13]. A CYP27C1 orthologue had not been identified in any agnathan species to date as the 3,4-retinoid dehydrogenase responsible for the synthesis of vitamin A2. Therefore, we searched for an orthologue in sea lamprey by using polymerase chain reaction (PCR) with degenerate primers based on alignments of the amino acid sequences of known CYP27C1 homologues from multiple vertebrate species. In this way, we were able to amplify a portion of the CYP27C1 transcript from adult sea lamprey eye cDNA (GenBank accession number MF163257). This transcript is predicted to encode a protein with 54% amino acid identity to zebrafish CYP27C1 (NP_001106808.2, AA100-538; electronic supplementary material, figure S3). Bioinformatic searches (BLASTP, Genbank) confirmed that, among all known and predicted sea lamprey protein sequences, this transcript shares the highest per cent identity with zebrafish CYP27C1.
In zebrafish (Danio rerio) and bullfrogs (Lithobates catesbeianus), CYP27C1 is specifically expressed in the retinal pigment epithelium (RPE) of the eye, and transcript levels are positively correlated with the presence of vitamin A2 [13]. Therefore, we predicted that adult lamprey, which have high levels of vitamin A2 in their eyes (figure 1c), would also show high levels of CYP27C1 expression, specifically in the RPE.
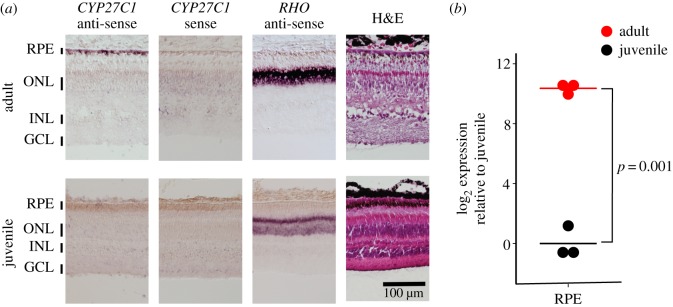
To investigate the expression of CYP27C1 in lamprey, we performed in situ hybridization (ISH) and quantitative PCR (qPCR) to compare the eyes of juveniles and adults. Consistent with our prediction, CYP27C1 is specifically expressed in the RPE of the adult upstream migrant and was not detectable in the juvenile (figure 4a). These qualitative differences were confirmed by qPCR analyses demonstrating that CYP27C1 is expressed at more than 1000-fold higher levels in adult RPE compared with juvenile RPE (Welch's t-test: t = −16.29, d.f. = 2.43, p = 0.0014; figure 4b). These results strongly suggest that CYP27C1 mediates formation of vitamin A2 in the eyes of adult upstream migrant lamprey.
Figure 4.
CYP27C1 is specifically expressed in the RPE of adult upstream migrant lamprey. (a) ISH of cross sections of retina and RPE from juvenile and adult lamprey. The CYP27C1 antisense probe labels the CYP27C1 transcript in the RPE of adults; only weak background signal was observed in the negative control (CYP27C1 sense probe), and the Rhodopsin (RHO) antisense probe (positive control) labels RHO in the ONL of both adults and juveniles. RPE, retinal pigment epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; H&E, haematoxylin and eosin staining. (b) qPCR analyses indicate that CYP27C1 transcript levels are significantly higher in the RPE of adult lamprey compared with juveniles.
3. Discussion
Our study provides the first electrophysiological characterization of the rod and cone photoreceptors of juvenile lamprey and indicates that this developmental stage possesses receptors that are physiologically similar to those of adult lamprey [18,19]. The spectral sensitivities of photoreceptors from upstream adults were however significantly red-shifted compared with the downstream juveniles. Consistent with earlier studies of lamprey visual pigments [16,17], we found that this red-shift is attributable to a switch in the visual pigment chromophore from 11-cis retinal (A1) to 11-cis 3,4-didehydroretinal (A2). Concomitant with the vitamin A1-to-A2 shift, we observed a dramatic increase in the expression of CYP27C1 in the RPE of adult lamprey compared with juveniles. Taken together, our data suggest that CYP27C1-mediating chromophore switching is an evolutionarily ancient mechanism of sensory plasticity.
Teleost fish and amphibians produce vitamin A2 in the eye through the controlled expression of CYP27C1 in the RPE [11,13]. Similarly, we find that CYP27C1 is highly expressed in the RPE of adult upstream migrant lamprey that use the vitamin A2 chromophore. Previous biochemical analyses of adult lamprey indicated that vitamin A2 is found only in the eye [17]. Therefore, the same pattern of tissue-specific expression of CYP27C1 seen in teleost fish and amphibians likely mediates the production of vitamin A2 in the eyes of adult lamprey and may be widespread among lampreys. Recent transcriptomic analysis of the eyes of another lamprey species (Geotria australis) shows a similar pattern of elevated CYP27C1 expression in upstream migrating adults (electronic supplementary material, figures S3 and S4) [24]. Thus, CYP27C1-mediated A1-to-A2 switching may be a mechanism of sensory plasticity conserved across a diversity of vertebrates.
Vertebrates evolved in the ocean and subsequently colonized freshwater habitats [25]. The light environment of fresh inland waterways, especially rivers and streams, is often turbid and red-shifted due to the presence of suspended sediments and dissolved organic matter. This turbidity likely represented a significant challenge to the visual systems of early vertebrates attempting to colonize such habitats. We therefore conclude that the CYP27C1-mediated vitamin A1-to-A2 switch in the eyes of early vertebrates may have been among the suite of adaptations that facilitated the early invasion of brackish and freshwater habitats.
4. Material and methods
4.1. Animals
Juvenile and adult sea lamprey (P. marinus) were provided to us by the Hammond Bay Biological Station of the US Geological Survey, Millersburg, MI, USA. Juvenile lamprey were captured by drift net in the St Marys River while in the process of migrating downstream to Lake Huron to begin the parasitic stage of the life cycle. Adult lamprey were captured in tributaries of Lake Huron (Ocqueoc River and Cheboygan River) in the process of their upstream spawning migration. Additional sea lamprey samples used for preliminary PCR analysis were obtained from the Connecticut River and reared at the Conte Anadromous Fish Research Laboratory in Turner Falls, MA, USA. Lamprey were kept in well-aerated tanks in cyclic 12 L/12 D h lighting in accordance with the rules and regulations of the NIH guidelines for research animals, as approved by the institutional animal care and use committee of the University of California, Los Angeles.
4.2. Analyses of retinoid content
We used HPLC to examine the retinoid content of eyes of juvenile and adult lamprey. The eyes were homogenized under dim light conditions in cold saline with a glass dounce. Retinaldehydes were then derivatized by treatment with hydroxylamine (Sigma, 255580) and extracted with hexane. The extract was dried under a stream of nitrogen, resuspended in 120 µl of hexane, and 100 µl was injected into an Agilent 1100 series HPLC equipped with a Zorbax RX-SIL column (4.6 × 250 mm, 5 µm, Agilent). The samples were eluted with a gradient mobile phase consisting of 0.5% ethyl acetate in hexane for 5 min then a ramp up to 10% ethyl acetate in hexane from 5 to 20 min, followed by isocratic conditions through 35 min. The column was held at 25°C, and the flow rate was 1.4 ml min−1 throughout the run. The samples were monitored with a photodiode array detector at 325, 350 and 380 nm, and retinoids were putatively identified by comparison to authentic standards or published accounts (electronic supplementary material, table S1) [26–29].
4.3. Single-cell spectral sensitivity and microspectrophotometry
Electrical responses of juvenile lamprey photoreceptor outer segments were recorded with suction electrodes as previously described for adult animals [18]. Light from a halogen lamp was passed through narrow band-pass interference filters and neutral absorption filters to provide stimuli of varying wavelengths and intensities. Spectral sensitivity was calculated cell by cell as the light required to elicit a criterion response at each tested wavelength and was normalized to the wavelength of peak sensitivity. MSP measurements of pigment absorbance were made as previously described for mouse rods [30], except that the measuring beam was confined to single lamprey rod or cone outer segments. The fitting of absorbance measurements to pigment nomograms was done for juvenile rods and cones with nomograms of Govardovskii et al. [20], and for adult rods and cones as in Kefalov et al. [21] with a weighted sum of nomograms by means of a program in R computing language (available in the accompanying Dryad repository: dx.doi.org/10.5061/dryad.kv3j7). Only the longer-wavelength α-bands of the nomograms were used for fitting, because absorbance at shorter wavelengths was complicated by scattering, the occurrence of pigment β-band absorption and the presence of photoproducts.
4.4. Analyses of CYP27C1 expression
A partial sequence of the sea lamprey orthologue of CYP27C1 was obtained via degenerate PCR. PCR primers (electronic supplementary material, table S2) were designed with the CODEHOP algorithm [31]; the input to the algorithm consisted of an alignment of the amino acid sequences of CYP27C1 from D. rerio (NP_001106808.2), Xenopus tropicalis (CAJ83899.1), Chelonia mydas (XP_007069504.1), Apteryx australis (XP_013816080.1), Monodelphis domestica (XP_001377301.3) and Callithrix jacchus (XP_002749285.1). Total RNA was extracted from adult RPE to generate cDNA as described below. PCR was performed with the Phusion Hot Start polymerase (NEB, M0536) following the manufacture's recommendations, and the resultant amplicons were sequenced via the Sanger method. Based on these initial sequencing results, additional PCR primers were designed (electronic supplementary material, table S2), and RACE PCR [32,33] was performed in an effort to obtain a full-length transcript.
CYP27C1 transcript levels in RPE of juvenile and adult lamprey were measured by qPCR. Total RNA was extracted from the RPE of four juvenile and four adult individuals with Trizol reagent (Invitrogen, 10296010) and used to generate cDNA with the SuperScript IV reverse transcriptase (ThermoFisher, 18090010) following the manufacturer's protocols. Primers corresponding to the 3′ portion of the coding sequences of CYP27C1 and GAPDH were selected (electronic supplementary material, table S2). CYP27C1 primer efficiency was determined by assaying a dilution series of mature lamprey RPE cDNA. The primers produced a single amplicon as indicated by melt curve analyses, and were 96% efficient at the threshold (Ct) levels used for quantification in the sample analyses. Measurements were made in three biological replicates with the Sybr® Green PCR master mix (Life Technologies, 4309155) and the Applied Biosystems StepOne real-time PCR system. The technical replicates of individual samples were averaged and expression was compared (ΔCt) relative to GAPDH.
The localization of the CYP27C1 transcript within the eyes of juvenile and adult lamprey was evaluated by ISH. The coding sequences of CYP27C1 and Rhodopsin (RHO) obtained from mature lamprey RPE cDNA were cloned by blunt-ended ligation into the BlueScript vector pBSK+ (electronic supplementary material, table S2) and then used as templates for synthesis of digoxigenin-labelled probes following established methods [34]. Whole eyes of juvenile and adult lamprey were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight and then embedded in Tissue-Tek OCT compound (Sakura). Twelve micrometre horizontal sections through the centre of each eye were then prepared. Each section was incubated overnight at 4°C with 30% hydrogen peroxide in PBS in order to bleach the melanin pigmentation of the RPE, which can obscure the ISH signal. The sections were then hybridized, developed and mounted as previously described [34].
Supplementary Material
Acknowledgements
We are grateful to Nicholas Johnson and the staff of the Hammond Bay Biological Station of the US Geological Survey for supplying juvenile and adult lamprey. We thank Susan Shen for careful reading of the manuscript. We thank Clint Makino for assistance with MSP experiments. We also thank Allison Loynd and Junwoo Suh for laboratory assistance.
Ethics
Experiments were conducted in accordance with the National Institutes of Health guidelines for research animals and approved by the institutional animal care and use committee of the University of California, Los Angeles.
Data accessibility
The data supporting the findings of this study are available in the electronic supplementary material and the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.kv3j7) [35].
Authors' contributions
M.B.T., M.C.C., G.L.F. and J.C.C. conceived the study, designed experiments and helped to analyse the data; A.M., M.B.T., G.E.P. and R.F. helped to design the experiments, performed them and analysed the data; J.M.E. and S.D.M. collected, reared and dissected anadromous lamprey samples; M.B.T., G.L.F. and J.C.C. wrote and all authors read and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by a contract from the Great Lakes Fishery Commission to G.L.F. and by grants from Research to Prevent Blindness (to J.C.C.) and the National Institutes of Health (EY001844 to G.L.F., EY001157 to M.C.C. and EY024958 and EY026672 to J.C.C.). M.B.T. was supported by a fellowship from the McDonnell Center for Cellular and Molecular Neurobiology at Washington University, St Louis.
Disclaimer
Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- 1.Jerlov NG. 1976. Marine optics. Amsterdam, The Netherlands: Elsevier Scientific. [Google Scholar]
- 2.Jokela-Määttä M, Smura T, Aaltonen A, Ala-Laurila P, Donner K. 2007. Visual pigments of Baltic Sea fishes of marine and limnic origin. Vis. Neurosci. 24, 389–398. (doi:10.1017/S0952523807070459) [DOI] [PubMed] [Google Scholar]
- 3.Lythgoe JN. 1979. The ecology of vision. Oxford, UK: Clarendon Press. [Google Scholar]
- 4.Lythgoe JN. 1984. Visual pigments and environmental light. Vision Res. 24, 1539–1550. (doi:10.1016/S0042-6989(84)80003-6) [DOI] [PubMed] [Google Scholar]
- 5.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Cheng CL, Novales Flamarique I. 2004. Opsin expression: new mechanism for modulating colour vision. Nature 428, 279 (doi:10.1038/428279a) [DOI] [PubMed] [Google Scholar]
- 7.Temple SE, Veldhoen KM, Phelan JT, Veldhoen NJ, Hawryshyn CW. 2008. Ontogenetic changes in photoreceptor opsin gene expression in coho salmon (Oncorhynchus kisutch, Walbaum). J. Exp. Biol. 211, 3879–3888. (doi:10.1242/jeb.020289) [DOI] [PubMed] [Google Scholar]
- 8.Bridges CDB. 1972. The rhodopsin–porphyropsin visual system. In Handbook of sensory physiology VII (ed. Dartnall HJA.), pp. 417–480. Berlin, Germany: Springer. [Google Scholar]
- 9.Beatty DD. 1966. A study of the succession of visual pigments in Pacific salmon (Oncorhynchus). Can. J. Zool. 44, 429–455. (doi:10.1139/z66-045) [DOI] [PubMed] [Google Scholar]
- 10.Toyama M, et al. 2008. Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species—a qualitative and comparative study. Photochem. Photobiol. 84, 996–1002. (doi:10.1111/j.1751-1097.2008.00344.x) [DOI] [PubMed] [Google Scholar]
- 11.Reuter TE, White RH, Wald G. 1971. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J. Gen. Physiol. 58, 351–371. (doi:10.1085/jgp.58.4.351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provencio I, Loew ER, Foster RG. 1992. Vitamin A2-based visual pigments in fully terrestrial vertebrates. Vision Res. 32, 2201–2208. (doi:10.1016/0042-6989(92)90084-V) [DOI] [PubMed] [Google Scholar]
- 13.Enright JM, et al. 2015. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr. Biol. 25, 3048–3057. (doi:10.1016/j.cub.2015.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuraku S, Kuratani S. 2006. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool. Sci. 23, 1053–1064. (doi:10.2108/zsj.23.1053) [DOI] [PubMed] [Google Scholar]
- 15.Hardisty MW. 2006. Lampreys: life without jaws. Ceredigion, UK: Forrest Text. [Google Scholar]
- 16.Crescitelli F. 1956. The nature of the lamprey visual pigment. J. Gen. Physiol. 39, 423–435. (doi:10.1085/jgp.39.3.423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wald G. 1957. The metamorphosis of visual systems in the sea lamprey. J. Gen. Physiol. 40, 901–914. (doi: 10.1085/jgp.40.6.901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morshedian A, Fain GL. 2015. Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr. Biol. 25, 484–487. (doi:10.1016/j.cub.2014.12.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asteriti S, Grillner S, Cangiano L. 2015. A Cambrian origin for vertebrate rods. eLife 4, e07166 (doi:10.7554/eLife.07166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. (doi:10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 21.Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. 2005. Breaking the covalent bond—a pigment property that contributes to desensitization in cones. Neuron 46, 879–890. (doi:10.1016/j.neuron.2005.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry JW, Bowmaker JK. 2000. Visual pigment reconstitution in intact goldfish retina using synthetic retinaldehyde isomers. Vision Res. 40, 2241–2247. (doi:10.1016/S0042-6989(00)00101-2) [DOI] [PubMed] [Google Scholar]
- 23.Harosi FI. 1994. An analysis of two spectral properties of vertebrate visual pigments. Vision Res. 34, 1359–1367. (doi:10.1016/0042-6989(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 24.Lamb TD, Patel H, Chuah A, Natoli RC, Davies WI, Hart NS, Collin SP, Hunt DM. 2016. Evolution of vertebrate phototransduction: cascade activation. Mol. Biol. Evol. 33, 2064–2087. (doi:10.1093/molbev/msw095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halstead LB. 1985. The vertebrate invasion of fresh-water. Phil. Trans. R. Soc. Lond. B 309, 243–258. (doi:10.1098/rstb.1985.0085) [Google Scholar]
- 26.Babino D, Perkins BD, Kindermann A, Oberhauser V, von Lintig J. 2015. The role of 11-cis-retinyl esters in vertebrate cone vision. FASEB J. 29, 216–226. (doi:10.1096/fj.14-261693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane MA, Napoli JL. 2010. Quantification of endogenous retinoids. Methods Mol. Biol. 652, 1–54. (doi:10.1007/978-1-60327-325-1_1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zonta F, Stancher B. 1984. High-performance liquid chromatography of retinals, retinols (vitamin A1) and their dehydro homologues (vitamin A2): improvements in resolution and spectroscopic characterization of the stereoisomers. J. Chromatogr. 301, 65–75. (doi:10.1016/S0021-9673(01)89179-2) [DOI] [PubMed] [Google Scholar]
- 29.Landers GM, Olson JA. 1988. Rapid, simultaneous determination of isomers of retinal, retinal oxime and retinol by high-performance liquid chromatography. J. Chromatogr. 438, 383–392. (doi:10.1016/S0021-9673(00)90269-3) [DOI] [PubMed] [Google Scholar]
- 30.Nymark S, Frederiksen R, Woodruff ML, Cornwall MC, Fain GL. 2012. Bleaching of mouse rods: microspectrophotometry and suction-electrode recording. J. Physiol. 590, 2353–2364. (doi:10.1113/jphysiol.2012.228627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose TM, Henikoff JG, Henikoff S. 2003. CODEHOP (COnsensus-DEgenerate hybrid oligonucleotide primer) PCR primer design. Nucleic Acids Res. 31, 3763–3766. (doi:10.1093/nar/gkg524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotto-Lavino E, Du G, Frohman MA. 2006. 3′ end cDNA amplification using classic RACE. Nat. Protoc. 1, 2742–2745. (doi:10.1038/nprot.2006.481) [DOI] [PubMed] [Google Scholar]
- 33.Scotto-Lavino E, Du G, Frohman MA. 2006. 5′ end cDNA amplification using classic RACE. Nat. Protoc. 1, 2555–2562. (doi:10.1038/nprot.2006.480) [DOI] [PubMed] [Google Scholar]
- 34.Enright JM, Lawrence KA, Hadzic T, Corbo JC. 2015. Transcriptome profiling of developing photoreceptor subtypes reveals candidate genes involved in avian photoreceptor diversification. J. Comp. Neurol. 523, 649–668. (doi:10.1002/cne.23702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morshedian A, Toomey MB, Pollock GE, Frederiksen R, Enright JM, McCormick SD, Cornwall MC, Fain GL, Corbo JC. 2017. Data from: Cambrian origin of the CYP27C1-mediated vitamin A1-to-A2 switch, a key mechanism of vertebrate sensory plasticity. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.kv3j7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Morshedian A, Toomey MB, Pollock GE, Frederiksen R, Enright JM, McCormick SD, Cornwall MC, Fain GL, Corbo JC. 2017. Data from: Cambrian origin of the CYP27C1-mediated vitamin A1-to-A2 switch, a key mechanism of vertebrate sensory plasticity. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.kv3j7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the electronic supplementary material and the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.kv3j7) [35].