Preface
Mammalian cells are surrounded by diverse nutrients including glucose, amino acids, various macromolecules and micronutrients, which they can import through transmembrane transporters and endolysosomal pathways. By utilizing different nutrient sources, cells gain metabolic flexibility to survive periods of starvation. Quiescent cells take up sufficient nutrients to sustain homeostasis. However, proliferating cells depend on growth factor-induced increases in nutrient uptake to support biomass formation. Here, we review cellular nutrient acquisition strategies and their regulation by growth factors and cell-intrinsic nutrient sensors. We further discuss how oncogenes and tumour suppressors promote nutrient uptake, thereby supporting cancer cell survival and growth.
Introduction
The uptake and metabolism of nutrients is central to life, as it provides matter for the generation energy and biomass. Many nutrients are reduced organic compounds, which can be oxidized to drive formation of ATP from ADP and inorganic phosphate. Glucose is catabolized to pyruvate with concomitant ATP production in glycolysis. Carbons derived from pyruvate as well as amino acids and fatty acids can feed into the tricarboxylic acid (TCA) cycle for complete oxidation to CO2, which yields large quantities of ATP through oxidative phosphorylation1. Continuous re-generation of ATP allows cells to counteract their entropic decay, for instance by maintaining quality control of macromolecules and powering ion pumps that create electrochemical gradients. While the metabolism of quiescent cells is optimized for high ATP yield, a cell’s metabolic needs dramatically alter when committing to growth and proliferation. Now, all components for doubling cellular mass must be acquired directly from extracellular sources or synthesized endogenously. To fulfil the metabolic demands of biomass formation, proliferating cells increase uptake of nutrients and, rather than oxidizing them to CO2, reprogram glycolysis and the TCA cycle into biosynthetic hubs to generate building blocks for macromolecular synthesis2.
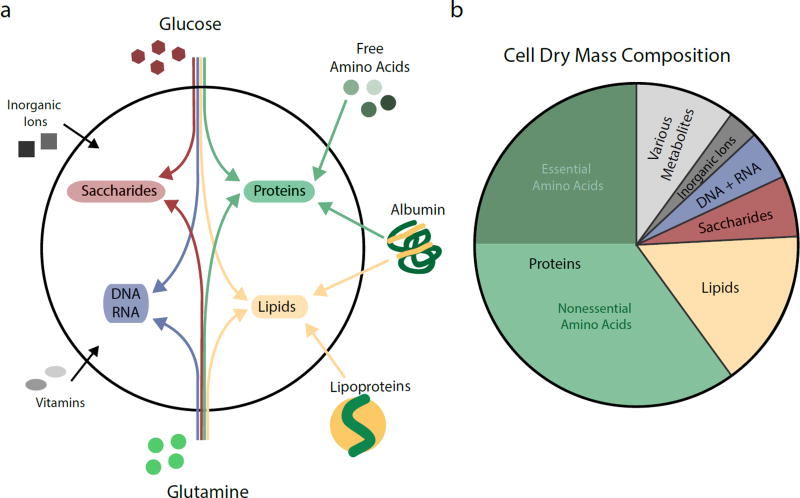
Unicellular organisms as well as multicellular plants tend to have loose nutritional requirements and can produce energy and macromolecular precursors from many different organic substrates or even simple sources of reduced carbon and nitrogen. In contrast, mammalian cells use only a few abundant nutrients such as glucose, glutamine and fatty acids for the bulk of ATP production and non-essential metabolite synthesis (Figure 1a). However, mammalian cells lack the biosynthetic capacity to produce the diversity of metabolites required for cellular functions and must acquire various essential nutrients from extracellular sources3. For example, mammalian cells cannot de novo synthesize 10 essential proteinogenic amino acids that together make up almost a quarter of cell dry mass (Figure 1b)4. At least two fatty acids are essential, alpha-linolenic acid and linoleic acid, which serve as precursors for membrane and signalling lipids. Mammalian cells further require low quantities of vitamins and various inorganic ions. The complex metabolic requirements of cell growth are reflected in the composition of plasma and interstitial fluids, which contain a wide range of low molecular weight nutrients and macromolecules5. To obtain these diverse nutrients, cells have evolved several import pathways, including cell surface nutrient transporters, receptor-mediated endocytosis and macropinocytosis of bulk solutes.
Figure 1. The Nutritional Requirements for Mammalian Cell Growth.
a, Contributions of major nutrients present in mammalian circulation towards the synthesis of cellular macromolecules. Nucleic acids (DNA and RNA) are synthesized intracellularly from glucose and glutamine. Other non-essential amino acids can also contribute to nucleotide production (not shown). Saccharides are derived from glucose, with nitrogen groups being donated by glutamine. Amino acids for protein synthesis can be imported in their free form or derived from catabolism of extracellular proteins. Non-essential amino acids can also be synthesized from glucose and glutamine. Extracellular lipids are delivered by lipoproteins and serum albumins. Most lipids are not essential for mammalian cells and can also be generated from glucose and glutamine carbons. Cells further require exogenous supply of a variety of essential micronutrients such as inorganic ions and vitamins. b, Fractional contribution of proteins, lipids, saccharides, nucleic acids (DNA and RNA), inorganic ions and metabolites to dry mass of a representative mammalian cell. The proportion of essential and non-essential amino acids contained within proteins are indicated.
Because cancer is in part a disease of dysregulated growth, transformed cells have increased demands for nutrients such as glucose and glutamine to support macromolecular synthesis2,6. However, solid tumour growth frequently creates regional nutrient deficiencies by outstripping the vascular supply. It is becoming clear that malignant cells can survive and grow in vascularly compromised environments by exploitin g the full array of nutrients available extracellularly, including low molecular weight nutrients as well as macromolecules and cellular debris. The capacity to enhance anabolic metabolism has emerged as a core feature of many oncogene and tumour suppressor pathways that is fundamental to their carcinogenic action7,8. At the same time, studying the metabolism of transformed cells has contributed significantly to the understanding of how cells regulate nutrient usage during physiological processes such as growth and adaptation to stress. Here, we review insights from cancer metabolism research concerning how mammalian cells acquire and use the diverse low molecular weight nutrients and macromolecules present in the extracellular space. We highlight cellular pathways that function in nutrient uptake, their regulation by signalling pathways and dysregulation during cancer development.
Growth Factor Signalling Controls Metazoan Nutrient Uptake
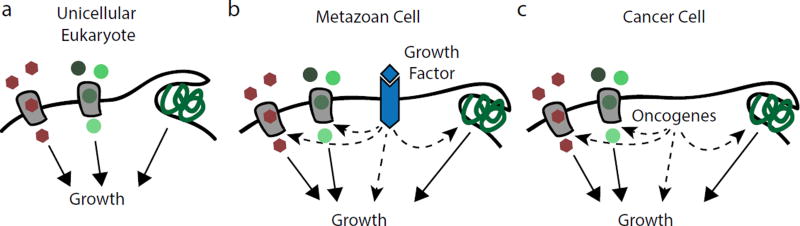
Unicellular organisms acquire nutrients as they become available in the environment and adjust the rates of anabolic pathways and macromolecular synthesis accordingly. For such organisms, nutrients not only supply biosynthetic substrates, but also serve as principal signals to engage in proliferation (Figure 2a). The discovery that growth factors initiate cell cycle entry in metazoan organisms led to the assumption that cellular metabolism could be disregarded as a regulator of animal cell proliferation. Rather, it was assumed that growth factor-induced proliferation raises a cell’s bioenergetic and biosynthetic demands, with nutrient uptake being increased as a passive consequence. However, it has emerged over the last 15 years that growth factors act as cell-extrinsic regulators of nutrient acquisition and usage in metazoan cells2,7 (Figure 2b). Downstream of growth factor-activated receptor tyrosine kinases, the phosphoinositide 3-kinase (PI3-kinase) and Ras signalling pathways are central regulators of cellular nutrient acquisition. PI3-kinase and Ras directly promote nutrient uptake to supply growing cells with sufficient resources for biomass formation, and concomitantly increases expression or activity of biosynthetic enzymes to allocate nutrients towards anabolism.
Figure 2. Coordination of Cell Growth and Nutrient Uptake.
Eukaryotic cells import low molecular weight nutrients such as glucose and amino acids through plasma membrane transporters and can also ingest bulk extracellular macromolecules through the non-selective endocytic pathway of macropinocytosis. a, Unicellular eukaryotes take up nutrients as they become available in the environment. In addition to supplying bioenergetic and biosynthetic pathways, nutrients function as direct signals for unicellular organisms to commit to growth and proliferation. b, The cells of metazoan organisms have lost the ability to regulate nutrient acquisition cell-autonomously; rather, they are instructed by growth factor signalling pathways to engage in nutrient uptake, thereby ensuring that nutrient supply matches the metabolic demands of cell growth. c, Components of growth factor signalling pathways are frequently mutated in cancer. These oncogenes cause cellular transformation in part by granting a cell autonomy over nutrient uptake and by increasing availability of precursors for macromolecular synthesis.
Because metazoan cells are commonly surrounded by nourishing body fluids, placing nutrient uptake under cell-extrinsic control constitutes a metabolic adaptation of multicellular animals to prevent excessive consumption of the body’s resources and suppress aberrant proliferation2,7. Cell-extrinsic regulation of nutrient uptake constitutes a fundamental barrier for cellular transformation: to employ the unchecked anabolic metabolism characteristic for cancer, a cell must acquire mutations that permit independent control of nutrient access (Figure 2c). The oncogenic potential of signalling pathways such as the PI3-kinase and Ras pathways in part lies in their ability to confer cellular autonomy for both cell cycle entry and nutrient acquisition, which conspire to drive tumour cell proliferation.
Influence of Intracellular Nutrient Sensors on Nutrient Acquisition
Like unicellular eukaryotes, metazoan cells also employ a variety of nutrient-responsive signalling networks to regulate nutrient acquisition and coordinate the use of metabolic resources. By surveying the abundance of energy and key metabolites, intracellular nutrient sensors play an important role in metabolic homeostasis and cell survival. For instance, the sterol response element binding protein (SREBP) pathway surveys cholesterol levels in endoplasmic reticulum membranes. When cellular cholesterol levels decrease, SREBP undergoes proteolytic cleavage and is released from the membrane. Subsequently, SREBP translocates to the nucleus and induces expression of lipoprotein receptors to increase cholesterol acquisition from extracellular sources and expression of lipogenic enzymes to enhance de novo biosynthesis9. In this manner, SREBP acts as an internal sensor that contributes to maintaining the balance of phospholipids and cholesterol in cellular membranes.
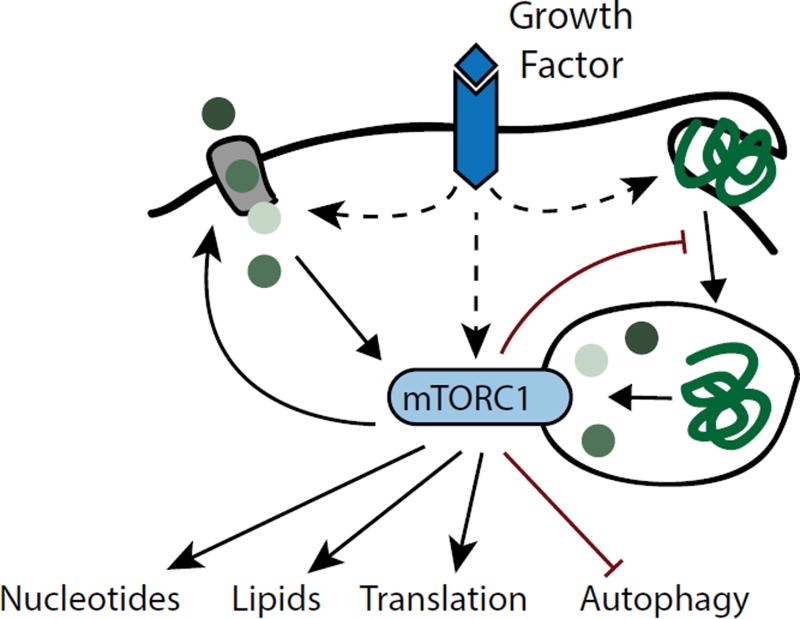
In response to cellular metabolite levels, nutrient sensors can also adjust the use of nutrients for energy production and macromolecular synthesis10. The kinase mechanistic target of rapamycin complex 1 (mTORC1) is a central coordinator of amino acid availability and cell growth11,12. mTORC1 activation requires abundant intracellular amino acids. Once active, mTORC1 enhances the consumption of amino acids in protein translation and also stimulates other anabolic processes including lipid and nucleotide synthesis (Figure 3). Concomitantly, mTORC1 suppresses macromolecular catabolism by blocking autophagy, which targets intracellular constituents for degradation in the lysosome. Many functions of mTORC1 are antagonized by AMP-activated protein kinase (AMPK), a sensor of cellular energy levels that becomes active when AMP levels rise13. AMPK initiates autophagy and fatty acid beta-oxidation to promote catabolism of macromolecules as bioenergetic substrates for ATP production. At the same time, AMPK suppresses the energetically costly synthesis of new macromolecules, in part by inhibiting mTORC1. The interplay between mTORC1 and AMPK is central to cellular metabolic homeostasis in fluctuating nutrient environments14.
Figure 3. Metabolic Control by the mTORC1 Signalling Pathway.
Full activation of mTORC1 kinase requires concerted inputs from growth factor signalling and intracellular amino acids. Once active, mTORC1 enhances cell growth by promoting protein translation as well as biosynthesis of lipids and nucleotides. mTORC1 also increases cell surface amino acid transporter levels, but suppresses lysosomal catabolism of proteins derived from endocytosis or autophagy, thereby rendering cells dependent on availability of free amino acids.
Intracellular nutrient sensors do not only respond to metabolite levels, but are commonly modulated by growth factor signalling pathways. For instance, activation of mTORC1 requires concerted inputs from intracellular amino acids and from the PI3-kinase effector, the serine/threonine kinase Akt11,12. Conversely, Akt antagonizes activation of AMPK15. These principles allow cells to integrate metabolic status and growth factor signals to regulate macromolecular synthesis and growth.
Nutrient Transporter-Mediated Uptake of Glucose and Amino Acids
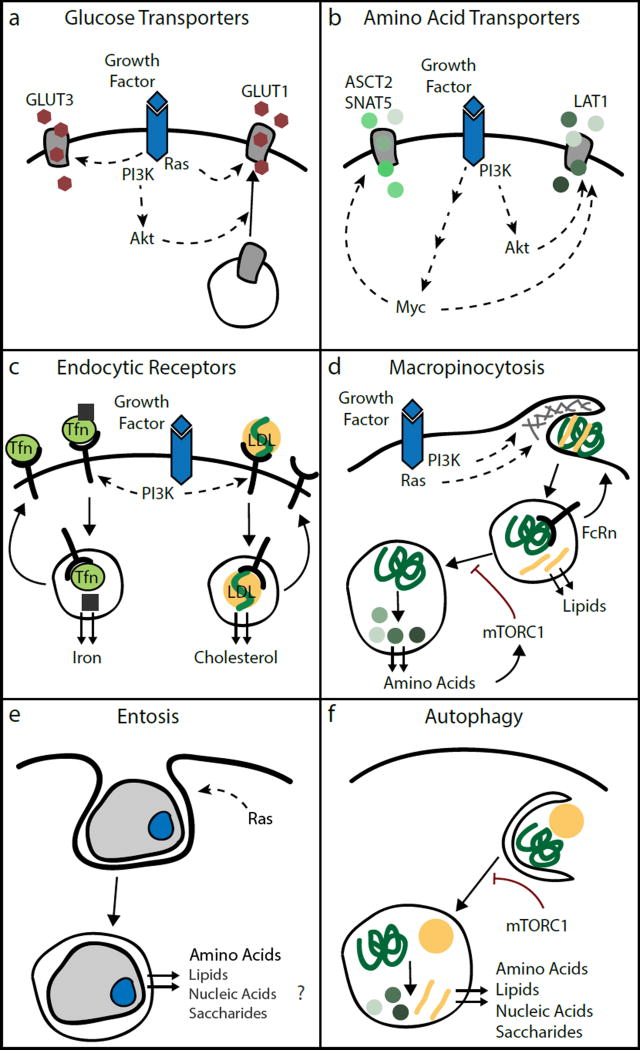
Glucose and amino acids are abundant in the extracellular fluids of mammalian organisms and constitute principal cellular nutrients. Due to their hydrophilic nature, these low molecular weight nutrients cannot diffuse across the plasma membrane, but rather are imported by cells through nutrient transporters of the solute carrier family. While quiescent cells express only basal levels of nutrient transporters and therefore are limited in their capacity to take up glucose and amino acids, cells increase their uptake upon growth factor stimulation16. Central regulators of cellular glucose uptake downstream of growth factors are PI3-kinase and its effector Akt (Figure 4a). Upon growth factor stimulation, activation of PI3-kinase and Akt effects cell surface translocation of various glucose transporters such as the insulin-regulated GLUT4 and the ubiquitously expressed GLUT116–20. In addition, Akt increases activity of the enzyme hexokinase, which phosphorylates glucose, resulting in its intracellular capture. PI3-kinase signalling also promotes glucose utilization by activation of several glycolytic enzymes, including phosphofructokinase and aldolase that collectively increase flux through glycolysis19,21–23. Similarly, Ras GTPases stimulate glucose metabolism by increasing expression of GLUT1, hexokinase and several glycolytic enzymes24–26. Mutations that constitutively activate PI3-kinase or Ras signalling, which are among the most common genetic events in human cancer, share the ability to enhance cellular glucose uptake and glycolytic flux independently of growth factor stimulation. Glucose uptake is also promoted by oncogenic mutations in upstream receptor tyrosine kinases; for instance, epidermal growth factor receptor mutations increase surface presentation of the glucose transporter GLUT327. These findings explain the century-old observation by German physiologist Otto Warburg that tumour cells display a marked increase in glucose consumption compared to their non-transformed counterparts28. High glucose uptake rates can be detected by 18F-deoxyglucose positron emission tomography (FDG-PET) in many different cancer types, which is now a commonly used diagnostic tool.
Figure 4. Growth Factor Signalling Regulates the Repertoire of Nutrient Uptake Pathways in Mammalian Cells.
a, Growth factor activation of PI3-kinase/Akt signalling induces trafficking of the glucose transporter GLUT1 from intracellular vesicles to the plasma membrane. GLUT1 is transcriptionally upregulated through Ras and Akt. Growth factor signalling can also increase membrane concentrations of GLUT3. b, PI3-kinase/Akt and the transcription factor Myc increase expression of various amino acid transporters including the glutamine transporters ASCT2 and SNAT5 and the neutral amino acid transporter LAT1. c, PI3-kinase upregulates receptor-mediated endocytosis of Tfn and LDL, which enhances cellular acquisition of iron and cholesterol. d, Ras and PI3-kinase initiate macropinocytosis for bulk ingestion of extracellular macromolecules and cellular debris. Macropinocytosis mediates uptake of albumin, which can release bound lipids in endosomes and is recycled through the neonatal Fc receptor (FcRn). If the cellular amino acid sensor, mTORC1, becomes inactive, lysosomal catabolism of ingested albumin increases to liberate its amino acid content. e, Cells can engulf neighbouring cells through entosis, and digest them in the lysosome to recover their amino acid content. Entosis could also supply other nutrients, although this remains to be addressed experimentally. f, Under favourable conditions, mTORC1 suppresses catabolism of cellular contents through autophagy. When mTORC1 becomes inactive upon nutrient deprivation or growth factor withdrawal, cells engulf cytosolic constituents and organelles in autophagosomes for delivery and subsequent catabolism in the lysosome. Autophagy allows recycling of amino acids, lipids, nucleic acids and saccharides from intracellular nutrient stores.
Although glucose uptake in mammalian cells is predominantly regulated by growth factors, it can be secondarily modulated by cell-intrinsic sensors. AMPK increases GLUT1 concentration in the plasma membrane by inhibiting its Thioredoxin-Interacting Protein (TXNIP)-mediated endocytosis29, and by phosphorylating GLUT1 directly increases its rate of glucose transport30,31. Activation of AMPK by a decline in cellular energy levels therefore improves availability of glucose as a bioenergetic substrate for restoring energy balance. Cells can also increase glucose uptake when ATP production through the mitochondrial respiratory chain is limited by decreased oxygen availability or produces harmful levels of reactive oxygen species. When electron buildup in the respiratory chain results in high mitochondrial production of reactive oxygen species and accumulation of TCA cycle intermediates, activity of the transcription factor hypoxia-inducible factor 1 alpha (HIF1alpha) increases, which in turn suppresses entry of glucose carbons into the TCA cycle and decreases oxidative phosphorylation32,33. Concomitantly, HIF1alpha induces expression of GLUT1 and lactate dehydrogenase, thereby increasing glucose uptake and glycolysis as an alternative means to generate ATP34–36.
Due to their biophysically diverse properties, amino acids require a variety of import systems with specificity for different amino acid classes. The regulation of amino acid transporters is still being elucidated, but growth factor signalling pathways clearly play an instructive role. The transcription factor Myc, which is induced by growth-promoting signalling pathways and amplified in various tumour types, induces expression of the glutamine transporters ASCT2 and SNAT5 (Figure 4b). Myc also upregulates glutaminase, the first enzyme of the glutaminolysis pathway, which funnels glutamine carbons into the TCA cycle37,38. Expression or cell surface presentation of several other amino acid transporters such as the neutral amino acid transporter LAT1 is induced by Myc as well as by PI3-kinase/Akt signalling38–40. At the same time, Myc and PI3-kinase/Akt stimulate ribosomal biogenesis and mTORC1-dependent translational initiation, respectively, thereby allocating the increased amino acid supply towards protein synthesis.
Receptor-Mediated Endocytosis of Nutrient Carriers
A variety of nutrients are insoluble in the aqueous environment of living organisms and must be transported through extracellular fluids by protein carriers. Cells acquire such nutrients through plasma membrane receptors, which recognize nutrient carriers and initiate their internalization for subsequent cargo release in endolysosomal compartments41. Receptor-mediated endocytosis constitutes the major pathway through which cells obtain cholesterol and iron (Figure 4c). The low-density lipoprotein (LDL) receptor mediates uptake of LDL, which is the principal extracellular carrier of cholesterol and also contains phospholipids as well as triglycerides. Transferrin (Tfn) receptors mediate endocytosis of Tfn, the systemic carrier of iron. Cells employ nutrient-sensing signalling pathways to monitor intracellular lipid and iron availability and adjust expression of LDL and Tfn receptors accordingly. When cells become cholesterol-depleted, SREBP transcription factors induce expression of the LDL receptor to enhance cellular LDL uptake9. Upon iron deficiency, iron regulatory protein 1 stabilizes Tfn receptor mRNA and increases translation of the receptor protein to enhance transferrin endocytosis42.
While LDL and Tfn receptor levels are adjusted to intracellular nutrient availability under basal conditions, their absolute level of expression and surface presentation is modulated by growth factors. Growth factor stimulation or expression of activated Akt leads to increased expression and surface presentation of the LDL receptor40,43. PI3-kinase/Akt signalling upregulates the LDL receptor at least in part by activating mTORC1, which potentiates SREBP-mediated gene expression44. Similarly, growth factors signal to PI3-kinase/Akt to increase expression of Tfn receptors, although the underlying mechanism remains to be determined40,45–47. Upregulation of the LDL receptor supports survival and proliferation of glioblastoma cells, and elevated Tfn receptor levels have been documented in several cancer types48,49. Together, this suggests that growth factor signalling pathways induce expression of LDL and Tfn receptors to increase nutrient supply for biomass formation, which could have nutritional benefits for transformed cells.
Macropinocytosis of Extracellular Proteins as an Amino Acid Source
While receptor-mediated endocytosis effects uptake of specific macromolecules, metazoan cells also possess a non-selective endocytic pathway for bulk ingestion of extracellular solutes, which is referred to as macropinocytosis50,51. Macropinocytosis is initiated by actin-driven protrusions of the plasma membrane that engulf portions of the environment for internalization into large endocytic vesicles. These macropinosomes can subsequently be trafficked to the lysosome, where digestive enzymes degrade their macromolecular cargo, which creates an intracellular nutrient source (Figure 4d). The conservation of macropinocytosis across eukaryotes suggests this endocytic pathway evolved together with the lysosomal system as a nutrient acquisition strategy ancestral eukaryotic cells50. Indeed, unicellular amoeboid eukaryotes such as Dictyostelium can satisfy their dietary requirements through macropinocytosis of extracellular macromolecules. In mammalian cells, actin-driven membrane ruffling and subsequent macropinosome formation is an immediate response to growth factor stimulation52,53. Downstream of growth factor-activated receptor tyrosine kinases, the intracellular signalling cascade that triggers macropinocytosis is centred around Ras and PI3-kinase, which induce membrane ruffles and macropinosome closure. Mammalian cells have little intrinsic macropinocytic activity without external stimuli. However, constitutively activated Ras GTPases induce macropinocytosis cell-autonomously in the absence of growth factors and cancer cell lines harbouring Ras mutations commonly display high macropinocytic activity54,55.
While glucose and amino acids are important nutrients for mammalian cells, macropinocytosis of extracellular proteins has emerged as another route for nutrient acquisition. By increasing macropinocytic activity, oncogenic Ras promotes the use of extracellular proteins as nutrients to decrease cellular dependence on exogenous supply of monomeric amino acids56. Ras-mutant cells can proliferate even in the complete absence of essential amino acids such as leucine by ingesting albumin, the most abundant protein of mammalian plasma57,58. PI3-kinase has been shown to also regulate macropinosome formation50. However, it remains to be investigated whether oncogenic activation of PI3-kinase signalling shares the property of oncogenic Ras to enhance the nutritional use of proteins. In vivo, tumours can siphon off a substantial fraction of circulating albumin59. Pancreatic cancers cells harbouring a K-Ras mutation display increased uptake and intracellular catabolism of albumin compared to surrounding normal tissue, which is blockezd by macropinocytosis inhibition56,60. These findings suggest that extracellular proteins play an important nutritional role for cancer cells that reside in poorly perfused tumour regions.
Diverse Nutritional Benefits of Endolysosomal Pathways
Due to its non-selective nature, macropinocytosis has the potential to supply nutrients other than amino acids. For instance, cells can use macropinocytosis to take up albumin, which functions as the main carrier of free fatty acids in mammalian plasma61. Of note, internalized albumin can escape lysosomal degradation because it binds at the acidic pH of endosomes to the neonatal Fc receptor, which recycles albumin back to the cell surface62. This raises the possibility that cells can macropinocytose albumin, acquire its lipid cargo and release it back into the extracellular space or designate albumin for lysosomal degradation to access its amino acid content. Moreover, macropinocytosis can mediate uptake of macromolecular structures such as membrane vesicles and apoptotic bodies63. These particles are abundant in damaged tissues and necrotic tumour regions and could supply a range of nutrients. Epithelial cells can also internalize whole neighbouring cells in a non-phagocytic form of engulfment referred to as entosis (Figure 4e). The internalized cell undergoes cell death and is catabolized by lysosomal hydrolases, releasing nutrients that sustain survival and proliferation of starved cells64. Cell-in-cell structures that resemble entotic vacuoles have been found in several human cancers, raising the possibility that entosis can confer a metabolic advantage in nutrient-deprived tumours. The likelihood of a cell to engulf its neighbour is increased by oncogenic activation of K-Ras65, suggesting that Ras signalling provides nutritional benefits by inducing recovery of nutrients delivered to the lysosome from both macropinocytosis and entosis.
When cells do not have access to extracellular nutrients, they engage in the self-catabolic process of macroautophagy66. During autophagy, double membrane vesicles called autophagosomes are formed, which engulf cytosolic constituents and organelles and subsequently fuse with lysosomes for cargo degradation by lysosomal enzymes (Figure 4f). Autophagy is frequently upregulated in nutrient-deprived regions of solid tumours67. Consistently, inhibiting autophagy induces cell death and prevents growth of solid tumours68,69. By recycling nutrients from intracellular macromolecules, autophagy is an important cellular strategy to sustain viability during limited starvation periods. However, unlike macropinocytosis, autophagy is not induced by growth factor signalling and cannot support cell proliferation in the absence of extracellular nutrients, but rather results in cellular atrophy70.
Coordination of Nutrient Transporters and Endolysosomal Pathways
As delineated above, growth factor activation of PI3-kinase and Ras instructs cells to both import glucose and amino acids through nutrient transporters and to ingest macromolecules through the endolysosomal system. If growth factors provide a general signal to engage in nutrient uptake, how does a cell coordinate different nutrient acquisition routes? Evidence suggests that mTORC1 helps regulate a cell’s preference for use of either monomeric amino acids or proteins to support growth. Concerted signals from mTORC1 and its upstream activator Akt promote expression and cell surface presentation of glucose and amino acid transporters19,20,40. At the same time, mTORC1 inhibits the autophagy initiator kinases Ulk1/2 to prevent catabolism of intracellular matter, and blocks catabolism of proteins that were internalized through macropinocytosis57,71,72. mTORC1 also limits lysosomal capacity by preventing nuclear translocation of MiT/TFE transcription factors, which induce expression of lysosomal biogenesis genes73–75. Thus, Akt/mTORC1 signalling defines a cellular state that is characterized by high rates of glucose and amino acid import through nutrient transporters with concomitant suppression of lysosomal catabolism of macromolecules (Figure 3). By suppressing macromolecular catabolism, mTORC1 helps cells maximize biomass formation, but also renders them dependent on extracellular supply of free amino acids. In contrast, when mTORC1 activity is reduced, cell surface levels of nutrient transporters decrease and enhanced lysosomal degradation of macropinocytosed proteins generates an intracellular amino acid source. Thereby, mTORC1 inhibition allows cells to sustain growth in nutrient deprived environments such as poorly vascularized tumour regions (Palm et al., 2015).
Nutritional Importance and Flexible Use of Glucose and Glutamine
Glucose and glutamine are key substrates for multiple metabolic pathways and while mammals can in principal synthesize them de novo, cells oftentimes depend on exogenous glucose and glutamine supply. A feature of many cancer cells and other rapidly proliferating cells such as observed during development or an immune response is the Warburg effect or aerobic glycolysis, which refers to the uncoupling of glycolysis from the TCA cycle despite abundant oxygen levels28. This metabolic state results from taking up large quantities of glucose and excreting the glucose carbons in excess of their biosynthetic and bioenergetic needs as lactate. Aerobic glycolysis increases the availability of glycolytic intermediates as precursors for various anabolic pathways when ATP needs of a cell are saturated2. Glutamine is the second most consumed nutrient of proliferating cells and is a major source of carbons to replenish TCA cycle intermediates76,77.
Despite the importance of glucose and glutamine, metabolic flexibility allows cells to compensate for deprivation of glucose or glutamine by enhanced use of the other nutrient. Cells can funnel carbons from either glucose or glutamine into the TCA cycle to drive ATP production through mitochondrial respiration78. Glucose-derived acetyl-CoA functions as a precursor for fatty acid and cholesterol synthesis. However, when acetyl-CoA synthesis from glucose is reduced, reductive carboxylation of glutamine-derived alpha-ketoglutarate can step in to generate acetyl-CoA for lipogenesis79,80. Some cancers also bypass the reliance on glucose or glutamine for acetyl-CoA production altogether by taking up exogenous acetate from which to synthesize acetyl-CoA81,82. In the absence of glucose, even some biosynthetic intermediates of glycolysis can be derived from glutamine carbons through action of the TCA cycle and a mitochondrial form of the glycolytic enzyme phosphoenolpyruvate carboxykinase83.
While glutaminolysis is the predominant way by which proliferating cells in culture replenish TCA cycle intermediates, analysis of in vivo tumour metabolism has demonstrated preferential use of glucose-derived pyruvate for TCA cycle anaplerosis through the action of pyruvate carboxylase84–87. TCA cycle anaplerosis from other carbon sources can support de novo glutamine synthesis, which allows naïve embryonic stem cells to proliferate in the complete absence of extracellular glutamine88. Beyond providing carbons for TCA cycle anaplerosis, glutamine is also a major source of reduced nitrogen for synthesis of amino acids, nucleotides and hexosamines. However, various cancers express high levels of branched-chain amino acid transferases, which allow cells to use leucine, isoleucine and valine as nitrogen sources89,90. Such metabolic adaptability provides a selective advantage for cancer cells that must survive or proliferate in the poorly perfused microenvironments of solid tumours where glucose and glutamine levels fluctuate.
Choice between Uptake and Biosynthesis of Non-Essential Amino Acids and Lipids
While cells must take up essential nutrients from the extracellular space, they can acquire non-essential metabolites from either exogenous sources or endogenous production. Most non-essential amino acids derive their carbon backbone from intermediates of glycolysis and the TCA cycle, and their nitrogen from either ammonia or transamination of any excess amino acid. Therefore, if glucose and glutamine are abundant, mammalian cells can synthesize other non-essential amino acids in sufficient quantities to engage in macromolecular synthesis and growth. Indeed, several TCA-cycle derived amino acids are preferentially produced intracellularly, regardless of their presence in the environment91. Cells sense non-essential amino acids shortages through the GCN2/ATF4 pathway, which is activated by uncharged tRNAs and induces expression of various amino acid biosynthesis enzymes. Because ATF4-deficient cells fail to induce biosynthetic programmes for asparagine and serine, they become auxotrophic for these normally non-essential amino acids92,93. Even when expressing asparagine synthetase, cells rely on exogenous asparagine supply to survive glutamine deprivation, because the amide nitrogen of asparagine is donated directly from glutamine93,94.
Under nutrient-rich conditions, proliferating cells produce significant quantities of fatty acids de novo. Consistently, increased expression of fatty acid synthase and enhanced lipogenesis have been observed in several cancer types95. However, the biosynthetic demands of lipogenesis are substantial and lipid production of cultured cells is suppressed by exogenous addition of lipids or lipoproteins96,97. Consistently, proliferating cells can take up extracellular lipids and use them as precursors for membrane lipids91,98. Unsaturated fatty acids become essential nutrients under hypoxia, because oxygen limitation impairs the activity of stearoyl-CoA desaturase 1 (SCD), the enzyme that introduces a double bond into fatty acids prior to incorporation into membrane phospholipids. As a consequence, deprivation of unsaturated fatty acids triggers apoptosis of lipogenic cancer cells subjected to hypoxia99. Oncogenic variants of K-Ras and H-Ras promote cellular uptake of lysophospholipids with an unsaturated fatty acid moiety, which support cell proliferation under hypoxia when SCD becomes inactive100. Evidence suggests that increased uptake of exogenous lipids is also selected for under some circumstances in vivo. For instance, over-expression of the scavenger receptor CD36, which mediates cellular influx of lipids from circulating lipoproteins, increases the metastatic potential of tumour cells, while genetic or pharmacological inhibition of CD36 suppresses metastasis formation101.
Because mammals lack the glyoxylate cycle, which is required for synthesis of glucose and amino acids from fatty acid carbons, the use of fatty acids in biosynthesis is limited to production of membrane lipids. However, fatty acid carbons are an efficient bioenergetic substrate, their oxidation generating twofold more ATP per mole than oxidation of glucose or amino acids. Fatty acid oxidation can provide sufficient ATP to rescue the viability of cells when glucose becomes limiting102. Even under nutrient-replete conditions, some cancer types will use fatty acid oxidation to maintain oxidative phosphorylation103,104.
Metabolic Cooperation in the Tumour Microenvironment
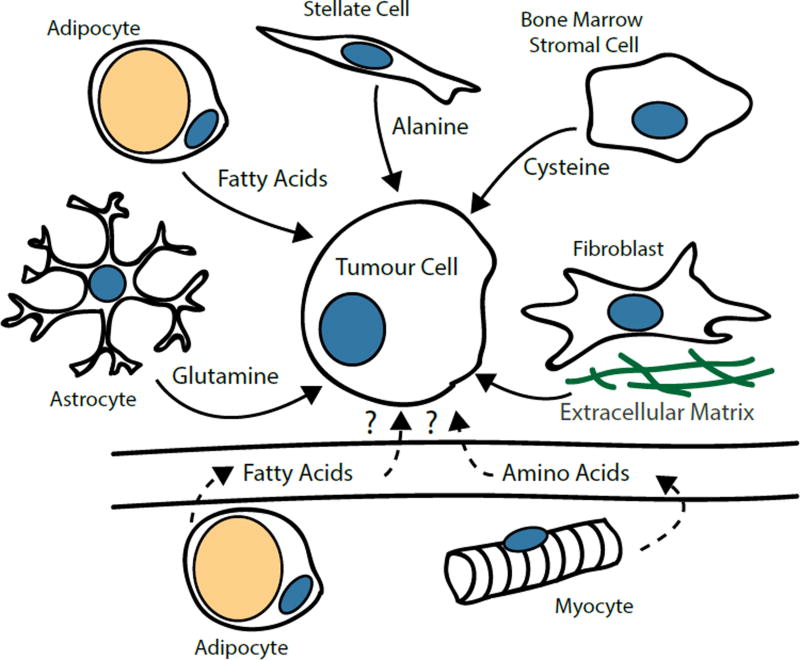
The organization of metazoan organisms into multiple cell types allows for functional compartmentalization of cellular metabolism; most cell types employ only a subset of the organism’s metabolic pathways and rely on nutrient supply by other tissues. For instance, the liver synthesizes glucose, triglycerides and plasma proteins, whereas adipose tissue stores triglycerides for future release as free fatty acids. While the importance of dedicated metabolic organs for interorgan nutrient exchange is well established, recent findings have demonstrated remarkable metabolic cooperation between cancer and stromal cells in the tumour microenvironment (Figure 4). Ovarian cancer cells induce lipolysis in nearby adipocytes, causing them to release fatty acids, which the cancer cells then acquire through upregulation of the fatty acid binding protein FABP4105. Glioblastoma-associated astrocytes express high levels of glutamine synthetase and support cancer cell proliferation by releasing glutamine into the tumour microenvironment106. Chronic myeloid leukaemia cells express only low levels of the xCT transporter, which mediates import of cystine, the oxidized dimer in which cysteine predominantly exists extracellularly. However, bone marrow stromal cells take up cystine in excess, causing them to secrete cysteine, which is taken up by tumour cells107. Pancreatic stellate cells secrete alanine, which is imported by tumour cells and converted to pyruvate as a bioenergetic substrate for the TCA cycle. Alanine release depends on stellate cell autophagy, suggesting it is at least in part derived from lysosomal protein catabolism108. Fibroblasts also provide proteins as nutrients directly in the form of extracellular matrix, which can be endocytosed and catabolized by adjacent epithelial cells109. Taken together, these studies reveal the surprising ability of malignant cells to supplement their metabolism with nutrients provided by neighbouring cells with complementary metabolic activities, which significantly contributes to tumour cell survival and proliferative capacity. Non-transformed cells oftentimes undergo phenotypic changes in the tumour microenvironment, which raises the possibility that tumor cells secrete factors that modulate the metabolism of neighbouring cells to their advantage7.
Metabolic crosstalk need not be restricted to cells within a tumour. Pancreatic cancer development is accompanied by increased plasma levels of branched-chain amino acids that originate from protein breakdown in other organs110. The growth of various tumours is associated with increased triglyceride breakdown in adipose tissue and elevated plasma levels of free fatty acids, and wasting of adipose lipid stores can be inhibited by blocking lipolytic enzymes111. It remains to be clarified whether the increase of circulating amino acids and fatty acids provides nutritional benefits for tumor cells. However, such metabolic interactions between a tumour and distant organs could contribute to the cancer-associated wasting syndrome, cachexia.
Concluding Remarks and Future Outlook
The study of cancer cell metabolism has begun to unravel how growth factor signals, or more specifically the oncogenes and tumour suppressors that constitute these pathways, regulates cellular nutrient uptake and usage. For instance, although macropinocytosis was the first endocytic pathway to be discovered in the early 20th century112, studying the induction of macropinocytosis by oncogenic Ras has only recently revealed its function in amino acid acquisition by mammalian cells56–58. Beyond their importance for tumour biology, insights from cancer metabolism have significantly contributed to the understanding of cellular metabolism in physiological contexts. For example, it has become clear that activated T-cells as well as proliferating cells in the developing embryo have metabolic traits reminiscent of the Warburg effect. Similarly, metabolic adaptations of cancer cells that proliferate in vascularly compromised tumours have foreshadowed investigations of immune cells that navigate nutrient deficiencies at sites of infection or in the tumour microenvironment.
Despite these advances, we remain with an incomplete picture of the regulation of cellular nutrient acquisition by signal transduction. Most studies focus on individual nutrients or cellular uptake pathways, but we lack an integrative view of how cells coordinate acquisition of the diverse nutrients required for growth. Many insights into cellular metabolism stem from the analysis of a single cell type or cancer or have focused on specific oncogenes. Yet the few comparative studies available suggest that cell type or tissue identity can profoundly influence cellular metabolism. Concentrations and fluxes of nutrients differ in interstitial fluids compared to the circulation, but measuring extracellular nutrient concentrations within tissues remains a technical challenge and the nutrient levels surrounding cells are thus poorly defined. Lastly, it remains an outstanding question how a cell’s preference for distinct nutrient acquisition strategies is regulated. For instance, proliferating mammalian cells tend to use glucose and free amino acids for biomass formation, despite being surrounded by protein-rich extracellular fluids. In contrast, proliferating embryonic cells of oviparous animals depend entirely on catabolism of extracellular macromolecules deposited in egg white and yolk. Understanding the factors that determine such differences in nutrient usage will be important to predict the efficacy of cancer therapeutics that target cellular metabolism, and shed further light on metabolic features of metazoan cells.
Figure 5. Metabolic Cooperation between Cancer Cells and Non-Transformed Cells.
Non-transformed cells in the tumor microenvironment can secrete a variety of metabolites that supply energy-producing and biosynthetic pathways of cancer cells. Nutrients released by normal cells can support survival and growth of cancer cells when the existing vascular supply becomes limiting. Cancer cells can also enter symbioses with non-transformed cells engaging in metabolic activities that are not active in cancer cells. For example, fatty acids released by adipocytes can serve as bioenergetic fuel for cancer cells. The generation of amino acids from autophagic degradation of intracellular proteins or the deposition of proteins as extracellular matrix can also provide nutrients. Astrocytes are capable of de novo glutamine synthesis and supply glutamine to glioblastoma cells. Bone marrow stromal cells import cystine and release it as cysteine, which is taken up by chronic myeloid leukaemia cells that do not express cystine transporters. Tumours can also induce catabolism of lipid stores in fat and structural proteins in muscle, resulting in elevated levels of fatty acids and amino acids in circulation, which might improve nutrient supply for cancer cells.
Acknowledgments
We thank members of the Thompson laboratory and in particular Lydia Finley and Tullia Lindsten for helpful discussions. WP is the recipient of the Genentech Foundation Hope Funds for Cancer Research Fellowship. Work in the Thompson laboratory is supported by a grant from NCI to CBT (R01 CA201318) and Cancer Center Support Grant P30CA008748. CBT is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. CBT also serves on the board of directors of Merck and Charles River Laboratories.
References
- 1.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. W.H. Freeman 6th edition. 2012 [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.) 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science (New York, N.Y.) 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 4.Alberts BM, et al. Molecular biology of the cell. Garland Science; 6th edition. 2014 [Google Scholar]
- 5.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. The New England journal of medicine. 2004;351:1548–1563. doi: 10.1056/NEJMcpc049016. [DOI] [PubMed] [Google Scholar]
- 6.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 7.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell metabolism. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Science advances. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 10.Chantranupong L, Wolfson RL, Sabatini DM. Nutrient-sensing mechanisms across evolution. Cell. 2015;161:67–83. doi: 10.1016/j.cell.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A, Hall MN. Nutrient sensing and TOR signaling in yeast and mammals. The EMBO journal. 2017 doi: 10.15252/embj.201696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews. Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annual review of pharmacology and toxicology. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 15.Hahn-Windgassen A, et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. The Journal of biological chemistry. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 16.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Molecular cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. This work shows that even quiescent mammalian cells require growth factor stimulation to take up sufficient glucose for maintaining bioenergetics at a level that supports cell survival. [DOI] [PubMed] [Google Scholar]
- 17.Cong LN, et al. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Molecular endocrinology (Baltimore, Md.) 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 18.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. The Journal of biological chemistry. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 19.Rathmell JC, et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Molecular and cellular biology. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barthel A, et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. The Journal of biological chemistry. 1999;274:20281–20286. doi: 10.1074/jbc.274.29.20281. [DOI] [PubMed] [Google Scholar]
- 21.Hu H, et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell. 2016;164:433–446. doi: 10.1016/j.cell.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes & development. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. The Journal of biological chemistry. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 24.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science (New York, N.Y.) 1987;235:1492–1495. doi: 10.1126/science.3103217. This paper reports the first link between oncogenic Ras and Src variants and increased glucose transporter expression, providing a molecular association between cellular transformation and enhanced glucose uptake. [DOI] [PubMed] [Google Scholar]
- 25.Gaglio D, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Molecular systems biology. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. This paper documents metabolic changes that occur during cancer development, demonstrating how a driver oncogene can globally reprogram cellular metabolism during tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makinoshima H, et al. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. The Journal of biological chemistry. 2014;289:20813–20823. doi: 10.1074/jbc.M114.575464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warburg O, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Zeitschr. 1924;152(1924):309–344. [Google Scholar]
- 29.Wu N, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Molecular cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbud W, et al. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Archives of biochemistry and biophysics. 2000;380:347–352. doi: 10.1006/abbi.2000.1935. [DOI] [PubMed] [Google Scholar]
- 31.Barnes K, et al. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) Journal of cell science. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- 32.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Lum JJ, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes & development. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seagroves TN, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Molecular and cellular biology. 2001;21:3436–3444. doi: 10.1128/mcb.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. The Journal of biological chemistry. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 36.Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. The Journal of biological chemistry. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- 37.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao P, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmada M, Speil A, Jeyaraj S, Bohmer C, Lang F. The serine/threonine kinases SGK1, 3 and PKB stimulate the amino acid transporter ASCT2. Biochemical and biophysical research communications. 2005;331:272–277. doi: 10.1016/j.bbrc.2005.03.159. [DOI] [PubMed] [Google Scholar]
- 40.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Molecular biology of the cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. This paper shows that Akt promotes survival of mammalian cells in part by maintaining plasma membrane presentation of a variety of nutrient transporters and endocytic receptors of nutrient carriers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews. Molecular cell biology. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 42.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Streicher R, et al. SREBP-1 mediates activation of the low density lipoprotein receptor promoter by insulin and insulin-like growth factor-I. The Journal of biological chemistry. 1996;271:7128–7133. doi: 10.1074/jbc.271.12.7128. [DOI] [PubMed] [Google Scholar]
- 44.Porstmann T, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell metabolism. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neckers LM, Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis RJ, Czech MP. Regulation of transferrin receptor expression at the cell surface by insulin-like growth factors, epidermal growth factor and platelet-derived growth factor. The EMBO journal. 1986;5:653–658. doi: 10.1002/j.1460-2075.1986.tb04263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galvez T, et al. siRNA screen of the human signaling proteome identifies the PtdIns(3,4,5)P3-mTOR signaling pathway as a primary regulator of transferrin uptake. Genome biology. 2007;8:R142. doi: 10.1186/gb-2007-8-7-r142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer discovery. 2011;1:442–456. doi: 10.1158/2159-8290.cd-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: their distribution and possible clinical relevance. Journal of clinical pathology. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloomfield G, Kay RR. Uses and abuses of macropinocytosis. Journal of cell science. 2016;129:2697–2705. doi: 10.1242/jcs.176149. [DOI] [PubMed] [Google Scholar]
- 51.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nature reviews. Molecular cell biology. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haigler HT, McKanna JA, Cohen S. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. The Journal of cell biology. 1979;83:82–90. doi: 10.1083/jcb.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunk U, Schellens J, Westermark B. Influence of epidermal growth factor (EGF) on ruffling activity, pinocytosis and proliferation of cultivated human glia cells. Experimental cell research. 1976;103:295–302. doi: 10.1016/0014-4827(76)90266-4. [DOI] [PubMed] [Google Scholar]
- 54.Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science (New York, N.Y.) 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 55.Amyere M, et al. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Molecular biology of the cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. This study establishes that macropinocytosis allows Ras-transformed cells to use extracellular proteins as an amino acid source, suggesting macropinocytosis induction contributes to the oncogenic properties of Ras mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palm W, et al. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259–270. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamphorst JJ, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer research. 2015;75:544–553. doi: 10.1158/0008-5472.can-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stehle G, et al. Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia. Critical reviews in oncology/hematology. 1997;26:77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 60.Davidson SM, et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nature medicine. 2017;23:235–241. doi: 10.1038/nm.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Vusse GJ. Albumin as fatty acid transporter. Drug metabolism and pharmacokinetics. 2009;24:300–307. doi: 10.2133/dmpk.24.300. [DOI] [PubMed] [Google Scholar]
- 62.Chaudhury C, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. The Journal of experimental medicine. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Current biology : CB. 2001;11:R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 64.Krajcovic M, Krishna S, Akkari L, Joyce JA, Overholtzer M. mTOR regulates phagosome and entotic vacuole fission. Molecular biology of the cell. 2013;24:3736–3745. doi: 10.1091/mbc.E13-07-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Q, et al. Competition between human cells by entosis. Cell research. 2014;24:1299–1310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabinowitz JD, White E. Autophagy and metabolism. Science (New York, N.Y.) 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Degenhardt K, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo JY, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes & development. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang S, et al. Pancreatic cancers require autophagy for tumor growth. Genes & development. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 71.Ganley IG, et al. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. The Journal of biological chemistry. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung CH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Molecular biology of the cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roczniak-Ferguson A, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Science signaling. 2012;5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Settembre C, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. The EMBO journal. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. The Journal of cell biology. 2007;178:93–105. doi: 10.1083/jcb.200703099. This paper is one of the first studies to demonstrate both the importance of glutamine for cell survival and the role of Myc overexpression in causing cellular glutamine addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan J, et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Molecular systems biology. 2013;9:712. doi: 10.1038/msb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mashimo T, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Comerford SA, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincent EE, et al. Mitochondrial Phosphoenolpyruvate Carboxykinase Regulates Metabolic Adaptation and Enables Glucose-Independent Tumor Growth. Molecular cell. 2015;60:195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 84.Sellers K, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. The Journal of clinical investigation. 2015;125:687–698. doi: 10.1172/jci72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marin-Valencia I, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell metabolism. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng T, et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davidson SM, et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell metabolism. 2016;23:517–528. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayers JR, et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science (New York, N.Y.) 2016;353:1161–1165. doi: 10.1126/science.aaf5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tonjes M, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nature medicine. 2013;19:901–908. doi: 10.1038/nm.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hosios AM, et al. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Developmental cell. 2016;36:540–549. doi: 10.1016/j.devcel.2016.02.012. This work provides comprehensive quantitation of how different nutrients contribute to macromolecular synthesis and biomass in proliferating mammalian cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye J, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ye J, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. The EMBO journal. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J, et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Molecular cell. 2014;56:205–218. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos CR, Schulze A. Lipid metabolism in cancer. The FEBS journal. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 96.Bailey JM. LIPID METABOLISM IN CULTURED CELLS. V. COMPARATIVE LIPID NUTRITION IN SERUM AND IN LIPID-FREE CHEMICALLY DEFINED MEDIUM. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 1964;115:747–750. doi: 10.3181/00379727-115-29026. [DOI] [PubMed] [Google Scholar]
- 97.Brown MS, Goldstein JL. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Louie SM, Roberts LS, Mulvihill MM, Luo K, Nomura DK. Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochimica et biophysica acta. 2013;1831:1566–1572. doi: 10.1016/j.bbalip.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young RM, et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes & development. 2013;27:1115–1131. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamphorst JJ, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pascual G, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 102.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 103.Camarda R, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nature medicine. 2016;22:427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Caro P, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tardito S, et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nature cell biology. 2015;17:1556–1568. doi: 10.1038/ncb3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang W, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature cell biology. 2012;14:276–286. doi: 10.1038/ncb2432. This paper gives an early example for metabolic coupling in a tumour microenvironment by demonstrating that leukaemia cells express only low levels of cystine transporters and rely on import of cysteine that is provided by stromal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sousa CM, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muranen T, et al. Starved epithelial cells uptake extracellular matrix for survival. Nature communications. 2017;8:13989. doi: 10.1038/ncomms13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mayers JR, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature medicine. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Das SK, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science (New York, N.Y.) 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 112.Lewis WH. Pinocytosis. Bull. Johns Hopkins Hosp. 1931;49:17–26. [Google Scholar]