Abstract
Background
The degree to which the chromosomal mediated iron acquisition system contributes to virulence of many bacterial pathogens is well defined. However, the functional roles of plasmid encoded iron acquisition systems, specifically Sit and aerobactin, have yet to be determined for Salmonella spp. In a recent study, Salmonella enterica strains isolated from different food sources were sequenced on the Illumina MiSeq platform and found to harbor the incompatibility group (Inc) FIB plasmid. In this study, we examined sequence diversity and the contribution of factors encoded on the IncFIB plasmid to the virulence of S. enterica.
Results
Whole genome sequences of seven S. enterica isolates were compared to genomes of serovars of S. enterica isolated from food, animal, and human sources. SeqSero analysis predicted that six strains were serovar Typhimurium and one was Heidelberg. Among the S. Typhimurium strains, single nucleotide polymorphism (SNP)-based phylogenetic analyses revealed that five of the isolates clustered as a single monophyletic S. Typhimurium subclade, while one of the other strains branched with S. Typhimurium from a bovine source. DNA sequence based phylogenetic diversity analyses showed that the IncFIB plasmid-encoded Sit and aerobactin iron acquisition systems are conserved among bacterial species including S. enterica. The IncFIB plasmid was transferred to an IncFIB plasmid deficient strain of S. enterica by conjugation. The transconjugant SE819::IncFIB persisted in human intestinal epithelial (Caco-2) cells at a higher rate than the recipient SE819. Genes of the Sit and aerobactin operons in the IncFIB plasmid were differentially expressed in iron-rich and iron-depleted growth media.
Conclusions
Minimal sequence diversity was detected in the Sit and aerobactin operons in the IncFIB plasmids present among different bacterial species, including foodborne Salmonella strains. IncFIB plasmid encoded factors play a role during infection under low-iron conditions in host cells.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-017-3954-5) contains supplementary material, which is available to authorized users.
Keywords: Salmonella enterica, Incompatibility group FIB plasmid, Iron acquisition systems (SitABCD and aerobactin), Human intestinal epithelial (Caco-2) cells, Virulence, Single nucleotide polymorphism (SNP), Whole genome sequencing (WGS)
Background
Salmonellosis, the leading bacterial foodborne illness in the United States, is associated with consumption of food contaminated with Salmonella spp. [1]. In the US alone, it is estimated that more than 1 million Salmonella infections occur annually, resulting in approximately 20,000 hospitalizations and 400 deaths [1] . S. enterica has been identified as the source of multiple outbreaks associated with meat and poultry products in the US and other countries [2]. Approximately 42% of reported cases occur in children under 10 years of age [3]. The most common manifestation of Salmonella infection is gastroenteritis. However, in severe cases, septicemia or organ system infections can develop [4]. Some S. enterica serovars cause more invasive infections than others; for example, a previous study demonstrated serovars Heidelberg and Typhimurium were more invasive, i.e., representing 13% and 6% of infections, respectively [5]. Thus, it is important to understand those factors contributing to the more severe manifestations of this disease.
Incompatibility group (Inc) FIB plasmids (also commonly known as ColV plasmids) can encode both virulence factors and antimicrobial resistance genes [6, 7]. These plasmids have been shown to contribute to the virulence of extra-intestinal pathogenic Escherichia coli (ExPEC) [7–9]. It has been demonstrated that horizontal gene transfer of IncFIB plasmid resulted in the emergence of a dominant avian clonal type of S. enterica, namely serovar Kentucky [10]. In the same study, investigators examined distribution of these plasmids among 902 Salmonella isolates from different poultry sources. The IncFIB plasmid was found to occur predominantly in serovar Kentucky (72.9% of isolates tested), followed by Typhimurium (15%) and Heidelberg (1.7%); the latter two serovars are among the most commonly associated with disease in humans [10]. Results of the study demonstrated that acquisition of the IncFIB plasmid by S. enterica serovar Kentucky significantly increased its ability to colonize the chicken cecum and cause extra-intestinal disease [10]. The potential for horizontal gene transfer of virulence and fitness related factors encoded by the IncFIB plasmid between Salmonella and other enteric bacteria exists in isolates recovered from food sources. This is a potential concern for human health as these virulent species of bacteria can be acquired by humans via contaminated foods.
A previous study carried out in our laboratory showed that IncFIB plasmids from Salmonella carry genes associated with virulence, including iron acquisition transport systems, such as the Fur (ferric uptake regulator), regulated iron ABC transporter (sitABCD) and the aerobactin iron acquisition (iucABCD-iutA) system [6]. Fur, a transcriptional and post-transcriptional regulator that senses iron in the environment, plays a crucial role in the colonization and pathogenicity in many bacteria [11–13]. The role of the chromosomal mediated Sit iron acquisition system in virulence of many bacterial pathogens is well defined [14, 15]. However, functional roles of iron acquisition transport systems encoded by the IncFIB plasmid have yet to be determined for Salmonella. The main objectives of this study were: i) to analyze and compare genomes of IncFIB plasmid containing S. enterica strains isolated from turkeys and chicken; ii) to determine the sequence diversity of IncFIB plasmid encoded Sit and aerobactin operons in different bacteria including Salmonella; iii) to evaluate the degree of contribution of IncFIB plasmid encoded factors in virulence of S. enterica.
Iron is an essential cofactor for various metabolic enzymes associated with important biological pathways in both microorganisms and host [16]. For example, iron influences butyrate production by modulating expression of butyryl-coenzyme A (CoA):acetate CoA-transferase enzyme of butyrate producer such as Roseburia intestinalis [17]. In the recent past, butyrate has been identified as an important metabolite for maintaining healthy microbiota in human gut [17]. Iron is also an important growth factor for pathogenic bacteria, with iron concentrations of 10−6 to 10−7 M required by most microorganisms to carry out metabolic processes, including electron transport, glycolysis, DNA synthesis, and defense against toxic reactive oxygen intermediates (ROI) [18]. In response to infection, eukaryotic hosts utilize strategies to limit iron availability to pathogens. Specific host proteins, such as transferrin, lactoferrin, and ferritin, generally bind to haem to form a complex within haemoprotein, resulting in unavailability of free iron [19, 20]. Additionally, iron is required by macrophages and/or other host cells for the production of ROI and reactive nitrogen intermediate (RNI) which, both aid in the elimination of pathogens [16]. On the other hand, pathogens have evolved to encode various iron acquisition systems in the chromosome and plasmids, including those mentioned above, to sequester iron from the host to establish an infection [21, 22]. For instances, it has shown that aerobactin defective mutants of avian pathogenic E. coli (APEC) and uropathogenic E. coli (UPEC) showed reduced virulence in a chicken infection model [9]. Previous study demonstrated that SitABCD iron acquisition system encoded by chromosome of S. Typhimurium required for complete virulence of this pathogen [15]. Plasmid encoded Sit and aerobactin iron acquisition systems homologs have been identified in Cronobacter spp. which possesses several virulence associated genes [23, 24].
Recently, we sequenced seven S. enterica isolates from different food sources such as turkey and chicken, six of which contained IncFIB plasmids [25]. In the present study, we used single nucleotide polymorphism (SNP)-based phylogenetic analysis to compare genome sequences of these isolates to selected S. enterica serovars isolated from food, animal, and human sources available on the public data base. Additionally, we conducted phylogenetic analysis of the IncFIB plasmid encoded Sit and aerobactin operons to determine sequence diversity of these factors. The expression of genes within these operons was also evaluated at the transcriptional level following growth in iron-rich or iron-depleted media. To delineate functional roles of IncFIB plasmid encoded factors in virulence, invasion, and persistence of S. enterica, human intestinal epithelial cells (Caco-2) were infected with IncFIB negative recipient SE819, transconjugant SE819::IncFIB, and wild type (plasmid donor) SE163A strains. The results provide data useful in understanding the functional roles of iron acquisition systems encoded by IncFIB plasmids of S. enterica and related pathogens.
Methods
Bacterial strains and growth medium
Bacterial isolates included in this study (Additional file 1: Table S1) were sub-cultured on sheep’s blood agar plates (Remel, Lenexa, KS). Single colonies were picked and grown overnight in Luria-Bertani (LB) medium supplemented with the following antibiotics: tetracycline (32 μg/mL); kanamycin (32 μg/mL); and nalidixic acid (64 μg/mL). Sodium azide (350 μg/mL) (Sigma-Aldrich, St. Louis, MO) was used in the media for conjugation. Ferric chloride (100 μM) and 2,2/−bipyridyl (200 μM) (Sigma-Aldrich) were added to LB broth to prepare iron-rich and iron-depleted growth media, respectively.
Whole genome sequencing (WGS)
Genomic DNA was extracted with the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA), and sequenced at the University of Arkansas for Medical Sciences DNA Sequencing Core Facility (Little Rock, AR). To construct DNA libraries, the Nextera XT DNA Sample Prep Kit was employed, following manufacturer’s instructions (Illumina, San Diego, CA). Sequencing was performed on the Illumina MiSeq with 2 × 250 paired-end reads [25]. CLC Genomics Workbench (version 8.5.1; Qiagen, Germantown, MD) was used for trimming and de novo assembly of paired-end reads. Contigs less than 200 nucleotides were excluded from analysis. Draft genomes of S. enterica were annotated with the Rapid Annotation using Subsystem Technology (RAST) [26], Pathosystems Resource Integration Center (PATRIC) [27], and NCBI Prokaryotic Pipeline (PGAP). More detailed descriptions of the sequencing experiments were reported by Khajanchi et al. (2016) [25]. The S. enterica WGS data were submitted to NCBI and the accession numbers were listed in Table 1. Serovars of the seven S. enterica isolates were predicted using SeqSero, a WGS based tool (www.denglab.info/SeqSero) [28].
Table 1.
Salmonella enterica isolates employed in the SNP analysis of this study
| Strain | Serovar | Isolation Country | State | Source | Isolation Date | Accession |
|---|---|---|---|---|---|---|
| SE163Ac | Typhimuriumb | USA | OH | Turkey diagnostic | 2002 | LSZD00000000 |
| SE696Ac | Typhimuriumb | USA | MW | Turkey processing | 2000 | LXHA00000000 |
| SE710Ac | Typhimuriumb | USA | ND | Turkey diagnostic | 1992 | LXGZ00000000 |
| SE819c | Heidelbergb | USA | MD | Turkey | 2002 | LSZE00000000 |
| SE397c | Typhimuriumb | USA | INAa | Chicken carcass | 1999 | LYRR00000000 |
| SE452c | Typhimuriumb | USA | INA | Ground turkey | 1999 | LYRS00000000 |
| SE478c | Typhimuriumb | USA | INA | Turkey | 1999 | LYRT00000000 |
| SESL476 | Heidelberg | USA | MN | Food | 2003 | NC_011083.1 |
| SECVM20752 | Heidelberg | USA | NC | Turkey | 2002 | AMNR00000000 |
| SE21381 | Heidelberg | USA | CT | Food | 2002 | AMMZ00000000 |
| SECVM24359 | Heidelberg | USA | MO | Turkey | 2003 | AMNP00000000 |
| SE29169 | Heidelberg | USA | CT | Food | 2003 | APIX00000000 |
| SE41563 | Heidelberg | USA | OR | Human | 2011 | AJGX00000000a |
| SE41565 | Heidelberg | USA | WA | Food | 2011 | AJHA00000000a |
| SE41567 | Heidelberg | USA | WA | Food | 2011 | AMNG00000000 |
| SE41573 | Heidelberg | USA | OH | Human | 2011 | AJGY00000000a |
| SE41576 | Heidelberg | USA | OH | Human | 2011 | AMNH00000000 |
| SE82–2052 | Heidelberg | USA | ME | Human | 1982 | AMMX00000000b |
| SEN1536 | Heidelberg | USA | GA | Food | 2004 | AKYN00000000 |
| SEN15757 | Heidelberg | USA | GA | Food | 2007 | AMND00000000 |
| SEN18393 | Heidelberg | USA | CT | Food | 2008 | AMNF00000000 |
| SEN19992 | Heidelberg | USA | CA | Food | 2009 | AMMC00000000 |
| SEN26457 | Heidelberg | USA | CO | Food | 2010 | AMNE00000000 |
| SEN29341 | Heidelberg | USA | MN | Food | 2011 | AMNJ00000000 |
| SEN4403 | Heidelberg | USA | CA | Food | 2005 | AMMT00000000 |
| SEN4496 | Heidelberg | USA | CO | Food | 2005 | AMMQ00000000 |
| SESARA31 | Heidelberg | USA | MD | Swine | 1987 | AMLV00000000 |
| SESARA32 | Heidelberg | USA | TX | Dog | 1986 | AMLU00000000 |
| SESARA37 | Heidelberg | USA | CO | Turkey | 1987 | AMLR00000000 |
| SEN13–01290 | Heidelberg | Canada | Quebec | Food | 2012 | CP012930.1 |
| SE12–4374 | Heidelberg | Canada | Quebec | Human | 2012 | CP012924.1 |
| SECFSAN002064 | Heidelberg | USA | INA | Human | 2012 | CP005995.1 |
| SECFSAN002069 | Heidelberg | USA | WA | Chicken | 2012 | CP005390.2 |
| SEB182 | Heidelberg | France | INA | Cattle | INA | CP003416.1 |
| SECVM_N41746 | Heidelberg | USA | NY | Food | 2012 | NZ_JYYX01000033.1 |
| SECVM_N42472 | Heidelberg | USA | GA | Food | 2012 | NZ_JYZU01000053.1 |
| SECVM_N37938 | Heidelberg | USA | NY | Food | 2011 | NZ_JYWR01000049.1 |
| SECVM_N30683 | Heidelberg | USA | NM | Food | 2011 | NZ_JYUW01000029.1 |
| SET000240 | Typhimurium | Japan | INA | Human | 2000 | AP011957.1 |
| SELT2 | Typhimurium | INA | INA | Human | 1940s | NC_003197.1 |
| SECFSAN001921 | Typhimurium | INA | INA | Food | INA | CP006048.1 |
| SECDC_H2662 | Typhimurium | INA | INA | Human | 1997 | CP014979.1 |
| SEUSDA-ARS-USMARC-1808 | Typhimurium | USA | INA | INA | INA | CP014969.1 |
| SERM9437 | Typhimurium | USA | CO | Food | 2009 | CP012985.1 |
| SESA972816 | Typhimurium | China | INA | Cattle | 2002 | CP007484.1 |
| SECDC_2009K-1640 | Typhimurium | USA | INA | Human | 2009 | CP014975.1 |
| SECDC_2010K-1587 | Typhimurium | USA | INA | Human | 2006 | CP014965.1 |
| EUSDA-ARS-USMARC-1810 | Typhimurium | USA | INA | Cattle | 2005 | CP014982.1 |
| EUSDA-ARS-USMARC-1880 | Typhimurium | USA | INA | Cattle | 2003 | CP014981.1 |
| SECVM29188 | Kentucky | USA | GA | Food | 2003 | CP001122.1 |
| SECDC191 | Kentucky | INA | INA | INA | INA | NZ_ABEI00000000.1 |
| SESK222-32B | Kentucky | INA | INA | Food | 2005 | NZ_JUIU00000000.1 |
aINA; information not available
b Salmonella serovars were predicted using SeqSero, a WGS-based tool [28]
cSeven S. enterica isolates (SE163A, SE696A, SE710A, SE452, SE478, SE397 and SE819) were sequenced in this study; the genome sequences of the rest of the 45 Salmonella isolates used for SNP analysis were obtained from a public data base
Single nucleotide polymorphism (SNP) analysis
A phylogenetic tree of the sequenced Salmonella genomes (n = 52) was constructed with the parsnp program (Harvest software) [29], which identifies core genomes across isolates and builds a phylogeny using maximum likelihood and core single nucleotide polymorphisms (SNPs). Seven S. enterica isolates (SE163A, SE696A, SE710A, SE452, SE478, SE397 and SE819) were sequenced in our study; the genome sequences of the rest of the 45 Salmonella enterica strains isolated from food, animal, and human sources were obtained from a public data base. Detail information of the isolates used in the SNP analysis was listed in the Table 1.
Phylogenetic analysis of iron acquisition systems
Composite phylogenetic trees were generated by averaging pair-comparisons of DNA sequences of the S. enterica and E. coli strains harboring IncFIB plasmids for each of the genes in either Sit (sitABCD) or aerobactin (iucABCD-iutA) operons, based on unweighted pair group means, employing the average (UPGMA) clustering algorithm, BioNumerics (version 7.6, Applied Maths, Kortrijk, Belgium).
Conjugation and in vitro passage
Conjugation experiments were performed as described previously [30], with some modifications that were specifically employed for this study. To obtain a transconjugant that only contain IncFIB plasmid in SE819, we performed the conjugation experiments in two steps. In the first conjugation experiment, plasmids present in the wild type S. enterica SE163A were transferred to the sodium azide resistant recipient E. coli J53 using a plate mating strategy [31]. Briefly, the donor and recipient strains were cross streaked on selective LB agar plates containing sodium azide (350 μg/mL) and kanamycin (32 μg/mL) and incubated at 37 °C. After approximately 48 h, the cells from the intersection were collected and re-streaked onto selective plates containing sodium azide (350 μg/mL) and kanamycin (32 μg/mL). Individual colonies were picked and sub-cultured onto MacConkey agar. Subsequently, colorless colonies were picked form selective plate and transconjugants were confirmed by PCR targeting of the plasmid specific genes present in different plasmids in the donor SE163A strain (IncFIB, IncA/C and IncX4) [10, 32]. Transconjugants containing IncFIB but lacking the other plasmids were selected and stored at −80 °C. In the second conjugation experiment, a single colony of E. coli J53::IncFIB (Additional file 1: Table S1) and S. enterica SE819 lacking IncFIB plasmid were grown in LB medium overnight at 37 °C. The donor (E. coli J53::IncFIB) and recipient (SE819) strains were mixed at a ratio of 1:1 and centrifuged at 7000 RPM for 5 min, and the pellet was spread onto LB agar and incubated at 37 °C for 4–5 h. Cells were harvested from the LB agar and spread onto selective MacConkey agar plates supplemented with kanamycin (32 μg/mL) and nalidixic acid (64 μg/mL). After incubation overnight at 37 °C, 15 transconjugants were screened by PCR for the presence of the IncFIB plasmid.
In addition to generation of IncFIB positive transconjugants, generation of IncFIB plasmid cured strains was attempted by serially passaging strains SE163A, SE417, and SE478 on LB agar plates for up to 34 days. A PCR template was prepared from a single colony at each passage and examined for presence or absence of the plasmid by PCR. Previous studies in our laboratory demonstrated that SE819 is a less virulent isolate lacking several virulence associated plasmids and successfully used as the recipient for conjugation studies [30, 32].
Growth kinetics
Wild type (SE163A), recipient (SE819), and transconjugant (SE819::IncFIB) strains were grown in LB overnight at 37 °C with shaking (180 RPM). The optical density (OD) was measured at 600 nm, using a Genesys 10UV spectrophotometer (Thermo Electron Corp., Madison, WI). To determine growth kinetics, the overnight cultures of these three strains were inoculated into fresh LB, LB supplemented with ferric chloride (iron-rich), and LB supplemented with 2,2/ bipyridyl (iron-depleted) media. The initial OD was adjusted to 0.5 for LB and iron-rich LB. A higher initial OD (3.0) was used in the case of iron-depleted LB to accommodate slow growth under iron deficient conditions. The cell suspensions were incubated with shaking (180 RPM) at 37 °C and the OD was measured in 1 h intervals for 8 h, followed by a final reading at 24 h. Three biological replicates for each sample and each condition were employed and experiments were repeated three times.
RNA isolation and reverse transcription (RT) PCR
Wild type, recipient, and transconjugant strains were grown in LB, iron-rich, and iron-depleted media supplemented with tetracycline. RNA was isolated from approximately 109 cells collected at mid-logarithmic phase with the Ribopure Bacterial RNA Isolation Kit (Ambion, Invitrogen, Carlsbad, CA), following manufacturer’s instructions. Genomic DNA was removed by treatment with DNase I and the RNA concentration was measured with a NanoDrop spectrometer (Thermo Scientific, Waltham, MA).
Quantitative reverse transcription-PCR (qRT-PCR) was performed to determine expression of the encoded iron acquisition genes on the IncFIB plasmid under iron-rich and iron-depleted growth conditions. cDNA was synthesized from 250 ng RNA using the iScript cDNA synthesis kit (BioRad, Hercules, CA), following manufacturer’s instructions. Primers were designed using the PrimerQuest tool (Integrated DNA Technologies, Coralville, IA) and are listed in Additional file 1: Table S2. The sitA primer was designed to detect only transcripts produced from plasmid encoded sitA and not from the chromosome. qRT-PCR was performed using iQ SYBR green super-mix and CFX touch real-time PCR detection system (Bio-Rad). The Salmonella gmk gene served as endogenous control, to normalize expression [33]. No-reverse-transcriptase (NRT) controls and no-template controls (NTC) were also used to monitor for genomic DNA contamination of the RNA. Differential gene expression and fold differences were calculated by relative quantification (ΔΔ threshold cycle [ΔΔCT]), using Bio-Rad CFX manager software. Two independent experiments using two biological replicates and four technical replicates for each strain were carried out.
Bacterial invasion assay
Bacterial invasion assays were performed using human intestinal epithelial cells (Caco-2) as described previously [32], with some modifications that were specifically employed for this study. Briefly, 105 Caco-2 cells/well were seeded in 24-well tissue culture plates and incubated at 37 °C overnight. Cells in one of the wells were counted using Cellometer Auto T4 (Nexcelom Bioscience, Lawrence, MA) and the Caco-2 cells were infected with SE819, transconjugant SE819::IncFIB, and wild type SE163A strains at multiplicity of infection (MOI) of 10. After incubation for 1 h at 37 °C, the cells were washed twice with PBS to remove bacteria that had not infected the Caco-2 cells and incubated with 200 μg/ml of gentamicin. After incubation for 1 h at 37 °C, the cells were washed twice with PBS and lysed with 0.1% chilled Triton X-100, followed by dilution and plating on TSA agar to obtain colony forming unit counts (CFUs) of bacteria following overnight incubation at 37 °C. Three replicates per strain were included and the experiments were repeated three times.
To determine influence of iron in invasion of bacteria in to host cells, wild-type (SE163A), recipient (SE819) and transconjugant (SE819::IncFIB) strains were grown overnight in iron-rich and iron-depleted media prior to infection of Caco-2 cells.
Bacterial persistence assay
Caco-2 cells were infected similar to the invasion assay. After 1 h incubation, the cells were washed twice with PBS and incubated with 100 μg/ml of gentamicin for 48 h. After incubation, cells were washed, lysed, and CFUs were counted as in the invasion assay procedure, with three replicates per strain and experiments carried out in triplicate.
Since variable number of adherence of Caco-2 cells was observed in each experiment, the % invasion and % persistence was determined per 106 Caco-2 cells in order to normalize the number of host cells in different experiments performed. Likewise, number of bacteria for 10 MOI was different for each experiment hence, to normalize this variation between the experiments; % of invasion and % of persistence were determined for each of the strain and plotted the graph as % invasion per 106 Caco-2 cells and % persistence per 106 Caco-2 cells.
Statistical analysis
Student’s t-test was used to determine statistically significant difference between sample groups, with a P-value ≤0.05 considered significant.
Results
SNP analysis
To understand the role of IncFIB plasmids and how the genes they carry contribute to Salmonella virulence, seven S. enterica strains that were isolated from turkeys and chicken in different geographic locations in the USA were sequenced (Table 1 and Additional file 1: Table S1) [25]. Six of the strains contained IncFIB plasmids and the other (SE819), a less virulent strain, was used as the recipient for conjugation. The isolates containing the IncFIB plasmids were predicted to be serovar Typhimurium using the WGS based Salmonella serotyping tool SeqSero, while SE819 was identified as serovar Heidelberg (Table 1). The genome sequences of the isolates were compared to determine evolutionary relatedness to previously sequenced genomes (n = 45) of S. enterica serovars isolated from various food, animal, and human sources (Table 1), using core genome SNP-based phylogenetic analyses. A SNP based evolutionary tree showed each serovar joined distinct phylogenetic clades, irrespective of presence or absence of an IncFIB plasmid (Fig. 1a). To understand the evolutionary relatedness of the S. Typhimurium isolates (n = 17) including the IncFIB positive strains from the current study, a S. Typhimurium-specific phylogenetic tree was constructed (Fig. 1b). It showed five of the IncFIB plasmid containing S. enterica strains isolated from turkey-associated sources (SE163A, SE696A, SE710A, SE452, SE478) clustered in a single monophyletic clade. The other S. enterica isolate from chicken (SE397) branched separately together with the S. Typhimurium strain (SEUSDA-ARS-USMARC-1880) isolated from a bovine source (Fig. 1b). These data indicate that the five S. enterica IncFIB plasmid-containing isolates from turkey associated sources have nearly identical chromosomal genetic relatedness.
Fig. 1.
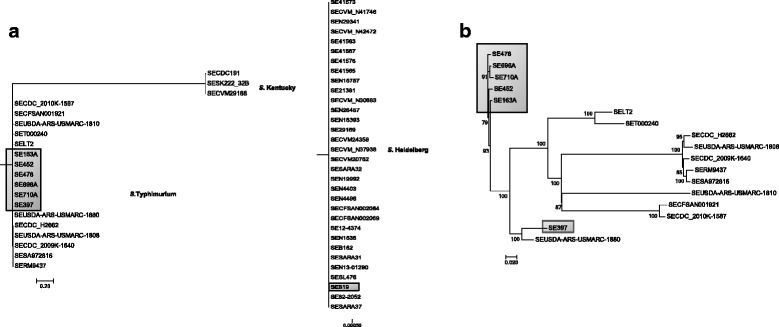
SNP based phylogenetic analysis of Salmonella enterica strains isolated from food, animal, and human sources. a SNP based evolutionary tree showed that each serovar joined distinct phylogenetic clades, irrespective of presence or absence of an IncFIB plasmid. b Five IncFIB containing S. Typhimurium isolates (SE163A, SE696A, SE710A, SE452, and SE478) clustered in one clade (shown in the box) and SE397 was found to be identical to S. Typhimurium USDA-1880. Seven S. enterica isolates (SE163A, SE696A, SE710A, SE452, SE478, SE397 and SE819) marked with text boxes were sequenced in our study; the genome sequences of the other isolates were obtained from a public data base. Harvest, a core genome SNP mining tool (https://www.cbcb.umd.edu/software/harvest), was used to generate the SNP tree. S. enterica SL476 was employed as a reference for SNP detection
Phylogenetic analysis of sitABCD and iucABCD-iutA iron acquisition systems
The IncFIB plasmids of Salmonella examined in this study encode the Fur regulated iron transporter (sitABCD) and aerobactin (iucABCD-iutA). The sitABCD iron transporter can be encoded both in a plasmid and on the chromosome, whereas the iucABCD-iutA iron acquisition system is plasmid-encoded. Interestingly, sequence annotation revealed all six of the IncFIB positive strains possessing the sit operon, both in the chromosome and IncFIB plasmid. Phylogenetic analysis of both plasmid and chromosome encoded sitABCD transporters from the different Salmonella isolates and E. coli strains were performed to investigate the genomic diversity of this system (Fig. 2). All chromosome-encoded sit operons of S. enterica clustered in one clade, including the reference LT2 S. Typhimurium strain, while the plasmid-encoded sit operon of different serovars of Salmonella and E. coli separated into a distinct clade (Fig. 2). To highlight the differences, PCR primers designed for the plasmid-encoded sit genes did not amplify chromosome sit genes, as evident by the lack of PCR products for S. enterica SE819. Our results indicate that plasmid-encoded Sit systems are conserved in both Salmonella and E. coli while sequence diversity was found between plasmid and chromosome encoded Sit operons in the tested S. enterica isolates (Fig. 2). The plasmid-encoded aerobactin iron transporter genes were also conserved in the different species of Salmonella and E. coli, with nearly identical sequences, i.e., ~99% identity (Fig. 3).
Fig. 2.
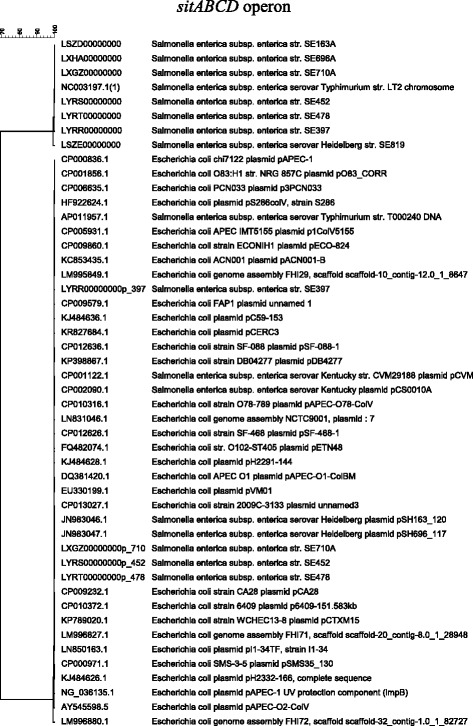
Phylogenetic analysis of the IncFIB plasmid and chromosome encoded sitABCD iron transporter. Seven S. enterica isolates (SE163A, SE696A, SE710A, SE452, SE478, SE397, and SE819) were sequenced in this study and the sitABCD sequences of the other isolates were from the NCBI database. All isolates, except for SE819, contained the IncFIB plasmid. The phylogenetic tree was generated using the UPGMA clustering algorithm
Fig. 3.

Phylogenetic analysis of the IncFIB plasmid encoded iucABCD-iutA iron transporter. Seven S. enterica isolates (SE163A, SE696A, SE710A, SE452, SE478, SE397, and SE819) were sequenced in this study and the iucABCD-iutA sequences of the other isolates were from the NCBI database. All isolates, except for SE819, contained the IncFIB plasmid. Since the iucABCD-iutA transporter is encoded only on a plasmid, the SE819 strain was excluded from analysis. The phylogenetic tree was generated using the UPGMA clustering algorithm
Conjugation and stability of the IncFIB plasmid
To examine the role of the IncFIB plasmid mediated iron acquisition systems of S. enterica, a transconjugant (SE819::IncFIB) was generated that contained the IncFIB virulence associated plasmid, in which S. enterica SE819 and SE163A served as recipient and donor, respectively. The presence of the IncFIB plasmid in the transconjugants was confirmed by PCR; transcripts of the IncFIB-associated genes were found in both donor and transconjugant, but not in the recipient strain.
The construction of the transconjugant was essential, since the IncFIB plasmids appear to be stable, in that after 25 to 34 passages SE163A, SE417, and SE478 had not lost the IncFIB plasmid, as evidenced by PCR amplification of the IncFIB replicon. Therefore, addition of the IncFIB plasmid to the less virulent strain was important for evaluating the impact of the plasmid on the virulence of S. enterica.
Growth kinetics
Growth kinetics of donor, recipient, and transconjugant strains were determined by growth in LB, LB supplemented with ferric chloride (iron-rich), and LB supplemented with 2,2/ bipyridyl (iron-depleted) (Fig. 4). In general, all grew better in the LB and iron-rich LB than in iron-depleted LB. Highest growth rates of the strains were observed under iron-rich conditions, compared to LB and iron-depleted LB. The donor/wild type S. enterica SE163A grew better in iron-depleted LB, compared to the recipient and transconjugant strains. Overall, the results showed the three S. enterica strains demonstrated different growth kinetics, depending on the presence of iron in the growth medium (Fig. 4).
Fig. 4.
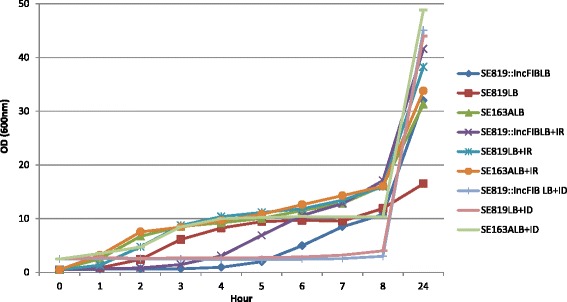
Growth kinetics of S. enterica strains in iron available in vitro growth media. Wild type (SE163A), recipient (SE819), and transconjugant (SE819::IncFIB) strains were grown in: LB; LB amended with ferric chloride (iron-rich); and LB amended with 2,2/ bipyridyl (iron-depleted). Each data point represents the average OD of three replicates. Three independent experiments were performed to examine growth kinetics of the strains. Results of a representative experiment are shown in the graph. ID = iron-depleted and IR = iron-rich
qRT-PCR
To determine gene expression of plasmid encoded Sit and aerobactin operons, the transcripts of the sitA, iucA, and iutA genes in wild type, transconjugant, and recipient strains grown in iron-rich and iron-depleted LB were examined using qRT-PCR. Increased level (~3 log) of sitA expression was found in the transconjugant strain grown under iron-depleted compared to iron-rich conditions (Fig. 5a). However, due to variation among replicates, the difference was not statistically significant. A significantly increased level of sitA expression was observed in SE163A in the iron-depleted growth medium, as compared with iron-rich (Fig. 5a). As expected, the sitA transcript was not detected in SE819, which lacked the plasmid. The expression of iucA in the transconjugant strain in iron-depleted conditions was significantly greater (~3 log), compared to iron-rich (Fig. 5b), while expression was similar for SE163A in both the iron-depleted and iron-rich media (Fig. 5b). Results of iutA gene expression were similar to iucA, with a ~ 2.5-fold difference of expression for transconjugants and similar levels of expression for S. enterica SE163A (Fig. 5c).
Fig. 5.
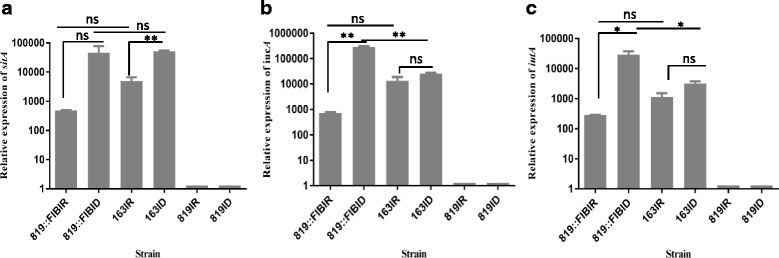
Gene expression of sitA, iucA, and iutA in S. enterica strains grown under low-iron and high-iron conditions. Differential gene expression was examined in the S. enterica donor (SE163A), recipient (SE819), and transconjugant (SE819::IncFIB) strains using qRT-PCR and SYBR green assay. a relative gene expression of sitA; b relative gene expression of iucA; c relative gene expression of iutA, shown in a representative experiment. The error bars show the standard error of mean for two biological replicates (consisting of four technical replicates) of each strain. gmk was used as a reference gene to normalize the expression of target genes in different samples grown under low and high iron conditions. SE819 strain which does not possess IncFIB plasmid used as control to normalize the background gene expressions. Student’s t-test was performed to determine the statistically significant difference between two groups of samples. A p value ≤0.05 was considered a significant difference between the two groups compared, as indicated by the asterisks (ns = not significant)
Invasion and persistence in Caco-2 cells
To assess the role of the IncFIB plasmid in invasion and persistence in host cells, human intestinal epithelial cells (Caco-2) were infected with recipient, transconjugant, and wild type strains of S. enterica. Significantly increased invasion and persistence of the wild type in Caco-2 cells was observed, compared to transconjugant and recipient strains. A slightly higher uptake was observed in the transconjugant, compared to the recipient (Fig. 6a). However, this difference was not statistically significant by Student’s t-test. A significantly higher number of transconjugants were found to persist in Caco-2 cells, compared to the recipient strain (Fig. 6b).
Fig. 6.
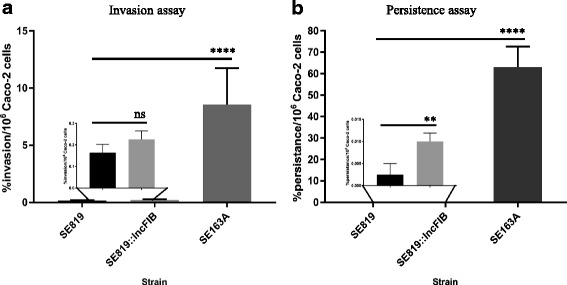
Invasion and persistence assays of different strains of S. enterica. Human intestinal epithelial cells (Caco-2) were infected with recipient SE819, transconjugant and wild type SE163A of S. enterica at MOI of 10. Bacterial invasion (a) and persistence assays (b) were performed 1 h and 48 h post infection, respectively. A slightly higher uptake and increased persistence was observed when Caco-2 cells were infected with the transconjugant strain as compared to the recipient SE819 strain. The error bars show the standard error of means for three biological replicates of three independent experiments. Student’s t-test was performed to determine the statistically significant difference between two groups of sample. A p value ≤0.05 was considered a significant difference between the two groups compared, as indicated by the asterisks (wild type vs. recipient and transconjugant vs. recipient). Statistically non-significant differences are indicated by “ns”
Both recipient and transconjugant strains invaded in Caco-2 cells at similar rates when grown in iron-rich and iron-depleted media prior to infection (Fig. 7a). Wild type strain invaded at higher rate in both iron-rich and iron-depleted conditions than the recipient and transconjugant, however, this difference was not statistically significant (Fig. 7a). When grown in iron-rich media, the transconjugant persisted at lower rate in Caco-2 cells as compared the recipient; however, this difference was not statistically significant. Whereas, in iron-depleted media, the transconjugant persisted significantly higher rate than the recipient (Fig. 7b). Interestingly, transconjugant persisted at higher rate when grown in the iron-depleted than the iron-rich media (Fig. 7b). Wild type strain persisted significantly higher rate in both iron-rich and iron-depleted conditions than the recipient and transconjugant. Unlike transconjugant, wild type strain showed similar persistence rate both in iron-rich and iron-depleted conditions (Fig. 7b).
Fig. 7.
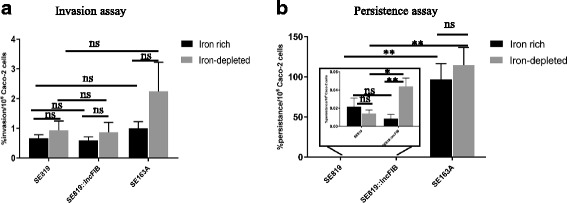
Invasion and persistence assays of different strains of S. enterica grown in iron-rich and iron-depleted conditions prior to infection. Recipient SE819, transconjugant and wild type SE163A of S. enterica strains were grown in iron-rich and iron-depleted media overnight prior to infect human intestinal epithelial cells (Caco-2) with at MOI of 10. Bacterial invasion (a) and persistence assays (b) were performed 1 h and 48 h post infection, respectively. The error bars show the standard error of means for 2–3 biological replicates of two independent experiments. Student’s t-test was performed to determine the statistically significant difference between two groups of sample. A p value ≤0.05 was considered a significant difference between the two groups compared, as indicated by the asterisks. Statistically non-significant differences are indicated by “ns”
Discussion
Dissemination of plasmid mediated antimicrobial resistance and virulence genes of bacterial pathogens is a persistent health concern [6, 34]. A better understanding of the contribution of specific types of plasmids to antimicrobial resistance and virulence is important for development of novel strategies to control the spread of these plasmids among foodborne pathogens. In the present study, the genetic background of S. enterica isolates containing IncFIB plasmids and the iron acquisition systems encoded by these plasmids were analyzed by both in silico analysis and laboratory based experiments.
SNP analysis of genomes of a number of S. enterica strains representing serovars Heidelberg, Typhimurium, and Kentucky isolated from food, animal, and human sources, revealed serovar specific clades (Fig. 1a), indicating paraphyletic relationships. More importantly, within the S. Typhimurium clade, five of the IncFIB plasmid containing turkey-associated isolates formed into a unique single monophyletic subclade distinct from the other sequences analyzed (Fig. 1b). It is interesting to note that the IncFIB plasmid was integrated into the chromosome of S. Typhimurium SET000240 after isolation from a human patient, indicating IncFIB encoded genes have the potential to be maintained as a plasmid with the ability to integrate into the chromosome [35]. Hence, this plasmid has the potential to disseminate by horizontal gene transfer into Salmonella and other pathogens.
Previous studies have shown that IncFIB plasmids have the potential to contribute to increased colonization and fitness in the chick embryo and/or other avian animal models when infected with avian pathogenic E. coli [36, 37] and S. Kentucky [10]. Hence, dissemination of the IncFIB plasmid into Salmonella serovars, such as Typhimurium and Heidelberg, which are known to cause human infections, may contribute to improved survival in food animals, resulting in increased opportunities for human infection via contaminated food products. In addition to encoding factors contributing to virulence, IncFIB plasmids in Salmonella often carry multiple antimicrobial resistance genes [6], which is problematic if they cause diseases requiring antimicrobial therapy. The identification and evaluation of specific factors relative to colonization, virulence, and plasmid dissemination are important for finding effective ways to control these foodborne pathogens and reduce spread of virulence and antimicrobial resistance plasmids in the food production environment and exposure to humans.
Iron is one of the key signaling elements that helps pathogens sense their environment as well as regulate gene expression to survive under reduced iron conditions in human and animal hosts [13, 38, 39]. Bacterial pathogens are known to possess iron transporter systems that facilitate survival under low iron conditions [9, 21, 22]. In the present study, the focus was on plasmid-encoded iron transporter systems encoded by sitABCD and iucABCD-iutA. sitABCD genes are present both in the chromosome and on the IncFIB plasmids of the six isolates whose genomes have been sequenced in this study; however, there appears to be significant sequence diversity between the two sitABCD operons (Fig. 2). Degree of virulence depending on whether the sitABCD transporters are encoded on both the chromosome and the plasmid versus one or the other may influence the virulence of pathogen. It is known that chromosome encoded sitABCD play role in virulence [15], however, currently there is scarcity of study evaluating the role of plasmid encoded sitABCD in virulence of S. enterica and other pathogens. Also, it remains to be determined whether presence of both sitABCD will contribute in virulence as synergistic manner. We speculate that plasmid and chromosomally encoded sitABCD transporters may exhibit differential expression within the host and the natural environment. Further research is needed to substantiate this conclusion.
The IncFIB plasmids demonstrated stability in in vitro culture, as evidenced by retention of the plasmid after repeated passaging. Host addiction genes (vagCD and ccdAB), encoded on this plasmid likely contribute to stability of the plasmid [40–42] and is indicative of the plasmid’s importance to the bacterial cell in food animal environments. While stably maintained in the bacterial host, copies of the plasmid are able to be transferred to recipients. The IncFIB plasmid was able to be transferred from Salmonella to E. coli and vice versa via conjugation, confirming the IncFIB to be a conjugative plasmid.
During the growth kinetics experiments, wild type, recipient, and transconjugant strains all showed slower and/or reduced growth under low iron conditions when compared to high iron conditions (Fig. 4), indicating the importance of iron for replication and growth of these Salmonella isolates and supporting results of previous studies [10, 43]. In addition, SE819 (recipient) and SE819::IncFIB (transconjugant) showed similar growth kinetics in vitro in iron-depleted media. The reason why transconjugant did not show any growth advantage in iron-depleted growth media is currently unknown. Since, wild type strain (SE163A) showed increased growth kinetics in iron-depleted media compared to the recipient and transconjugants; therefore additional factors may require in addition to IncFIB plasmid to obtain growth advantages in iron-depleted condition. Additional experiments are essential to test this hypothesis.
Expression of the plasmid encoded sitA, iucA, and iutA genes were greater under iron-depleted conditions compared with iron-rich conditions in the transconjugant SE819::IncFIB, while increased sitA transcript was observed only under iron-depleted conditions in the case of SE163A. iucA and iutA of SE163A were expressed similarly under both iron-rich and iron-depleted growth media. The cause of the observed difference in expression profiles of wild type and the transconjugant remains unknown, but is likely related to differences in the bacterial genomes or other plasmids present in the wild type strain. Botteldoom et al. [33] investigated the expressions of the different housekeeping genes (16S rRNA, rpo D and gmk) of Salmonella enterica in different growth conditions by qRT-PCR. They demonstrated that expression of gmk was more stable than the other two housekeeping genes in various growth conditions tested. In our initial experiment, we examined both 16S rRNA and gmk as reference genes for normalizations; we also observed that expression of gmk was stable than 16S rRNA in different growth conditions. Therefore, in the further experiments, we used gmk as the reference gene to normalize the gene expressions in qRT-PCR.
Fur is an iron regulatory component and is a well-studied transcription repressor of target gene(s) repression in response to iron [44–46]. Fur binds to consensus regions upstream of target genes Fur-box [47, 48]. A previous study identified consensus sequences of Fur-box in the S. Typhimurium genome [48] and several studies have suggested that Fur regulates directly or indirectly the many virulence factors of pathogens, including shiga-like toxins in E. coli [47], colonization capacity of Helicobacter pylori in the human stomach [13], biofilm formation of Vibrio species, and Type 3 secretion systems of Shigella and S. Typhimurium [11]. The chromosome encoded sitABCD in S. Typhimurium is regulated by Fur, an iron transporter required for virulence [15]. The mechanisms of regulation of plasmid-encoded genes of sitABCD and iucABCD-iutA operons in response to iron have yet to be determined.
In the invasion assays, a slightly higher percentage uptake of the transconjugant strain was observed compared to the recipient (Fig. 6a). Similarly, the percentage of persistence in the host was significantly higher in the transconjugant than recipient (Fig. 6b), indicating IncFIB plasmid contributes to invasion and persistence. However, the specific mechanisms are not known. Additionally, the wild type strain SE163A showed significantly higher invasion and persistence in Caco-2 cells compared to transconjugant and recipient (Fig. 6a & b). In addition, transconjugant persisted at higher rate when grown in iron-depleted than the iron-rich media prior to infection while, wild type strain showed similar persistence rate in both iron-rich and iron-depleted conditions (Fig. 7b). The distinct persistence of wild type and tansconjugant when grown in iron-rich and iron-depleted conditions prior to infection of Caco-2 cells may be due to the difference in the genetic backbone of these strains. From these observations we speculate that factors encoded on the IncFIB plasmid contribute synergistically to facilitate uptake and persistence in the host. Whereas additional virulence factors, which are either encoded by other virulence associated plasmid(s) or reside on the chromosome, contribute to increased infectivity and fitness within host cells. However, we have to interpret of these findings cautiously as wild type strain possesses several virulence associated plasmids which is absent in the recipient strain. Additionally, substantial nucleotides sequence diversity were found in the different virulence associated genes examined such as phoQ, sdiA, sinH, avrA, ssaQ, sopB, bcfC, htpG, iroN, eutR, ssaC, sifA, and spaN encoded on the chromosome between SE819 (recipient) and other six isolates including wild type strain SE163A (data not shown). Future studies would be helpful to examine the role of plasmid encoded factors associated with IncX4, IncA/C, and/or IncI1 plasmids often co-located with IncFIB plasmids, in both invasion and the persistence of host cells, by generating transconjugants containing combinations of plasmids.
Conclusions
In conclusion, it has been shown that there is minimal sequence diversity in the iron acquisition systems of IncFIB plasmids present among different bacterial species, including foodborne Salmonella serovars, suggesting that similar plasmid encoded virulence factor(s) may disseminate among bacterial pathogens. The results of the study show that the IncFIB plasmids contribute to an increased ability to persist in intestinal epithelial cells as well as the virulence potential of an organism. Thus, because IncFIB plasmids have the potential to carry both virulence and antimicrobial resistance genes, they represent a health concern since transfer of a single plasmid to a susceptible bacterial strain can render it both more virulent and resistant to multiple antimicrobial agents.
Acknowledgements
The authors thank Dr. R. Doug Wagner for providing the Caco-2 cells used in this research. The opinions expressed in this manuscript are solely the responsibility of the authors and do not necessarily represent the official views and policy of the US Food and Drug Administration or National Institutes of Health. Reference to any commercial materials, equipment, or process does not in any way constitute approval, endorsement, or recommendation by the Food and Drug Administration.
Funding
The FDA Commissioner’s Fellowship Program is acknowledged for this research project and support for BKK. The University of Arkansas for Medical Sciences (UAMS) Sequencing Facility is funded in part by NIH Grant no. UL1TR000039 and the UAMS Translational Research Institute and Center for Microbial Pathogenesis and Host Inflammatory Responses Grant no. P20GM103625.
Availability of data and materials
The S. enterica WGS data have been deposited to NCBI (BioProject: PRJNA312617).
Abbreviations
- IncFIB
Incompatibility group (Inc) FIB plasmid
- S. enterica
Salmonella enterica
- SNP
Single nucleotide polymorphism
- WGS
Whole genome sequencing
Additional file
Salmonella spp. that were sequenced and constructed for this research. Table S2. Primers employed for qRT-PCR (PDF 66 kb)
Authors’ contributions
BK, SF, SZ: designed and coordinated the study, carried out experiments and wrote the manuscript; NH, SY, JH: performed SNP analyses and wrote the manuscript; CC, RC: provided scientific data interpretation and wrote the manuscript. All authors read and approved the submitted manuscript.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-017-3954-5) contains supplementary material, which is available to authorized users.
Contributor Information
Bijay K. Khajanchi, Email: bijay.khajanchi@fda.hhs.gov
Steven L. Foley, Email: steven.foley@fda.hhs.gov
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folster JP, Pecic G, Rickert R, Taylor J, Zhao S, Fedorka-Cray PJ, Whichard J, McDermott P. Characterization of multidrug-resistant Salmonella enterica serovar heidelberg from a ground turkey-associated outbreak in the United States in 2011. Antimicrob Agents Chemother. 2012;56(6):3465–3466. doi: 10.1128/AAC.00201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scallan E, Mahon BE, Hoekstra RM, Griffin PM. Estimates of illnesses, hospitalizations and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J. 2013;32(3):217–221. doi: 10.1097/INF.0b013e31827ca763. [DOI] [PubMed] [Google Scholar]
- 4.Wilmshurst P, Sutcliffe H. Splenic abscess due to Salmonella heidelberg. Clin Infect Dis. 1995;21(4):1065. doi: 10.1093/clinids/21.4.1065. [DOI] [PubMed] [Google Scholar]
- 5.Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, Medus C, Cronquist A, Angulo FJ. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. 2008;198(1):109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 6.Han J, Lynne AM, David DE, Tang H, Xu J, Nayak R, Kaldhone P, Logue CM, Foley SL. DNA sequence analysis of plasmids from multidrug resistant Salmonella enterica serotype Heidelberg isolates. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TJ, Siek KE, Johnson SJ, Nolan LK. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J Bacteriol. 2006;188(2):745–758. doi: 10.1128/JB.188.2.745-758.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguero ME, de la Fuente G, Vivaldi E, Cabello F. ColV increases the virulence of Escherichia coli K1 strains in animal models of neonatal meningitis and urinary infection. Med Microbiol Immunol. 1989;178(4):211–216. doi: 10.1007/BF00202554. [DOI] [PubMed] [Google Scholar]
- 9.Gao Q, Wang X, Xu H, Xu Y, Ling J, Zhang D, Gao S, Liu X. Roles of iron acquisition systems in virulence of extraintestinal pathogenic Escherichia coli: salmochelin and aerobactin contribute more to virulence than heme in a chicken infection model. BMC Microbiol. 2012;12:143. doi: 10.1186/1471-2180-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson TJ, Thorsness JL, Anderson CP, Lynne AM, Foley SL, Han J, Fricke WF, McDermott PF, White DG, Khatri M, et al. Horizontal gene transfer of a ColV plasmid has resulted in a dominant avian clonal type of Salmonella enterica serovar Kentucky. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porcheron G, Dozois CM. Interplay between iron homeostasis and virulence: fur and RyhB as major regulators of bacterial pathogenicity. Vet Microbiol. 2015;179(1–2):2–14. doi: 10.1016/j.vetmic.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Mey AR, Wyckoff EE, Kanukurthy V, Fisher CR, Payne SM. Iron and fur regulation in Vibrio cholerae and the role of fur in virulence. Infect Immun. 2005;73(12):8167–8178. doi: 10.1128/IAI.73.12.8167-8178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst FD, Bereswill S, Waidner B, Stoof J, Mader U, Kusters JG, Kuipers EJ, Kist M, van Vliet AH, Homuth G. Transcriptional profiling of Helicobacter pylori fur- and iron-regulated gene expression. Microbiology. 2005;151(Pt 2):533–546. doi: 10.1099/mic.0.27404-0. [DOI] [PubMed] [Google Scholar]
- 14.Sabri M, Leveille S, Dozois CM. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology. 2006;152(Pt 3):745–758. doi: 10.1099/mic.0.28682-0. [DOI] [PubMed] [Google Scholar]
- 15.Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35(5):1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 16.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2(12):946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 17.Dostal A, Lacroix C, Bircher L, Pham VT, Follador R, Zimmermann MB, Chassard C. Iron modulates butyrate production by a child gut microbiota in vitro. MBio. 2015;6(6):e01453–e01415. doi: 10.1128/mBio.01453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg ED. Iron and infection. Microbiol Rev. 1978;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111(5):603–606. doi: 10.1016/S0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- 20.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. doi: 10.1016/S0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 21.Di Lorenzo M, Stork M. Plasmid-Encoded Iron Uptake Systems. Microbiol Spectr. 2014;2(6):PLAS-0030-2014. [DOI] [PubMed]
- 22.Runyen-Janecky LJ. Role and regulation of heme iron acquisition in gram-negative pathogens. Front Cell Infect Microbiol. 2013;3:55. doi: 10.3389/fcimb.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco AA, Hu L, Grim CJ, Gopinath G, Sathyamoorthy V, Jarvis KG, Lee C, Sadowski J, Kim J, Kothary MH, et al. Characterization of putative virulence genes on the related RepFIB plasmids harbored by Cronobacter spp. Appl Environ Microbiol. 2011;77(10):3255–3267. doi: 10.1128/AEM.03023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grim CJ, Kothary MH, Gopinath G, Jarvis KG, Beaubrun JJ, McClelland M, Tall BD, Franco AA. Identification and characterization of Cronobacter iron acquisition systems. Appl Environ Microbiol. 2012;78(17):6035–6050. doi: 10.1128/AEM.01457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khajanchi BK, Han J, Gokulan K, Zhao S, Gies A, Foley SL. Draft Genome Sequences of Four Salmonella enterica Strains Isolated from Turkey-Associated Sources. Genome Announc. 2016;4(5):e01122–16. [DOI] [PMC free article] [PubMed]
- 26.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014;42(Database issue):D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Yin Y, Jones MB, Zhang Z, Deatherage Kaiser BL, Dinsmore BA, Fitzgerald C, Fields PI, Deng X. Salmonella serotype determination utilizing high-throughput genome sequencing data. J Clin Microbiol. 2015;53(5):1685–1692. doi: 10.1128/JCM.00323-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treangen TJ, Ondov BD, Koren S, Phillippy AM. The harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15(11):524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaldhone P, Nayak R, Lynne AM, David DE, McDermott PF, Logue CM, Foley SL. Characterization of Salmonella enterica serovar Heidelberg from turkey-associated sources. Appl Environ Microbiol. 2008;74(16):5038–5046. doi: 10.1128/AEM.00409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley DE, Taylor DE, Cohen DR. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol. 1980;143(3):1466–1470. doi: 10.1128/jb.143.3.1466-1470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gokulan K, Khare S, Rooney AW, Han J, Lynne AM, Foley SL. Impact of plasmids, including those encodingVirB4/D4 type IV secretion systems, on Salmonella enterica serovar Heidelberg virulence in macrophages and epithelial cells. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botteldoorn N, Van Coillie E, Grijspeerdt K, Werbrouck H, Haesebrouck F, Donne E, D'Haese E, Heyndrickx M, Pasmans F, Herman L. Real-time reverse transcription PCR for the quantification of the mntH expression of Salmonella enterica as a function of growth phase and phagosome-like conditions. J Microbiol Methods. 2006;66(1):125–135. doi: 10.1016/j.mimet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Foley SL, Johnson TJ, Ricke SC, Nayak R, Danzeisen J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol Mol Biol Rev. 2013;77(4):582–607. doi: 10.1128/MMBR.00015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izumiya H, Sekizuka T, Nakaya H, Taguchi M, Oguchi A, Ichikawa N, Nishiko R, Yamazaki S, Fujita N, Watanabe H, et al. Whole-genome analysis of Salmonella enterica serovar typhimurium T000240 reveals the acquisition of a genomic island involved in multidrug resistance via IS1 derivatives on the chromosome. Antimicrob Agents Chemother. 2011;55(2):623–630. doi: 10.1128/AAC.01215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs PS, Maurer JJ, Nolan LK, Wooley RE. Prediction of chicken embryo lethality with the avian Escherichia coli traits complement resistance, colicin V production, and presence of the increased serum survival gene cluster (iss) Avian Dis. 2003;47(2):370–379. doi: 10.1637/0005-2086(2003)047[0370:POCELW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Ginns CA, Benham ML, Adams LM, Whithear KG, Bettelheim KA, Crabb BS, Browning GF. Colonization of the respiratory tract by a virulent strain of avian Escherichia coli requires carriage of a conjugative plasmid. Infect Immun. 2000;68(3):1535–1541. doi: 10.1128/IAI.68.3.1535-1541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litwin CM, Calderwood SB. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6(2):137–149. doi: 10.1128/CMR.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Silva Neto JF, Lourenco RF, Marques MV. Global transcriptional response of Caulobacter crescentus to iron availability. BMC Genomics. 2013;14:549. doi: 10.1186/1471-2164-14-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mnif B, Vimont S, Boyd A, Bourit E, Picard B, Branger C, Denamur E, Arlet G. Molecular characterization of addiction systems of plasmids encoding extended-spectrum beta-lactamases in Escherichia coli. J Antimicrob Chemother. 2010;65(8):1599–1603. doi: 10.1093/jac/dkq181. [DOI] [PubMed] [Google Scholar]
- 41.Engelberg-Kulka H, Glaser G. Addiction modules and programmed cell death and antideath in bacterial cultures. Annu Rev Microbiol. 1999;53:43–70. doi: 10.1146/annurev.micro.53.1.43. [DOI] [PubMed] [Google Scholar]
- 42.Pullinger GD, Lax AJ. A Salmonella dublin virulence plasmid locus that affects bacterial growth under nutrient-limited conditions. Mol Microbiol. 1992;6(12):1631–1643. doi: 10.1111/j.1365-2958.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 43.Nugent SL, Meng F, Martin GB, Altier C. Acquisition of Iron is Required for growth of Salmonella spp. in tomato fruit. Appl Environ Microbiol. 2015;81(11):3663–3670. doi: 10.1128/AEM.04257-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjarnason J, Southward CM, Surette MG. Genomic profiling of iron-responsive genes in Salmonella enterica serovar typhimurium by high-throughput screening of a random promoter library. J Bacteriol. 2003;185(16):4973–4982. doi: 10.1128/JB.185.16.4973-4982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hantke K. Members of the fur protein family regulate iron and zinc transport in E. coli and characteristics of the fur-regulated fhuF protein. J Mol Microbiol Biotechnol. 2002;4(3):217–222. [PubMed] [Google Scholar]
- 46.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 47.Calderwood SB, Mekalanos JJ. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Lorenzo V, Wee S, Herrero M, Neilands JB. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The S. enterica WGS data have been deposited to NCBI (BioProject: PRJNA312617).


