Abstract
The ideal neuroprosthetic interface permits high-quality neural recording and stimulation of the nervous system while reliably providing clinical benefits over chronic periods. Although current technologies have made notable strides in this direction, significant improvements must be made to better achieve these design goals and satisfy clinical needs. This article provides an overview of the state of neuroprosthetic interfaces, starting with the design and placement of these interfaces before exploring the stimulation and recording platforms yielded from contemporary research. Finally, we outline emerging research trends in an effort to explore the potential next generation of neuroprosthetic interfaces.
Keywords: neural engineering, brain-machine interface, prosthetic, challenges, recording, stimulation
I. INTRODUCTION
Prosthetic devices have existed for millennia. The first prostheses were solely cosmetic, one of the earliest examples being an artificial Egyptian toe from the fifteenth century BC.1 In the sixteenth century, these devices became more functional when Ambroise Paré engineered an artificial hand actuated by a system of springs and catches.1,2 Prosthetic technology continues to evolve today due to improvements in our fundamental understanding of the body and advancements in biomedical engineering.3,4 Pioneering work in the late 1960s by Fetz revealed that, given feedback, monkeys could learn to consciously control the firing rate of their own cortical neurons.5 The next year, Humphrey et al found that recordings from neurons could be used to predict, and thus potentially drive, arm movements.6 Subsequent groundbreaking studies demonstrated just that; in 1999, Chapin et al. showed that rats could control a robot arm’s trajectory via recordings from the motor cortex.7 These early works laid the foundation for the field of neuroprostheses, devices designed to interact with the nervous system and restore function. The subject has long since developed into a multi-disciplinary field to address losses of motility, sensation, and quality and ease of living due to injury or disease. For instance, the first commercially available cochlear implant was developed in 1972 based on early work by Djourno et al. nearly 20 years prior; it remains the most successful clinical neuroprosthesis.8 Moreover, deep brain stimulation (DBS) has been a popular option for treating Parkinson’s disease for nearly 30 years.8,9 Perhaps the most technologically advanced upper-limb prosthesis, the DEKA arm, boasts numerous degrees of freedom (DoF) and a control scheme that allows for simultaneous coordination of multiple joints.10–12 Similarly, the modular prosthetic limb developed in the Johns Hopkins’ Applied Physics Lab has 26 total DoF and a distributed processor network for real-time motor coordination. Mechanically, these artificial limbs behave with comparable dexterity to their human counterparts.10,13 However, many of these technological achievements have not transitioned well to clinical deployment. Upper-limb prosthesis rejection rates average 23–26% for adults, with varying rejection rates for different subtypes.10,14,15 In fact, users generally prefer less advanced devices, attributing their choices to the low practicality and durability, unwieldiness, and lack of nonvisual or tactile feedback in more advanced prostheses.10,16 Given these trends, prosthesis adoption does not appear driven by mechanical design or dexterity. Rather, the limiting factor in current prosthetic systems is the quality of the neuroprosthetic interface (NI), defined here as any platform designed to facilitate communication between the nervous system and a prosthetic device.
Neuroprosthetic interfaces can record from and/or stimulate select areas of the nervous system, traditionally via electrodes placed near the cells or tissues of interest.3,4,17 When localized to the central nervous system (i.e., the brain and spinal cord), such devices are referred to as brain–machine interfaces (BMIs) or brain-computer interfaces (BCIs).3,18,19 For our purposes, “neuroprosthetic interface” encompasses both BMIs and interfaces outside the central nervous system (CNS), for instance, with the peripheral nervous system (PNS). An ideal interface would allow the user to directly control the output behavior of the prosthesis (e.g., the movement of an artificial arm) while receiving relevant sensory information from the device. This essentially recreates the control-feedback loop found in an intact limb, where the nervous system propagates information via electrical signals, or action potentials, throughout the body.17,20,21 These signals provide output for controlled muscle contractions and input/feedback from sensory organs (e.g., texture, temperature, position), creating a bidirectional pathway through which we explore and manipulate our environment. It is generally accepted that introducing this input–output behavior in prosthetic devices would yield better, more natural integration with their users, thereby improving their practicality, adoption rates, and positive human impact.22,23
Broadly speaking, direct communication with the nervous system is one of the primary goals in neuroengineering to date.17,24 Recording neural activity can reveal how the nervous system encodes information, as well as user intent such as motor commands.19,25 Similarly, electrical stimulation (generally via current injection) can induce different neuronal behaviors depending on the type and intensity of stimulation, as well as the intended target. Stimulation of the subthalamic nucleus, for example, can reduce the tremors characteristic of Parkinson’s disease.21,26–28 Both recording and stimulation of the nervous system have clear clinical benefits; however, the complexity of the nervous system has made a clear best standard for neuroprosthetic interfaces difficult to determine. 18,29 The incomplete understanding surrounding nervous system repair and neural modulation exacerbates these difficulties.17,28 The effects of electrical stimulation (and different patterns thereof) on neuronal activity are still undergoing extensive study.30,31 Even more “well-established” strategies experience hurdles; for instance, although the incidence of hardware issues in DBS is low, a decline in verbal fluency is a common post-surgical complication.32 Ongoing research efforts have become more interdisciplinary as the design challenges for NIs become better defined. Among them are biocompatibility, decoding neural information, spatial and temporal precision of recording and stimulation, signal fidelity, and chronic efficacy in vivo.3,33–35 However, as clinicians, engineers, and researchers address these hurdles, NIs have been applied to controlling wheelchairs and computer programs, moving artificial limbs, and restoring basic sensory feedback.17,36–43 Future NIs may even serve to improve and restore memory, with current research showing promise in rats and nonhuman primates.44,45
In this review, we present a broad overview of the development of neuroprosthetic interfaces. We begin by outlining the basic design constraints for a neuroprosthetic interface, as well as important considerations for NI placement (i.e., within the CNS or PNS). We then outline current NI strategies from the literature, emphasizing achievements and ongoing challenges in the field. These strategies range from noninvasive recording techniques to electrodes implanted directly within the brain, and each type of NI has advantages and disadvantages. These studies preface a discussion of promising areas of future development, in which researchers are exploring novel ways (e.g., ultrasound and infrared light) to stimulate and monitor the nervous system.
II. NI DESIGN OBJECTIVES
The specific application of an NI dictates its physical and functional parameters and introduces specific design constraints. In general, however, all platforms share the same goal: robust, clinically viable communication with the nervous system. This goal underlies the rationale for three universal design objectives that applies to all NIs: (1) biocompatibility, (2) high-resolution/selectivity, and (3) long-term reliability/stability. While the aesthetic of the interface (e.g., its appearance and size) does not apply as a design objective in the context of the goal described, it is worth mentioning as an important factor in users’ comfort with and ultimate acceptance of a neuroprosthesis.
First, NIs must be biocompatible; that is, they must minimally disrupt the function of otherwise healthy tissue. This applies not only to their physical properties (e.g., material composition and stiffness), but any effects of their function. Stimulating electrodes, for example, must deliver safe amounts of current into the surrounding tissue without inducing irreversible redox reactions, which can damage both the electrode and the host.21 Biocompatibility must be considered in the context of the target tissue because the response to a material is often dependent upon where it is implanted (e.g., brain versus peripherally). These safety thresholds are dependent on not only the electrode type and size but also the stimulation parameters (e.g., waveform, duty cycle, pulse frequency, and width); thus, they may well vary across different NIs, as indicated in a more in-depth review of stimulation thresholds by Cogan et al.46 Equally important is the method of delivery into the body, if the device is to be implanted. Both short-term trauma (as seen in needle deliveries) and the longer-term foreign body response can adversely affect NI function.35 A common problem in implantable, electrode-bearing NIs is encapsulation of the electrodes in fibrous tissue, which ultimately compromises the ability of the device to stimulate and record as impedances and other biophysical properties change.33–35 Thus, both the interface and its delivery method must be designed with tissue reactivity in mind. Moreover, this biocompatibility should persist for a lifetime. Although this goal has yet to be fully realized, research achievements to date suggest it is far from infeasible; a 2010 study reported 7 years of recording from the monkey cortex with a microwire array. Visual prostheses have been implanted for more than 10 years in humans, and electrodes to correct foot-drop in patients with hemiplegia have been implanted for up to 12 years.47–49 These and other longitudinal studies continue to provide invaluable insights into the longterm biocompatibility of NIs,
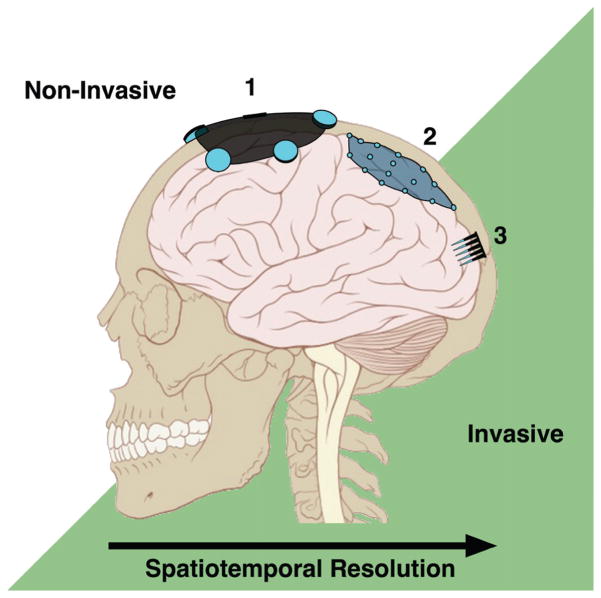
Second, the interface should be designed such that (1) interference from other tissues (either electrically active or non-electrically active) is minimized and (2) the target area is close enough for high-resolution spatial and temporal recording, and/or selective stimulation. The interface is dependent on both the placement of the NI and its ability to isolate the target signals from surrounding electrical activity. In many cases, smaller microelectrodes may be advantageous, with greater flexibility in placement and less contact with non-target cells. The precision with which an NI can stimulate or record from the target cells is integral to its functionality. Cochlear implants, for example, permit many individuals to perceive speech but not music; literature suggests that an increased number of active electrodes may aid in better melody recognition.50–52 Moreover, there is often a tradeoff between recording/stimulation selectivity (i.e., the electrode proximity to the tissue) and the foreign body response to more invasive NIs (Fig. 1).
FIG. 1.
Signal resolution and NI placement. In general, the more invasive the NI the higher the accessible spatial and temporal resolution. Scalp-mounted EEG electrodes (1) and ECoG electrodes under the dura (2) record gross cortical oscillations, while intracortical electrode arrays (3) can detect single-cell activity. (Adapted from Lynch & Jaffe, 2006.)
Finally, an ideal NI must exhibit consistent behavior once implanted, both physically and functionally. For instance, physically, NIs may experience micro-motion relative to the brain that could exacerbate a foreign body response and increase the distance between the electrode and the target neurons. Moreover, implanted NIs may suffer from wire breakage, delamination, and insulation breakdown over long periods of time in vivo (i.e., “wear and tear” issues).34,53–55 These NIs can affect the consistency of recordings, meaning more invasive NIs must often be recalibrated regularly. From a functional standpoint, consistency often pertains to the impedance of the electrodes. Related to this finding, as noted above, astrocytes and macrophages surround NI electrodes post-implantation and increase the distance between the active recording/stimulation site and the target neurons.56 This process may effectively increase impedance or change the phase and frequency response thereof, dropping the signal-to-noise ratio (impairing or eliminating recording capabilities) while increasing the amount of current needed to effectively stimulate the target. Even optogenetic stimulation (addressed in more detail below) may suffer, as gliosis may obstruct the transmission of light into tissue.33–35 Moreover, the foreign-body response may result in a decreased neuronal density in the vicinity of the active region (potentially due to neuronal degeneration), further inhibiting NI function over time.33,34,57,58 Interestingly, the presence of microglia has not been directly correlated with electrode performance, although other biotic factors (intraparenchymal bleeding, neurotoxic proteins) are currently being explored as potential contributors to chronic degradation in NI performance.54,55,59 Hence, chronic NI function is directly tied to its biocompatibility. Arguably, maintaining reliable performance for the long term is the most important consideration for NIs and the most challenging obstacle to date.
III. NI PLACEMENT: CENTRAL OR PERIPHERAL?
Typically, NIs can be categorized by their location in the body; that is, whether they interact with the CNS (BMIs, as described above; spinal cord) or the PNS. We present a brief discussion of localization in the CNS versus PNS before exploring each in more depth.
A. CNS Versus PNS Placement
In the brain, recording BMIs offer levels of spatial resolution down to multi- or single-neuron (or unit) recordings.19,60 Several groups have used these recordings of neural activity to drive motor commands. In 2006, Hochberg et al. reported motor cortex recordings for up to 11 months using an implanted microelectrode array in human spinal cord injury (SCI) patients, who could move computer cursors and robotic arms.61 Since then, at least one patient with locked-in syndrome and two with amyotrophic lateral sclerosis (ALS) have been able to communicate using an intracortical NI linked to a computer.62,63 Similarly, at least one human implanted with a microelectrode array has demonstrated cursor control for 1,000 days, nearly 3 years post-implantation.64 Control signals recorded from the brain have been used to drive direct stimulation of intact, but paralyzed muscle groups in both nonhuman primates and, more recently, a quadriplegic human.65,66 The high spatial resolution of these BMIs requires the implantation of electrodes in the otherwise non-injured brain. However, electrode implantation in the CNS may result in inflammation and fibrous encapsulation of the device, often leading to signal instability as previously outlined.33,35 To decode commands from neural recordings, BMI software implements a learning algorithm to decode the user’s intent from specific patterns of neural activity. 19 With extensive training and calibration, users can learn to modulate these patterns and control a target device. Such systems often require regular tuning and recalibration to account for the signal instability often encountered by more invasive BMIs, limiting utility and practicality.
As the end point of the brain’s connection to the environment, the PNS presents an opportunity to leverage the processing power of the CNS rather than straining to decipher it. For instance, PNS interfaces present the benefit of leveraging specialized brain networks and, in particular, spinal cord feedback loops to control and fine-tune movement. Using this approach, motor commands can be recorded directly from motor neurons/axons or the muscles they innervate. Myoelectric prostheses, for example, detect the electrical activity from residual muscles to drive motor commands. As the electrical signals from muscle have an amplitude several orders of magnitude larger than those of nerves, they can be detected through the skin (i.e., noninvasively), and are easier to record and decode. For these reasons, myoelectric prostheses are the most widely available advanced prosthetic system, although they present their own issues. Namely, they offer a limited number of command channels (open and close), and experience signal inconsistency due to skin movement and sweat interfering with the surface electrodes over time.16 Implantable myoelectric sensors (IMES) are a potential alternative, eliminating the aforementioned issues to provide more consistent signal quality and greater number of control channels.67–69 Early clinical trials show promise for IMES, with subjects performing more complex tasks with IMES-driven prostheses. 67 Similarly, afferent nerve fibers can be stimulated to deliver sensory information to the host, with stimulation of different neuronal subtypes resulting in different perceptions (e.g., pressure, texture, and proprioception).70 Targeted muscle reinnervation (TMR) reroutes nerves to distinct muscle groups, leveraging the larger signal amplitudes of muscles to decode motor commands from surface recordings. Interestingly, multiple reports suggest that a similar approach (targeted sensory innervation) may provide sensory restoration by redirecting nerves to sensory nerve fascicles to innervate predefined areas of skin; post-innervation, the skin above the can be stimulated, recreating a sense of touch for the otherwise missing limb.71–74
NIs in the PNS may thus be subjected to less of the computational burden of signal interpretation than those in the CNS. However, the PNS presents its own challenges. The computational benefit of recording from the PNS (namely, that the signals come “processed” from the CNS for optimal movement control) is also its drawback: the signal may be stereotyped and require time and training to map to other types of control. Also, interference from surrounding musculature when making neural recordings, as well as signal from muscle being much coarser that that from single units (e.g., neurons or axons), limits the complexity of the command signal. Moreover, in patients in whom the pathway from the brain to the peripheral nerve is compromised (e.g., SCI or ALS patients), the PNS is not a viable location for the interface, although this can be circumvented by stimulating the PNS through commands recorded remotely from the brain.66
Finally, peripheral nerves must innervate a living target to survive and maintain useful activity. Following peripheral nerve injury, the distal axon segments undergo Wallerian degeneration and distal support cells provide a supportive environment for axon regeneration and reinnervation (see the 2011 review article by Pfister et al.).75 However, this environment is temporary, and numerous conditions (among them the size of the injury, time needed for repair, and the condition of the proximal nerve segment) often prevent reinnervation of the distal segment before it degrades.76,77 NIs that present a living target may better take advantage of placement in the PNS, conceptually similar to TMR noted above. Moreover, early stages of tissue-engineered “biohybrid” platforms are discussed later in the review.
There is no single best option for NI placement, as each comes with its own unique benefits and challenges. Often the particular deficit and/or desired efficacy of a device determines the location. However, the needs and condition of the patient often inform or provide constraints regarding where an NI would function best. Even given efficacy and patient-specific parameters, there may be more than one suitable site for NI implantation. With design objectives and placement options in mind, we now turn toward an overview of different NI strategies, as well as notable accomplishments and common issues in the field.
IV. CURRENT INTERFACES IN THE CNS
Generally speaking, BMIs extract and relay information from the brain or spinal cord to some output that “replaces, restores…or improves natural CNS output.”19 For instance, when disease and/or trauma compromise neuromuscular pathways, NI systems intercept neural activity directly from the sensorimotor cortex, bypassing the faulty pathways entirely.25 Individuals with a diminished capacity for voluntary movement (as seen in ALS, SCI, and similar conditions) can thus use BMIs to communicate and interact with their environment through an external device. Such subjects have controlled computer cursors in two-dimensional and three-dimensional spaces, have used computer applications, have browsed the Internet, and have moved motorized wheelchairs.40,43,78–83 BMIs also see use in rehabilitation, with patients controlling robotic orthotics and muscle stimulators to reinforce or support movement.84–87 Electrical stimulation of the spinal cord is also being explored to restore movement and coordination to muscles paralyzed from SCI.88–90 Notably, Harkema et al. have demonstrated that epidural stimulation of the spinal cord in SCI patients can recruit local neural circuitry and give them the ability to stand with minimal support; at least one study participant regained some degree of conscious control over lower limb movement and bladder function.91,92
The most common BMIs can be described on a spectrum of invasiveness: generally, the more invasive the system, the higher the signal resolution (Fig. 1). The three most prominent recording methods in the CNS are scalp electroencephalography (EEG), electrocorticography (ECoG), which records from the cortical surface (also called intracranial EEG, or iEEG), and intracortical and depth electrodes. ECoG, intracortical electrodes, and depth electrodes also provide opportunities for stimulation and have led to the early development of bidirectional BMIs, that is, systems capable of both recording and stimulation to “close the loop” with direct sensory stimulation, rather than auditory or visual feedback. There is also a growing body of literature on transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), non-invasive techniques that can increase or decrease activity in specific cortical regions.93 In the context of NIs, TMS and tDCS have been applied to improve motor learning, for example, when calibrating control schemes for a BMI.93–95
A. EEG
Originally developed in 1929 by Hans Berger, EEG uses electrodes placed on the scalp to detect oscillations from neural activity summed over different regions of the brain.19,25 Researchers discovered that participants could learn to consciously affect these oscillations once provided with some form of feedback, usually visual or auditory cues.96,97 This biofeedback, combined with EEG’s noninvasiveness and simplicity, has made EEG the most common BMI for clinical use.41 Current EEG arrays are designed to monitor specific types and frequencies of brain waves. With training, these waves can be used as command signals.4,41,80,96,98,99 One set of examples includes event-related brain potentials (ERPs), which arise due to specific, task-dependent stimuli. The P300, for example, is an ERP that occurs when the user is presented with the item they were focusing on among other related items. For example, a P300-BCI for communication may present the user with different letters until the P300 is detected, indicating the user has seen the desired letter, and build up words sequentially.98 Among their many applications, EEG-BMIs have been used to control computer programs, write text, move a computer cursor in three dimensions, direct powered wheelchairs, and trigger functional electrical stimulation of otherwise paralyzed muscles.4,100–102
Clinically, the noninvasiveness of EEG is beneficial, but it invariably limits the bandwidth of accessible brain activity. Although increased understanding of EEG signals and analysis techniques may somewhat reduce these limits, current spatial resolution is restricted to averages of neural activity across relatively large areas of the brain. Historically, the maximum transfer rate for EEG-BMI was believed to be approximately 2 words per minute, although Chen et. al. recently demonstrated a significant improvement to approximately 12 words per minute.13,103 Other biopotentials arising from muscle activity (EMG), eye movement (EOG), and the filtering effect of the layers of tissue between the brain and the scalp electrodes can also confound the target signal, which is on the scale of microvolts.4,101,104,105 Many EEG-BMIs require repeated calibration due to signal variation within and across experiments, although research into adaptive classification algorithms to address these issues is ongoing.106,107 Overall, EEG remains a powerful and evolving tool in rehabilitation and assistive technology.4,13,36,40,41,78,84,86,101,108–110
B. ECoG/iEEG
For ECoG recordings, electrodes (usually embedded in a flexible grid) are placed underneath the skull, either above the dura mater (epidural) or underneath it in direct contact with the cortical surface (Fig. 2).41,97,111 Compared with EEG, ECoG offers higher spatial resolution, greater spectral frequency, and a generally improved signal-to-noise ratio.112–114 These advantages have been leveraged in numerous studies demonstrating the potential of ECoG as a BMI with higher resolution than EEG and less of the immune response than seen by intracortical electrodes.115 ECoG is most often seen in seizure intervention. During surgery, subdural arrays are used to record seizure activity, map the cortex, and target seizure foci before therapeutic resection; they may also be used to study the effects of DBS.116,117 Achievements in ECoG-BMIs include computer cursor control in one, two, and three dimensions, using recordings of motor imagery to select different characters, and more recently, real-time control of a prosthetic limb with a movement prediction accuracy of 69.2 percent.113,114,118,119 In 2013, the Fetz group reported successfully using an ECoG grid to elicit sensations by stimulating the somatosensory cortex in two human patients.120 Although not as spatially specific as intracortical electrodes, differing stimulation frequencies and amplitudes were perceived as changes in the sensation’s intensity.120
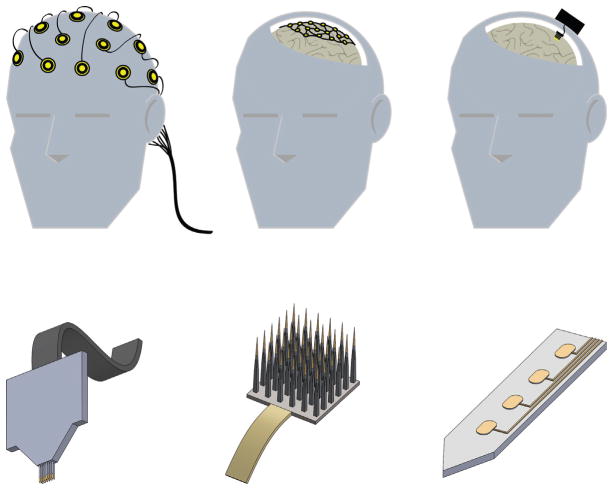
FIG. 2.
Neuroprosthetic interfaces in the CNS. Top: Placement and invasiveness for prominent BMI approaches. Current interfaces interact with the CNS at the scalp (left), the brain surface (middle), or from within the brain (right). Bottom: Examples of intracortical/penetrating neural electrodes. Intracortical NIs may take the form of microwire assemblies (left), arrays (middle), or flat shanks with multiple active recording/stimulation sites (right; center ellipses portray active sites).
The benefits of ECoG stem from its proximity to the brain compared to scalp EEG: direct cortical contact eliminates the signal attenuation and filtering that result from recording through the skull. These advantages come at the cost of increased invasiveness, as ECoG requires surgery to expose the brain and position the electrodes.19,97,114 As such, clinical studies have generally been limited to epilepsy patients already undergoing surgery for seizure intervention. 115 Clinical studies have shown that, with control schemes adopted from EEG, ECoG-BMIs require shorter learning periods while delivering comparable accuracy.19,114 However, due to the above restrictions, these studies have historically been limited to short-term trials. The recently FDA-approved Neuropace system, which uses subdural electrodes to detect seizure activity, may provide opportunities for longer studies.121 Whether these benefits persist over time remains to be seen, although a 2010 study by Chao et al. showed near-constant predictive accuracy over multiple months in monkeys.122 Historically, ECoG has been considered “middle ground” on the spectrum of BMIs. The electrode placement allows recording of finer-scale activity than EEG without penetrating the blood-brain barrier, potentially preserving long-term signal quality.114,115,123 Active efforts to improve ECoG platforms include more flexible arrays, electrode miniaturization, and low-impedance electrode coatings—all designed to improve biocompatibility, signal resolution, and selectivity. 124 While these improvements are beneficial for NIs in general, craniotomies must be performed for the positioning of ECoG arrays on the brain. As such, human ECoG trials are primarily acute, and the ratio of risk to benefit for ECoG use may well vary depending on the patient. While the behavior of ECoG arrays over time in humans has not been fully elucidated, longer-term animal studies with subdural electrodes and the development of wireless, fully implantable ECoG devices show promise for ECoG as applied to NIs.115,122,125–128 Significant progress must also be made in ECoG signal acquisition and analysis to account for the amplitude attenuation at higher frequencies (i.e., gamma bands, which can exceed 100 Hz) and to better capture signals from subcortical areas. These higher frequencies have been strongly correlated with critical cognitive and motor processes, and although still largely untapped, they may prove valuable in next-generation BCI systems.114 In short, more detailed risk–benefit analyses must be conducted before accurate comparisons can be made.
C. Intracortical and Depth Electrodes
Electrodes implanted within the cortex offer the highest spatial and temporal resolution recording, and they are capable of recording single units (the action potentials of individual neurons) as well as field potentials (activity across several neurons).97 Current intracortical electrodes include flat shanks and shank arrays with multiple electrically active sites, platform arrays with several fixed electrodes (e.g., the well-known Utah electrode array and its FDA-approved iteration in BrainGate trials), microwire assemblies, and DBS electrodes, which are implanted in subcortical structures (Fig. 1).26,34,97,104,129 The high resolution and signal-to-noise ratio (SNR) of intracortical NIs compared with EEG and ECoG makes them ideal for the real-time detection required for smooth prosthesis control.130 Moreover, strategic positioning of the active sites offers selective recording from different neuronal populations. Simultaneous recordings from cortical and subcortical regions have been performed in rodents, primates, and humans.59 Beyond recording, intracortical and depth electrodes can be placed with high spatial specificity, making them ideal for directly stimulating specific areas of the brain. CNS stimulation can be applied to suppress epileptic seizures, mitigate symptoms of Parkinson’s disease (as seen with DBS), and this procedure may be beneficial in treating certain psychiatric conditions.26,28,35,131 Potential also exists for restoring sensory function using this technique. Several studies in non-human primates show that sensory information can be delivered via intracortical stimulation, often as part of a closed-loop BMI.132–134 Perhaps one of the greatest examples of sensory restoration is that of cochlear implants, which are designed to stimulate the auditory nerve tonotopically. Worldwide, more than 100,000 people with hearing loss use cochlear implants to compensate for their impairments, and they can even hear and understand speech.135 Similarly, retinal stimulation can elicit visual percepts in patients with vision loss.136,137 Ongoing development of electrodes and stimulation parameters has provided basic letter recognition, orientation, and improved visual acuity in blind patients.138–140 The first commercially approved retinal prosthesis, Argus II, has been shown to improve hand–eye coordination, mobility, and letter recognition, although the quality of these benefits varies as the stimulation complexity increases.137,138,141
The high spatiotemporal resolution of intracortical and depth electrodes is achieved through exposing and/or penetrating the brain, though these are significantly more invasive processes than those required for EEG or even ECoG. Moreover, the quality of intracortical recordings tends to degrade over long periods of time. While Hochberg et al. reported recordings from the motor cortex over 1 year postimplantation, they reported decreased signal amplitude and channel count.61,97 Even when chronic recordings are attained, extremely labor-intensive recalibration has been necessary due to signal/spike drift from day to day. Studies on single-unit stability show that individual neurons may vary their firing patterns significantly across recordings; one such study reporting only 39% of the units remaining stable after 15 days.142,143 Thus, arguably the most significant design problem for intracortical electrodes is the stability of the interface over both acute and chronic time scales.33,34 This stability is hindered by a host of factors acting on a range of time scales. In the short term, the initial mechanical trauma of electrode insertion and the presence of a foreign body lead to scar formation surrounding the implant, inflammation, and a decrease in neuron density surrounding the electrode.33,53 Over time, implanted electrodes may suffer from mechanical failures (e.g., broken connectors, insulation fracture and delamination). 54,55,59 There may also be so-called micro-motion effects due to differences in mechanical properties between electrodes and brain tissue that become relevant due to head motion and/or pulsatile motion due to the patient’s heartbeat. Electrode arrays fixed to the skull can also experience motion relative to the brain that may shift them away from optimal recording locations.34,53,104,144 All of these factors can consequently affect signal quality during chronic implantation. However, the exact effects of the immune response on signal quality are ambiguous. Studies report high variability in both immune response and device functionality across and within several types of intracortical recording electrodes.34,35 Strategies to overcome these biocompatibility and biostability challenges include finding insertion speeds to minimize initial trauma, delivering immunosuppressive and anti-inflammatory agents, incorporating bioactive molecules to encourage cell adhesion, and constructing electrodes from softer materials.34,145–151 Additionally, downstream correction in silico may account for signal drift through auto-recalibration, as suggested by Bishop in 2014, or offset correction algorithms, as demonstrated by Homer et al.152,153 Still, no consensus has been reached regarding the kind of cortical activity that is best for intracortical recordings. The benefits of capturing individual spikes versus the summed activity of several neurons (local field potentials, or LFPs) are still being compared. LFPs may be more stable, and they have been shown to yield similar performance to single-unit activity in predicting movement.154,155 Finally, current BMIs are limited by the number of electrodes and active sites that can be incorporated onto one device, which in turn limits the number of distinct neural signals that may be desired for multidimensional input/output. The Argus II described above has 60 electrodes and covers 11° by 19° in the visual field, compared to the full field of human vision, which is roughly 180° by 135°, including peripheral vision.141,156,157 This limitation in NI bandwidth is a key target of the DARPA Neural Engineering System Design (NESD) initiative, which aims to record from and stimulate 1,000,000 and 100,000 neurons, respectively.158
D. Bidirectional Systems
Systems capable of simultaneous (or near-simultaneous) electrical stimulation and recording of neural activity are also being explored. Based on temporal and spatial proximity of the stimulation and recording region(s), this approach introduces the additional challenge of the electrical stimulation itself interfering with the quality of the recording; however, there have been several notable successes in bidirectional neural interfaces. In 2011, O’Doherty et al. implanted microwires into the primary motor and somatosensory cortices of monkeys. By stimulation of the latter cortex, they were able to provide tactile feedback as the monkeys directed a computer cursor (using motor cortex recordings) to explore different objects.134 Alessandro Vato et al. demonstrated bidirectional BMI in rodents; early validation showed it capable of stimulating the brain upon detection of some target activity.159 Vato et al. also demonstrated a bidirectional BMI algorithm that maps the state of an external device (e.g., position) into a series of electrical impulses while transforming recordings from the motor cortex into a tunable output force. Such a paradigm could conceivably be applied toward the operation of an artificial limb. The algorithm was tested against simulations of cortical neural data, proving robust results even when the data were artificially degraded to mimic declining BMI sensitivity.160 Similarly, Dr. Fetz’s research group at the University of Washington has conducted extensive research into linked microrecording-to-microstimulation systems.161–163 Here, the Neurochip and its successor, the Neurochip 2, were designed to deliver intracortical stimulation upon detection of certain neural activity. Both platforms have been implanted in freely moving monkeys. As of 2011, the Neurochip 2 was able to record (via ECoG) and stimulate the motor cortex in a closed loop.163
Additionally, Brian Litt and other researchers have reported on extensive work in developing iEEG systems for seizure intervention.164,165 One such device, the Neuropace RNS System, uses iEEG to record cortical activity and stimulates targeted regions to suppress epileptic seizures upon the detection of abnormal activity. Through careful feature selection and algorithm design and training on large datasets, these platforms can be tuned to detect precursor seizure activity specific to each participant. The RNS is FDA approved, although studies comparing the behavior of closed-loop versus open-loop stimulation devices (which simply stimulate target regions regardless of cortical activity) are ongoing.164–166
E. Challenges in the CNS
There is a clear tradeoff between spatiotemporal resolution and invasiveness for CNS interfaces (see Figs. 1 and 2). Thus far, the output capabilities of noninvasive BMIs are limited by their resolution and the ability to account for variation in the target signal features; the latter requires extensive training to learn alternative, potentially non-intuitive control schemes.83,106 Variability in performance accuracy across BMIs has been well documented; sources range from user fatigue and concentration to the type and severity of the user’s disability.4,98,167 Although EEG-BCIs have been used for gross motor commands, the narrow bandwidth and latency currently prevent real-time, fine movement control for prosthetic limbs.4 While the tailored electrode placement of invasive BMIs allows for higher-resolution recording, the highly variable in vivo stability and long-term behavior of the current widely used technologies in the field still pose substantial developmental hurdles to their clinical adoption.33,35
Overall, intracortical electrodes will require significant improvements and further optimization before widespread use ensues. However, early studies in humans have shown that subjects implanted with intracortical arrays can operate computer applications and prosthetic devices, such as the DEKA Arm (reported by Hochberg et al. in 2012).61,168 Additionally, the bandwidth of ECoG allows for the detection of key neural information related to motor intent and certain disorders without directly penetrating the blood–brain barrier. Moreover, advances in flexible electronics (e.g., thin-film electrodes) have yielded ECoG arrays that conform to the brain without compressing the tissue.125,169–171 However, implanting these arrays requires large craniotomies for grid placement; thus, human trials with ECoG arrays have generally been limited to acute studies for patients already undergoing treatments (primarily for epilepsy), with a few recent trials in other patient populations.119,172,173 More chronic studies have been performed in nonhuman primates. A 2011 study in rhesus macaques showed stable power spectra and evoked potentials over 8 weeks.125 Thus, while ECoG-based BCIs may in time provide tangible benefits for NI users, the effects of chronic implantation on both interface function and the host brain must be further characterized.
V. CURRENT INTERFACES IN THE PNS
Peripheral nervous system interfaces (PNSIs) target the peripheral nerves located throughout the body for electrical stimulation and recording.71,174 Like BMIs, peripheral interface research aims to recover bodily functions that are lost when trauma or disease-related damage occurs to the nerves supplying the area and/or the end targets themselves. Although peripheral nerves exhibit regenerative abilities when transected, this process is slow, and the target area may atrophy in the interim.174 By recording the activity from the proximal nerve stumps, peripheral interfaces can provide motor commands for a neuroprosthesis; similarly, information can be translated into electrical impulses to stimulate the nerve, replicating tactile and proprioceptive sensation.71,74,175–177 Challenges in the PNS overlap significantly with those in the CNS, namely, the tradeoff between signal selectivity and tissue disruption, although the fascicular organization of peripheral nerves can be leveraged here. Targeted muscle reinnervation, originally developed by Todd Kuiken, involves rerouting nerves to isolated portions of healthy muscle and using the resultant EMG signals to control prostheses.72,178 While not directly contacting the PNS, TMR leverages the larger signal amplitude of EMGs, the ease of accessing muscle relative to the nervous system, and preexisting methods of recording muscle activity.71,178 These benefits are crucial to the success of TMR, and several research efforts have demonstrated that patients undergoing TMR exhibit real-time multi-joint control of prosthetic limbs and perform daily tasks more naturally.72,178 Ongoing clinical trials in TMR will likely lead to further improvements. Another approach is the regenerative peripheral nerve interface (RPNI), developed by Paul Cederna et al., wherein nerve fascicles are implanted into muscle grafts, each with its own electrode.179 Although it is still being optimized, RPNI has proven viable for more than a year in a rodent model, with muscle reinnervation and consistently detectable muscle activity during implantation. 179–182
Functional electrical stimulation (FES) is another application wherein peripheral nerves are stimulated; clinical trials have proven FES useful in correcting foot-drop, regulating respiration, and restoring grasp for tetraplegics.71,100,183 Stimulation of the PNS also has applications in regulating bladder dysfunction, incontinence, and has even been implicated in stroke protection in animal models and immune system modulation in at least one human study.184–188 The NIH’s SPARC initiative (Stimulating Peripheral Activity to Relieve Conditions) is focused on supporting these and similar research efforts in “peripheral neuromodulation,” i.e., influencing organ function via stimulation of the PNS. As with BMIs, peripheral interfaces can be broadly differentiated by their invasiveness (Fig. 3).
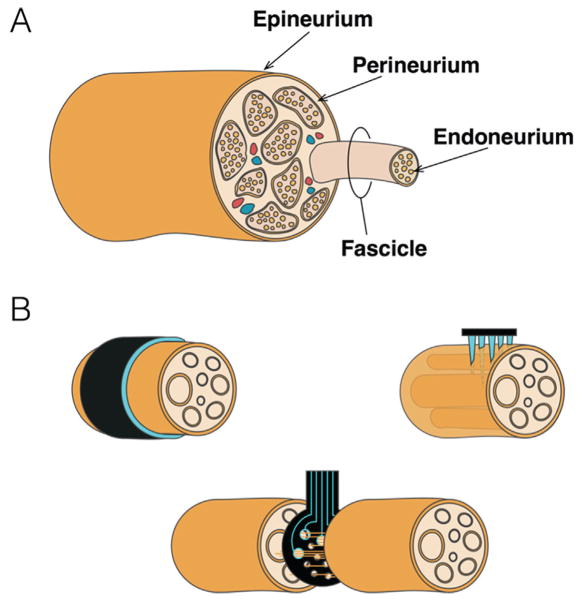
FIG. 3.
NIs in the Peripheral Nerve. (A) General overview of peripheral nerve anatomy. Individual axons are bundled in endoneurium and in turn, into discrete fascicles, supplied via blood vessels embedded in the perineurium. Fascicles in turn are bundled and protected by the dense connective tissue of the epineurium. (B) Peripheral interface electrodes range from extraneural (upper left), to penetrating/intraneural (upper right), to regenerative (bottom).
A. Extraneural
Extraneural electrodes reside outside the epineurium (Fig. 3) and are the least invasive of the PNSIs. They take a number of forms, although they generally consist of a biocompatible insulator such as silicone containing at least two electrical contacts.71,189 Some are sutured directly to the epineurium, while others are designed to wrap around or enclose the nerve. Cuff electrodes, the most widely investigated peripheral interface electrode, are favored for their ease of implantation and fascicular selectivity. Studies have demonstrated that certain cuff electrode designs can record and stimulate chronically, and they have already been used in relieving pain, managing incontinence via stimulation, and controlling hand-grasp prostheses, with some studies lasting several months.190–193 Using multiple cuffs or several contacts within a cuff enables stimulation of different nerve fascicles. The somatotopic organization of axons within these fascicles allows for the selective, graded activation of distinct muscle groups in the upper limb.3,194–198 A prevalent application of extraneural NIs is vagus nerve stimulation (VNS), an invaluable technique that has been clinically approved to treat symptoms of drug-resistant epilepsy and, more recently, chronic depression.199,200 The therapeutic potential for VNS in Alzheimer’s disease, migraines, heart disease, and other psychiatric conditions is also being explored.200 Similarly, cuff electrodes have been implanted in human amputees, providing consistent tactile sensations throughout the “phantom” limb for more than a year, yielding measured improvements in dexterity with their prostheses.201 Flat-interface nerve electrodes (FINEs) increase the surface area of the target nerve and consequent selectivity by flattening it; the amount of damage was force dependent with no detectable damage below a certain threshold. Moreover, the flattening of the nerve increases the number of accessible fascicles near the epineurium.71,202,203 Computational models of the FINE strongly suggest its potential for highly selective stimulation in FES applications; a theory since borne out by numerous animal and clinical trials.204–206
B. Intraneural
Intraneural electrodes penetrate the epineurium and generally the perineurium as well, contacting the target fascicles directly. Electrodes have been designed for both longitudinal and transverse insertion into the nerve, although longitudinal intrafascicular electrodes (LIFEs) are more common and have been implanted in human subjects.71,176,207 The Utah array, originally developed for cortical applications, has also been adapted for experimental use to record from and stimulate peripheral nerves.208 They experience higher selectivity and signal-to-noise ratios, as well as a lower stimulus current threshold (in general), as there is less leakage into neighboring tissues.209 The selectivity is such that specific fascicles and portions of single fascicles can be independently stimulated.210 Intrafascicular electrodes have been shown to record action potentials for months at a time in cats with minimal tissue reactivity, although due to their needle structure and penetration into the perineurium, they are capable of damaging the nerve and can elicit secondary inflammatory responses.3,208,211,212 Some researchers have sought to mitigate these issues by replacing the metal wires with polymer filaments, which more closely match the stiffness of neural tissue and exhibit limited electrode drift.71,213,214 An animal study has demonstrated that LIFEs can be used as part of a closed-loop system for controlling ankle position.215 Further research and experimentation may allow for similar developments in humans. A feasibility study has already shown that LIFEs can record viable motor command signals and transmit stimuli encoding joint angle and force when implanted in the median nerve.176 Similarly, a researcher volunteered to have a 100-electrode array implanted in his median nerve for approximately 3 months and experienced force feedback from the sensors of a prosthetic hand and controlled it in turn; however, only a fraction of the electrodes remained functional by the end of the study due to wire failure.175 As with intracortical electrodes, the long-term stability of these devices must be further evaluated and improved upon.
C. Regenerative
Regenerative electrodes (commonly called sieve electrodes) are placed across transected nerves, allowing the regenerating nerve to extend axons through the electrode itself. Contacts around some of the holes allow for recording from and stimulation of axons and groups of axons. Several regenerative electrodes also possess guidance tubes for proper placement of the nerve trunk ends.189 Sieve electrodes made from polyimide reduce (but do not eliminate) the compressive damage of more rigid materials like silicon, which can lead to axonopathy.216 Moreover, polyimide electrodes have proven biocompatibility, with no detectable inflammation over 12 months of implantation in a rat study and similar results in others.217,218 Recently, Gore et al. reported successful motor axon growth through a PDMS regenerative electrode in rats, with reinnervation of distal muscle confirmed through recording during locomotion.219 However, a significant drawback with sieve electrodes is that the target nerve must be transected, which may cause some degree of cell death in the projecting spinal motor and dorsal root ganglia neurons as well as introduce often significant time periods for the axons to regenerate to appropriate targets. Moreover, post-transection, fascicular organization of the target nerve changes dramatically—smaller sensory and autonomic fibers regenerate through the sieve faster than larger (e.g., motor fibers), which in some cases may have diminished or absence of regrowth.217,220,221 This process is reflected, for example, in a low degree of reinnervation seen in distal muscle.217,220 Due to the invasiveness of the procedure and the time needed for the nerve to heal, research with regenerative electrodes has been limited mainly to animal studies.189,221–226 However, some of these studies indicate that such electrodes may be chronically viable. Srinivasan et al. recorded action potentials from rats for up to 5 months post-implantation. 225 efforts to optimize sieve electrodes in vivo are ongoing, and pores ranging from 30 to 65 nm in diameter appear to best support nerve regeneration. 71,227,228 Similarly, Bellamkonda et al. noted that a pore length of 3 mm ensured that at least one node of Ranvier (where the action potential presents the largest detectable extracellular signal) would be in the NI.225 Additionally, Kung et al. created a “regenerative peripheral nerve interface,” wherein a transected nerve innervates distinct muscle units, each of which was paired with an electrode.180 Thus far, the interface has yielded reliable behavior (i.e., recorded compound muscle action potentials from the muscle units) for up to 7 months in a rodent model. 180 Notably, studies have used polyethylene glycol (PEG) to reconnect damaged nerve fibers at the axonal level.229–231 Immediately following trauma, PEG therapy has been shown to rapidly restore functional connections in animal models; as such, it may be a viable method to limit the damage of nerve transection and improve acute and chronic functionality of sieve electrodes.229–233
D. Challenges in the PNS
Extraneural electrodes must contend with the relatively large biopotentials of adjacent tissues such as EMG, although insulation like that seen in cuff electrodes at least partially mitigates this issue.71,234 Extraneural selectivity is mainly limited to fascicles near the epineurium, where the electrode contacts the nerve. FINEs as described above increase electrical access to the deeper fascicles of the nerve. Also, the slowly penetrating interfasicular nerve electrode (SPINE) extends blunt electrical contacts into the epineurium without piercing the perineurium. However, SPINEs have only undergone acute studies, and their influence on the nerve over longer periods has not yet been investigated.71,235 Intraneural and regenerative electrodes offer high selectivity and have been proven stable, some for months at a time. However, the stability of these electrodes must be further studied and optimized before clinical trials become widespread. Fibrous encapsulation of these electrodes is common, and while potentially beneficial for physical stability and maintenance of intimate contact, the resulting signal attenuation can lower the efficacy of implanted devices.3 Additionally, the material properties of peripheral interface electrodes must be carefully tailored (as is the case with all electrodes), as overly rigid or tightly fitting electrodes can damage the nerve by compressing it or cutting off the blood supply.71 This is a particularly important consideration in cases of nerve interface in motile limbs requiring sliding and often stretching of the nerve, as device “catch points” may lead to nerve injury and impede blood flow.
A related phenomenon is the loss of electrode functionality due to connection failure, which has generally been observed in longer studies.175,236 This problem is also complicated by the range of motion experienced by peripheral tissues (i.e., limb movement), which necessitates the use of flexible materials that will remain in contact with the target area without damaging the nerve. As TMR uses surface EMG sensors to detect the rerouted motor commands, it must contend with the interference from movement and sweat, which negatively impact signal fidelity through the skin.13
Reverse recruitment is another issue facing PNSIs, notably for FES applications. In the intact neuromuscular pathway, motor units are activated in order of increasing size; that is, smaller axons become active before larger ones (i.e., the size principle). The more fatigue-resistant motor units are recruited before the fatigue-sensitive ones during normal muscle contractions.174,237 However, muscles activated via FES often fatigue quickly due to violation of the natural size principle, with the early recruitment of the larger fibers due to nonspecific electrical stimulation of the nerve.238–241 Some efforts to address this effect include varying stimulation intensities, frequencies, pulse widths, and “alternating” paradigms, wherein different fascicles are stimulated in sequence to avoid overtaxing any one group of motor units.238,242,243 As with BMIs, evaluating and improving the long-term in vivo function of peripheral interfaces is crucial to harnessing their full potential.
VI. FUTURE INTERFACES: NOVEL PARADIGMS
A. Optogenetics
Optogenetics involves the incorporation of opsins, or light-sensitive ion channel proteins, into cells via transgenesis or viral vectors. Cells expressing these opsins are then subject to targeted stimulation by illumination that in turn leads to changes in membrane potential.60,244 Once opsins are introduced, it is possible to stimulate targeted neurons with pulses of light rather than current injection. Stimulation can be inhibitory or excitatory depending on the current passed through the channel, and selectivity has high spatial control; specific cell types can be targeted via transfection methods, and only transfected cells will be susceptible to optical stimulation.60,245,246 Although optogenetics technology has been extensively developed for neuronal probing and control in vivo, recent efforts have been made to integrate optogenetics into neural interface technology. Current research in this area is focused on studying how best to implement this powerful tool for neural interfaces and on combining it with existing interface technologies.247,248 Bryson et al. have demonstrated “orderly recruitment” of muscles innervated with light-sensitive motoneurons.247,249–251 In this case, “orderly” refers to concordance with the size principle: the preferential recruitment of smaller muscle units before larger, more easily fatigued units. This specificity is physiologically advantageous but, as noted above, is currently unaccounted for in electrical stimulation. Additionally, optogenetic stimulation of the auditory nerve and retina has been realized, and optogenetic stimulation arrays are being developed for use in a closed-loop neuroprosthetic system.245,248,252 Implanted nerve cuffs offer a potential method of long-term interface, and they are being investigated in animal studies.249 Similarly, Steinberg et al. and others have begun exploring optogenetics as a therapeutic strategy for improving functional stroke recovery.253–255 Optogenetics may even serve as a potential extension of current DBS, using light to selectively target neural circuitry.256,257
In a similar vein, neurons transduced with genetically encoded calcium indicators (GECIs) or voltage sensitive dyes (GEVSDs) can produce proteins that fluoresce during neuronal signaling.258–260 These sensors enable the direct visualization of electrical activity in real time, providing an invaluable opportunity that has already been used to supplement and improve upon conventional recording methods. 59,169,258 The combination of optogenetics and GECIs in a closed loop has already been realized, with the GECI R-GECO1 and a channelrhodopsin allowing for reliable, simultaneous activation and imaging both in vivo and in C. elegans with little overlap.261 It is feasible to consider that future neural interfaces will use optogenetics and calcium/voltage sensing to interact with neural tissue without physical contact or direct current injection. As with all NI platforms, optogenetics poses its own unique challenges. Host parenchyma must be transfected with adeno-associated or lentiviruses to express photoresponsive proteins, or ex vivo tranfected neurons must be injected into the brain (as described below). Additionally, light waveguides must be implanted in the brain in order to access deeper structures, and a fully implantable laser light system with sufficient power has yet to be developed (although LED optrodes can be used).262 Finally, visible light cannot penetrate far into the brain before being scattered; this is being addressed with near-infrared (NIR) light, which can penetrate significantly deeper into tissue. Nanoparticles designed to absorb NIR light and in turn emit appropriate wavelengths to activate channelrhodopsins in target regions of the nervous system are being pursued.263,264 Tsien et al. also demonstrated that channelrhodopsins sensitive to far-red light (above 600 nm) can be activated noninvasively because tissue absorbs and scatters such wavelengths less effectively than blue light.265 Chronic function is also of concern because many optical probes are relatively rigid compared to the brain, and have experienced both glial encapsulation and reductions in signal quality over weeks to months, similar to other NI platforms.266
B. Magnetoencephalography (MEG)
Electrical activity induces magnetic fields that can be recorded via MEG. Similar to EEG, the magnetic fields can be consciously altered by users, allowing for real-time recording and control schemes based on these varying patterns, and there is evidence that MEG confers greater spatiotemporal resolution recording than EEG.267,268 Thus far, MEG has been applied to control both virtual and physical prosthetic hands (alongside visual feedback of the hand’s position) and drive the operation of virtual software by translating user-modified signals into computer mouse clicks.267,269–271 The primary limiting factor for MEG in the context of NIs is the environment required; participants are placed in a magnetically shielded room and must remain still to avoid artifacts from body and head movements.
C. Ultrasound
A potential noninvasive stimulation and recording methodology is transcranial Doppler ultrasound (TCD), which measures cerebral blood flow velocities. Different states of mental activity can be reflected by changes in blood flow velocities, which are then detected with TCD.272 While TCD carries an inherent latency compared to other NIs (5–10 seconds between a change in mental state and the corresponding change in blood flow velocity), it is robust against electrical artifacts and is more portable than MEG.272,273 Early research has shown TCD is fairly accurate in distinguishing distinct mental states (83–86% in a 2011 study by Myrden et al.), and it has been applied at least once to communicate via control of a virtual keyboard.272,273 Ultrasound has also been used to evoke sensation through stimulation of peripheral nerves; moreover, neuropathic tissue has been shown as more responsive to ultrasound than healthy nerve, potentially offering a noninvasive way to identify neuropathic conditions. 274,275 Interestingly, focused ultrasound is also capable of inducing conduction block in peripheral nerves and is actively being explored as a potential treatment for pain and spasticity.276–278
D. Transcranial Magnetic Stimulation
TMS consists of magnetic pulses directed to the brain. By varying the parameters of the pulse train and the coils, specific areas of the cortex can be targeted for excitation or inhibition.279,280 As a non-invasive technique, TMS has been an invaluable research tool for mapping the cortex, studying information processing, and investigating brain plasticity in humans.279 Interestingly, TMS can elicit visual percepts in subjects through stimulation of the occipital cortex, providing a way to map and evaluate functional differences in the visual cortex for both seeing and blind subjects.281,282 Within the context of NIs, TMS has been used in combination with EEG to “link” two human brains by delivering movement commands or phosphenes (perceptions of light caused by non-visual stimuli) to one participant based on recorded activity of the other.283,284
E. Infrared Nerve Stimulation
Extraneural optical nerve stimulation (also called infrared nerve stimulation, or INS) using pulses of infrared light has been suggested as an alternative method to interface with neural tissue.285–289 INS utilizes low-energy, pulsed light to reliably stimulate neural structures. INS parameters (radiation wavelength, irradiation time, and energy) can significantly alter the light-tissue interaction, and the careful selection of parameters may provide a level of selectivity superior to that of electrical stimulation.290 The feasibility, safety, and selectivity of INS have been well described and characterized in a number of animal models. Notably, fascicular infrared stimulation of the rat sciatic nerve elicited muscle responses with a selectivity previously only achieved with intraneural microelectrodes.288 The development of INS-based interfaces may offer significant advantages over electrical stimulation; the wireless stimulation and high spatial resolution allowing for finer activation of the peripheral nerve. Recently, INS has been applied to the CNS, and has been shown to effectively evoke excitation and inhibition in the motor and somatosensory cortex, auditory and vestibular systems, cortical column, and the primary visual cortex in nonhuman primates.285,291,292 Kuo et al. also reported on the stimulation of the subthalamic nucleus in a rodent model, with stimulation resulting in increased dopamine, suggesting that INS may be a potential therapeutic platform for Parkinson’s disease and other dopamine-related conditions. 293 Finally, early trials in humans have shown that INS can be used to stimulate human dorsal root ganglia.294 Although the research surrounding INS is promising, clinical applications of INS would require constant stimulation at 12–15 Hz, placing it above the upper threshold for injury.295 An alternative to bypass these limitations has been proposed that combines INS with extraneural stimulation via nerve cuff.296–298 This “electro-optical” paradigm uses INS to precondition the nerve, making it more excitable and amenable to electrical stimulation. 296–298
Another point of concern is the mechanism(s) of action for INS. Research suggests a photo-thermal mechanism, wherein the absorption of photons heats the water in targeted neural tissue, generating a transient temperature gradient.286 This gradient has been shown to generate depolarizing currents in neuronal membranes by increasing their capacitance.299 However, the exact workings are still not fully known and are likely location-specific: INS of the cochlea, for example may involve a photo-mechanical (through thermal expansion) or photo-acoustic (via laser-induced stress waves) effect.300
F. Biohybrid Microsystems
The first “biohybrid” neural interface, devised by Kennedy et al., was a glass cone electrode containing neurotrophic factors to elicit ingrowth of host neurites.110,301,302 In these so-called “neurotrophic electrodes,” the activity of the neurites was recorded within the cone. Due to actual ingrowth and integration between the electrode and host neurites, it was believed that this strategy would provide a more stable interface. In another more recent approach, Mark Allen et al. constructed electrodes composed primarily of extracellular matrix (ECM), the network of proteins that surrounds cells in vivo.303,304 As ECM is mechanically and biologically compliant with the brain, electrodes composed primarily of ECM may decrease the immune response and mechanical mismatch seen by traditional rigid, non-organic electrodes. Allen et al. fabricated collagen/Matrigel-based electrodes and implanted them in a rodent model, demonstrating multi-unit recordings over 5 weeks and reduced glial scarring compared with synthetic electrodes at 16 weeks.303
In alternative approaches, a number of research groups have begun creating advanced biohybrid neural interfaces by incorporating living cells and tissues into implantable devices.29,39,305–307 These efforts are intended to improve the integration of implants with the host nervous system. One such approach involves coating intracortical electrodes with live cells, as explored by Purcell et al. in 2009 and de Faveri et al. more recently.308,309 The results of Purcell et al suggested an initial “neuroprotective effect” from the neural stem cell–seeded electrodes, mitigating the tissue response 1 week post-implantation. However, increased neuronal loss was reported after 6 weeks, possibly due to the degradation of the hydrogel surrounding the cells.308 De Faveri et al. coated microelectrodes with neurons and astrocytes within fibrin hydrogel, demonstrating high signal quality and a diminished astrocytic response up to 30 days post-implantation.309 Electrochemical testing of the coated microelectrodes showed no significant changes in their impedance, attributed to water and ion absorption from the surrounding solution. The fibrin’s small swelling profile and controllable thickness allowed de Faveri et al. to reach a signal-to-noise comparable to the bare microelectrodes for 85% of their cortical recordings, although the fibrin coating did result in an increased distance between the electrode and recording site.309
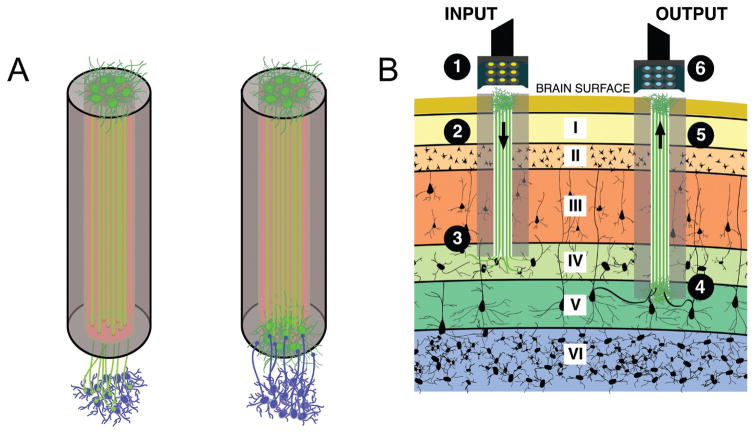
We are pursuing another biohybrid strategy for chronic BMI utilizing advanced micro-tissue engineering techniques to create the first biological “living electrodes.” These living electrodes are composed of discrete population(s) of neurons connected by long engineered axonal tracts that penetrate the brain to a prescribed depth for integration with local neurons/axons, with the latter portion remaining externalized on the brain surface where functional information is gathered using less invasive means (e.g., ECoG). In this radical paradigm, only the biological component of these constructs penetrates the brain, thus attenuating a chronic foreign body response. This strategy is founded on the plasticity of both endogenous and tissue-engineered neural networks, whereby neurons have the intrinsic ability to sense (through dendritic inputs) and respond to (via axonally transmitted action potentials) local activity. Toward this end, we have developed three-dimensional micro-tissue–engineered neural networks (micro-TENNs).310–313 By localizing the neuronal somata at one or both ends of hydrogel micro-columns, we are able to create long encapsulated axonal tracts within a miniature form factor in vitro that can then be precisely microinjected en masse into the brain. Initially, micro-TENNs have been developed for the physical reconstruction of long-distance neural circuitry lost due to trauma or disease (Fig. 4).311–314
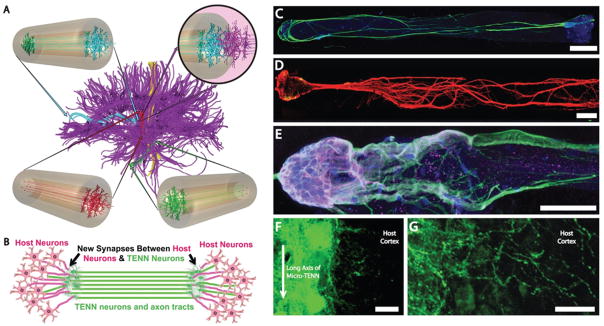
FIG. 4.
Micro-tissue engineered neural networks (Micro-TENNs), consisting of discrete neuronal population(s) with long axonal tracts within a biocompatible micro-column. Micro-TENNs are used for the direct reconstruction of long-distance axonal pathways after CNS degradation. (A) Diffusion tensor imaging representation of the human brain demonstrating the connectome comprised of long-distance axonal tracts connecting functionally distinct regions of the brain. Unidirectional (red, green) micro-TENNs and bidirectional (blue) micro-TENNs can bridge various regions of the brain (blue: corticothalamic pathway, red: nigrostriatal pathway, green: entorhinal cortex to hippocampus pathway) and synapse with host axons (purple; top right). (B) Conceptual representation of a micro-TENN forming local synapses with host neurons to form a new functional relay to replace missing or damaged axonal tracts. (C) Confocal reconstruction of a bidirectional micro-TENN, consisting of two populations of neurons spanned by long axonal tracts within a hydrogel micro-column stained via immunocytochemistry to denote axons (b-tubulin III; green), and cell nuclei (Hoechst; blue). (D) Confocal reconstruction of a unidirectional micro-TENN consisting of a single neuron population (MAP2; green) extending axons (tau; red) longitudinally (adapted from (Cullen et al., 2012)). (E) Confocal reconstruction of a unidirectional micro-TENN, stained via immunocytochemistry to denote neuronal somata/dendrites (MAP2; purple), neuronal somata/axons (tau; green) and cell nuclei (Hoechst; blue). (F) Confocal reconstruction of a transplanted GFP+ micro-TENN showing lateral outgrowth in vivo. (G) Confocal reconstruction showing GFP+ processes extending from a transplanted micro-TENN into the cortex of a rat. Scale bars: 300 μm in C, 250 μm in D, 100 μm in E, 20 μm in F and G. (Reprinted from Struzyna LA, Harris JP, Katiyar KS, Chen HI, Cullen DK, with permission from the Editorial Office of Neural Regeneration Research, Copyright 2015.)
More recently, we have applied similarly engineered axonal constructs to serve as living electrodes by synapsing with specific cortical regions and transmitting information in one or both directions (Fig. 5). Paired with an ECoG array, these micro-TENNs could relay information from the cortex to the surface and vice versa, eliminating the need for a non-organic chronic foreign body within the brain. Similarly, transducing the micro-TENNs with channelrhodopsins and/or genetically encoded calcium indicators would allow for targeted optical stimulation and recording as previously described. As living constructs, micro-TENNs offer a way to bypass the electrode size and number limitations of current NI platforms via “biological multiplexing”;each micro-TENN axon can synapse with multiple host neurons, offering a powerful method to reach a multitude of neurons and several target regions with a single construct. Moreover, through careful selection of the neurons used to create micro-TENNs and customized cell- and tissue-engineering techniques, we may influence the specific host neuronal subtypes with which the living electrode neurons form synapses, thereby adding a level of specificity to local stimulation and recording that is not currently attainable with conventional NI systems. To date, micro-TENNs have been implanted in the rat brain, and neural survival, maintenance of axonal architecture, and synaptic integration with the host have been demonstrated.310 Thus, in addition to serving as “living electrodes,” these versatile constructs may serve as living scaffolds to promote regeneration of host axons along the micro-TENN axons or may physically “wire-in” to replace lost host circuitry. 314,315 Although it is actively being developed and tested, our biohybrid “living electrode” strategy may result in a neural interface with a specificity, spatial density, and long-term fidelity greater than that possible with artificial microelectronic or optical substrates alone.
FIG. 5.
Micro-TENNs as “living electrodes.” (A) Unidirectional micro-TENNs (left) synapse with the host (blue cells) and deliver inputs to targeted cortical regions, while bidirectional micro-TENNs (right) may be synapsed by the host and transmit cortical activity. Relevant signal propagation denoted by arrows. (B) Conceptual schematic of the micro-TENNs as “living electrodes” in vivo. Left: Input paradigm. An LED array (1) optically stimulates a unidirectional micro-TENN with channelrhodopsin-positive neurons (2), which synapse with host Layer IV neurons (3). Right: Output paradigm. A microelectrode array (4) records from the neurons of a bidirectional micro-TENN (5), which are synapsed by host neurons from Layer V (6). Roman numerals denote cortical layers.
In the PNS, biohybrid neural interfaces are also promising to enable robust integration between peripheral axons and electronics to drive the next generation of robotic prosthetic devices.29,39,306 In the case of loss-of-limb, it is surgically advantageous to avoid implanting electrodes in the otherwise non-injured brain or spinal cord. Thus, as described previously for the case of TMR and RPNI, a neural interface with the peripheral nerves originally serving the lost limb would be ideal, as this is the final point of motor output and primary sensory input. However, PNS axons necessitate a living target for innervation, and residual muscle may not be available or may not provide the signal complexity for fine motor control. Therefore, we employ a blend of tissue engineering and micro-electrical techniques to facilitate direct integration with host axons to allow for complex command signals while enabling a vehicle for proprioceptive and other sensory feedback. We previously demonstrated that these tissue engineered constructs serve as a replacement end target and drive host axon regeneration into intimate contact with micro-electrodes. 39,306 Our current efforts are aimed toward testing the ability of these constructs to transmit electrical signals, and establishing the mechanism of action by which they allow for integration with host axons. Collectively, our biohybrid approaches operate at the intersection of neuroscience and engineering to establish preformed implantable neural networks as a complimentary alternative to conventional hardware/electrode-based interfaces. Thus, these approaches potentially represent a paradigm-shift for chronic neural interface with the CNS or PNS.
VII. CONCLUSION
The nervous system connects the individual to their environment, and its breakdown is often physically and emotionally devastating. This is far from a rare issue—in 2010 more than 20% of the United States population reported having some disability, with the majority related to motor impairments in the upper or lower body.316 Nearly 2 million people in the United States are amputees due to trauma or disease alone, with almost 200,000 people receiving amputations per year.10,317 The Amyotrophic Lateral Sclerosis Association reports a prevalence of 30,000 ALS patients in the United States; the number of people living with other neuromuscular disorders is several orders of magnitude larger.316,318 Fortunately, neuroprosthesis research has yielded a host of new technologies that can promote neural regeneration, enable communication, and restore mobility post-injury. These successes have their roots in multidisciplinary research, crossing disparate domains in engineering, neuroscience, and medicine. It follows that future interfaces will likely use some combination of both established and newly developed strategies to address the challenges faced by current interfaces—immunoreactivity, chronic stability, and selectivity, among others—to integrate more seamlessly with the human body. As NIs improve, prosthesis adoption will increase accordingly as patients feel more in tune with their devices. Concurrently, ongoing research in this space will lead to new applications and platforms for rehabilitation, therapeutics, and the restoration of quality of life.
Acknowledgments
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (Grant No. DGE1321851). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation. This material was also supported in part by the Clinical Center of the National Institutes of Health (Grant No. U01NS094340). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was conducted as part of the Penn Medicine Neuroscience Center at the University of Pennsylvania.
References
- 1.Thurston AJ. Paré and prosthetics: the early history of artificial limbs. ANZ J Surg. 2007;77(12):1114–9. doi: 10.1111/j.1445-2197.2007.04330.x. [DOI] [PubMed] [Google Scholar]
- 2.Hernigou P. Ambroise Paré IV: the early history of artificial limbs (from robotic to prostheses) Int Orthop. 2013;37(6):1195–7. doi: 10.1007/s00264-013-1884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: biochallenges and engineered solutions. Annu Rev Biomed Eng. 2009;11:1–24. doi: 10.1146/annurev-bioeng-061008-124927. [DOI] [PubMed] [Google Scholar]
- 4.Mak JN, Wolpaw JR. Clinical applications of brain-computer interfaces: current state and future prospects. IEEE Rev Biomed Eng. 2009;2:187–99. doi: 10.1109/RBME.2009.2035356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fetz EE. Operant conditioning of cortical unit activity. Science (80-) 1969 Feb 28;163(3870):955–8. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey DR, Schmidt EM, Thompson WD. Predicting measures of motor performance from multiple cortical spike trains. Science (80-) 1970;170(3959):758–62. doi: 10.1126/science.170.3959.758. [DOI] [PubMed] [Google Scholar]
- 7.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MAL. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 2(7):664–70. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 8.Roche JP, Hansen MR. On the horizon: cochlear implant technology. Otolaryngol Clin North Am. 2015;48(6):1097–116. doi: 10.1016/j.otc.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beitz JM. Parkinson’s disease: a review. Front Biosci. 2014;(3):65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 10.Resnik L, Meucci MR, Lieberman-Klinger S, Fantini C, Kelty DL, Disla R, Sasson N. Advanced upper limb prosthetic devices: Implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710–7. doi: 10.1016/j.apmr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Resnik L, Fantini C, Disla R, Etter K, Lieberman S. Using a virtual reality environment (VRE) to facilitate training with an advanced upper limb prosthesis. 2011:2–3. doi: 10.1682/jrrd.2010.07.0127. [DOI] [PubMed] [Google Scholar]
- 12.Resnik L, Borgia M. User ratings of prosthetic usability and satisfaction in VA study to optimize DEKA arm. J Rehabil Res Dev. 2014;51(1):15–26. doi: 10.1682/JRRD.2013.02.0056. [DOI] [PubMed] [Google Scholar]
- 13.Kung T, Bueno R, Alkhalefah GK, Langhals NB, Urbanchek MG, Cederna PS. Innovations in prosthetic interfaces for the upper extremity. Plast Reconstr Surg. 2013;132(6):1515–23. doi: 10.1097/PRS.0b013e3182a97e5f. [DOI] [PubMed] [Google Scholar]
- 14.Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Orthot Int. 2007;31(3):236–57. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- 15.Biddiss E, Chau T. Upper-limb prosthetics: critical factors in device abandonment. Am J Phys Med Rehabil. 2007;86(12):977–87. doi: 10.1097/PHM.0b013e3181587f6c. [DOI] [PubMed] [Google Scholar]
- 16.Schultz AE, Kuiken TA. Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities. PM R. 2011;3(1):55–67. doi: 10.1016/j.pmrj.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Lega BC, Serruya MD, Zaghloul Ka. Brain-machine interfaces: electrophysiological challenges and limitations. Crit Rev Biomed Eng. 2011;39(1):5–28. doi: 10.1615/critrevbiomedeng.v39.i1.20. [DOI] [PubMed] [Google Scholar]
- 18.Cullen DK, Pfister B. State of the art and future challenges in neural engineering: strategies for nervous system repair foreword/editors’ commentary (volume 2) Crit Rev Biomed Eng. 2011;39(2):79–80. doi: 10.1615/critrevbiomedeng.v39.i2.10. [DOI] [PubMed] [Google Scholar]
- 19.Wolpaw JR. Handbook of clinical neurology. 1. Vol. 110. Elsevier; 2013. Brain-computer interfaces; pp. 67–74. [DOI] [PubMed] [Google Scholar]
- 20.Berne RM, Koeppen BM, Stanton BA. Berne & Levy physiology. Philadelphia, PA: Mosby/Elsevier; 2010. [Google Scholar]
- 21.Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 22.Pisotta I, Perruchoud D, Ionta S. Hand-in-hand advances in biomedical engineering and sensorimotor restoration. J Neurosci Methods. 2015;246:22–9. doi: 10.1016/j.jneumeth.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Shimada S, Fukuda K, Hiraki K. Rubber hand illusion under delayed visual feedback. PLoS One. 2009;4(7):1–5. doi: 10.1371/journal.pone.0006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatsopoulos N, Donoghue J. The science of neural interface systems. Annu Rev Neurosci. 2009;32:249–66. doi: 10.1146/annurev.neuro.051508.135241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113(6):767–91. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 26.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8(1):67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 27.Berenyi A, Belluscio M, Mao D, Buzsaki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science (80-) 2012;337(6095):735–7. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ, Jr, Moro E, Vitek JL, Weaver FM, Gross RE, DeLong MR. Deep brain stimulation for parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2010;7:199–203. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cullen DK, Smith DH. Bionic connections. Sci Amer. 2013;308(1):52–7. doi: 10.1038/scientificamerican0113-52. [DOI] [PubMed] [Google Scholar]
- 30.Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63(4):508–22. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logothetis NK, Augath M, Murayama Y, Rauch A, Sultan F, Goense J, Oeltermann A, Merkle H. The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci. 2010;13(10):1283–91. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- 32.Fukaya C, Yamamoto T. Deep brain stimulation for Parkinson’s disease: recent trends and future direction. Neurol Med Chir (Tokyo) 2015;55(5):422–31. doi: 10.2176/nmc.ra.2014-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris JP, Tyler DJ. Biological, mechanical, and technological considerations affecting the longevity of intracortical electrode recordings. Crit Rev Biomed Eng. 2013;41(6):435–56. [PubMed] [Google Scholar]
- 34.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Tresco PA, Winslow BD. The challenge of integrating devices into the central nervous system. Crit Rev Biomed Eng. 2011;39(1):29–44. doi: 10.1615/critrevbiomedeng.v39.i1.30. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharyya S, Konar A, Tibarewala DN. A differential evolution based energy trajectory planner for artificial limb control using motor imagery EEG signal. Biomed Signal Process Control. 2014;11(1):107–13. [Google Scholar]
- 37.Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7(11):1032–43. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- 38.Galán F, Nuttin M, Lew E, Ferrez PW, Vanacker G, Philips J, del Millán JR. A brain-actuated wheelchair: asynchronous and noninvasive brain–computer interfaces for continuous control of robots. Clin Neurophysiol. 2008;119(9):2159–69. doi: 10.1016/j.clinph.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Kameswaran N, Cullen DK, Pfister BJ, Ranalli NJ, Huang JH, Zager EL, Smith DH. A novel neuroprosthetic interface with the peripheral nervous system using artificially engineered axonal tracts. Neurol Res. 2008;30(10):1063–7. doi: 10.1179/174313208X362541. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann T, Herweg A, Kübler A. Toward brain-computer interface based wheelchair control utilizing tactually-evoked event-related potentials. J Neuroeng Rehabil. 2014;11:7. doi: 10.1186/1743-0003-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebedev MA, Nicolelis MaL. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29(9):536–46. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, Cipriani C, Denaro L, Denaro V, Di Pino G, Ferreri F, Guglielmelli E, Hoffmann KP, Raspopovic S, Rigosa J, Rossini L, Tombini M, Dario P. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol. 2010;121(5):777–83. doi: 10.1016/j.clinph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Yu T, Li Y, Long J, Li F. A hybrid brain-computer interface-based mail client. Comput Math Methods Med. 2013:2013. doi: 10.1155/2013/750934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampson RE, Song D, Opris I, Santos LM, Shin DC, Gerhardt GA, Marmarelis VZ, Berger TW, Deadwyler SA. Facilitation of memory encoding in primate hippocampus by a neuroprosthesis that promotes task specific neural firing. J Neural Eng. 2013;10(6):66013. doi: 10.1088/1741-2560/10/6/066013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berger TW, Hampson RE, Song D, Goonawardena A, Marmarelis VZ, Deadwyler SA. A cortical neural prosthesis for restoring and enhancing memory. J Neural Eng. 2011;8(4):46017. doi: 10.1088/1741-2560/8/4/046017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cogan SF, Ludwig KA, Welle CG, Takmakov P. Tissue damage thresholds during therapeutic electrical stimulation. J Neural Eng. 2016;13(2):021001. doi: 10.1088/1741-2560/13/2/021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krüger J, Caruana F, Volta RD, Rizzolatti G. Seven years of recording from monkey cortex with a chronically implanted multiple microelectrode. Front Neuroeng. 2010;3:6. doi: 10.3389/fneng.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delbeke J. Electrodes and chronic optic nerve stimulation. Biocybern Biomed Eng. 2011;31(3):81–94. [Google Scholar]
- 49.Waters RL, McNeal DR, Faloon W, Clifford B. Functional electrical stimulation of the peroneal nerve for hemiplegia. Long-term clinical follow-up. J Bone Joint Surg Am. 1985;67(5):792–3. [PubMed] [Google Scholar]
- 50.Singh S, Kong Y-Y, Zeng F-G. Cochlear implant melody recognition as a function of melody frequency range, harmonicity, and number of electrodes. Ear Hearing. 2009;30:160–8. doi: 10.1097/AUD.0b013e31819342b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson NR, Pisoni DB, Miyamoto RT. Cochlear implants and spoken language processing abilities: review and assessment of the literature. Restor Neurol Neurosci. 2010;28(2):237–50. doi: 10.3233/RNN-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong Y-Y, Cruz R, Jones JA, Zeng F-G. Music perception with temporal cues in acoustic and electric hearing. Ear Hear. 2004;25(2):173–85. doi: 10.1097/01.aud.0000120365.97792.2f. [DOI] [PubMed] [Google Scholar]
- 53.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6):66014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9(5):056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 55.Prasad A, Xue QS, Dieme R, Sankar V, Mayrand RC, Nishida T, Streit WJ, Sanchez JC. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front Neuroeng. 2014;7(February):2. doi: 10.3389/fneng.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravikumar M, Sunil S, Black J, Barkauskas DS, Haung AY, Miller RH, Selkirk SM, Capadona JR. The roles of blood-derived macrophages and resident microglia in the neuroinflammatory response to implanted Intracortical microelectrodes. Biomater. 2014;35:8049–64. doi: 10.1016/j.biomaterials.2014.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, McCreery DB, Carter RR, Bullara LA, Yuen TGH, Agnew WF. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Rehabil Eng. 1999;7(3):315–26. doi: 10.1109/86.788468. [DOI] [PubMed] [Google Scholar]
- 58.McConnell GC, Rees HD, Levey AI, Gutekunst C-A, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6(5):056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 59.Gunasekera B, Saxena T, Bellamkonda R, Karumbaiah L. Intracortical Recording Interfaces: Current Challenges to Chronic Recording Function. ACS Chem Neurosci. 2015 doi: 10.1021/cn5002864. [DOI] [PubMed] [Google Scholar]
- 60.Gilja V, Chestek CA, Diester I, Henderson JM, Deisseroth K, Shenoy KV. Challenges and opportunities for next-generation intracortically based neural prostheses. IEEE Trans Biomed Eng. 2011;58(7):1891–9. doi: 10.1109/TBME.2011.2107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 62.Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, Sorice BL, Perge JA, Jarosiewicz B, Hochberg LR, Shenoy KV, Henderson JM. Clinical translation of a high-performance neural prosthesis. Nat Med. 2015;21(10):6–8. doi: 10.1038/nm.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bacher D, Jarosiewicz B, Masse NY, Stavisky SD, Simeral JD, Newell K, Oakley EM, Cash SS, Friehs G, Hochberg LR. Neural point-and-click communication by a person with incomplete locked-in syndrome. Neurorehabil Neural Repair. 2015;29(5):462–71. doi: 10.1177/1545968314554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simeral JD, Kim S-P, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng. 2011;8(2):25027. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016:1–13. doi: 10.1038/nature17435. advance on. [DOI] [PubMed] [Google Scholar]
- 66.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456(7222):639–42. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasquina PF, Evangelista M, Carvalho AJ, Lockhart J, Griffin S, Nanos G, McKay P, Hansen M, Ipsen D, Vandersea J, Butkus J, Miller M, Murphy I, Hankin D. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand. J Neurosci Methods. 2014;244:85–93. doi: 10.1016/j.jneumeth.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker JJ, Yatsenko D, Schorsch JF, DeMichele GA, Troyk PR, Hutchinson DT, Weir RF, Clark G, Greger B. Decoding individuated finger flexions with implantable myoelectric sensors. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:193–6. doi: 10.1109/IEMBS.2008.4649123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlatter S, Engineering B, Island R. Implantable myoelectric sensors for intramuscular EMG recording. IEEE Trans Biomed Eng. 2009;56(1):2009. doi: 10.1109/TBME.2008.2005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber DJ, Bauman MJ, Fisher L. Spinal nerve interfaces for bidirectional communication with prosthetic limbs. EmbcEmbsOrg. 2012;485(7398):7398. [Google Scholar]
- 71.Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprotheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–58. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- 72.Kuiken T, Li G, Lock B, Lipschutz RD, Miller L, Stubblefield K, Englehart K. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301(6):619–28. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marasco PD, Schultz AE, Kuiken TA. Sensory capacity of reinnervated skin after redirection of amputated upper limb nerves to the chest. Brain. 2009;132(6):1441–8. doi: 10.1093/brain/awp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hebert JS, Olson JL, Morhart MJ, Dawson MR, Marasco PD, Kuiken TA, Ming Chan K. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation. IEEE Trans Neural Syst Rehabil Eng. 2014;22(4):765–73. doi: 10.1109/TNSRE.2013.2294907. [DOI] [PubMed] [Google Scholar]
- 75.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39(2):81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 76.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98(1):16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Di Pino G, Denaro L, Vadalà G, Marinozzi A, Tombini M, Ferreri F, Papalia R, Accoto D, Guglielmelli E, Di Lazzaro V, Denaro V. Invasive neural interfaces: the perspective of the surgeon. J Surg Res. 2014;188(1):77–87. doi: 10.1016/j.jss.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Cincotti F, Mattia D, Aloise F, Bufalari S, Astolfi L, De Vico Fallani F, Tocci A, Bianchi L, Marciani MG, Gao S, Millan J, Babiloni F. High-resolution EEG techniques for brain-computer interface applications. J Neurosci Methods. 2008;167(1):31–42. doi: 10.1016/j.jneumeth.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 79.McCane LM, Sellers EW, McFarland DJ, Mak JN, Carmack CS, Zeitlin D, Wolpaw JR, Vaughan TM. Brain-computer interface (BCI) evaluation in people with amyotrophic lateral sclerosis. Amyotroph lateral scler frontotemporal degener 2014. 2013 Nov;:1–9. doi: 10.3109/21678421.2013.865750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nijboer F, Furdea A, Gunst I, Mellinger J, McFarland DJ, Birbaumer N, Kübler A. An auditory brain-computer interface (BCI) J Neurosci Methods. 2008;167(1):43–50. doi: 10.1016/j.jneumeth.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sellers EW, Vaughan TM, Wolpaw JR. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler. 2010;11(5):449–55. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- 82.Taylor DM, Tillery SIH, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296(5574):1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 83.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci USA. 2004;101(51):17849–54. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boord P, Barriskill A, Craig A, Nguyen H. Brain-computer interface—FES integration: towards a hands-free neuroprosthesis command system. Neuromodulation. 2004;7(4):267–76. doi: 10.1111/j.1094-7159.2004.04212.x. [DOI] [PubMed] [Google Scholar]
- 85.Contreras-Vidal JL, Grossman RG. NeuroRex: A clinical neural interface roadmap for EEG-based brain machine interfaces to a lower body robotic exoskeleton. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS; 2013; pp. 1579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfurtscheller G, Guger C, Müller G, Krausz G, Neuper C. Brain oscillations control hand orthosis in a tetraplegic. Neurosci Lett. 2000;292(3):211–4. doi: 10.1016/s0304-3940(00)01471-3. [DOI] [PubMed] [Google Scholar]
- 87.Millán JDR, Rupp R, Müller-Putz GR, Murray-Smith R, Giugliemma C, Tangermann M, Vidaurre C, Cincotti F, Kübler A, Leeb R, Neuper C, Müller K-R, Mattia D. Combining brain-computer interfaces and assistive technologies: State-of-the-art and challenges. Front Neurosci. 2010;4:1–15. doi: 10.3389/fnins.2010.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lobel DA, Lee KH. Brain machine interface and limb reanimation technologies: Restoring function after spinal cord injury through development of a bypass system. Mayo Clin Proc. 2014;89(5):708–14. doi: 10.1016/j.mayocp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Zimmermann JB, Jackson A. Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Front Neurosci. 2014;8:1–8. doi: 10.3389/fnins.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grahn PJ, Mallory GW, Michael Berry B, Hachmann JT, Lobel DA, Luis Lujan J. Restoration of motor function following spinal cord injury via optimal control of intraspinal microstimulation: toward a next generation closed-loop neural prosthesis. Front Neurosci. 2014;8(SEP):1–12. doi: 10.3389/fnins.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One. 2015;10(7):1–20. doi: 10.1371/journal.pone.0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–47. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmena JM, Cohen LG. Handbook of clinical neurology. 1. Vol. 109. Elsevier; 2012. Brain–machine interfaces and transcranial stimulation. future implications for directing functional movement and improving function after spinal injury in humans; pp. 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soekadar SR, Witkowski M, Cossio EG, Birbaumer N, Cohen LG. Learned EEG-based brain self-regulation of motor-related oscillations during application of transcranial electric brain stimulation: feasibility and limitations. Front Behav Neurosci. 2014;8:93. doi: 10.3389/fnbeh.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dutta A, Paulus W, Nitsche MA. Facilitating myoelectric-control with transcranial direct current stimulation: a preliminary study in healthy humans. J Neuroeng Rehabil. 2014;11(1):13. doi: 10.1186/1743-0003-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Birbaumer N, Cohen LG. Brain-computer interfaces: communication and restoration of movement in paralysis. J Physiol. 2007;579(Pt 3):621–36. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Donoghue JP. Bridging the brain to the world: a perspective on neural interface systems. Neuron. 2008;60(3):511–21. doi: 10.1016/j.neuron.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 98.Piccione F, Giorgi F, Tonin P, Priftis K, Giove S, Silvoni S, Palmas G, Beverina F. P300-based brain computer interface: reliability and performance in healthy and paralysed participants. Clin Neurophysiol. 2006;117(3):531–7. doi: 10.1016/j.clinph.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 99.Neuper C, Scherer R, Wriessnegger S, Pfurtscheller G. Motor imagery and action observation: Modulation of sensorimotor brain rhythms during mental control of a brain-computer interface. Clin Neurophysiol. 2009;120(2):239–47. doi: 10.1016/j.clinph.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 100.Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R. Thought—control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351(1):33–6. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 101.Müller-Putz GR, Scherer R, Pfurtscheller G, Rupp R. EEG-based neuroprosthesis control: a step towards clinical practice. Neurosci Lett. 2005;382(1–2):169–74. doi: 10.1016/j.neulet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Long J, Li Y, Wang H, Yu T, Pan J. Control of a simulated wheelchair based on a hybrid brain computer interface. Engineering in Medicine and Biology Society (EMBC), 2012 Ann Int Conf IEEE; 2012; pp. 6727–30. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Wang Y, Nakanishi M, Gao X, Jung T-P, Gao S. High-speed spelling with a noninvasive brain–computer interface. Proc Natl Acad Sci. 2015;112(44):E6058–67. doi: 10.1073/pnas.1508080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patil PG, Turner DA. The development of brain-machine interface neuroprosthetic devices. Neurotherapeutics. 2008;5(1):137–46. doi: 10.1016/j.nurt.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolpaw JR. Brain–computer interface. Encycl Neurosci. 2009:429–37. [Google Scholar]
- 106.Blakely T, Miller KJ, Zanos SP, Rao RPN, Ojemann JG. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurgical Focus. 2009;27:E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- 107.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51(6):1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 108.Cincotti F, Mattia D, Aloise F, Bufalari S, Schalk G, Oriolo G, Cherubini A, Marciani MG, Babiloni F. Non-invasive brain-computer interface system: towards its application as assistive technology. Brain Res Bull. 2008;75(6):796–803. doi: 10.1016/j.brainresbull.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scherberger H. Neural control of motor prostheses. Curr Opin Neurobiol. 2009;19(6):629–33. doi: 10.1016/j.conb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz AB, Cui XT, Weber DJ, Moran DW. Brain-controlled interfaces: movement restoration with neural prosthetics. Neuron. 2006;52(1):205–20. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 111.Lachaux JP, Rudrauf D, Kahane P. Intracranial EEG and human brain mapping. J Physiol Paris. 2003;97(4–6):613–28. doi: 10.1016/j.jphysparis.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 112.Leuthardt EC, Schalk G, Roland J, Rouse A, Moran DW. Evolution of brain-computer interfaces: going beyond classic motor physiology. Neurosurg Focus. 2009;27(1):E4–E4. doi: 10.3171/2009.4.FOCUS0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schalk G, Miller KJ, Anderson NR, Wilson JA, Smyth MD, Ojemann JG, Moran DW, Wolpaw JR, Leuthardt EC. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1(2):63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 115.Schalk G, Leuthardt EC. Brain-computer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng. 2011;4:140–54. doi: 10.1109/RBME.2011.2172408. [DOI] [PubMed] [Google Scholar]
- 116.Hill NJ, Gupta D, Brunner P, Gunduz A, Adamo MA, Ritaccio A, Schalk G. Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J Vis Exp. 2012;(64):1–5. doi: 10.3791/3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Panov F, Levin E, de Hemptinne C, Swann NC, Qasim S, Miocinovic S, Ostrem JL, Starr PA. Intraoperative electrocorticography for physiological research in movement disorders: principles and experience in 200 cases. J Neurosurg. 2016:1–10. doi: 10.3171/2015.11.JNS151341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang W, Collinger JL, Degenhart AD, Tyler-Kabara EC, Schwartz AB, Moran DW, Weber DJ, Wodlinger B, Vinjamuri RK, Ashmore RC, Kelly JW, Boninger ML. An electrocorticographic brain interface in an individual with tetraplegia. PLoS One. 2013;8(2):1–8. doi: 10.1371/journal.pone.0055344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yanagisawa T, Hirata M, Saitoh Y, Goto T, Kishima H, Fukuma R, Yokoi H, Kamitani Y, Yoshimine T. Real-time control of a prosthetic hand using human electrocorticography signals. J Neurosurg. 2011;114(6):1715–22. doi: 10.3171/2011.1.JNS101421. [DOI] [PubMed] [Google Scholar]
- 120.Johnson LA, Wander JD, Sarma D, Su DK, Fetz EE, Ojemann JG. Direct electrical stimulation of the somatosensory cortex in humans using electrocorticography electrodes: a qualitative and quantitative report. J Neural Eng. 2013;10(3):036021. doi: 10.1088/1741-2560/10/3/036021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun FT, Morrell MJ. The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev Med Devices. 2014;11(6):563–72. doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- 122.Chao ZC, Nagasaka Y, Fujii N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front Neuroeng. 2010;3:3. doi: 10.3389/fneng.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng. 2009;6(3):036003. doi: 10.1088/1741-2560/6/3/036003. [DOI] [PubMed] [Google Scholar]
- 124.Castagnola E, Ansaldo A, Maggiolini E, Ius T, Skrap M, Ricci D, Fadiga L. Smaller, softer, lower-impedance electrodes for human neuroprosthesis: a pragmatic approach. Front Neuroeng. 2014;7:8. doi: 10.3389/fneng.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thongpang S, Richner TJ, Brodnick SK, Schendel A, Kim J, Wilson JA, Hippensteel J, Krugner-Higby L, Moran D, Ahmed AS, Neimann D, Sillay K, Williams JC. A micro-electrocorticography platform and deployment strategies for chronic BCI applications. Clin EEG Neurosci. 2011;42(4):259–65. doi: 10.1177/155005941104200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schendel AA, Thongpang S, Brodnick SK, Richner TJ, Lindevig BD, Krugner-Higby L, Williams JC. A cranial window imaging method for monitoring vascular growth around chronically implanted micro-ECoG devices. J Neurosci Methods. 2013;218(1) doi: 10.1016/j.jneumeth.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Henle C, Raab M, Cordeiro JG, Doostkam S, Schulze-Bonhage A, Stieglitz T, Rickert J. First long term in vivo study on subdurally implanted Micro-ECoG electrodes, manufactured with a novel laser technology. Biomed Microdevices. 2011;13(1):59–68. doi: 10.1007/s10544-010-9471-9. [DOI] [PubMed] [Google Scholar]
- 128.Mestais CS, Charvet G, Sauter-Starace F, Foerster M, Ratel D, Benabid AL. WIMAGINE: Wireless 64-channel ECoG recording implant for long term clinical applications. IEEE Trans Neural Syst Rehabil Eng. 2015;23(1):10–21. doi: 10.1109/TNSRE.2014.2333541. [DOI] [PubMed] [Google Scholar]
- 129.Akamatsu N, Tsuji S. Deep brain stimulation for epilepsy. Brain Nerve. 2011;63(4):365–9. [PubMed] [Google Scholar]
- 130.Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100(19):11041–6. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13(6):696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Klaes C, Shi Y, Kellis S, Minxha J, Revechkis B, Andersen RA. A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback. J Neural Eng. 2014;11:56024. doi: 10.1088/1741-2560/11/5/056024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tabot GA, Dammann JF, Berg JA, Tenore FV, Boback JL, Vogelstein RJ, Bensmaia SJ. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc Natl Acad Sci USA. 2013;110(45):18279–84. doi: 10.1073/pnas.1221113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, Nicolelis MAL. Active tactile exploration using a brain–machine–brain interface. Nature. 2011;479(7372):228–31. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lim HH, Lenarz M, Lenarz T. Auditory midbrain implant: a review. Trends Amplif. 2009;13(3):149–80. doi: 10.1177/1084713809348372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G, de Juan E. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 2003;43(24):2573–81. doi: 10.1016/s0042-6989(03)00457-7. [DOI] [PubMed] [Google Scholar]
- 137.Pezaris JS, Eskandar EN. Getting signals into the brain: visual prosthetics through thalamic microstimulation. Neurosurg Focus. 2009;27(1):E6–E6. doi: 10.3171/2009.4.FOCUS0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barry MP, Dagnelie G, Group AIIS. Use of the Argus II retinal prosthesis to improve visual guidance of fine hand movements. Invest Ophthalmol Vis Sci. 2012;53(9):5095–101. doi: 10.1167/iovs.12-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yanai D, Weiland JD, Mahadevappa M, Greenberg RJ, Fine I, Humayun MS. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am J Ophthalmol. 2007;143(5):820–7. doi: 10.1016/j.ajo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 140.Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel V-P, Gekeler F, Greppmaier U, Harscher A, Kibbel S, Koch J, Kusnyerik A, Peters T, Stingl K, Sachs H, Stett A, Szurman P, Wilhelm B, Wilke R. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011;278(1711):1489–97. doi: 10.1098/rspb.2010.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stronks HC, Dagnelie G. The functional performance of the Argus II retinal prosthesis. Expert Rev Med Devices. 2014;11(1):23–30. doi: 10.1586/17434440.2014.862494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carmena JM. Stable Ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci. 2005;25(46):10712–6. doi: 10.1523/JNEUROSCI.2772-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dickey AS, Suminski A, Amit Y, Hatsopoulos NG. Single-unit stability using chronically implanted multielectrode arrays. J Neurophysiol. 2009;102(2):1331–9. doi: 10.1152/jn.90920.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rousche PJ, Normann RA. Chronic recording capability of the utah intracortical electrode array in cat sensory cortex. J Neurosci Methods. 1998;82(1):1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 145.Harris AR, Morgan SJ, Chen J, Kapsa RMI, Wallace GG, Paolini AG. Conducting polymer coated neural recording electrodes. J Neural Eng. 2013;10(1):016004. doi: 10.1088/1741-2560/10/1/016004. [DOI] [PubMed] [Google Scholar]
- 146.Shain W1, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE Trans Neural Syst Rehabil Eng. 2003;11(2):186–8. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- 147.George PM1, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26(17):3511–9. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 148.Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim DH, Martin DC. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomater. 2007;28(8):1539–52. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Green RA, Lovell NH, Poole-Warren LA. Cell attachment functionality of bioactive conducting polymers for neural interfaces. Biomater. 2009;30(22):3637–44. doi: 10.1016/j.biomaterials.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 150.Bae WJ, Ruddy BP, Richardson AG, Hunter IW, Bizzi E. Cortical recording with polypyrrole microwire electrodes. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5794–7. doi: 10.1109/IEMBS.2008.4650531. [DOI] [PubMed] [Google Scholar]
- 151.McClain MA, Clements IP, Shafer RH, Bellamkonda RV, LaPlaca MC, Allen MG. Highly compliant, microcable neuroelectrodes fabricated from thin-film gold and PDMS. Biomed Microdevices. 2011;13(2):361–73. doi: 10.1007/s10544-010-9505-3. [DOI] [PubMed] [Google Scholar]
- 152.Bishop W, Chestek CC, Gilja V, Nuyujukian P, Foster JD, Ryu SI, Shenoy KV, Yu BM. Self-recalibrating classifiers for intracortical brain-computer interfaces. J Neural Eng. 2014;11(2):026001. doi: 10.1088/1741-2560/11/2/026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Homer ML, Perge JA, Black MJ, Harrison MT, Cash SS, Hochberg LR. Adaptive offset correction for intracortical brain-computer interfaces. IEEE Trans Neural Syst Rehabil Eng. 2014;22(2):239–48. doi: 10.1109/TNSRE.2013.2287768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ajiboye AB, Simeral JD, Donoghue JP, Hochberg LR, Kirsch RF. Prediction of imagined single-joint movements in a person with high-level tetraplegia. IEEE Trans Biomed Eng. 2012;59(10):2755–65. doi: 10.1109/TBME.2012.2209882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Murphy MD, Guggenmos DJ, Bundy DT, Nudo RJ. Current challenges facing the translation of brain computer interfaces from preclinical trials to use in human patients. Front Cell Neurosci. 2015;9:1–14. doi: 10.3389/fncel.2015.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Howard IP, Rogers BJ. Oxford psychology series. New York: Oxford University Press; 1996. Binocular vision and stereopsis; p. 746. [Google Scholar]
- 157.Spector RH. Visual Fields. In: Walker H, Hall W, Hurst J, editors. Clinical methods: the history, physical, and laboratory examinations. 3. Boston: Butterworths; 1990. pp. 565–72. [PubMed] [Google Scholar]
- 158.DARPA. DARPA-BAA-16-09: Neural Engineering System Design (NESD) DARPA; 2016. [Google Scholar]
- 159.Angotzi GN, Boi F, Zordan S, Bonfanti A, Vato A. A programmable closed-loop recording and stimulating wireless system for behaving small laboratory animals. Sci Rep. 2014;4:5963. doi: 10.1038/srep05963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vato A, Szymanski FD, Semprini M, Mussa-Ivaldi FA, Panzeri S. A bidirectional brain-machine interface algorithm that approximates arbitrary force-fields. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fetz EE. Sensorimotor rehabilitation: at the crossroads of basic and clinical sciences. 1. Vol. 218. Elsevier B.V; 2015. Restoring motor function with bidirectional neural interfaces; pp. 241–252. [DOI] [PubMed] [Google Scholar]
- 162.Mavoori J, Jackson A, Diorio C, Fetz E. An autonomous implantable computer for neural recording and stimulation in unrestrained primates. J Neurosci Methods. 2005;148(1):71–7. doi: 10.1016/j.jneumeth.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 163.Zanos S, Richardson AG, Shupe L, Miles FP, Fetz EE. The neurochip-2: an autonomous head-fixed computer for recording and stimulating in freely behaving monkeys. IEEE Trans Neural Syst Rehabil Eng. 2011;19(4):427–35. doi: 10.1109/TNSRE.2011.2158007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stacey WC, Litt B. Devices for Seizure Control. 2008;4(4):190–201. doi: 10.1038/ncpneuro0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.D’Alessandro M, Esteller R, Vachtsevanos G, Hinson A, Echauz J, Litt B. Epileptic seizure prediction using hybrid feature selection over multiple intracranial EEG electrode contacts: a report of four patients. IEEE Trans Biomed Eng. 2003;50(5):603–15. doi: 10.1109/tbme.2003.810706. [DOI] [PubMed] [Google Scholar]
- 166.Howbert JJ, Patterson EE, Stead SM, Brinkmann B, Vasoli V, Crepeau D, Vite CH, Sturges B, Ruedebusch V, Mavoori J, Leyde K, Sheffield WD, Litt B, Worrell GA. Forecasting seizures in dogs with naturally occurring epilepsy. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0081920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schalk G. Can Electrocorticography (ECoG) support robust and powerful brain-computer interfaces? Front Neuroeng. 2010;3:9. doi: 10.3389/fneng.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372–5. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuzum D, Takano H, Shim E, Reed JC, Juul H, Richardson AG, de Vries J, Bink H, Dichter MA, Lucas TH, Coulter DA, Cubukcu E, Litt B. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat Commun. 2014;5:5259. doi: 10.1038/ncomms6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14(12):1599–605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bink H, Lai Y, Saudari SR, Helfer B, Viventi J, Van der Spiegel J, Litt B, Kagan C. Flexible organic electronics for use in neural sensing. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:5400–3. doi: 10.1109/IEMBS.2011.6091335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bundy DT, Wronkiewicz M, Sharma M, Moran DW, Corbetta M, Leuthardt EC. Using ipsilateral motor signals in the unaffected cerebral hemisphere as a signal platform for brain-computer interfaces in hemiplegic stroke survivors. J Neural Eng. 2012;9(3):036011. doi: 10.1088/1741-2560/9/3/036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spüler M, Walter A, Ramos-Murguialday A, Naros G, Birbaumer N, Gharabaghi A, Rosenstiel W, Bogdan M. Decoding of motor intentions from epidural ECoG recordings in severely paralyzed chronic stroke patients. J Neural Eng. 2014;11(6):066008. doi: 10.1088/1741-2560/11/6/066008. [DOI] [PubMed] [Google Scholar]
- 174.del Valle J, Navarro X. International review of neurobiology. 1. Vol. 109. Elsevier; 2013. Interfaces with the peripheral nerve for the control of neuroprostheses; pp. 63–83. [DOI] [PubMed] [Google Scholar]
- 175.Warwick K, Gasson M, Hutt B, Goodhew I, Kyberd P, Andrews B, Teddy P, Shad A. The application of implant technology for cybernetic systems. Arch Neurol. 2003;60(10):1369–73. doi: 10.1001/archneur.60.10.1369. [DOI] [PubMed] [Google Scholar]
- 176.Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs. J Hand Surg Am. 2004;29(4):605–15. doi: 10.1016/j.jhsa.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 177.Hebert JS, Chan KM, Dawson MR. Cutaneous sensory outcomes from three transhumeral targeted reinnervation cases. Prosthet Orthot Int. 2016 doi: 10.1177/0309364616633919. [DOI] [PubMed] [Google Scholar]
- 178.Kuiken TA, Dumanian GA, Lipshutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–53. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 179.Langhals NB, Woo SL, Moon JD, Larson JV, Leach MK, Cederna PS, Urbanchek MG. Electrically stimulated signals from a long-term regenerative peripheral nerve interface. Conf Proc IEEE Eng Med Biol Soc. 2014;48109:1989–92. doi: 10.1109/EMBC.2014.6944004. [DOI] [PubMed] [Google Scholar]
- 180.Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133(6):1380–94. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 181.Sando IC, Leach MK, Woo SL, Moon JD, Cederna PS, Langhals NB, Urbanchek MG. Regenerative peripheral nerve interface for prostheses control: electrode comparison. J Reconstr Microsurg. 2015 Oct 26;32(03):194–9. doi: 10.1055/s-0035-1565248. 2016. [DOI] [PubMed] [Google Scholar]
- 182.Woo SL, Urbanchek MG, Leach MK, Moon JD, Cederna P, Langhals NB. Quantification of muscle-derived signal interference during monopolar needle electromyography of a peripheral nerve interface in the rat hind limb. Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual Int Conf of IEEE; 2014; pp. 4382–5. [DOI] [PubMed] [Google Scholar]
- 183.Popović MB. Control of neural prostheses for grasping and reaching. Med Eng Phys. 2003;25(1):41–50. doi: 10.1016/s1350-4533(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 184.Choudhary M, van Mastrigt R, van Asselt E. Inhibitory effects of tibial nerve stimulation on bladder neurophysiology in rats. Springerplus. 2016;5(1):35. doi: 10.1186/s40064-016-1687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schober MS, Sulkowski JP, Lu PL, Minneci PC, Deans KJ, Teich S, Alpert SA. Sacral nerve stimulation for pediatric lower urinary tract dysfunction: development of a standardized pathway with objective urodynamic outcomes. J Urol. 2015;194(6):1721–6. doi: 10.1016/j.juro.2015.06.090. [DOI] [PubMed] [Google Scholar]
- 186.Sulkowski JP, Nacion KM, Deans KJ, Minneci PC, Levitt MA, Mousa HM, Alpert SA, Teich S. Sacral nerve stimulation: a promising therapy for fecal and urinary incontinence and constipation in children. J Pediatr Surg. 2014;50(10):1644–7. doi: 10.1016/j.jpedsurg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 187.Ay I, Nasser R, Simon B, Ay H. Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stimul. 2015;9(2):166–73. doi: 10.1016/j.brs.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, Huang A, Lam K, Simon B, Baker DG. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation Technol Neural Interface. 2016;2015 doi: 10.1111/ner.12398. n/a – n/a. [DOI] [PubMed] [Google Scholar]
- 189.Hoffmann KP, Koch KP, Stieglitz T. Implantable microelectrodes as an interface to the peripheral nervous system. Biomed Tech. 2005;50(1):845–84. [Google Scholar]
- 190.Rodríguez FJ, Ceballos D, Schüttler M, Valero A, Valderrama E, Stieglitz T, Navarro X. Polyimide cuff electrodes for peripheral nerve stimulation. J Neurosci Methods. 2000;98(2):105–18. doi: 10.1016/s0165-0270(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 191.Inmann A, Haugland M. Functional evaluation of natural sensory feedback incorporated in a hand grasp neuroprosthesis. Med Eng Phys. 2004;26(6):439–47. doi: 10.1016/j.medengphy.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 192.Matzel K, Stadelmaier U, Hohenfellner M, Hohenberger W. Chronic sacral spinal nerve stimulation for fecal incontinence: Long-term results with foramen and cuff electrodes. Dis Colon Rectum. 2001;44(1):59–66. doi: 10.1007/BF02234822. [DOI] [PubMed] [Google Scholar]
- 193.Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J Neurosci Methods. 1996;64(1):95–103. doi: 10.1016/0165-0270(95)00123-9. [DOI] [PubMed] [Google Scholar]
- 194.Polasek KH, Hoyen HA, Keith MW, Kirsch RF, Tyler DJ. Spiral nerve cuff electrodes for an upper extremity neuroprosthesis. Annu Int Conf IEEE Eng Med Biol - Proc; 2006; pp. 3584–7. [DOI] [PubMed] [Google Scholar]
- 195.Polasek KH, Hoyen HA, Keith MW, Tyler DJ. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Trans Neural Syst Rehabil Eng. 2007;15(1):76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- 196.Gustafson KJ, Pinault GC, Neville JJ, Syed I, Davis JA, Jr, Jean-Claude J, Triolo RJ. Fascicular anatomy of human femoral nerve: implications for neural prostheses using nerve cuff electrodes. J Rehabil Res Dev. 2009;46(7):973–84. doi: 10.1682/jrrd.2008.08.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gustafson KJ, Grinberg Y, Joseph S, Triolo RJ. Human distal sciatic nerve fascicular anatomy: Implications for ankle control using nerve-cuff electrodes. J Rehabil Res Dev. 2012;49(2):309. doi: 10.1682/jrrd.2010.10.0201. [DOI] [PubMed] [Google Scholar]
- 198.Bäumer P, Weiler M, Bendszus M, Pham M. Somatotopic fascicular organization of the human sciatic nerve demonstrated by MR neurography. Neurology. 2015;84(17):1782–7. doi: 10.1212/WNL.0000000000001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lulic D, Ahmadian A, Baaj AA, Benbadis SR, Vale FL. Vagus nerve stimulation. Neurosurg Focus. 2009;27(3):E5. doi: 10.3171/2009.6.FOCUS09126. [DOI] [PubMed] [Google Scholar]
- 200.Bewernick B, Schlaepfer TE. Update on neuromodulation for treatment-resistant depression. F1000Research. 2015;4(0):1–10. doi: 10.12688/f1000research.6633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides longterm stable natural touch perception. Sci Transl Med. 2014;86(257):257ra138–257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tyler DJ, Durand DM. Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Ann Biomed Eng. 2003;31(6):633–42. doi: 10.1114/1.1569263. [DOI] [PubMed] [Google Scholar]
- 203.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10(4):294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 204.Schiefer MA, Triolo RJ, Tyler DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng. 2008;16(2):195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schiefer MA, Polasek KH, Triolo RJ, Pinault GCJ, Tyler DJ. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J Neural Eng. 2010;7(2):26006. doi: 10.1088/1741-2560/7/2/026006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schiefer MA, Freeberg M, Pinault GJ, Anderson J, Hoyen H, Tyler DJ, Triolo RJ. Selective activation of the human tibial and common peroneal nerves with a flat interface nerve electrode. J Neural Eng. 2013;10(5):056006. doi: 10.1088/1741-2560/10/5/056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens Bioelectron. 2010;26(1):62–9. doi: 10.1016/j.bios.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 208.Branner A, Normann RA. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res Bull. 2000;51(4):293–306. doi: 10.1016/s0361-9230(99)00231-2. [DOI] [PubMed] [Google Scholar]
- 209.Nannini N, Horch K. Muscle recruitment with intrafascicular electrodes. IEEE Trans Biomed Eng. 1991;38(8):769–76. doi: 10.1109/10.83589. [DOI] [PubMed] [Google Scholar]
- 210.Yoshida K, Horch K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. Biomed Eng IEEE …. 1993;40(5):492–4. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]
- 211.Rice ASC, McMahon SB, Wall PD. The electrophysiological consequences of electrode impalement of peripheral nerves in the rat. Brain Res. 1993;631(2):221–6. doi: 10.1016/0006-8993(93)91538-4. [DOI] [PubMed] [Google Scholar]
- 212.Lefurge T, Goodall E, Horch K, Stensaas L, Schoenberg A. Chronically implanted intrafascicular recording electrodes. Ann Biomed Eng. 1991;19(2):197–207. doi: 10.1007/BF02368469. [DOI] [PubMed] [Google Scholar]
- 213.Lawrence SM, Dhillon GS, Jensen W, Yoshida K, Horch KW. Acute peripheral nerve recording characteristics of polymer-based longitudinal intrafascicular electrodes. IEEE Trans Neural Syst Rehabil Eng. 2004;12(3):345–8. doi: 10.1109/TNSRE.2004.831491. [DOI] [PubMed] [Google Scholar]
- 214.Lawrence SM, Larsen JO, Horch KW, Riso R, Sinkjr T. Long-term biocompatibility of implanted polymerbased intrafascicular electrodes. J Biomed Mater Res. 2002;63(5):501–6. doi: 10.1002/jbm.10303. [DOI] [PubMed] [Google Scholar]
- 215.Yoshida K, Horch K. Closed-loop control of ankle position using muscle afferent feedback with functional neuromuscular stimulation. IEEE Trans Biomed Eng. 1996;43(2):167–76. doi: 10.1109/10.481986. [DOI] [PubMed] [Google Scholar]
- 216.Lago N, Udina E, Ramachandran A, Navarro X. Neurobiological assessment of regenerative electrodes for bidirectional interfacing injured peripheral nerves. IEEE Trans Biomed Eng. 2007;54(6):1129–37. doi: 10.1109/TBME.2007.891168. [DOI] [PubMed] [Google Scholar]
- 217.Lago N, Ceballos D, Rodríguez JF, Stieglitz T, Navarro X. Long-term assessment of axonal regeneration through polyimide regenerative electrodes to interface the peripheral nerve. Biomater. 2005;26(14):2021–31. doi: 10.1016/j.biomaterials.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 218.Klinge PM1, Vafa MA, Brinker T, Brandis A, Walter GF, Stieglitz T, Samii M, Wewetzer K. Immunohistochemical characterization of axonal sprouting and reactive tissue changes after long-term implantation of a polyimide sieve electrode to the transected adult rat sciatic nerve. Biomater. 2001;22(17):2333–43. doi: 10.1016/s0142-9612(00)00420-8. [DOI] [PubMed] [Google Scholar]
- 219.Gore RK, Choi Y, Bellamkonda R, English A. Functional recordings from awake, behaving rodents through a microchannel based regenerative neural interface. J Neural Eng. 2015;12(1):016017. doi: 10.1088/1741-2560/12/1/016017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Negredo P, Castro J, Lago N, Navarro X, Avendaño C. Differential growth of axons from sensory and motor neurons through a regenerative electrode: a stereological, retrograde tracer, and functional study in the rat. Neuroscience. 2004;128(3):605–15. doi: 10.1016/j.neuroscience.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 221.Bradley RM, Smoke RH, Akin T, Najafi K. Functional regeneration of glossopharyngeal nerve through micromachined sieve electrode arrays. Brain Res. 1992;594(1):84–90. doi: 10.1016/0006-8993(92)91031-9. [DOI] [PubMed] [Google Scholar]
- 222.Akin T, Najafi K, Smoke RH, Bradley RM. A micromachined silicon sieve electrode for nerve regeneration applications. IEEE Trans Biomed Eng. 1994;41(4):305–13. doi: 10.1109/10.284958. [DOI] [PubMed] [Google Scholar]
- 223.Mensinger AF, Anderson DJ, Buchko CJ, Johnson MA, Martin DC, Tresco PA, Silver RB, Highstein SM. Chronic recording of regenerating VIIIth nerve axons with a sieve electrode. J Neurophysiol. 2000;83(1):611–5. doi: 10.1152/jn.2000.83.1.611. [DOI] [PubMed] [Google Scholar]
- 224.Kawada T, Zheng C, Tanabe S, Uemura T, Sunagawa K, Sugimachi M. A sieve electrode as a potential autonomic neural interface for bionic medicine. Conf Proc IEEE Eng Med Biol Soc. 2004;6:4318–21. doi: 10.1109/IEMBS.2004.1404202. [DOI] [PubMed] [Google Scholar]
- 225.Srinivasan A, Tahilramani M, Bentley JT, Gore RK, Millard DC, Mukhatyar VJ, Joseph A, Haque AS, Stanley GB, English AW, Bellamkonda RV. Microchannel-based regenerative scaffold for chronic peripheral nerve interfacing in amputees. Biomaterials. 2015;41:151–65. doi: 10.1016/j.biomaterials.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stieglitz T, Beutel H, Meyer J-U. A flexible, light-weight multichannel sieve electrode with integrated cables for interfacing regenerating peripheral nerves. Sensors Actuators A Phys. 1997;60(1–3):240–3. [Google Scholar]
- 227.FitzGerald JJ, Lacour SP, McMahon SB, Fawcett JW. Microchannel electrodes for recording and stimulation: in vitro evaluation. IEEE Trans Biomed Eng. 2009;56(5):1524–34. doi: 10.1109/TBME.2009.2013960. [DOI] [PubMed] [Google Scholar]
- 228.Wallman L, Zhang Y, Laurell T, Danielsen N. The geometric design of micromachined silicon sieve electrodes influences functional nerve regeneration. Biomater. 2001;22(10):1187–93. doi: 10.1016/s0142-9612(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 229.Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, Schallert T, Thayer WP. The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res. 2015;230:207–30. doi: 10.1002/jnr.23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:1–13. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP. Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surg Res. 2012;177(2):392–400. doi: 10.1016/j.jss.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, Estler CJ, Boydston EA, Schallert T, Bittner GD. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol. 2010;104(2):695–703. doi: 10.1152/jn.01051.2009. [DOI] [PubMed] [Google Scholar]
- 233.Donaldson J, Shi R, Borgens R. Polyethylene glycol rapidly restores physiological functions in damaged sciatic nerves of guinea pigs. Neurosurgery. 2002;50(1):147–56. doi: 10.1097/00006123-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 234.FitzGerald JJ, Lago N, Benmerah S, Serra J, Watling CP, Cameron RE, Tarte E, Lacour SP, McMahon SB, Fawcett JW. A regenerative microchannel neural interface for recording from and stimulating peripheral axons in vivo. J Neural Eng. 2012;9(1):016010. doi: 10.1088/1741-2560/9/1/016010. [DOI] [PubMed] [Google Scholar]
- 235.Tyler DJ, Durand DM. A slowly penetrating interfascicular nerve electrode for selective activation of peripheral nerves. IEEE Trans Rehabil Eng. 1997;5(1):51–61. doi: 10.1109/86.559349. [DOI] [PubMed] [Google Scholar]
- 236.Bradley RM, Cao X, Akin T, Najafi K. Long-term chronic recordings from peripheral sensory fibers using a sieve electrode array. J Neurosci Methods. 1997;73(2):177–86. doi: 10.1016/s0165-0270(97)02225-5. [DOI] [PubMed] [Google Scholar]
- 237.Cope TC, Pinter MJ. The size principle: still working after all these years. Physiology. 1995;10:280–6. [Google Scholar]
- 238.Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. 2012;85(2):201–15. [PMC free article] [PubMed] [Google Scholar]
- 239.Maffiuletti Na. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110(2):223–34. doi: 10.1007/s00421-010-1502-y. [DOI] [PubMed] [Google Scholar]
- 240.Chou LW, Binder-Macleod SA. The effects of stimulation frequency and fatigue on the force-intensity relationship for human skeletal muscle. Clin Neurophysiol. 2007;118(6):1387–96. doi: 10.1016/j.clinph.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dreibati B, Lavet C, Pinti A, Poumarat G. Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann Phys Rehabil Med. 2010;53(4):266–77. doi: 10.1016/j.rehab.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 242.Decker MJ, Griffin L, Abraham LD, Brandt L. Alternating stimulation of synergistic muscles during functional electrical stimulation cycling improves endurance in persons with spinal cord injury. J Electromyogr Kinesiol. 2010;20(6):1163–9. doi: 10.1016/j.jelekin.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 243.Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol. 2011;111(10):2399–407. doi: 10.1007/s00421-011-2128-4. [DOI] [PubMed] [Google Scholar]
- 244.Kushibiki T, Okawa S, Hirasawa T, Ishihara M. Optogenetics: novel tools for controlling mammalian cell functions with light. Int J Photoenergy. 2014;2014 [Google Scholar]
- 245.Lebedev MA, Tate AJ, Hanson TL, Li Z, O’Doherty JE, Winans JA, Ifft PJ, Zhuang KZ, Fitzsimmons NA, Schwarz DA, Fuller AM, An JH, Nicolelis MAL. Future developments in brain-machine interface research. Clinics (Sao Paulo) 2011;66(Suppl 1):25–32. doi: 10.1590/S1807-59322011001300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liske H, Qian X, Anikeeva P, Deisseroth K, Delp S. Optical control of neuronal excitation and inhibition using a single opsin protein, ChR 2. Sci Rep. 2013;3:3110. doi: 10.1038/srep03110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Iyer SM, Delp SL. Optogenetic regeneration. Science (80-) 2014;344(6179):44–5. doi: 10.1126/science.1253088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Humphreys L, Ferrández JM, Fernández E. Long-term modulation and control of neuronal firing in excitable tissue using optogenetics. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics) 2011;6686 LNCS(PART 1):266–73. [Google Scholar]
- 249.Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J, Greensmith L, Lieberam I. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science (80-) 2014;344(6179):94–7. doi: 10.1126/science.1248523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Llewellyn ME, Thompson KR, Deisseroth K, Delp SL. Orderly recruitment of motor units under optical control in vivo. Nat Med. 2010;16(10):1161–5. doi: 10.1038/nm.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19(2):169–75. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- 253.Cheng MY, Wang EH, Steinberg GK. Optogenetic approaches to study stroke recovery. ACS Chem Neurosci. 2014;5(12):1144–5. doi: 10.1021/cn500216f. [DOI] [PubMed] [Google Scholar]
- 254.Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci. 2014;111(35):12913–8. doi: 10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cheng MY, Aswendt M, Steinberg GK. Optogenetic approaches to target specific neural circuits in post-stroke recovery. Neurotherapeutics. 2015;13(2):325–40. doi: 10.1007/s13311-015-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lüscher C, Pascoli V, Creed M. Optogenetic dissection of neural circuitry: from synaptic causalities to blue prints for novel treatments of behavioral diseases. Curr Opin Neurobiol. 2015;35:95–100. doi: 10.1016/j.conb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 257.Luscher C, Pollak P. Optogenetically inspired deep brain stimulation: linking basic with clinical research. Swiss Med Wkly. 2016;146:w14278. doi: 10.4414/smw.2016.14278. [DOI] [PubMed] [Google Scholar]
- 258.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dreosti E, Odermatt B, Dorostkar MM, Lagnado L. A genetically encoded reporter of synaptic activity in vivo. Nat Methods. 2009;6(12):883–9. doi: 10.1038/nmeth.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–40. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, Patel R, Pulver SR, Wardill TJ, Fischer E, Schüler C, Chen TW, Sarkisyan KS, Marvin JS, Bargmann CI, Kim DS, Kügler S, Lagnado L, Hegemann P, Gottschalk A, Schreiter ER, Looger LL. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wu F, Stark E, Im M, Cho IJ, Yoon ES, Buzsáki G, Wise KD, Yoon E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. J Neural Eng. 2013;10(5):056012. doi: 10.1088/1741-2560/10/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.He L, Zhang Y, Ma G, Tan P, Li Z, Zhang S, Wu X, Jing J, Fang S, Zhou L, Wang Y, Huang Y, Hogan PG, Han G, Zhou Y. Near-infrared photoactivatable control of Ca 2+ signaling and optogenetic immunomodulation. Elife. 2015;4:160. doi: 10.7554/eLife.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hososhima S, Yuasa H, Ishizuka T, Hoque MR, Yamashita T, Yamanaka A, Sugano E, Tomita H, Yawo H. Nearinfrared (NIR) up-conversion optogenetics. Sci Rep. 2015;5:16533. doi: 10.1038/srep16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nature Neurosci. 2013;16:1499–508. [Google Scholar]
- 266.Pashaie R, Anikeeva P, Lee JH, Prakash R, Yizhar O, Prigge M, Chander D, Richner TJ, Williams J. Optogenetic brain interfaces. IEEE Rev Biomed Eng. 2014;7:3–30. doi: 10.1109/RBME.2013.2294796. [DOI] [PubMed] [Google Scholar]
- 267.Mellinger J, Schalk G, Braun C, Preissl H, Rosenstiel W, Birbaumer N, Kübler A. An MEG-based brain-computer interface (BCI) Neuroimage. 2007;36(3):581–93. doi: 10.1016/j.neuroimage.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shih JJ, Krusienski DJ, Wolpaw JR. Mayo Clinic Proceedings. Vol. 87. Elsevier Inc; 2012. Brain-computer interfaces in medicine; pp. 268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fukuma R, Yanagisawa T, Yorifuji S, Kato R, Yokoi H, Hirata M, Saitoh Y, Kishima H, Kamitani Y, Yoshimine T. Closed-loop control of a neuroprosthetic hand by magnetoencephalographic signals. PLoS One. 2015;10(7):1–13. doi: 10.1371/journal.pone.0131547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McClay W, Yadav N, Ozbek Y, Haas A, Attias H, Nagarajan S. A Real-time magnetoencephalography braincomputer interface using interactive 3D visualization and the Hadoop Ecosystem. Brain Sci. 2015;5(4):419–40. doi: 10.3390/brainsci5040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Foldes ST, Weber DJ, Collinger JL. MEG-based neurofeedback for hand rehabilitation. J Neuroeng Rehabil. 2015;12(1):85. doi: 10.1186/s12984-015-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lu J, Mamun KA, Chau T. Online transcranial Doppler ultrasonographic control of an onscreen keyboard. Front Hum Neurosci. 2014;8:199. doi: 10.3389/fnhum.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Myrden AJB, Kushki A, Sejdić E, Guerguerian A-M, Chau T. A brain-computer interface based on bilateral transcranial Doppler ultrasound. PLoS One. 2011;6(9):e24170. doi: 10.1371/journal.pone.0024170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tych RE, Gofeld M, Jarvik JG, Kliot M, Loeser JD, McClintic AM, Ollos RJ, Pederson KD, Sparks RE, Terman GW, Mourad PD. Neuropathic tissue responds preferentially to stimulation by intense focused ultrasound. Ultrasound Med Biol. 2013;39(1):111–6. doi: 10.1016/j.ultrasmedbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Saffari CJWN., JR Ultrasonic stimulation of peripheral nervous tissue: an investigation into mechanisms. J Phys Conf Ser. 2015;581(1):12003. [Google Scholar]
- 276.Juan EJ, González R, Albors G, Ward MP, Irazoqui P. Vagus nerve modulation using focused pulsed ultrasound: potential applications and preliminary observations in a rat. Int J Imaging Syst Technol. 2014 Mar 1;24(1):67–71. doi: 10.1002/ima.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Foley JL, Little JW, Vaezy S. Image-Guided High-intensity focused ultrasound for conduction block of peripheral nerves. Ann Biomed Eng. 2007;35(1):109–19. doi: 10.1007/s10439-006-9162-0. [DOI] [PubMed] [Google Scholar]
- 278.Lee Y-F, Lin C-C, Cheng J-S, Chen G-S. Nerve conduction block in diabetic rats using high-intensity focused ultrasound for analgesic applications. Br J Anaesth. 2015;114(5):840–6. doi: 10.1093/bja/aeu443. [DOI] [PubMed] [Google Scholar]
- 279.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–50. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 280.Aliğauskienė M, Truffert A, Vaičienė N, Magistris MR. Transcranial magnetic stimulation in clinical practice. Medicina (B Aires) 2005;41(10):813–24. [PubMed] [Google Scholar]
- 281.Fernandez E, Alfaro A, Tormos JM, Climent R, Martínez M, Vilanova H, Walsh V, Pascual-Leone A. Mapping of the human visual cortex using image-guided transcranial magnetic stimulation. Brain Res Brain Res Protoc. 2002;10(2):115–24. doi: 10.1016/s1385-299x(02)00189-7. [DOI] [PubMed] [Google Scholar]
- 282.Gothe J, Brandt SA, Irlbacher K, Roricht S, Sabel BA, Meyer B-U. Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain. 2002;125(3):479–90. doi: 10.1093/brain/awf045. [DOI] [PubMed] [Google Scholar]
- 283.Rao RP, Stocco A, Bryan M, Sarma D, Youngquist TM, Wu J, Prat CS. A direct brain-to-brain interface in humans. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Grau C, Ginhoux R, Riera A, Nguyen TL, Chauvat H, Berg M, Amengual JL, Pascual-Leone A, Ruffini G. Conscious brain-to-brain communication in humans using non-invasive technologies. PLoS One. 2014;9(8):1–6. doi: 10.1371/journal.pone.0105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chernov M, Roe AW. Infrared neural stimulation: a new stimulation tool for central nervous system applications. Neurophotonics. 2014;1(1):011011. doi: 10.1117/1.NPh.1.1.011011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Richter C-P, Matic AI, Wells JD, Jansen ED, Walsh JT. Neural stimulation with optical radiation. Laser Photon Rev. 2011;5(1):68–80. doi: 10.1002/lpor.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10(6):064003. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 288.Wells J, Kao C, Mariappan K, Albea J, Jansen ED, Konrad P, Mahadevan-Jansen A. Optical stimulation of neural tissue in vivo. Opt Lett. 2005;30(5):504–6. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 289.Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Methods. 2007;163(2):326–37. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Izzo AD, Walsh JT, Jr, Jansen ED, Bendett M, Webb J, Ralph H, Richter CP. Optical parameter variability in laser nerve stimulation: a study of pulse duration, repetition rate, and wavelength. IEEE Trans Biomed Eng. 2007;54(6):1108–14. doi: 10.1109/TBME.2007.892925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Goyal V, Rajguru S, Matic AI, Stock SR, Richter CP. Acute damage threshold for infrared neural stimulation of the cochlea: functional and histological evaluation. Anat Rec. 2012;295(11):1987–99. doi: 10.1002/ar.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Cayce JM, Friedman RM, Chen G, Jansen ED, Mahadevan-Jansen A, Roe AW. Infrared neural stimulation of primary visual cortex in non-human primates. Neuroimage. 2014;84:181–90. doi: 10.1016/j.neuroimage.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kuo JR, Lin SS, Liu J, Chen SH, Chio CC, Wang JJ, Liu JM. Deep brain light stimulation effects on glutamate and dopamine concentration. Biomed Opt Express. 2014;6(1):23. doi: 10.1364/BOE.6.000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cayce JM, Wells JD, Malphrus JD, Kao C, Thomsen S, Tulipan NB, Konrad PE, Jansen ED, Mahadevan-Jansen A. Infrared neural stimulation of human spinal nerve roots in vivo. Neurophotonics. 2015;2(1):15007. doi: 10.1117/1.NPh.2.1.015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wells JD, Thomsen S, Whitaker P, Jansen ED, Kao CC, Konrad PE, Mahadevan-Jansen A. Optically mediated nerve stimulation: identification of injury thresholds. Lasers Surg Med. 2007;39(6):513–26. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 296.Duke AR, Cayce JM, Malphrus JD, Konrad P, Mahadevan-Jansen A, Jansen ED. Combined optical and electrical stimulation of neural tissue in vivo. J Biomed Opt. 2015;14(6):060501. doi: 10.1117/1.3257230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Duke AR, Lu H, Jenkins MW, Chiel HJ, Jansen ED. Spatial and temporal variability in response to hybrid electro-optical stimulation. J Neural Eng. 2012;9(3):036003. doi: 10.1088/1741-2560/9/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Duke AR, Peterson E, Mackanos MA, Atkinson J, Tyler D, Jansen ED. Hybrid electro-optical stimulation of the rat sciatic nerve induces force generation in the plantarflexor muscles. J Neural Eng. 2012;9(6):066006. doi: 10.1088/1741-2560/9/6/066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shapiro MG, Homma K, Villarreal S, Richter C-P, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tan X, Rajguru S, Young H, Xia N, Stock SR, Xiao X, Richter C-P. Radiant energy required for infrared neural stimulation. Sci Rep. 2015;5:13273. doi: 10.1038/srep13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kennedy PR, Mirra SS, Bakay RAE. The cone electrode: ultrastructural studies following long-term recording in rat and monkey cortex. Neurosci Lett. 1992;142(1):89–94. doi: 10.1016/0304-3940(92)90627-j. [DOI] [PubMed] [Google Scholar]
- 302.Bartels J, Andreasen D, Ehirim P, Mao H, Seibert S, Wright EJ, Kennedy P. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. J Neurosci Methods. 2008;174(2):168–76. doi: 10.1016/j.jneumeth.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shen W, Karumbaiah L, Liu X, Saxena T, Chen S, Patkar R, Bellamkonda RV, Allen MG. Extracellular matrixbased intracortical microelectrodes: toward a microfabricated neural interface based on natural materials. Microsystems Nanoeng. 2015;1:15010. doi: 10.1038/micronano.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shen W, Struzyna LA, Cullen DK, Allen MG. Fabrication of neural microelectrode arrays from extracellular matrix. Solid-State Sensors, Actuators, and Microsystems Technical Digest. Hilton Head Workshop. 2016 [Google Scholar]
- 305.Cullen DK, Patel AR, Doorish JF, Smith DH, Pfister BJ. Developing a tissue-engineered neural-electrical relay using encapsulated neuronal constructs on conducting polymer fibers. J Neural Eng. 2008;5(4):374–84. doi: 10.1088/1741-2560/5/4/002. [DOI] [PubMed] [Google Scholar]
- 306.Cullen DK, Wolf JA, Smith DH, Pfister BJ. Neural tissue engineering for neuroregeneration and biohybridized interface microsystems in vivo (Part 2) Crit Rev Biomed Eng. 2011;39(3):241–59. doi: 10.1615/critrevbiomedeng.v39.i3.40. [DOI] [PubMed] [Google Scholar]
- 307.Cullen DK, Wolf JA, Vernekar VN, Vukasinovic J, La-Placa MC. Neural tissue engineering and biohybridized microsystems for neurobiological investigation in vitro (Part 1) Crit Rev Biomed Eng. 2011;39(3):201–40. doi: 10.1615/critrevbiomedeng.v39.i3.30. [DOI] [PubMed] [Google Scholar]
- 308.Purcell EK, Seymour JP, Yandamuri S, Kipke DR. In vivo evaluation of a neural stem cell-seeded prosthesis. J Neural Eng. 2009;6(2):26005. doi: 10.1088/1741-2560/6/2/026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.De Faveri S, Maggiolini E, Miele E, De Angelis F, Cesca F, Benfenati F, Fadiga L. Bio-inspired hybrid microelectrodes: a hybrid solution to improve long-term performance of chronic intracortical implants. Front Neuroeng. 2014;7:7. doi: 10.3389/fneng.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Huang JH, Cullen DK, Browne KD, Groff R, Zhang J, Pfister BJ, Zager EL, Smith DH. Long-term survival and integration of transplanted engineered nervous tissue constructs promotes peripheral nerve regeneration. Tissue Eng Part A. 2009 Jul 10;15(7):1677–85. doi: 10.1089/ten.tea.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Struzyna LA, Wolf JA1, Mietus CJ, Adewole DO, Chen HI, Smith DH, Cullen DK. Rebuilding brain circuitry with living micro-tissue engineered neural networks. Tissue Eng. 2015;21:2744–56. doi: 10.1089/ten.tea.2014.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cullen DK, Tang-Schomer MD, Struzyna LA, Patel AR, Johnson VE, Wolf JA, Smith DH. Microtissue engineered constructs with living axons for targeted nervous system reconstruction. Tissue Eng Part A. 2012;18:120817094501006. doi: 10.1089/ten.tea.2011.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Harris JP, Struzyna LA, Murphy PL, Adewole DO, Kuo E, Cullen DK. Advanced biomaterial strategies to transplant preformed micro-tissue engineered neural networks into the brain. J Neural Eng. 2016;13(1):016019. doi: 10.1088/1741-2560/13/1/016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Struzyna LA, Katiyar K, Cullen DK. Living scaffolds for neuroregeneration. Curr Opin Solid State Mater Sci. 2014;18(6):308–18. doi: 10.1016/j.cossms.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Struzyna LA, Harris JP, Katiyar KS, Chen HI, Cullen DK. Restoring nervous system structure and function using tissue engineered living scaffolds. Neural Regen Res. 2015;10(5):679–85. doi: 10.4103/1673-5374.156943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Brault MW. Americans with disabilities: 2010 household economic studies. US Census Bur. 2012;423:1–24. [Google Scholar]
- 317.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 318.Media Relations TALSA. The ALS Association: fighting Lou Gehrig disease on multiple fronts. Rare Dis. 2013;1:e24910. doi: 10.4161/rdis.24910. [DOI] [PMC free article] [PubMed] [Google Scholar]