Abstract
Background
Primary invasive cutaneous aspergillosis is a rare fungal infection that occurs mostly in immunocompromised patients. Newborns of very low birth weight present a high risk for this type of infection due to an immaturity of the cutaneous barrier and of the immune system.
Case presentation
We describe here a case of simultaneous invasive cutaneous aspergillosis in two preterm twins. Two male preterm bichorionic biamniotic twins (A & B) were born at a general hospital by spontaneous normal delivery at 24 weeks and 6 days of gestation. They were transferred to our hospital where they receive surfactant, antibiotics and hydrocortisone. Six days later, twin A showed greenish lesions in the umbilical region. The spectrum of antibiotic therapy was broadened and fluconazole was added. The umbilical catheters of the two twins were removed and replaced by epicutaneo-cava venous catheters and the cultures were positive for Aspergillus fumigatus. Fluconazole was replaced in both twins by liposomal amphotericin B and the incubators were changed. The serum galactomannan was also positive for both twins. At day 10, yellowish lesions appeared in the abdominal region in twin B. He died on day 18 following complications related to his prematurity. Concerning the twin A, serum galactomannan was negative on day 30; liposomal amphotericin B was stopped 1 week later, with a relay by econazole (cream). His condition improved and on day 66 he was transferred for follow-up at the general hospital where he was born.
Conclusion
The source of contamination by A. fumigatus was not identified, but other similar cases from the literature include construction work at or near the hospital, oximeter sensors, latex finger stalls, non-sterile gloves, humidifying chambers of incubators, bedding and adhesive tapes. The skin fragility of preterm newborns is an excellent potential entry point for environmental fungal infections. These cases highlight the importance of suspecting primary cutaneous aspergillosis in extremely low birth weight neonates with rapidly progressive necrotic lesions.
Keywords: Primary cutaneous aspergillosis, Newborn, Aspergillus, A. fumigatus, Preterm, Premature, Invasive aspergillosis
Background
Primary cutaneous aspergillosis (PCA) is an uncommon fungal infection that occurs mostly in preterm infants and other immunocompromised populations. Neonates of extremely low birth weight are at high risk for this type of infection because of decreased qualitative immune defenses and defects in the skin barrier [1]. We describe here the case of simultaneous abdominal invasive cutaneous aspergillosis in preterm twins, confirmed by fungal culture and high levels of Aspergillus galactomannan antigen in the serum.
Case presentation
Two male preterm bichorionic biamniotic twins were born (day 0) at a general hospital by spontaneous normal delivery at 24 weeks and 6 days of gestation. The 31-year-old mother did not have any particular medical history and did not receive prophylactic antibiotic treatment or antenatal steroids. The pregnancy was obtained by in vitro fertilization and was uneventful until delivery. The second twin (Twin A) was born 3 h after his brother (Twin B). Their birth weights were 614 g (Twin A) and 760 g (Twin B) and the Apgar scores were 5–10 (Twin A) and 3–7 (Twin B) at 1 and 10 min, respectively. Central umbilical vein catheters were placed in both twins after birth. They were immediately intubated and given one dose of surfactant, before being transferred to the Neonatal Intensive Care Unit (NICU) of Hautepierre University Hospital (Strasbourg, France) for further management.
Twin A: evolution
On admission in NICU, the twin A received one additional dose of surfactant for hyaline membrane disease. An early neonatal infection following a presumed chorioamniotitis was suspected and systemic antibiotic therapy was started with amoxicillin and amikacin, without antifungal prophylaxis. He was treated with low doses of hydrocortisone during the first 10 days of life (0.5 mg/kg, twice a day for 7 days and then once a day for 3 days). This treatment was conducted in application of a new protocol after preliminary results of a study evaluating the efficacy of hydrocortisone in preventing bronchopulmonary dysplasia in very preterm neonates [2].
On day 3 after birth, antibiotics were discontinued because of a normalized C reactive protein (CRP). Six days after birth, twin A presented crusted and greenish cutaneous lesions on the abdomen and at the umbilicus. Intravenous amikacin, cefotaxime, vancomycin and fluconazole (8 mg/kg/d) were initiated. The umbilical catheter was removed in both twins and replaced by a peripheral venous catheter in the axillary vein. On day 8, the culture of this umbilical catheter (sent by the clinical department for bacteriological analysis only) was positive for Staphylococcus epidermidis identified by MALDI-TOF mass spectrometry (Brüker Daltonics, Ettlingen, Germany), and for molds, with a count of 20 to 40 fungal colonies on Columbia agar with 5% sheep’s blood (Oxoid, Dardilly, France). Moreover, on the same day, the cultures of swabs from a skin lesion and from the umbilical cord were also positive with more of 40 fungal colonies on ChromID™ Candida agar (bioMérieux, Marcy l’Etoile, France), macroscopically and microscopically compatible with Aspergillus spp. Fluconazole was therefore replaced on day 8 with liposomal amphotericin B (5 mg/kg/d) with continuation of vancomycin. Incubators were replaced for both twins. A. fumigatus was subsequently identified by phenotypic characteristics after culture on 2% malt extract agar (in-house) in all of these samples. Many colonies of a susceptible strain of Candida guilliermondii identified by MALDI-TOF mass spectrometry were also found in the culture of a skin lesion. The level of serum Aspergillus galactomannan antigen (enzymatic immuno-assay Platelia® Aspergillus Ag Bio-Rad, Marnes-la-Coquette, France) was very high on day 9. Indeed, it reached a value >5 (index) with a threshold of positivity of 0.5 (index). A. fumigatus was also found in low quantities in urine samples collected on day 9, but the culture of tracheal aspirations as well as blood cultures remained negative.
A second smear of the abdominal cutaneous lesions was collected on day 14. The culture revealed only the presence of S. epidermidis and Enterococcus faecalis, without fungi. The index values of serum Aspergillus galactomannan antigen decreased progressively, and were negative on day 30. The treatment with amphotericin B was stopped 7 days later, following the clinical improvement of the lesions, the negative fungal culture and the confirmation of the negative antigenemia on another sample. A relay by a topical treatment with econazole cream was started. A last smear of the healing abdominal skin lesions was collected on day 48 and the culture was sterile. Globally, management of some complications linked to premature birth were delayed because of the infectious context in the twin B, but the outcome was favorable. On day 66, the twin A was transferred back to the neonatal department of the general hospital where he was born (Fig. 1).
Fig. 1.
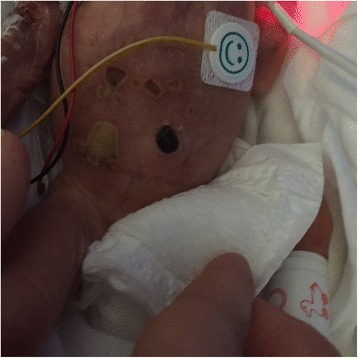
Abdominal lesions of twin A (Day 10)
Twin B: evolution
On admission to the NICU, the twin B received three additional doses of surfactant for hyaline membrane disease. He was treated with amoxicillin and amikacin until the CRP normalized on day 6 and with low doses of hydrocortisone during the first 10 days of life as part of the same protocol as his brother.
Six days after birth, his brother (twin A) presented abdominal cutaneous lesions leading to the removal of the umbilical catheter in both twins and their replacement by peripheral venous catheters in the axillary vein. On day 8, the culture of the umbilical catheter of twin B was also positive for S. epidermidis and for molds later identified as A. fumigatus, with a count of 20 to 40 fungal colonies on bacteriological media. Antifungal treatment with liposomal amphotericin B was started, in the hypothesis of a similar infection with A. fumigatus affecting the two brothers, even though twin A presented no skin lesion at this time. Incubators were replaced for both twins. The level of serum Aspergillus galactomannan antigen was very high on day 8, also reaching a value >5 (index). No other determination of the serum Aspergillus galactomannan antigen was determined for twin B. Like for his brother, A. fumigatus was found in low quantities in urine samples collected on day 9, but the culture of tracheal aspirations as well as blood cultures remained negative. On day 10, an erythematous yellowish cutaneous lesion appeared on the abdomen of twin B around the umbilicus. This lesion later became crusted. An empiric antibiotherapy with vancomycin was initiated with continued treatment with liposomal amphotericin B. However, the culture of a smear of these lesions remained sterile. A treatment by cefotaxime was added on day 11, replaced by imipenem on day 16 after identification of Bacillus cereus in a positive blood-culture.
Besides these infectious complications, the twin B suffered from several complications linked to premature birth. In the face of clinical deterioration with multiple organ failures despite appropriate treatments, a multidisciplinary decision in agreement with the parents was made on day 17 to limit active treatments. The twin B died the following day. No autopsy was performed (Fig. 2).
Fig. 2.
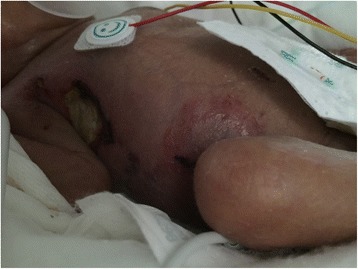
Abdominal lesions of twin B (Day 17)
Epidemiological investigation
After diagnosis of invasive aspergillosis in the twin A, an epidemiological investigation was undertaken to identify the source of contamination by Aspergillus and to prevent any outbreak of further cases of suspected nosocomial aspergillosis. On day 12, multiple samples had been collected in the hospital room of the twins and in the diaper storage area. Samples were also collected in the transfer incubator used the first day of life of twin B and in his incubator used until day 8, date of the diagnosis of the fungal cutaneous infection. This investigation did not enable finding the source of A. fumigatus. No epidemiological investigation was performed at the hospital where the twins were born. There was no construction work or renovation going on at this time in both hospitals’ surroundings, and no other cases were diagnosed in the following months (Fig. 3).
Fig. 3.
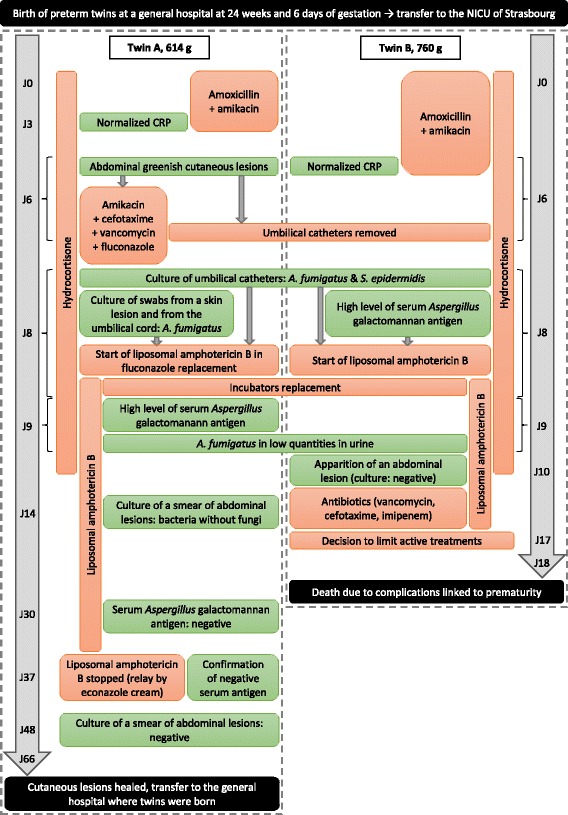
Timeline
Literature review and discussion
Including the two present cases, 41 cases of primary cutaneous aspergillosis have been published so far in reviews [3–6] and clinical cases of infection in preterm neonates (Tables 1 and 2), with a mean of gestational age of 25 weeks (23–32) and a mean of birth weight of 720 g (420–1500).
Table 1.
Demographics, risk factors and clinical aspects of primary cutaneous aspergillosis in preterm neonates
| N° [Ref] | Gestation (weeks) | Sex | Birth weight (g) | Age (days) | Antibiotics (days) | Steroids (days) | Type of skin lesions | Site of skin lesions |
|---|---|---|---|---|---|---|---|---|
| 1 [11] | 26 | F | 800 | 14 | Yes (7) | NR | Single erythematous nodule with pustules | Left mid-back |
| 2 [12] | 25 | M | 635 | 5 | Yes | NR | Erythematous papules with central pustules becoming necrotic ulcers | Lower back, legs |
| 3 [19] | 25 | F | 785 | 6 | Yes (6) | Yes (1) | Crusted lesions with erythematous borders | Back, axillae |
| 4 [19] | Preterm | M | 670 | 7 | Yes | No | NR | NR |
| 5 [33] | 24 | M | 710 | 6 | Yes | NR | Two ulcerations surrounded by multiple abscesses | Back |
| 6 [15] | 27 | M | 1500 | 8 | Yes (7) | Yes (2) | Indurated pustule surrounded by erythema becoming eroded and with marginal pustules | Foot |
| 7 [34] | 26 | M | 825 | 10 | NR | NR | Maculopapular rash becoming pustular lesions | NR |
| 8 [35] | 25 | NR | 615 | 7 | NR | NR | NR | NR |
| 9 [3] | 26 | M | 960 | 30 | Yes | Yes (8) | Dark-red plaque and pustules | Leg |
| 10 [13] | 32 | F | 1470 | 13 | Yes (7) | NR | Indurated abscess with erythematous overlying skin | Axilla |
| 11 [16] | 25 | M | 530 | 3 | Yes | Yes | Greyish-white lesions becoming eschars | Thighs, penis |
| 12 [16] | 29 | M | 440 | 6 | Yes | Yes | Pustules becoming eschars | Thighs, penis |
| 13 [16] | 26 | M | 540 | 5 | Yes | Yes | Erythema becoming eschars | Genital area |
| 14 [16] | 26 | M | 715 | 8 | Yes | No | Erythema leading to skin defects | Penis, genital area, abdomen |
| 15 [7] | Preterm | M | 900 | 17 | Yes (17) | No | Ulcers with black eschars | Cheek, nose, ear |
| 16 [20] | Preterm | NR | 792 | 3 | NR | NR | Necrotic skin lesions | Abdomen |
| 17 [13] | Preterm | NR | 420 | 7 | NR | NR | Necrotic skin lesions | Abdomen |
| 18 [21] | 29 | NR | 1300 | 14 | Yes | NR | Erythematous skin lesion becoming necrotic ulcers | Near the mouth |
| 19 [5] | 25 | M | 750 | 13 | Yes (7) | Yes | Erythematous lesions with a central pustule and yellow crust becoming necrotic eschars | Scrotum, buttock |
| 20 [36] | 24 | NR | 510 | 12 | Yes (12) | NR | Abrasion becoming necrotic eschar surrounded by erythema | Abdomen, chest, back |
| 21 [25] | 26 | M | 825 | NR | Yes | Yes | Necrotic purpura and erythema with satellite lesions | Neck, abdomen, lower extremity |
| 22 [25] | 28 | F | 580 | NR | Yes | No | Necrotic ulcers becoming eschar | Sacrum |
| 23 [25] | 26 | M | 580 | NR | Yes | Yes | Crusted erosion becoming eschar | Chest tube site |
| 24 [25] | 27 | F | 720 | NR | Yes | Yes | Necrotic purpura | Face, lower extremity |
| 25 [25] | 24 | F | 570 | NR | Yes | No | Necrotic lesions | Back |
| 26 [37] | 24 | M | 653 | 6 | Yes (6) | NR | Dark crusted efflorescences surrounded by erythema | Back |
| 27 [32] | 24 | F | 600 | 5 | Yes | NR | Periumbilical smoky-grey removable patches, erythema, maceration and pustules | Umbilicus, back |
| 28 [32] | 23 | M | 540 | 7 | Yes | NR | Umbilical smoky-grey removable patches, erythema and maceration | Umbilicus, leg, abdomen, chest |
| 29 [38] | 24 | M | 651 | 10 | Yes (10) | NR | Moist white to yellow abrasion becoming a necrotic lesion | Back |
| 30 [39] | 27 | F | 750 | 12 | Yes | NR | Erythema becoming necrotic lesions | Back |
| 31 [17] | 24 | M | 600 | 6 | Yes | NR | Confluent blisters with central necrosis surrounded by erythema | Back, axillae, perineum |
| 32 [18] | 24 | M | 760 | 3 | NR | NR | Purpuric and ulcerated rash becoming verrucous and crusted necrotic lesions | Back, chest, limbs |
| 33 [18] | 23 | M | 610 | 10 | Yes (10) | NR | Circular papules with white eschars | Back |
| 34 [18] | 24 | M | 590 | 10 | Yes (5) | NR | Circular maculopapular lesions | Back, axillae, neck |
| 35 [40] | 27 | F | 730 | 10 | NR | NR | Erythematous plaque becoming hemorrhagic ulceration with abscesses | Arm, trunk, abdomen |
| 36 [41] | 25 | F | 490 | 33 | NR | NR | Small hyper-pigmented nodule with a smaller satellite lesion | Hip |
| 37 [42] | 24 | M | 750 | 5–12 | Yes (10d) | NR | Vesicles becoming nodules with yellow crusted central necrosis evolving into ulcers | Inguinal area |
| 38 [43] | 25 | M | NR | 8 | Yes | NR | Single circular crusted papule with surrounding erythema | Left hip |
| 39 [44] | 25 | M | 550 | 4 | Yes (4d) | NR | Erythema with elevated edges, central ulceration and white-greyish exudate | Cervical region |
| 40 Twin A | 24 | M | 614 | 6 | Yes (3d) | Yes (10) | Crusted greenish ulcerations | Umbilicus, abdomen |
| 41 Twin B | 24 | M | 760 | 10 | Yes (6d) | Yes (10) | Erythema becoming crusted yellowish ulcerations | Umbilicus, abdomen |
NR not reported
Table 2.
Diagnosis, therapeutic management and outcome of primary cutaneous aspergillosis in preterm neonates
| N° [Ref] | Aspergillus species | Mode of diagnosis | Antifungal therapy (duration) | Surgery | Outcome |
|---|---|---|---|---|---|
| 1 [11] | A. flavus | Stain / culture | None | Excision | Died (unrelated, age 38d) |
| 2 [12] | A. flavus | Stain / culture | NR | NR | |
| 3 [19] | A. fumigatus | Stain / culture | AmB (60 mg/kg TD) and 5-FC (5d) | Cured/survived (FU 1 mo) | |
| 4 [19] | A. fumigatus | Stain / culture | None | Died (age 7d) | |
| 5 [33] | A. fumigatus | Stain / culture | LAmB (100,5 mg/kg TD) | Cured/survived (FU 3 mo) | |
| 6 [15] | A. fumigatus | Culture only | AmB (15.8 mg/kg TD) | Cured/survived (FU 40 d) | |
| 7 [34] | A. fumigatus | Culture only | AmB and 5-FC (4w) | Cured/survived (endophtalmitis 3 w after end of treatment) | |
| 8 [35] | A. niger | Stain / culture | AmB (40.5 mg/kg TD) | Cured/survived (NR) | |
| 9 [3] | A. fumigatus | Stain / culture | AmB (6.7 mg/kg TD) | Cured/survived (FU 1 y) | |
| 10 [13] | Aspergillus sp. | Culture only | AmB (32 mg TD/46d) and 5-FC (2w) | Abscess drainage | Cured/survived (age 3 mo) |
| 11 [16] | Aspergillus sp. | Stain only (autopsy) | None | Died (related; age 7 d) | |
| 12 [16] | A. fumigatus | Culture only | None | Died (related; age 10 d) | |
| 13 [16] | A. fumigatus, A. flavus | Culture / serum GM (+ stain at autopsy) | 5-FC (100 mg/kg/d/NR) | Died (related; time to death NR) | |
| 14 [16] | A. fumigatus | Culture / serum GM | LAmB (1.4–2.8 mg/kg/d/19d) | Cured/survived (age 20 mo) | |
| 15 [7] | A. niger | Culture only | AmB (0.25–1.0 mg/kg/d/6d) | Died (likely unrelated, time to death NR) | |
| 16 [20] | A. flavus | Stain / culture | NR | Died (unrelated; age 12 d) | |
| 17 [13] | A. flavus | Culture only | AmB 7d | Died (likely related; age 17 d) | |
| 18 [21] | A. flavus | Stain / culture | AmB | Died (related; time to death NR) | |
| 19 [5] | A. fumigatus | Stain / culture | AmB (1.5 mg/kg/d/22d) | Died (unrelated; age 40 d) | |
| 20 [36] | A. flavus | Stain / culture | LAmB (5 mg/kg/d /NR, after empiric AmB and fluconazole) | Died (likely related, time to death NR) | |
| 21 [25] | A. fumigatus, A. flavus | Stain / culture | AmB | Died (time to death NR) | |
| 22 [25] | A. flavus | Stain / culture | AmB | Died (time to death NR) | |
| 23 [25] | A. flavus | Stain / culture | AmB and 5-FC | Cured/survived (age NR) | |
| 24 [25] | A. flavus | Stain / culture | AmB | Died (time to death NR) | |
| 25 [25] | A. fumigatus | Stain / culture | AmB | Cured/survived (age NR) | |
| 26 [37] | A. fumigatus | Culture only | AmB (32 mg/kg TD, 40 d) | Cured/survived (age 146 d) | |
| 27 [32] | A. fumigatus | Culture only | LAmB (28d), then voriconazole (55d). Topical gentian violet and cyclopiroxolamine | Cured/survived (age NR) | |
| 28 [25] | A. niger | Culture only | LAmB (5d), then voriconazole (28d). Topical gentian violet and cyclopiroxolamine | Cured/survived (age NR) | |
| 29 [38] | A. fumigatus | Stain/culture | AmB lipid complex (7 mg/kg/d/3d), then LAmB (5 mg/kg/d/3w) + voriconazole (8 mg/kg/d/7w) + caspofungin (2 mg/kg/d/1w) replaced by micafungin (8 mg/kg/d/6w) | Cured/survived (19 mo) | |
| 30 [39] | A. fumigatus | Stain/culture | LAmB (5 mg/kg/d/10d) | Died (unrelated; age 16 d) | |
| 31 [17] | A. fumigatus | Culture (+ stain at autopsy) | LAmB (3,5 mg/kg/d/2d) | Died (related; age 13 d) | |
| 32 [18] | A. fumigatus | Stain / culture | AmB lipid complex (1d) | Died (likely related; age 6 d) | |
| 33 [18] | A. fumigatus | Stain / culture | AmB lipid complex alone then + caspofungin (total: 22d), then posaconazole (29d) | Cured/survived (age NR) | |
| 34 [18] | A. fumigatus | Stain / culture | AmB lipid complex (15d), then oral posaconazole (21d) | Cured/survived (age NR) | |
| 35 [40] | A. flavus | Stain / culture | LAmB (3 mg/kg/d/28d), then itraconazole (1 mg/kg/d/30d) | Cured/survived (age 3 y) | |
| 36 [41] | A. fumigatus | Stain / culture | LAmB (3 mg/kg/d/8d) | Excision | Cured/survived (age 3 y) |
| 37 [42] | A. fumigatus | Stain / culture | LAmB (5 mg/kg/d/14d) | Excision | Cured/survived (age 20 mo) |
| 38 [43] | A. fumigatus | Stain / culture | LAmB (21d) | Cured/survived (age NR) | |
| 39 [44] | A. fumigatus, A. nidulans | Culture / serum GM | LAmB (5 mg/kg/d/11d) alone then + voriconazole (6 mg/kg/d/2d, stopped because of toxic serum concentrations) | Died (unrelated; age 22 d) | |
| 40 Twin A | A. fumigatus | Culture / serum GM | LAmB (5 mg/kg/d/29d) | Cured/survived (age 66 d) | |
| 41 Twin B | A. fumigatus | Culture / serum GM | LAmB (5 mg/kg/d/9d) | Died (likely unrelated, age 18 d) |
NR not reported, GM galactomannan, AmB Amphotericin B deoxycholate, LAmB liposomal Amphotericin B, 5-FC 5-fluorocytosine, TD Total dose; FU Time to follow-up after end of treatment
Preterm infants have an important predisposition to neonatal PCA [4, 7]. This may be the result of a functionally immature immune system and immature skin barrier [1]. Indeed, their skin presents an increased vulnerability to minor trauma associated with intensive care. The skin of newborns is so fragile that a minor friction can allow Aspergillus spores to enter through a breach in the epithelium [1]. Another PCA risk factor is neutropenia, with an infrequent occurrence in neonates with aspergillosis [4]. This population may rather have a predisposition to aspergillosis through a qualitative defect in macrophages and neutrophil chemotaxis, phagocytosis and microbicidal activity, especially under severe illness and stress [1]. Other known risk factors include glucocorticoid administration and prior use of antibiotics [4, 5]. Macrophages normally ingest and kill Aspergillus spores and prevent germination, whereas normal neutrophil granulocytes stop hyphal growth and dissemination, and eradicate mycelia [8]. Glucocorticoids have been shown to impair the number as well as the fungicidal activity of both neutrophils and macrophages against Aspergillus [8–10]. Here, both twins received hydrocortisone during their first 10 days of life in order to prevent bronchopulmonary dysplasia. The exact role of antibiotics in the pathogenesis of PCA is unclear, but they may contribute to the infection by disturbing the skin flora ecology [11–14]. In the present report, Aspergillus infection first appeared 6 days after the birth of twin A, so after only a short cure of antibiotics. Prior exposure to antibiotics was documented in 34 reported cases of PCA (83%) and prior exposure to glucocorticoid in 12 cases (29%).
The mean age of neonates when first lesions appeared was 10 days, ranging from 3 to 33 days. Clinical presentations of PCA are not specific (Table 1). Typically, PCA begins as erythematous papule(s) or plaque(s). The skin lesions progress to pustules and to yellow crusted ulcerations like in our cases, and eventually to necrotic black eschars. An atypical form was described in the literature as a single indurated abscess with erythematous overlying skin [13]. Thus, any new skin lesion in a neonate at risk should raise the suspicion of PCA.
A. fumigatus is the species most frequently encountered in this type of infection (26 cases, 63%), followed by A. flavus (12 cases, 29%) and A. niger (3 cases, 7%). In 2 reported cases, 2 different species of Aspergillus sp. have been identified. The diagnosis was limited to Aspergillus sp. in one other case, one being only “suggested” after tissue staining. Except for the latter case, all infections were diagnosed at least with a positive culture. There was positive Aspergillus galactomannan antigenemia in 5 cases (12%) and positive tissue staining in 28 cases (68%), only after autopsy in 3 cases.
In most reported cases of neonatal PCA, the source of contamination was not discovered [4]. Construction in hospital areas close to immunocompromised patients is a well-described risk factor for Aspergillus infections. Construction work at or near the hospital was documented in 12 reported cases (29%). Other presumed or documented sources include oximeter sensors [15], latex finger stalls [16], non-sterile gloves [17], humidifying chambers of incubators [18], bedding [19] and adhesive tapes [3, 7, 11, 20, 21]. These sources of Aspergillus are also causes of maceration that can lead to small breaches in the skin, allowing Aspergillus spores to invade [1]. No hypothesis was made about the source of infection or the portal of entry in 21 of the reported cases (51%).
In the present report, the infection appeared shortly after birth and approximatively at the same time in both twins, suggesting a common source of contamination soon after birth, or maybe even during delivery. The umbilical localization is also quite uncommon and raises the question of a potential mother-to-child transmission. Aspergillus species are ubiquitous in nature and have been isolated from the human vaginal tract [22]. In cattle, they may infect the placenta and cause abortion [23]. In a report from the former U.R.S.S., human intra-uterine infection has been suggested on the basis of histopathologic findings in 6 stillborn fetuses [24]. However, a real proof for this route of infection could not be provided. Although contact transmission can occur, airborne transmission of Aspergillus spores is the major route of infection. In this case, no samples from the mother were sent to the microbiology laboratory before or after delivery.
The father of twins worked in the waste management sector and had regular skin-to-skin contact with twins after their birth, which could have been a potential source of contamination; this hypothesis was not explored further. The epidemiological investigation did not enable finding the source of contamination and no construction work was in progress at that time. Central umbilical catheters were positive for A. fumigatus in both twins before the occurrence of the cutaneous lesions in twin B, suggesting that theses catheters played a role in the infection. Skin maceration was another risk factor since the lesions appeared on the abdomen under the diapers. Besides the catheters and the diapers, contaminated materials used for both siblings or cross-contamination by medical personnel are the most likely sources. The contamination might have occurred at the outside hospital, during transport, or in the ICU.
Primary cutaneous aspergillosis can be a site-specific entity or the initial point of invasive or disseminated disease. In the literature, autopsy findings enabled to conclude to a disseminated aspergillosis in 6 of the 10 cases for which an autopsy was performed [13, 16–18, 21, 25]. In the present cases, no autopsy was performed in the case of twin B. Levels of serum Aspergillus galactomannan antigen were very high in both twins on days 8–9 and they remained positive for 3 weeks in the case of twin A. False positive serum Aspergillus galactomannan levels have been described outside of an invasive aspergillosis context in cases of intestinal fungal colonization, cross-reactivity with unidentified serum compounds or in cases of bacteremia or fungemia [26–28]. No stool samples from our two patients had been collected and there were no bacteremia or fungemia episodes concomitant with the positivity of the galactomannan. Furthermore, twins A and B had received 3 and 6 days of amoxicillin right after birth respectively, an antibiotic linked to false positive serum Aspergillus galactomannan levels [28, 29]. However in the case of twin A, the serum Aspergillus galactomannan levels were found positive on day 9, meaning 6 days after interruption of the amoxicillin treatment, and the negativation of this marker was in concordance with his clinical evolution. These data together with the ulcerative aspect of the skin lesions are arguments in favor of an invasive aspergillosis in both twins. In addition, A. fumigatus was found in low quantity in the urine of both twins at the same time as the cutaneous infection and this could be an element in favor of a disseminated infection. However, a contamination of urine samples by Aspergillus via compresses in the diapers can’t be excluded.
Most of the reported cases of PCA have been treated primarily with systemic deoxycholate amphotericin B. It remains the first-line therapy with a dose of 1 mg/kg/day for both suspected and proven primary invasive cutaneous aspergillosis in neonates, sometimes in combination with 5-fluorocytosine [4, 30, 31]. The experience with liposomal amphotericin B (Ambisome®) as a treatment of PCA is still limited in neonates of low birth weight. However, liposomal amphotericin B was the treatment given in the last reported cases of PCA. The treatment used in the present report is one of the alternative therapies with a dosage of 5 mg/kg/day [4, 30]. In cases of PCA refractory to amphotericin B, successful treatment with voriconazole has been reported in the literature [32]. Surgical debridement may also improve survival [30]. However, surgery is often not possible because neonates tend not to tolerate skin surgery well, and lesions can be extensive [1]. Surgical intervention was performed only in four of reported cases (10%). Prompt diagnosis and treatment maximize the chances of a favorable outcome. Nineteen neonates (46%) died after PCA in reported cases, with the death related or likely related to aspergillosis in eight cases (42% of dead patients). In the present report, invasive PCA was rapidly diagnosed and an adapted treatment was started promptly, but one of the two brothers died because of several other complications linked to premature birth.
Conclusion
The skin fragility of preterm newborns is an excellent potential entry point for environmental fungal infections. These cases highlight the importance of suspecting PCA in extremely low birth weight neonates with rapidly progressive necrotic lesions. An empiric antifungal treatment should be started promptly at the first signs of cutaneous infection, just after samples collection. In case of twins, the brother should be very closely monitored.
Acknowledgments
Funding
Not applicable.
Availability of data and materials
Not applicable.
Abbreviations
- 5-FC
5-fluorocytosine
- AmB
Amphotericin B deoxycholate
- CRP
C reactive protein
- FU
Time to follow-up after end of treatment
- GM
Galactomannan
- LAmB
liposomal amphotericin B
- NICU
Neonatal Intensive Care Unit
- NR
Not reported
- PCA
Primary cutaneous aspergillosis
- TD
Total dose
Authors’ contributions
FG drafted the paper and contributed to the content of the manuscript. OK, LD and DA made an overview of the clinical presentation of the patient and provided the images. RH contributed to the literature review and overview of the manuscript. JD, EC and VB critically reviewed the paper. MS supervised and contributed to the conception and design of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the parents of the patients for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Competing interests
The Authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Floriane Gallais, Email: floriane.gallais@chru-strasbourg.fr.
Julie Denis, Email: julie.denis@chru-strasbourg.fr.
Olfa Koobar, Email: olfakoobar@gmail.com.
Laurence Dillenseger, Email: laurence.palpacuer@chru-strasbourg.fr.
Dominique Astruc, Email: Dominique.Astruc@chru-strasbourg.fr.
Raoul Herbrecht, Email: Raoul.Herbrecht@chru-strasbourg.fr.
Ermanno Candolfi, Email: candolfi@unistra.fr.
Valérie Letscher-Bru, Email: letscher@unistra.fr.
Marcela Sabou, Email: amsabou@unistra.fr.
References
- 1.Walsh TJ. Editorial response: primary Cutaneous Aspergillosis—an emerging infection among Immunocompromised patients. Clin Infect Dis. 1998;27:453–457. doi: 10.1086/514718. [DOI] [PubMed] [Google Scholar]
- 2.Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet. 2016;387:1827–1836. doi: 10.1016/S0140-6736(16)00202-6. [DOI] [PubMed] [Google Scholar]
- 3.Papouli M, Roilides E, Bibashi E, Andreou A. Primary cutaneous aspergillosis in neonates: case report and review. Clin Infect Dis Off Publ Infect Dis Soc Am. 1996;22:1102–1104. doi: 10.1093/clinids/22.6.1102. [DOI] [PubMed] [Google Scholar]
- 4.Groll AH, Jaeger G, Allendorf A, Herrmann G, Schloesser R, von Loewenich V. Invasive pulmonary Aspergillosis in a critically ill neonate: case report and review of invasive Aspergillosis during the first 3 months of life. Clin Infect Dis. 1998;27:437–452. doi: 10.1086/514717. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff CA, Hebert AA. Neonatal primary Cutaneous Aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439–444. doi: 10.1046/j.1525-1470.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- 6.Tatara AM, Mikos AG, Kontoyiannis DP. Factors affecting patient outcome in primary cutaneous aspergillosis. Medicine (Baltimore). 2016;95:e3747. [DOI] [PMC free article] [PubMed]
- 7.Amod FC, Coovadia YM, Pillay T, Ducasse G. Primary cutaneous aspergillosis in ventilated neonates. Pediatr Infect Dis J. 2000;19:482–483. doi: 10.1097/00006454-200005000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985;76:1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:4870–4877. doi: 10.1128/iai.61.11.4870-4877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granstein RD, First LR, Sober AJ. Primary cutaneous aspergillosis in a premature neonate. Br J Dermatol. 1980;103:681–684. doi: 10.1111/j.1365-2133.1980.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 12.Roth JG, Troy JL, Esterly NB. Multiple Cutaneous ulcers in a premature neonate. Pediatr Dermatol. 1991;8:253–255. doi: 10.1111/j.1525-1470.1991.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta M, Weinberger B, Whitley-Williams PN. Cutaneous aspergillosis in a neonate. Pediatr Infect Dis J. 1996;15:464–465. doi: 10.1097/00006454-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Walmsley S, Devi S, King S, Schneider R, Richardson S, Ford-Jones L. Invasive Aspergillus infections in a pediatric hospital: a ten-year review. Pediatr Infect Dis J. 1993;12:673–682. doi: 10.1097/00006454-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Perzigian RW, Faix RG. Primary cutaneous aspergillosis in a preterm infant. Am J Perinatol. 1993;10:269–271. doi: 10.1055/s-2007-994737. [DOI] [PubMed] [Google Scholar]
- 16.Singer S, Singer D, Rüchel R, Mergeryan H, Schmidt U, Harms K. Outbreak of systemic aspergillosis in a neonatal intensive care unit. Mycoses. 1998;41:223–227. doi: 10.1111/j.1439-0507.1998.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 17.Stock C, Veyrier M, Magnin-Verschelde S, Duband S, Lavocat M-P, Teyssier G, et al. Primary cutaneous aspergillosis complicated with invasive aspergillosis in an extremely preterm infant: case report and literature review. Arch Pédiatrie Organe Off Sociéte Fr Pédiatrie. 2010;17:1455–1459. doi: 10.1016/j.arcped.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Etienne KA, Subudhi CPK, Chadwick PR, Settle P, Moise J, Magill SS, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit. J Hosp Infect. 2011;79:344–348. doi: 10.1016/j.jhin.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Rowen JL, Correa AG, Sokol DM, Hawkins HK, Levy ML, Edwards MS. Invasive aspergillosis in neonates: report of five cases and literature review. Pediatr Infect Dis J. 1992;11:576–582. doi: 10.1097/00006454-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 20.James MJ, Lasker BA, McNeil MM, Shelton M, Warnock DW, Reiss E. Use of a repetitive DNA probe to type clinical and environmental isolates of Aspergillus flavus from a cluster of cutaneous infections in a neonatal intensive care unit. J Clin Microbiol. 2000;38:3612–3618. doi: 10.1128/jcm.38.10.3612-3618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson V, Ortíz D, Newton OA, Nandí E. Disseminated and cutaneous aspergillosis in a premature infant: a fatal nosocomial infection. Pediatr Dermatol. 2001;18:366–367. doi: 10.1046/j.1525-1470.2001.1946f.x. [DOI] [PubMed] [Google Scholar]
- 22.Colino GD. Rua de Corrao IJ, Martinez EA. unusual mycotic findings in cytodiagnosis. Acta Cytol. 1976;20:288–289. [PubMed] [Google Scholar]
- 23.Jensen HE, Krogh HV, Schønheyder H. Bovine mycotic abortion--a comparative study of diagnostic methods. Zentralblatt Für Veterinärmedizin Reihe B J Vet Med Ser B. 1991;38:33–40. doi: 10.1111/j.1439-0450.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 24.Popova NI. Intrauterine destruction of the brain by molds. Arkh Patol. 1976;38:48–52. [PubMed] [Google Scholar]
- 25.Katta R, Bogle MA, Levy ML. Primary cutaneous opportunistic mold infections in a pediatric population. J Am Acad Dermatol. 2005;53:213–219. doi: 10.1016/j.jaad.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Siemann M, Koch-Dörfler M, Gaude M. False-positive results in premature infants with the Platelia Aspergillus sandwich enzyme-linked immunosorbent assay. Mycoses. 1998;41:373–377. doi: 10.1111/j.1439-0507.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 27.Mennink-Kersten MASH, Klont RR. Warris a, op den camp HJM, Verweij PE. Bifidobacterium lipoteichoic acid and false ELISA reactivity in Aspergillus antigen detection. Lancet Lond Engl. 2004;363:325–327. doi: 10.1016/S0140-6736(03)15393-7. [DOI] [PubMed] [Google Scholar]
- 28.Maertens J, Theunissen K, Verhoef G, Van Eldere J. False-positive Aspergillus galactomannan antigen test results. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;39:289–290. doi: 10.1086/422151. [DOI] [PubMed] [Google Scholar]
- 29.Mattei D, Rapezzi D, Mordini N, Cuda F, Lo Nigro C, Musso M, et al. False-positive Aspergillus galactomannan enzyme-linked immunosorbent assay results in vivo during amoxicillin-clavulanic acid treatment. J Clin Microbiol. 2004;42:5362–5363. doi: 10.1128/JCM.42.11.5362-5363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 31.Smolinski KN, Shah SS, Honig PJ, Yan AC. Neonatal cutaneous fungal infections. Curr Opin Pediatr. 2005;17:486–493. doi: 10.1097/01.mop.0000171320.91677.55. [DOI] [PubMed] [Google Scholar]
- 32.Frankenbusch K, Eifinger F, Kribs A, Rengelshauseu J, Roth B. Severe primary cutaneous aspergillosis refractory to amphotericin B and the successful treatment with systemic voriconazole in two premature infants with extremely low birth weight. J Perinatol Off J Calif Perinat Assoc. 2006;26:511–514. doi: 10.1038/sj.jp.7211532. [DOI] [PubMed] [Google Scholar]
- 33.Lackner H, Schwinger W, Urban C, Müller W, Ritschel E, Reiterer F, et al. Liposomal amphotericin-B (AmBisome) for treatment of disseminated fungal infections in two infants of very low birth weight. Pediatrics. 1992;89:1259–1261. [PubMed] [Google Scholar]
- 34.van den Anker JN. Wildervanck de Blecourt-Devilee M, Sauer PJ. Severe endophthalmitis after neonatal skin lesions with positive cultures of Aspergillus fumigatus. Eur J Pediatr. 1993;152:699–700. doi: 10.1007/BF01955254. [DOI] [PubMed] [Google Scholar]
- 35.Rowen JL, Atkins JT, Levy ML, Baer SC, Baker CJ. Invasive fungal dermatitis in the < or = 1000-gram neonate. Pediatrics. 1995;95:682–687. [PubMed] [Google Scholar]
- 36.Herron MD, Vanderhooft SL, Byington C, King JD. Aspergillosis in a 24-week newborn: a case report. J Perinatol Off J Calif Perinat Assoc. 2003;23:256–259. doi: 10.1038/sj.jp.7210876. [DOI] [PubMed] [Google Scholar]
- 37.Andresen J, Nygaard EA, Størdal K. Primary cutaneous aspergillosis (PCA)--a case report. Acta Paediatr Oslo Nor 1992. 2005;94:761–762. doi: 10.1111/j.1651-2227.2005.tb01978.x. [DOI] [PubMed] [Google Scholar]
- 38.Santos RP, Sánchez PJ, Mejias A, Benjamin DK, Walsh TJ, Patel S, et al. Successful medical treatment of cutaneous aspergillosis in a premature infant using liposomal amphotericin B, voriconazole and micafungin. Pediatr Infect Dis J. 2007;26:364–366. doi: 10.1097/01.inf.0000258698.98370.89. [DOI] [PubMed] [Google Scholar]
- 39.Erişir-Oygucu S, Akcan AB, Oygür N. Primary cutaneous aspergillosis in an extremely low birth weight preterm. Turk J Pediatr. 2009;51:621–623. [PubMed] [Google Scholar]
- 40.Manzoni P, Rizzollo S, Monetti C, Carbonara C, Priolo C, Mastretta E, et al. Neonatal cutaneous disseminated aspergillosis in a preterm extremely-low-birth-weight infant with favourable outcome at 3-year follow-up: a case report. Early Hum Dev. 2012;88 Suppl 2:S65–S68. doi: 10.1016/S0378-3782(12)70018-X. [DOI] [PubMed] [Google Scholar]
- 41.Klotz D, Kneitz H, Wirbelauer J. Primary cutaneous aspergillosis in an extremely low birth weight preterm infant. Hautarzt Z Für Dermatol Venerol Verwandte Geb. 2013;64:664–665. doi: 10.1007/s00105-013-2580-7. [DOI] [PubMed] [Google Scholar]
- 42.Rogdo B, Kahlert C, Diener PA, Micallef J. Primary cutaneous aspergillosis in a preterm neonate. BMJ Case Rep. 2014;2014 [DOI] [PMC free article] [PubMed]
- 43.Simpson CL, Boos MD, Castelo-Soccio L. A crusted papule in a premature neonate. Cutaneous fungal infection. JAMA Pediatr. 2015;169:1173–1174. doi: 10.1001/jamapediatrics.2015.1359. [DOI] [PubMed] [Google Scholar]
- 44.Frick MA, Boix H, Longueira FC, Martin-Gomez MT, Rodrigo-Pendás JÁ, Soler-Palacin P. Primary Cutaneous Aspergillosis in a preterm infant. Pediatr Infect Dis J. 2016;35:704–6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


