Abstract
In neuronal cells, presenilin-dependent γ-secretase activity cleaves amyloid precursor proteins to release Aβ peptides, and also catalyzes the release of the intracellular domain of the transmembrane receptor Notch. Accumulation of aberrant Aβ peptides appears to be causally related to Alzheimer's disease. Inhibition of Aβ peptide production is therefore a potential target for therapeutic intervention. Notch proteins play an important role in cell fate determination in many different organisms and at different stages of development, for example in mammalian T cell development. We therefore addressed whether structurally diverse γ-secretase inhibitors impair Notch function by studying thymocyte development in murine fetal thymic organ cultures. Here we show that high concentrations of the most potent inhibitors blocked thymocyte development at the most immature stage. In contrast, lower concentrations or less potent inhibitors impaired differentiation at a later stage, most notably suppressing the development of CD8 single-positive T cells. These phenotypes are consistent with an impairment of Notch signaling by γ-secretase inhibitors and define a strict Notch dose dependence of consecutive stages during thymocyte development.
The enzyme γ-secretase catalyzes the generation of amyloid beta peptides, Aβ(1–40) and Aβ(1–42), from amyloid precursor protein (APP) by a mechanism known as regulated intramembrane proteolysis (reviewed in ref. 1). The aberrant production of these peptides causes their deposition as plaques in the brain, which have been associated with the pathology of Alzheimer's disease (AD). Accumulating evidence has recently linked γ-secretase activity to the transmembrane proteins presenilin-1 and -2 (PS1 and PS2), mutations of which have been found to cause AD in the majority of familial cases of the disease (2). A similarity of presenilins to aspartyl proteases has become apparent as a result of several recent observations: First, γ-secretase activity was inhibited by mutations of either one of two highly conserved aspartate residues in adjacent helices of PS-transmembrane domains, which may constitute a catalytic domain (3–5). Second, pharmacological γ-secretase inhibitors representing aspartyl protease transition-state analogs directly bind to PS1 and PS2, suggesting that presenilins indeed contain the catalytic domain of a γ-secretase complex (6–8). Finally, the critical aspartates of presenilins are embedded in a motif that is strikingly homologous to the active sites of bacterial polytopic aspartyl proteases, the type-4 prepilin peptidases (TFPP; ref. 9).
While these properties have stimulated a search for γ-secretase/presenilin inhibitors for the treatment or prevention of AD, genetic and functional evidence indicates that presenilins are also required for the function of members of the Notch protein family. Notch-1 is a transmembrane receptor that is involved in cell fate decisions in many species (reviewed in refs. 10 and 11). It is synthesized as a large precursor protein that is proteolytically processed at three sites. The final step involves a γ-secretase, which cleaves at the intramembrane domain to release the Notch intracellular domain (NICD). This NICD in turn translocates to the nucleus where it functions as a transcriptional coactivator for the CSL transcription factor family. Although the γ-secretases involved in the processing of APP and Notch have not formally been shown to be identical, presenilins are clearly important for both proteolytic events (reviewed in ref. 12). Loss-of-function mutations of presenilin homologs in Caenorhabditis elegans or Drosophila mimicked Notch deficiencies (13–15). In the mouse, the combined inactivations of PS1 and PS2 caused embryonic lethality and skeletal and neural defects highly reminiscent of Notch-1 knockout phenotypes (16, 17). Aβ production and Notch processing at the γ-site were strongly inhibited in PS1−/− and in PS1−/−PS2−/− cells (18–21) or by mutations of the strictly conserved aspartate residues in PS1 or PS2 (3–5, 22, 23).
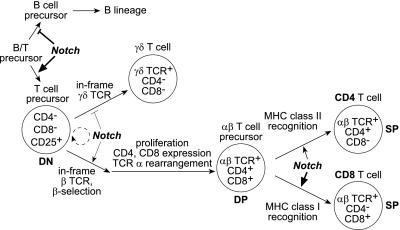
Although these experiments demonstrate a close connection between presenilins, γ-secretase, and Notch function, potential developmental defects of γ-secretase inhibition might not be of concern in the treatment of elderly AD patients. However, Notch family members also play a role in the adult hematopoietic system. Several Notch family members are expressed in hematopoietic stem cells and are potentially important for the self-renewal of these cells, which is stimulated by Notch ligand (24–27). Notch-1 has been found to play a role in B versus T cell differentiation (Fig. 1). The conditional inactivation of Notch-1 in hematopoietic precursor cells resulted in a block of T cell development at the earliest stage, coupled with aberrant expansion of immature B cells in the thymus (28). Conversely, bone marrow (BM) transfers performed with cells expressing a constitutively active form of Notch-1 resulted in a loss of B-lineage cells and the production of immature T cells in the BM (29).
Figure 1.
A model for Notch involvement in T-lymphoid development (modified from ref. 33). Notch signaling in lymphoid precursor cells in the bone marrow and/or in the thymus favors T-lymphoid over B-lymphoid differentiation. Notch also appears to bias the early differentiation of double-negative (DN) T cell precursors toward the αβ T cell lineage. Finally, Notch promotes positive selection of double-positive (DP) to single-positive (SP) cells with a predominant effect on the selection and/or survival of CD8 cells (see text for details and references).
The Notch pathway affects T cell development even after T-lineage commitment (Fig. 1). Transgenic expression of an activated form of Notch-3 caused the expansion of CD4−CD8− [double-negative (DN)], immature thymocytes, and impaired their progression to the CD4+CD8+ [double-positive (DP)] stage (30). In contrast, an activated form of Notch-1 in thymocytes promoted the differentiation of DP to CD4+ and CD8+ [single-positive (SP)] cells and increased the ratio of CD8+ over CD4+ SP cells in the thymus (31, 32). Constitutively active Notch-1 also appears to favor αβ over γδ T cell development (33). Surprisingly, conditional inactivation of Notch-1 in immature thymocytes did not perturb T cell development (34). Because the expression patterns of Notch-1, -2, and -3 overlap in the thymus (35, 36), this result may reflect redundancy in Notch signaling pathways. It is also not clear to what extent Notch-signaling in T cells depends on presenilins and γ-secretase activity, because Notch proteins may exert at least part of their signaling function without a release of the NICD (37). The inactivation of an upstream signal, such as cleavage by γ-secretase, might interfere with the function of all Notch family members. To elucidate the importance of γ-secretase activity in controlling thymocyte maturation, we studied small molecule inhibitors that blocked Aβ and NICD production in neuronal cells for their effects on thymocyte development.
Materials and Methods
Fetal Thymic Organ Cultures.
Embryos from timed pregnancies of C57BL/6 (Taconic Farms) or OT1 T cell antigen receptor (TCR) transgenic females (C57BL/6 genetic background; ref. 38) were isolated at day 14 of gestation. Thymic lobes were harvested and cultured by using standard techniques (39). Pairs of lobes were split onto transwell plates (Costar) for control and test conditions as indicated in the text. After 3 days in culture, half of the medium was replaced, maintaining the initial culture conditions. Typically, lobes were harvested after 6 days in culture and minced between frosted glass slides to prepare single-cell suspensions. Cells were counted and analyzed by flow cytometry. Control cells for flow cytometry were derived from thymi of adult C57BL/6 or OT1 TCR transgenic mice.
Flow Cytometric Analysis.
1 × 105 to 1 × 106 cells were stained with saturating concentrations of antibodies at 4°C for 30 min, using combinations of the following mouse-specific antibodies: Phycoerythrin (PE)-conjugated anti-CD4 and FITC-conjugated anti-CD8 (Caltag Laboratories, South San Francisco, CA), biotinylated (BIO) anti-CD3ɛ, BIO-anti-γδTCR, FITC-anti-CD25, PE-anti-CD44, BIO-anti-B220, and BIO-anti-Vα2 (PharMingen). Biotinylated antibodies were visualized by tricolor-conjugated streptavidin (SA-TRI; Caltag Laboratories). Data were collected on a FACScan flow cytometer (Becton Dickinson). All analyses were performed by using REPROMAC 2.3 software (TrueFacts Software, Seattle).
Results
γ-Secretase Inhibition Impairs the Development of CD8 T Cells.
PS1 and PS2 are abundantly expressed in thymocytes and splenocytes of adult mice, as detected by Western blot analysis or by surface and intracellular staining in flow cytometric analyses (data not shown). The genetic inactivation of PS1 and PS2 in mice causes early lethality (16, 17), which precludes the study of T cell development in these animals. We therefore took advantage of pharmacological γ-secretase inhibitors to study thymocyte development in fetal thymic organ cultures (FTOC). T cell precursor cells from the fetal liver initially populate the thymic lobes on day 12 of embryonic development. At embryonic day 14, the lobes still contain mostly immature DN thymocytes that, upon isolation and culturing of these lobes, recapitulate differentiation and proliferation, closely following the in vivo developmental pattern (40). After 6 days in culture, the lobes contain DN, DP, and SP thymocytes with a CD4/8 profile already very similar to that seen in thymocytes from adult animals.
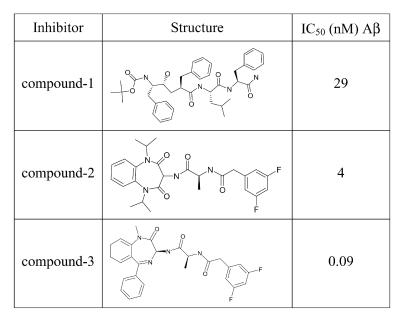
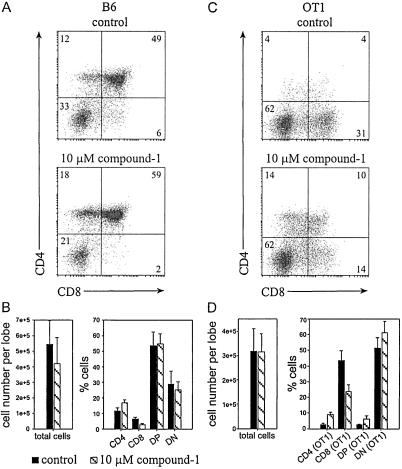
We cultured individual thymic lobes in the absence or presence of the γ-secretase inhibitor compound 1 (L-685,458; refs. 6, 41, and 42), which has been show to bind to PS1 and PS2 and to inhibit γ-secretase activity in neuronal cells with a half-maximal inhibitory concentration (IC50) of 29 nM (Fig. 2). The lobes were harvested after 6 days in culture and analyzed by flow cytometry. Fig. 3A (Upper) shows the CD4/8 expression profile of a C57BL/6 control lobe cultured in medium with 0.1% DMSO (as a solvent control), which is also representative of lobes cultured in standard medium without DMSO (data not shown). A large number of immature DN cells has progressed to the DP and SP stages, showing a 1.8-fold excess of CD4 over CD8 SP cells on average. Lobes treated with 10 μM compound 1 yielded slightly lower cell numbers, but still supported thymocyte differentiation to the SP stage (Fig. 3A Lower). However, the ratio of CD4 and CD8 cell numbers was consistently increased, showing a 5.6-fold excess of CD4 over CD8 SP cells. The bar diagrams in Fig. 3B graphically summarize the results of this experiment.
Figure 2.
Structure and activity of selected γ-secretase inhibitors. IC50 (nM) Aβ refers to the compound concentration required to inhibit 50% of Aβ(1–40) formation in human neuroblastoma cells.
Figure 3.
γ-Secretase inhibition impairs accumulation of CD8 SP cells in FTOC. C57BL/6 (B6) or OT1 TCR transgenic (OT1) thymocytes were isolated from FTOC after 6 days of culture in the absence (control) or presence of 10 μM compound 1. All cells were stained with PE anti-CD4 and FITC anti-CD8. In the case of OT1, cells were additionally stained with BIO anti-Vα2/SA-TRI to detect cells expressing the transgenic TCR. Flow cytometric profiles for B6 (A) and OT1 (C) are shown as two-dimensional dot blots for the CD4/8 staining with each dot representing a live cell. Numbers in quadrants indicate percentages of cells within respective thymic subpopulations. (B) Bar diagrams summarize B6 results for total cell recovery per lobe and for the relative representation of thymocyte subpopulations, presenting mean values and standard deviations (SD) obtained for nine control and five inhibitor-treated samples within this experiment, which is a representative example of five independent experiments. (D) Bar diagrams summarize OT1 results analogous to B, except that the analysis of thymic subpopulations was electronically restricted to cells expressing the OT1 TCR (Vα2-positive cells). Presented are the means and SD obtained for five control and five inhibitor-treated samples within this experiment, which is representative of three independent experiments.
In a time course experiment (data not shown), inhibitor treatment did not alter thymocyte development on day 3. Extended cultures up to day 12 in the presence of 10 μM compound 1 showed a change in CD4/8 ratios similar to what was seen on day 6, but the cell recovery from individual lobes was considerably reduced (≈30% of controls).
To evaluate the effects of γ-secretase inhibitors in more detail, we used thymocytes from the OT1 TCR-transgenic mouse model. The OT1 TCR specifically recognizes an ovalbumin peptide (SIINFEKL) in the context of H-2Kb MHC class I (38). In the absence of peptide antigen, thymocytes are positively selected to the CD8+ T cell compartment. Fig. 3C presents the CD4/8 profiles for FTOCs from OT1 TCR mice, cultured for 6 days in the absence or presence of 10 μM compound 1. As expected, control lobes effectively selected CD8 SP cells. In contrast, inhibitor treatment again strongly reduced the number of CD8 cells, whereas DP and CD4 SP cells became much more abundant.
The increased representation of CD4 cells could result from the assembly of TCRs containing products of rearranged endogenous α chain genes, changing the specificity of the TCR and permitting selection to the CD4 lineage by MHC class II molecules. Alternatively, increased numbers of bona fide OT1 TCR-expressing cells could be aberrantly selected to the CD4 lineage as a result of decreased γ-secretase activity. We therefore electronically restricted the analysis to cells that expressed high levels of the transgenic TCR (recognized by antibodies to the Vα2 chain; Fig. 3D). Clearly, most of the CD4 and DP cells isolated from the inhibitor-treated lobes still expressed the OT1 TCR, arguing for their aberrant selection. The representation of CD8 cells remained dramatically reduced.
These results, combined with previous results for the time course experiment with C57BL/6 FTOCs, suggested that compound 1 might act primarily after progression to the DP stage. We tested this hypothesis by culturing OT1 FTOCs for 3 days in control medium, followed by a 5-day incubation in medium containing 10 μM compound 1. As expected, even this delayed inhibitor treatment perturbed the CD4/8 ratio almost as efficiently as continuous inhibitor treatment for 8 days (unpublished observation).
Increasing Concentrations of a Potent γ-Secretase Inhibitor Shift the Block of Thymocyte Differentiation to Early Developmental Stages.
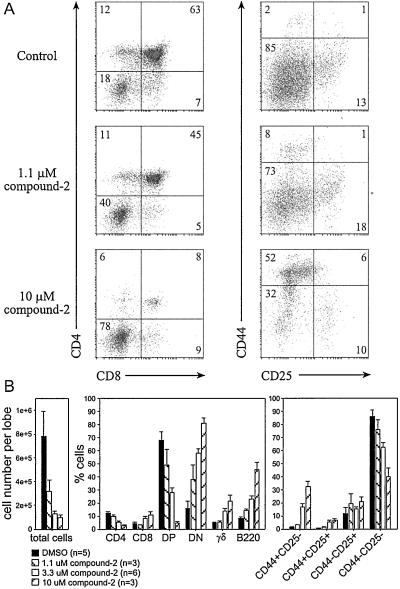
Considering the efficient block of T cell development by the conditional inactivation of Notch-1 (28), the change in CD4/8 cell ratios provoked by compound 1 presented a surprisingly mild phenotype. Consequently, we chose a second, more potent γ-secretase inhibitor (Fig. 2; patent WO28268) for further C57BL/6 FTOC experiments, and applied increasing concentrations to the cultures (Fig. 4). The results of 6-day cultures in the presence of 1.1 μM compound 2 were comparable with those observed when using 10 μM compound 1 (Fig. 3), except that we recovered fewer cells and found reduced numbers of DP and CD4 cells as well as CD8 cells (Fig. 4 A Top and Middle and B Left and Center). The reduction in CD8 cells became more apparent as we electronically excluded γδ T cells from the analysis, because γδ T cells expressing CD8α-homodimers contributed a larger percentage of cells under these conditions than in control samples (data not shown). Concentrations of 3.3 μM and 10 μM compound 2 further reduced the cell recovery per lobe (Fig. 4B Left). Moreover, the differentiation of immature DN cells to the DP or SP stages was impaired in a dose-dependent manner, resulting in a profound block at the DN stage in the presence of 10 μM compound 2. This early arrest of the αβ T cell lineage was accompanied by an increase in the relative representation of γδ T cells and cells expressing the B lineage marker CD45R/B220. We also noted that a seemingly increased number of CD8 SP cells under these conditions was due to the increased appearance of large, immature CD8 SP cells that are considered to be cells in transition from the DN to the DP stage (Fig. 4 A Bottom and B Center). To characterize this more profound developmental block, we investigated the expression of the surface markers CD44 and CD25, typically used to distinguish stages of differentiation within the DN compartment. Precursor cells mature from CD44+25− to CD44+25+ and subsequently extinguish CD44 expression. CD25 expression is normally lost upon successful signaling from the pre-TCR, leading to the appearance of CD44−25− cells that rapidly gain CD4 and CD8 expression (43). Our analysis revealed that the arrest of thymocyte development occurred at the earliest, CD44+25− stage of differentiation (Fig. 4 A and B, Right). The result was again strictly dependent on the concentration of the inhibitor.
Figure 4.
Potent γ-secretase inhibition causes an early block of T cell development. C57BL/6 FTOC cells were analyzed after 6 days in culture in the absence or presence of 1.1, 3.3, or 10 μM compound 2 γ-secretase inhibitor. (A) Flow cytometric profiles of live cells stained with a combination of PE anti-CD4, FITC anti-CD8, and BIO anti-TCRγδ/SA-TRI or PE anti-CD44, FITC anti-CD25, and BIO anti-B220/SA-TRI. Numbers in quadrants indicate percentages of cells within respective subpopulations. (B) Bar diagrams summarize results for the total cell recovery and thymocyte subpopulations of fetal lobes from littermates under the respective conditions. Shown is a representative example of three independent experiments, presenting means and SD for the indicated sample numbers (n). After determining the number of harvested cells per lobe, groups of two or three thymic lobes treated with 10 μM compound 2 were pooled to provide sufficient cells for flow cytometry, in which case n indicates the number of pools. All other lobes were continuously handled separately.
The striking effect of 10 μM compound 2 could even be appreciated by microscopic inspection of the cultured lobes. After an initial expansion up to the third day in culture, the inhibitor-treated lobes remained much smaller (≈1/3) than controls and their surface appeared smooth, whereas control lobes developed a humped surface.
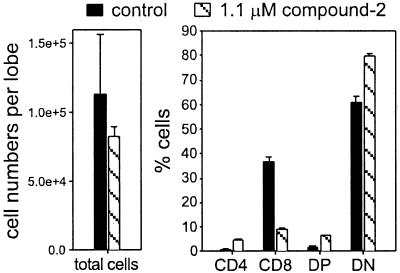
We confirmed this concentration-dependent effect of compound 2 in OT1 TCR transgenic FTOCs. Compound 2 at 10 μM again blocked the DN to DP transition (data not shown). The effect of 1.1 μM compound 2 on the CD4/8 cell ratio was emphasized in OT1 FTOCs: it strongly suppressed the accumulation of CD8 SP cells (Fig. 5). We also allowed thymocytes to mature from DN to DP cells in control medium for 3 days, followed by treatment with low (1 μM) or high (10 μM) concentrations of compound 2 (see Fig. 7, which is published as supplemental data on the PNAS web site, www.pnas.org). As expected, once the thymocytes passed the transition to the DP stage, this potent inhibitor changed the CD4/8 cell ratio similarly to experiments including high concentrations of compound 1.
Figure 5.
Low concentrations of a potent γ-secretase inhibitor affect the accumulation of CD8 SP cells similarly to high concentrations of a less potent inhibitor. OT1 TCR transgenic thymocytes were isolated from FTOC after 6 days of culture in the absence (control) or presence of 1.1 μM compound 2. All cells were stained with PE anti-CD4, FITC anti-CD8, and BIO anti-Vα2/SA-TRI. The bar diagrams summarize results for total cell recovery per lobe and for the relative representation of thymocyte subpopulations. Presented are mean values and the range of results obtained for two control and two inhibitor-treated samples, which is a representative example of three similar experiments.
Structurally Different γ-Secretase Inhibitors Differ in Potency but Consistently Affect Thymocyte Development in a Concentration-Dependent Manner.
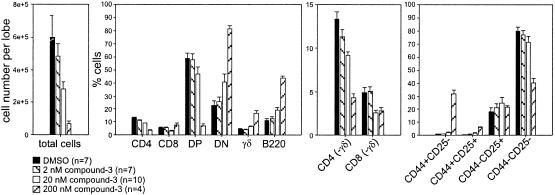
To demonstrate that the observed phenotypes were specifically caused by the inhibition of γ-secretase activity, we tested another structurally distinct γ-secretase inhibitor, compound 3 (Fig. 2; ref. 8), that exhibited very high potency in amyloid Aβ cleavage assays. Consistent with our results for high concentrations of compound 2, high concentrations of compound 3 dramatically reduced cell numbers and arrested T cell development at the DN, CD44+25− stage. This effect was again accompanied by the increased relative representation of γδ T cells and B220-expressing precursor cells. In a titration experiment, this highly potent compound blocked development at concentrations of 200 nM (Fig. 6). A 10-fold dilution to 20 nM permitted progression to SP cells, but again the CD4/8 cell ratio was shifted, decreasing the representation of CD8 cells, especially if γδ T cells were excluded (Fig. 6, third panel). A further dilution to a concentration of 2 nM allowed T cell development to proceed seemingly unimpaired.
Figure 6.
Structurally diverse γ-secretase inhibitors consistently affect thymocyte development in a concentration-dependent manner. C57BL/6 FTOC cells, cultured in various concentrations of the γ-secretase inhibitor compound 3 (0, 2, 20, and 200 nM) for 6 days, were analyzed as described in Fig. 4. CD4(−γδ) and CD8(−γδ) refers to SP cell numbers determined after electronically excluding TCRγδ-positive cells. Shown is a representative example of four experiments, presenting means and SEM for the indicated sample numbers (n). Sample numbers refer to individual thymic lobes except for the condition with 200 nM compound 3, where it refers to the number of pools consisting of 2–3 lobes used for flow cytometric analysis.
We analyzed a series of 12 additional, structurally diverse inhibitors with different potencies, which recapitulated the entire spectrum of results described for the selected inhibitors presented in this report and confirmed that high concentrations of the most potent inhibitors blocked early T cell development, whereas lower concentrations or less potent inhibitors impaired the development of CD8 cells (unpublished results).
Discussion
PS1, PS2, and Notch-1 inactivations in mice have been analyzed in great detail for their effects on embryonic development, but the early death of PS1−/−PS2−/− and Notch-1−/− animals has precluded comprehensive analyses of the importance of these genes in various lineages. The recent discovery of potent γ-secretase inhibitors, which specifically bind to PS-proteins, enabled us to study the role of γ-secretase/PS-function during T cell development. Although these inhibitors are not suitable for in vivo administration, the compounds could be applied to FTOCs, which faithfully recapitulate thymocyte development in vitro, and present a well established alternative to whole-animal studies. The versatility of this system allowed us to test many different conditions, using structurally distinct inhibitors at various concentrations, and to evaluate their effects on different stages of T cell development.
Most strikingly, the potent inhibition of γ-secretase (conditions including 10 μM compound 2 or 200 nM compound 3) blocked thymocyte development at the earliest, DN CD44+25− stage, which is highly reminiscent of the phenotype observed following conditional inactivation of Notch-1 in hematopoietic precursor cells (28). These samples contained a high percentage of apoptotic or dead cells (data not shown); however, we conclude from the selective absence of the more mature but not the immature stages that these inhibitors are not generally toxic to thymocytes. This conclusion is underscored by the fact that adult thymocytes, cultured overnight in the presence of the same inhibitor concentrations, do not show increased cell death compared with controls (data not shown). Moreover, our preliminary results indicate that fetal thymic lobes, cultured for 3 days in high concentrations of compound 2 or 3, do recover and permit differentiation of thymocytes to the SP stage if transferred and cultured in control medium for an additional 5 days. Most likely, the inhibition of γ-secretase impairs differentiation and/or proliferation signals and thereby leads to apoptosis. This notion is supported by the fact that an activated form of Notch-1 prevents glucocorticoid- or TCR-induced cell death of thymocytes in vitro (44, 45). γδ T cells do not go through a period of extensive proliferation during their differentiation and do not depend on β-selection, a differentiation signal for immature cells that successfully express a TCR β chain paired with a pre T α chain (46). These cells may therefore be less affected by the inhibitor treatment. Furthermore, we detected an increased relative representation of immature precursor cells, expressing the pan-B cell marker CD45R/B220 on their surface. Preliminary analysis revealed that about half of these cells also express CD19, another marker that is specifically expressed during early stages of B cell development. However, we could not detect IgM expression (typical of mature B cells) on these cells, suggesting that they represent early B-lineage cells (data not shown).
Although this dramatic arrest in development is most likely caused by a complete inhibition of Notch signaling, we still find a small number of immature SP and DP cells in these cultures, which may have developed from precursor cells that have already received a maturation signal before the isolation of lobes on day 14. This interpretation is supported by our observation that even a 2-μM concentration of the most potent compound 3 (which is a 10-fold higher concentration than shown in this report) does not further improve the block of differentiation. Lower concentrations of these potent inhibitors, or high concentrations of a less potent inhibitor, permit thymocytes to progress in their differentiation program. However, these conditions caused a reduction of SP cells, especially of CD8 cells.
These phenotypes are consistent with most predictions for the effect of Notch inactivation by γ-secretase inhibition, but they also shed light on somewhat controversial interpretations from previous studies of Notch. Robey and colleagues (31) found that expression of a constitutively activated form of Notch-1 in thymocytes strongly increased the number of CD8 cells with a concomitant decrease in CD4 cells, suggesting a role of Notch-1 in the CD4 versus CD8 lineage decision. Based on studies that reduced Notch-1 expression to 10% of normal by employing anti-Notch-1 antibodies or antisense RNA, Yasutomo et al. (47) argued however that Notch-1 was only required for CD8 cell maturation following TCR-directed lineage commitment. Studies by Deftos and colleagues (32, 44) indicated that high-level expression of another constitutively active form of Notch-1 increased maturation of both SP subpopulations, albeit with a dominance of CD8 cells. In contrast, very recent findings by Wolfer et al. (34) in Notch-1-deficient thymocytes argue against a role for Notch-1 in any of those events.
Our results now position these disparate observations along a continuum of Notch effects. A complete block of Notch activity arrests T cell development at the earliest stage, low activity still allows the transition from DN to DP stages, intermediate activity permits positive selection to the SP stage, and only high-level activity supports the accumulation of CD8 SP cells. Although there is specificity in the function of Notch proteins, which may normally be enhanced by their differential expression, the transcriptional effects of these proteins clearly overlap (30, 32). We suspect that conditional inactivation of Notch-1 in immature thymocytes permits other Notch isoform signaling pathways to predominate, a phenomenon that could not occur in cells exposed to γ-secretase inhibitors.
Detailed analysis of the effect of γ-secretase inhibition on gene transcription may permit assignment of the observed effects to specific target gene functions. A strong candidate for such a target gene is Hes-1, the inactivation of which caused an arrest of T cell development at the DN stage (48). It is more likely, however, that different, but possibly overlapping, subsets of target genes will be responsible for the observed changes at consecutive stages of differentiation.
In summary, pharmacological γ-secretase inhibitors affect thymopoiesis at multiple stages. Our ability to correlate potency of these inhibitors in amyloid precursor protein (APP) cleavage assays with efficacy in FTOC strongly suggests that these effects are mediated by the block of NICD generation from Notch proteins. Notch has been implicated through studies in transgenic mice and mouse mutants in at least four lymphoid differentiation events: (i) B versus T cell determination, (ii) maturation of CD44+25− precursor cells, (iii) differentiation of all SP cells, and (iv) accumulation of CD8 SP cells (Fig. 1). Our results within a single experimental system now show that these serial stages of T cell development become increasingly dependent on γ-secretase and Notch activation. Intriguingly, those events most closely associated with proliferation are the least sensitive to γ-secretase inhibition. Lastly, we note that the substantial difference in potency of γ-secretase inhibitors on Aβ generation in neuronal cells as compared with Notch function in T cells may provide an important therapeutic window for γ-secretase inhibition in the treatment of AD. The development of compounds that are suitable for whole-animal studies will permit direct assessment of thymopoiesis.
Supplementary Material
Acknowledgments
We thank Drs. Alan Nadin, Ian Churcher, and Chris Claiborne for the synthesis of the described inhibitors, and Drs. Jules Shafer, Timothy Harrison, and Luis Castro for their support of the research effort. We are very grateful to Erin Armstead for her excellent assistance in maintaining our mouse colony, and to Stephen Matheravidathu and Richard Wnek in the Merck Cell Analysis Facility for expert help with flow cytometry. Special thanks to our colleagues for helpful discussions and for critically reading the manuscript.
Abbreviations
- AD
Alzheimer's disease
- DN
double-negative
- DP
double-positive
- FTOC
fetal thymic organ culture
- NICD
Notch intracellular domain
- SP
single-positive
- TCR
T cell antigen receptor
- PE
phycoerythrin
- PS
presenilin
Note Added in Proof.
Independent studies by Hadland and colleagues yielded similar results (49).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Brown M S, Ye J, Rawson R B, Goldstein J L. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 2.Haass C, De Strooper B. Science. 1999;286:916–919. doi: 10.1126/science.286.5441.916. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe M S, Xia W, Ostaszewski B L, Diehl T S, Kimberly W T, Selkoe D J. Nature (London) 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 4.Steiner H, Duff K, Capell A, Romig H, Grim M G, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, et al. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- 5.Kimberly W T, Xia W, Rahmati T, Wolfe M S, Selkoe D J. J Biol Chem. 2000;275:3173–3178. doi: 10.1074/jbc.275.5.3173. [DOI] [PubMed] [Google Scholar]
- 6.Li Y M, Xu M, Lai M T, Huang Q, Castro J L, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil J G, et al. Nature (London) 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 7.Esler W P, Kimberly W T, Ostaszewski B L, Diehl T S, Moore C L, Tsai J Y, Rahmati T, Xia W, Selkoe D J, Wolfe M S. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 8.Seiffert D, Bradley J D, Rominger C M, Rominger D H, Yang F, Meredith J E, Jr, Wang Q, Roach A H, Thompson L A, Spitz S M, et al. J Biol Chem. 2000;275:34086–34091. doi: 10.1074/jbc.M005430200. [DOI] [PubMed] [Google Scholar]
- 9.Steiner H, Kostka M, Romig H, Basset G, Pesold B, Hardy J, Capell A, Meyn L, Grim M L, Baumeister R, et al. Nat Cell Biol. 2000;2:848–851. doi: 10.1038/35041097. [DOI] [PubMed] [Google Scholar]
- 10.Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 11.Weinmaster G. Curr Opin Genet Dev. 2000;10:363–369. doi: 10.1016/s0959-437x(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 12.Kopan R, Goate A. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- 13.Levitan D, Greenwald I. Nature (London) 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- 14.Ye Y, Lukinova N, Fortini M E. Nature (London) 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- 15.Struhl G, Greenwald I. Nature (London) 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 16.Donoviel D B, Hadjantonakis A K, Ikeda M, Zheng H, Hyslop P S, Bernstein A. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. Proc Natl Acad Sci USA. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 19.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm J S, Schroeter E H, Schrijvers V, Wolfe M S, Ray W J, et al. Nature (London) 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 20.Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner B A. Proc Natl Acad Sci USA. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- 22.Ray W J, Yao M, Mumm J, Schroeter E H, Saftig P, Wolfe M, Selkoe D J, Kopan R, Goate A M. J Biol Chem. 1999;274:36801–36807. doi: 10.1074/jbc.274.51.36801. [DOI] [PubMed] [Google Scholar]
- 23.Berezovska O, Jack C, McLean P, Aster J C, Hicks C, Xia W, Wolfe M S, Kimberly W T, Weinmaster G, Selkoe D J, Hyman B T. J Neurochem. 2000;75:583–593. doi: 10.1046/j.1471-4159.2000.0750583.x. [DOI] [PubMed] [Google Scholar]
- 24.Karanu F N, Murdoch B, Gallacher L, Wu D M, Koremoto M, Sakano S, Bhatia M. J Exp Med. 2000;192:1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai S, Fero J, Bartelmez S. Blood. 2000;96:950–957. [PubMed] [Google Scholar]
- 26.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear W S, Bernstein I D. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 27.Milner L A, Bigas A. Blood. 1999;93:2431–2448. [PubMed] [Google Scholar]
- 28.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald H R, Aguet M. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 29.Pui J C, Allman D, Xu L, DeRocco S, Karnell F G, Bakkour S, Lee J Y, Kadesch T, Hardy R R, Aster J C, Pear W S. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 30.Bellavia D, Campese A F, Alesse E, Vacca A, Felli M P, Balestri A, Stoppacciaro A, Tiveron C, Tatangelo L, Giovarelli M, et al. EMBO J. 2000;19:3337–3348. doi: 10.1093/emboj/19.13.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 32.Deftos M L, Huang E, Ojala E W, Forbush K A, Bevan M J. Immunity. 2000;13:73–84. doi: 10.1016/s1074-7613(00)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Washburn T, Schweighoffer E, Gridley T, Chang D, Fowlkes B J, Cado D, Robey E. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 34.Wolfer A, Bakker T, Wilson A, Nicolas M, Ioannidis V, Littman D R, Wilson C B, Held W, MacDonald H R, Radtke F. Nat Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 35.Hasserjian R P, Aster J C, Davi F, Weinberg D S, Sklar J. Blood. 1996;88:970–976. [PubMed] [Google Scholar]
- 36.Felli M P, Maroder M, Mitsiadis T A, Campese A F, Bellavia D, Vacca A, Mann R S, Frati L, Lendahl U, Gulino A, Screpanti I. Int Immunol. 1999;11:1017–1025. doi: 10.1093/intimm/11.7.1017. [DOI] [PubMed] [Google Scholar]
- 37.Wesley C S, Saez L. J Biol Chem. 2000;275:9099–9101. doi: 10.1074/jbc.275.13.9099. [DOI] [PubMed] [Google Scholar]
- 38.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 39.Ramsdell F. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Vol. 1. New York: Wiley; 1992. pp. 3.18.1–3.18.10. [Google Scholar]
- 40.Ceredig R. J Immunol. 1988;141:355–362. [PubMed] [Google Scholar]
- 41.Li Y M, Lai M T, Xu M, Huang Q, DiMuzio-Mower J, Sardana M K, Shi X P, Yin K C, Shafer J A, Gardell S J. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. . (First Published May 9, 2000; 10.1073/pnas.110126897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shearman M S, Beher D, Clarke E E, Lewis H D, Harrison T, Hunt P, Nadin A, Smith A L, Stevenson G, Castro J L. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- 43.Godfrey D I, Zlotnik A. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 44.Deftos M L, He Y W, Ojala E W, Bevan M J. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jehn B M, Bielke W, Pear W S, Osborne B A. J Immunol. 1999;162:635–638. [PubMed] [Google Scholar]
- 46.Hayday A C, Barber D F, Douglas N, Hoffman E S. Semin Immunol. 1999;11:239–249. doi: 10.1006/smim.1999.0180. [DOI] [PubMed] [Google Scholar]
- 47.Yasutomo K, Doyle C, Miele L, Germain R N. Nature (London) 2000;404:506–510. doi: 10.1038/35006664. [DOI] [PubMed] [Google Scholar]
- 48.Tomita K, Hattori M, Nakamura E, Nakanishi S, Minato N, Kageyama R. Genes Dev. 1999;13:1203–1210. doi: 10.1101/gad.13.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadland B K, Manley N R, Su D-m, Longmore G D, Moore C L, Wolfe M S, Schroeter E H, Kopan R. Proc Natl Acad Sci USA. 2001;96:7487–7491. doi: 10.1073/pnas.131202798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.