Abstract
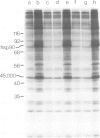
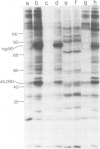
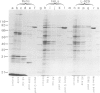
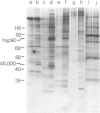
Double-stranded DNA (dsDNA) induces the transfer of phosphate from ATP to several proteins in extracts of widely divergent eukaryotic cells. Extracts of HeLa cells, rabbit reticulocytes, Xenopus eggs and Arbacia eggs all show dsDNA-dependent protein phosphorylation. The mechanism is specific for dsDNA and will not respond to either RNA or single-stranded DNA. One of the proteins which is phosphorylated in response to dsDNA has a subunit mol. wt. of 90 000 and has been identified as a heat-shock protein (hsp90). Although mouse cell extracts were shown to contain hsp90, they failed to show a dsDNA-dependent protein phosphorylation. The observation that dsDNA can modulate the phosphorylation of a set of proteins raises the possibility that dsDNA may play a role as a cellular regulatory signal.
Full text
PDF
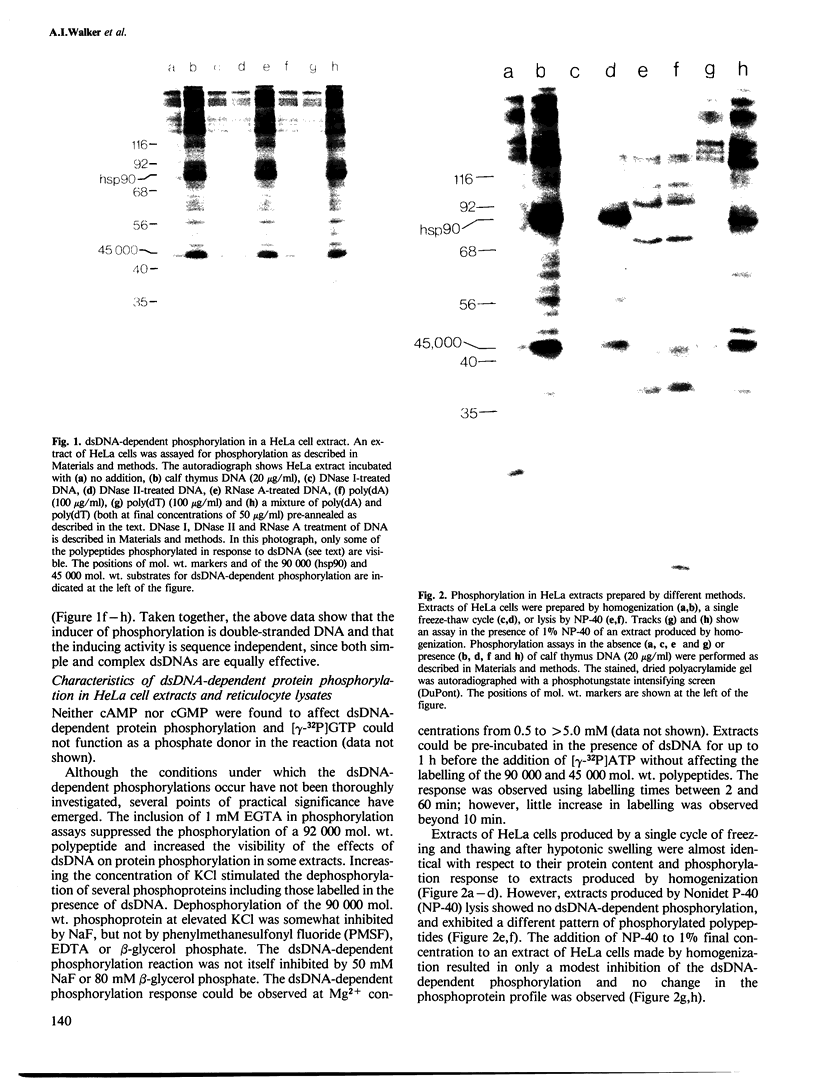
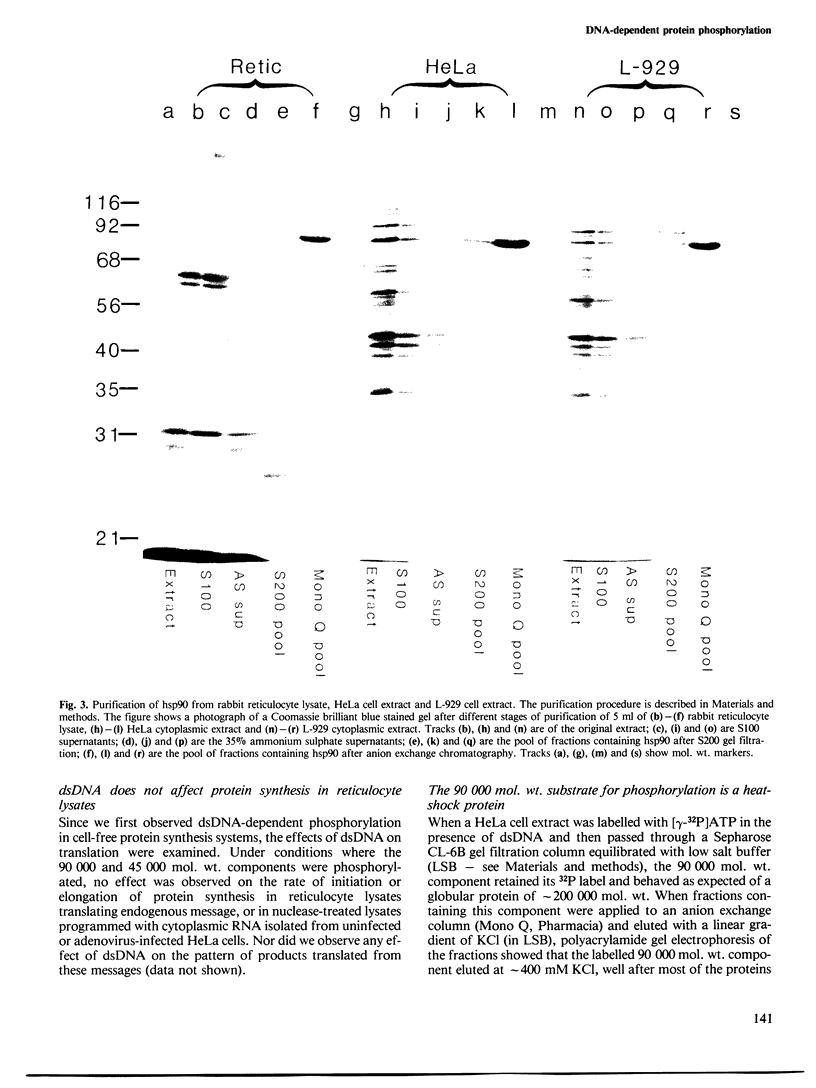




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger D. G., Bray S. J., Hunt T. Studies of the kinetics and ionic requirements for the phosphorylation of ribosomal protein S6 after fertilization of Arbacia punctulata eggs. Dev Biol. 1984 Jan;101(1):192–200. doi: 10.1016/0012-1606(84)90129-5. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Bouche G., Amalric F., Zalta J. P. Effects of heat shock on nuclear and nucleolar protein phosphorylation in Chinese hamster ovary cells. Eur J Biochem. 1980 Jul;108(2):399–404. doi: 10.1111/j.1432-1033.1980.tb04735.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. Phosphorylation and the control of protein synthesis. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):127–134. doi: 10.1098/rstb.1983.0045. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy I. M., Burdon R. H., Leader D. P. Heat shock causes diverse changes in the phosphorylation of the ribosomal proteins of mammalian cells. FEBS Lett. 1984 Apr 24;169(2):267–273. doi: 10.1016/0014-5793(84)80331-2. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Historical perspectives on protein phosphorylation and a classification system for protein kinases. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):3–11. doi: 10.1098/rstb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Li H. C. Phosphoprotein phosphatases. Curr Top Cell Regul. 1982;21:129–174. [PubMed] [Google Scholar]
- Mace D. C., Alberts B. M. The complex of T4 bacteriophage gene 44 and 62 replication proteins forms an ATPase that is stimulated by DNA and by T4 gene 45 protein. J Mol Biol. 1984 Aug 5;177(2):279–293. doi: 10.1016/0022-2836(84)90457-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]