Abstract
Background
Northern fowl mites (Ornithonyssus sylviarum) are obligate hematophagous ectoparasites of both feral birds and poultry, particularly chicken layers and breeders. They complete their entire life-cycle on infested birds while feeding on blood. Infestations of O. sylviarum are difficult to control and resistance to some chemical classes of acaricides is a growing concern. The contact susceptibility of O. sylviarum to a new active ingredient, fluralaner, was evaluated, as well as other compounds representative of the main chemical classes commonly used to control poultry mite infestations in Europe and the USA.
Methods
Six acaricides (fluralaner, spinosad, phoxim, propoxur, permethrin, deltamethrin) were dissolved and serially diluted in butanol:olive oil (1:1) to obtain test solutions used for impregnation of filter paper packets. A carrier-only control was included. Thirty adult northern fowl mites, freshly collected from untreated host chickens, were inserted into each packet for continuous compound exposure. Mite mortality was assessed after incubation of the test packets for 48 h at 75% relative humidity and a temperature of 22 °C.
Results
Adult mite LC50 /LC99 values were 2.95/8.09 ppm for fluralaner, 1587/3123 ppm for spinosad, 420/750 ppm for phoxim and 86/181 ppm for propoxur. Permethrin and deltamethrin LC values could not be calculated due to lack of mortality observed even at 1000 ppm.
Conclusions
Northern fowl mites were highly sensitive to fluralaner after contact exposure. They were moderately sensitive to phoxim and propoxur, and less sensitive to spinosad. Furthermore, the tested mite population appeared to be resistant to the pyrethroids, permethrin and deltamethrin, despite not being exposed to acaricides for at least 10 years.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-017-2289-z) contains supplementary material, which is available to authorized users.
Keywords: Acaricide, Poultry, Control, Fluralaner, Spinosad, Phoxim, Propoxur, Permethrin, Deltamethrin, Ornithonyssus sylviarum
Background
Two major ectoparasite species severely affect the poultry industry worldwide: the northern fowl mite, Ornithonyssus sylviarum, and the poultry red mite, Dermanyssus gallinae [1, 2]. Both mite species are obligate hematophagous parasites able to complete their life-cycles within about 1 week under optimal conditions [2–4]. Mite populations can become dense very quickly in commercial poultry facilities reducing hen performance and profitability [5, 6]. They differ mainly in that all stages of O. sylviarum mites live on the host full-time, occupying and laying eggs in the fluffy feathers mostly of the vent region [1] (Fig. 1), while D. gallinae lives predominantly off-host, hidden in cracks and crevices, and comes out nocturnally to feed on the birds [2]. Classical approaches to treat mite infestations mostly include the use of acaricidal sprays applied to the environment or to the host itself [6]. However, a complicating factor for both mite species is that they can persist without hosts for weeks and perhaps months in the environment [7, 8]. Their very small size makes them a difficult target for spray treatments and subsequent disinfestation of poultry houses between flocks. In addition, these acaricidal sprays must penetrate the feather layer from under the birds (vent region) to treat O. sylviarum on-host, which make it difficult to spray birds in enriched-cage or cage-free systems.
Fig. 1.
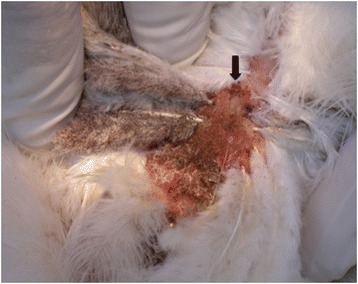
Northern fowl mites (Ornithonyssus sylviarum) on the vent region of an infested hen. A representative cluster of feeding mites indicated by an arrow
In North America, O. sylviarum is the most prevalent ectoparasite of commercial laying hen operations [1]. Control efficiency is threatened by serious mite resistance to a shrinking arsenal of acaricidal compound classes [9], especially for the synthetic pyrethroids, but also against carbamates and organophosphates. In the USA synthetic on-host control chemicals for O. sylviarum 15 years ago included the carbamate carbaryl, the organophosphate mixture tetrachlorvinphos/dichlorvos, and permethrin. Currently only permethrin is widely allowed for use, tetrachlorvinphos or its mixture with dichlorvos are used in some states, and carbaryl is no longer allowed [10]. Alternatives to traditional acaricides, such as botanical products or inert dusts, have been explored for both O. sylviarum and D. gallinae control, with inconsistent results; botanical products are notoriously variable, while silica dusts can be difficult to deploy effectively [2, 10, 11]. Entomopathogenic fungi, especially Beauveria bassiana and Metarhizium anisopliae, have promise for control of D. gallinae and perhaps of O. sylviarum, although results have been mixed [12–14]. It is critical that we explore other options, including new synthetic acaricides [2].
Twenty-first century developments of new classes of acaricidal compounds like isoxazolines, have restored optimism that safe and effective pest control could be maintained for crop, premise protection, and animal health. Isoxazolines work by binding to invertebrate GABA and glutamate channels [15], but act at previously unrecognized sites. This mitigates cross-resistance to other chemotypes, and differing target sites between arthropods and mammals result in selective toxicity and mechanistically based safety [16]. Isoxazolines, including fluralaner, afoxalaner and sarolaner, are under development and are of increasing importance in the control of external parasites in dogs and cats, including mites [17–20]. The present study was conducted to determine if fluralaner possesses contact activity against O. sylviarum, and compare this activity with that of other acaricides commonly used against mite infestations of poultry.
Methods
The northern fowl mites used in the tests originated from a long-term colony maintained on a flock of untreated hens without any acaricide exposure for over 10 years at the University of California, Riverside Agricultural Operations property adjacent to the main campus (Animal Use Protocol A20150009, University of California, Riverside). The mites were originally collected from commercial hen infestations in California, between 2000 and 2005.
The larval packet test method, a common bioassay for ixodid tick larvae, was used for mite acaricide exposure [21, 22]. Whatman #1 filter paper (GE Healthcare UK Ltd., Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) was cut into 10 × 8 cm pieces. The filter paper pieces were labeled using a #2 graphite pencil according to the test material, dose, and replication number and were placed onto a larger piece of Labmat material (Bel-Art, Thermo Fisher Scientific, Inc., Waltham, Massachusetts, USA) which had a plastic backing to protect the lab counter from contamination. The filter paper rectangles were treated with compound solution of the acaricides (see below) and allowed to dry for 48 h before adding mites.
The carrier solvent, also used for the control packets, was a 1:1 mixture of 1-butanol (ACS reagent grade, 99.4% pure, Sigma-Aldrich Inc., Milwaukee, Wisconsin, USA) and pure olive oil (100%, Stater Brothers Markets, San Bernardino, California, USA). Fluralaner (10 mg/l) was supplied by Merck Animal Health, Summit, New Jersey, USA. Permethrin was purchased from Sigma-Aldrich Inc., Milwaukee, Wisconsin, USA. Other compounds were purchased from VHS Labs, Manchester, New Hampshire, USA. All compounds were used as technical grade acaricides.
The treatments tested included a carrier control and the acaricide treatments (i) fluralaner, 96.2% purity; (ii) spinosad, 94% purity; (iii) phoxim, 98% purity; (iv) propoxur, 99.5% purity; (v) permethrin, 98.8% purity; and (vi) deltamethrin, 99.5% purity. Stock solutions were prepared for fluralaner (1000 ppm), spinosad (4000 ppm), phoxim (2000 ppm), propoxur (1000 ppm), permethrin (1000 ppm), deltamethrin (1000 ppm). Preliminary tests (three replications per concentration) were done using the stock solutions and two sequential 10× dilutions (e.g. fluralaner at 1000 ppm, 100 ppm and 10 ppm) to establish approximate active ranges for northern fowl mites. Because the 1000 ppm solutions and below of permethrin and deltamethrin showed no mortality (see below), they were not tested further. Five concentrations over informative concentration ranges then were prepared by serial dilution from the stock solutions for the remaining test materials as follows: (i) fluralaner at 1, 2, 5, 10 and 20 ppm: (ii) spinosad at 200, 400, 1000, 2000 and 4000 ppm; (iii) phoxim at 250, 500, 1000, 1500 and 2000 ppm; and (iv) propoxur at 31, 63, 125, 250 and 500 ppm. Each filter paper received 800 μl of one of the test solutions by pipetting small drops of it evenly on the filter paper and allowing the liquid to absorb and evenly saturate the entire filter paper.
Forty eight hours after treatment of the filter paper, mites were collected from hens by aspirating them from vent feathers into glass Pasteur pipettes [8]. Mites were transported back to the laboratory and exposed to the test packets within 3 h of removal from the hens. Mites were tapped from the pipettes onto a 15 × 15 cm metal sheet placed on an electronic chill table (Bioquip model 1431, Rancho Dominguez, California, USA) where they remained for a few minutes until the adult mites (largest life stage) were immobilized by cold and could be counted into the test packets. Packets (Fig. 2) were initially folded and then clipped closed on two sides, leaving one packet side open. Immobilized adult mites were added singly or in small groups through the open side of the test packets using a small paint brush. Test packets were placed on the chill table with the open end down during the mite counting process. Once in a packet the mites almost immediately warmed and became active, but moved quickly away from the chill table and toward the sealed (warmer) end of the test packet. This allowed more mites to be added to the bottom (colder side) of each packet until 30 mites per packet had been added. The last side was folded and clipped shut, enclosing the mites in the packet.
Fig. 2.
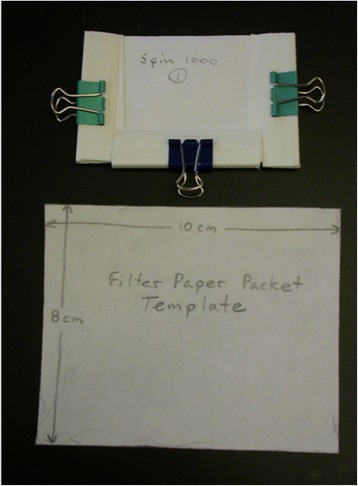
Test packets (clipped to enclose mites above and unfolded below) used to expose Ornithonyssus sylviarum to tested acaricides
Mites were held in the packets on a screen support surface, placed in a plastic holding box, and positioned above a 2 cm deep layer of saturated NaCl solution. The lid was replaced in order to hold the relative humidity at a constant 75% inside the box [23], which is a comfortable humidity for the mites [8]. A separate plastic humidity box was used for each test material to avoid any cross-contamination. The mites were held for 48 h at approximately 22 °C (room temperature). After 48 h each packet was opened to assess the mite mortality. Adult mite mortality was scored under a dissecting microscope, and the adult mites (alive or dead) were removed using a paint brush. Mites were considered dead if they failed to move after gentle stimulation with a probe.
To minimize potential differences over time in mite condition as a factor, a single group of mites was usually assayed with several acaricides (plus a carrier control) on a given test day. However, time constraints limited testing to setting up or scoring 33 packets per day. There were always three carrier-treated controls, and those were used to correct for control mortality. Three compounds were tested at a time in addition to the control, with five concentrations and two test packets (replications) per concentration and compound. Each compound was evaluated in at least two full trials of this type. The fluralaner was evaluated in three full trials.
The mortality of mites tested for each compound concentration was calculated using the Henderson & Tilton formula [24]. If a treatment’s mortality was less than the control, it was counted as zero mortality.
where n is the number of mites, T is a treated packet and Co is a control packet.
Statistical analyses were conducted using Minitab Version 17 (Minitab Inc., State College, PA, USA). The corrected % adult mite mortality was analyzed using probit analysis, which generated LC50 and LC99 values and 95% confidence intervals (CI) for each trial and acaricide. Lack of overlap in the 95% CI indicated statistically significant differences.
Results
Preliminary testing for three concentrations over a 100-fold concentration range of the compounds tested showed the rough limits of activity. The results obtained in the range finding test led to the concentrations used for final testing. Control mite mortality was 20% in those tests. The two pyrethroid compounds showed negligible raw mortality even at 1000 ppm (the highest concentration tested), i.e. 12% for permethrin and 14% for deltamethrin. The pyrethroids were therefore not tested further. Of the remaining test compounds fluralaner showed 100% mortality at 1000 and 100 ppm, and 99% at 10 ppm. Mortality for spinosad was 98% at 4000 ppm, 29% at 400 ppm and 11% at 40 ppm. Phoxim showed 100% mortality at 2000 ppm, 20% at 200 ppm and 14% at 20 ppm. Propoxur had 100% mortality at 1000 ppm, 80% at 100 ppm and 10% at 10 ppm.
The adult mite mortality data caused by the five selected doses of each compound are shown in Table 1. Raw mite mortality data are supplied in Additional file 1: Table S1. There was generally good agreement between the trials for each tested compound. Control mortality for each trial varied from 6 to 19%. Most packets had the expected mite number. Since O. sylviarum are small and exact counts were done, a few packets experienced mite escapes. In those very few cases, the data from that packet were excluded from analysis, but remaining data were adequate to allow statistical analyses. In a few packets small numbers (< 5) of crushed mites (e.g. killed when the packet was folded closed) were excluded entirely from the analysis, reducing the total number of mites in that packet accordingly. In addition, if a treatment’s mortality was less than the control, it was counted as zero mortality.
Table 1.
Corrected % adult Ornithonyssus sylviarum mortality at 48 h as a function of acaricide concentrations in test packets
| Acaricide | Trial date | Dose (ppm) | Control mortality (%) | Corrected mortality (%) |
|---|---|---|---|---|
| Fluralaner | 3-Apr-15 | 0 | 11.90 | |
| 1 | 2.94 | |||
| 2 | 34.98 | |||
| 5 | 83.98 | |||
| 10 | 100.00 | |||
| 20 | 98.62 | |||
| 20-Mar-15 | 0 | 5.60 | ||
| 1 | 40.52 | |||
| 2 | 41.04 | |||
| 5 | 92.86 | |||
| 10 | 100.00 | |||
| 20 | 100.00 | |||
| 8-Mar-15 | 0 | 12.10 | ||
| 1 | 0.00a | |||
| 2 | 29.58 | |||
| 5 | 81.04 | |||
| 10 | 100.00 | |||
| 20 | 100.00 | |||
| Trial Average | 1 | 14.48 | ||
| 2 | 35.20 | |||
| 5 | 82.02 | |||
| 10 | 100.00 | |||
| 20 | 99.54 | |||
| Spinosad | 20-Feb-15 | 0 | 15.60 | |
| 200 | 0.00a | |||
| 400 | 0.00a | |||
| 1000 | 25.00 | |||
| 2000 | 78.29 | |||
| 4000 | 95.92 | |||
| 27-Feb-15 | 0 | 18.60 | ||
| 200 | 0.00a | |||
| 400 | 0.00a | |||
| 1000 | 20.83 | |||
| 2000 | 83.88 | |||
| 4000 | 100.00 | |||
| Trial Average | 200 | 0.00 | ||
| 400 | 0.00 | |||
| 1000 | 22.92 | |||
| 2000 | 81.09 | |||
| 4000 | 97.96 | |||
| Phoxim | 20-Feb-15 | 0 | 15.60 | |
| 250 | 0.68 | |||
| 500 | 83.10 | |||
| 1000 | 96.05 | |||
| 1500 | 100.00 | |||
| 27-Feb-15 | 0 | 18.60 | ||
| 250 | 6.88 | |||
| 500 | 87.91 | |||
| 1000 | 100.00 | |||
| 1500 | 100.00 | |||
| 2000 | 100.00 | |||
| Trial Average | 250 | 3.78 | ||
| 500 | 85.51 | |||
| 1000 | 98.03 | |||
| 1500 | 100.00 | |||
| 2000 | 100.00 | |||
| Propoxur | 20-Feb-15 | 0 | 15.56 | |
| 31 | 0.00a | |||
| 63 | 44.70 | |||
| 125 | 67.22 | |||
| 250 | 100.00 | |||
| 500 | 100.00 | |||
| 27-Feb-15 | 0 | 18.60 | ||
| 31 | 1.67 | |||
| 63 | 42.64 | |||
| 125 | 89.76 | |||
| 250 | 100.00 | |||
| 500 | 100.00 | |||
| Trial Average | 31 | 0.84 | ||
| 63 | 43.67 | |||
| 125 | 78.49 | |||
| 250 | 100.00 | |||
| 500 | 100.00 |
Trials were conducted in spring 2015 and used for probit analysis. Control packets indicated by “0 ppm” dose
aTreatments had less mortality than controls and are marked as no mortality
Table 2 shows LC50 and LC99 values. Fluralaner was the most active molecule, with an LC50 of 2.95 ppm and an LC99 of 8.09 ppm. Spinosad was the least toxic compound on an active concentration basis, with an LC50 of 1587 ppm and LC99 of 3123 ppm. Phoxim LC50 was 420 ppm and its LC99 was 750 ppm. Propoxur LC50 was 86 ppm and its LC99 was 181 ppm.
Table 2.
Probit analysis results for tests of fluralaner and other tested acaricidal compounds tested against adult Ornithonyssus sylviarum
| Acaricide | Trial | LC50 (ppm) | 95% CI | LC99 (ppm) | 95% CI |
|---|---|---|---|---|---|
| Fluralaner | 1 | 3.48 | 3.06–3.93 | 9.87 | 8.81–11.32 |
| 2 | 1.95 | 1.56–2.31 | 7.22 | 6.20–8.83 | |
| 3 | 3.41 | 3.12–3.73 | 7.18 | 6.50–8.14 | |
| Mean | 2.95 | 8.09 | |||
| Spinosad | 1 | 1697 | 1562–1849 | 3595 | 3271–4026 |
| 2 | 1477 | 1381–1579 | 2651 | 2418–2880 | |
| Mean | 1587 | 3123 | |||
| Phoxim | 1 | 451 | 414–490 | 890 | 805–1015 |
| 2 | 389 | 366–412 | 609 | 569–663 | |
| Mean | 420 | 750 | |||
| Propoxur | 1 | 94 | 86–103 | 209 | 186–203 |
| 2 | 78 | 72–84 | 153 | 140–171 | |
| Mean | 86 | 181 |
Discussion
The severe impact of D. gallinae infestations in the poultry industry, particularly in Europe, has led to several assessments of laboratory susceptibility and field efficacy of acaricides for its control, especially over the last two decades [2]. This is the first recent testing of acaricidal contact activity for O. sylviarum. Earlier bioassay-type laboratory testing has been conducted on single O. sylviarum isolates [25–27]. Extensive field surveys were done between 1999 and 2001 of acaricide susceptibility of 26 O. sylviarum field populations collected from commercial layer operations in southern California [9]. At that time O. sylviarum was already widely and extremely resistant to the class of synthetic pyrethroids (permethrin), and significant field resistance was noted (relative to a susceptible population) to representatives of the carbamates (carbaryl) and the organophosphates (the tetrachlorvinphos/ dichlorvos combination). Resistance issues are similarly serious for D. gallinae, and some level of resistance, or tolerance, exists for most classes of acaricidal active compounds, as reviewed by Abbas et al. [28].
Spinosad, a representative compound of the semi-synthetic class of spinosyns, is being marketed for the control of O. sylviarum, but in this study the compound had rather marginal contact activity for O. sylviarum relative to other compounds tested. Our calculated LC99 (3123 ppm) was approximately three times the commercial product label rate for O. sylviarum of 0.1% (1035 ppm) [29]. Several things should be noted, however. First, spinosad often has delayed toxicity against arthropods [30], although one recent study on D. gallinae showed high mortality by 2.5 h post-treatment [31]. Secondly, in vitro testing is not necessarily indicative of field efficacy, and we tested 48 h-old residues of spinosad on filter paper, as opposed to fresh applications against mites on a host. We are not aware of published in vitro or in vivo tests of spinosad against O. sylviarum. George et al. [31] showed 3–4 weeks activity of spinosad against D. gallinae at 1940–3880 ppm (treated metal plate residues) and suggested applying 3880 ppm (3.88 g/l) to the point of liquid runoff as a residual field application rate. Those authors also saw maximum effect at 2 weeks post-treatment and suggested spinosad activity was greater at high mite densities. The 3880 ppm rate [31] and the high use rate recommended for spinosad by the manufacturer for Dermanyssus (60 ml/7 l) [32] are above our estimated LC99 value for spinosad on O. sylviarum.
With a new mode of action and predominantly systemic activity, the isoxazoline ectoparasiticides have seen growing use in companion animals for the treatment of flea and tick infestations, and have been tested for a wide variety of blood-feeding arthropods [33], skin-dwelling mammal mites such as Sarcoptes scabei [17, 20] and a few non- blood-feeders such as blow fly or mosquito larvae [15]. The latter study showed the lower threshold for significantly increased mortality for most target arthropods occurred at somewhere around 1 ppm. It is difficult sometimes to discern whether toxicity was entirely based on ingestion (as it clearly was in some membrane blood-feeding tests) or whether fluralaner might also cause morbidity or mortality on contact alone.
A study by Williams et al. [34], showed likely in vitro contact activity of fluralaner against larvae of the brown dog tick Rhipicephalus sanguineus, but that was by immersion, which also could result in some small amount of oral/internal exposure. In the present study the mites should not have ingested any residues of fluralaner, except perhaps by grooming appendages that came into contact with it on the filter paper. The LC99 for adult O. sylviarum is only about 8 ppm.
Conclusions
Northern fowl mites were highly susceptible to fluralaner by contact in this study. This mite resides primarily in the fluffy feathers of the vent region of chickens, where protonymphs and adults blood feed. This ectoparasite causes considerable economic damage to egg laying hens, caused by blood-feeding and the subsequent immune responses [6]. Pesticide sprays are currently the primary method of controlling O. sylviarum. The pesticides must be sprayed from underneath the birds at high pressures to effectively treat the mites living in the vent feathers. As birds are moved into alternative cages with solid floors and other structures, or cage-free environments, this type of treatment will become difficult to execute. Fluralaner has a higher contact activity than other acaricides we tested for O. sylviarum. This activity, together with its expected systemic activity, as demonstrated for it and other isoxazolines against other mite species [16, 19], would make fluralaner a valuable addition to the poultry mite control palette.
Acknowledgments
We very much appreciate the careful technical efforts of K. Chau and N. Wong in conduct of the research.
Funding
The studies were funded through a grant-in-aid (#S14096–00) from Merck Animal Health, Madison, NJ, USA, to BAM.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Raw mite mortality data are provided in Additional file 1.
Additional file
Raw northern fowl mite mortality data for trials of acaricides using filter paper packets. Second column has the pesticide and its concentration in ppm. Abbreviations: Cont, carrier control (butanol/olive oil); Spin, spinosad; Prop, propoxur; Phox, phoxim; Flur, fluralaner; Perm, permethrin; Delt, deltamethrin. (XLSX 52 kb)
Authors’ contributions
BAM and ACM established the final methods and design and executed the experiments. BAM conducted the statistical analysis and prepared the first paper draft. HZ, AH, FJ and AFS assisted with preliminary design of the study. All authors read and approved the final manuscript.
Ethics approval
All animals housed under Animal Use Protocol A20150009, University of California, Riverside.
Consent for publication
Not applicable.
Competing interests
BAM and ACM have no competing interests. HZ, AH, FJ and AFS are employees of Merck/MSD Animal Health Innovation GmbH, and these studies were conducted as part of a research program to evaluate the efficacy of fluralaner against the northern fowl mite.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-017-2289-z) contains supplementary material, which is available to authorized users.
Contributor Information
Bradley A. Mullens, Email: bradley.mullens@ucr.edu
Amy C. Murillo, Email: amy.murillo@ucr.edu
Hartmut Zoller, Email: hartmut.zoller@msd.de.
Anja R. Heckeroth, Email: anja.heckeroth@msd.de
Faris Jirjis, Email: faris.jirjis@merck.com.
Annie Flochlay-Sigognault, Email: annie.flochlay-sigognault@merck.com.
References
- 1.Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101–106. doi: 10.1146/annurev.en.35.010190.000533. [DOI] [PubMed] [Google Scholar]
- 2.Sparagano OAE, George DR, Harrington DWJ, Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Ann Rev Entomol. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- 3.Sikes RK, Chamberlain RW. Laboratory observations on three species of bird mites. J Parasitol. 1954;40:691–697. doi: 10.2307/3273713. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch JB, Owen JP. Arrhenotoky and oedipal mating in the northern fowl mite (Ornithonyssus sylviarum) (Acari: Gamasida: Macronyssidae) Parasit Vectors. 2012;5:281. doi: 10.1186/1756-3305-5-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpinen O, Roepstorff A, Permin A, Norgaard-Nielsen G, Lawson LG, Simonsen HB. Influence of Dermanyssus gallinae and Ascaridia galli infections on behavior and health of laying hens (Gallus gallus domesticus). Br Poult Sci. 2005;46:26–34. [DOI] [PubMed]
- 6.Mullens BA, Owen JP, Kuney DR, Szijj CE, Klingler KA. Temporal changes in distribution, prevalence and intensity of northern fowl mite (Ornithonyssus sylviarum) parasitism in commercial caged laying hens, with a comprehensive economic analysis of parasite impact. Vet Parasitol. 2009;160:116–130. doi: 10.1016/j.vetpar.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 7.Nordenfors H, Hoglund J, Uggla A. Effects of temperature and humidity on oviposition, molting, and longevity of Dermanyssus gallinae (Acari: Dermanyssidae) J Med Entomol. 1999;36:68–72. doi: 10.1093/jmedent/36.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Chen BL, Mullens BA. Temperature and humidity effects on off-host survival of the northern fowl mite (Acari: Macronyssidae) and the chicken body louse (Phthiraptera: Menoponidae) J Econ Entomol. 2008;101:637–646. doi: 10.1093/jee/101.2.637. [DOI] [PubMed] [Google Scholar]
- 9.Mullens BA, Velten RK, Hinkle NC, Kuney DR, Szijj CE. Acaricide resistance in northern fowl mite (Ornithonyssus sylviarum) populations on caged layer operations in southern California. Poultry Sci. 2004;83:365–374. doi: 10.1093/ps/83.3.365. [DOI] [PubMed] [Google Scholar]
- 10.Registered Pesticides Database. UCR, Riverside, CA. 2015. http://veterinaryentomology.ucr.edu/vet_pesticides.html. Accessed 1 Mar 2017.
- 11.Martin CD, Mullens BA. Housing and dustbathing effects on northern fowl mites (Ornithonyssus sylviarum) and chicken body lice (Menacanthus stramineus) on hens. Med Vet Entomol. 2012;26:323–333. doi: 10.1111/j.1365-2915.2011.00997.x. [DOI] [PubMed] [Google Scholar]
- 12.Tavassoli M, Ownag A, Pourseyed SH, Mardani K. Laboratory evaluation of three strains of the entomopathogenic fungus Metarhizium anisopliae for controlling Dermanyssus gallinae. Avian Pathol. 2008;37:259–263. doi: 10.1080/03079450802043718. [DOI] [PubMed] [Google Scholar]
- 13.Mullens BA, Soto D, Martin CD, Callaham BL, Gerry AC. Northern fowl mite (Ornithonyssus sylviarum) control evaluations using liquid formulations of diatomaceous earth, kaolin, sulfur, azadirachtin and Beauveria bassiana on caged laying hens. J Appl Poult Res. 2012;21:111–116. doi: 10.3382/japr.2011-00402. [DOI] [Google Scholar]
- 14.Immediato D, Figueredo LA, Iatta R, Camarda A, Nogueira de Luna RL, Giangaspero A, et al. Essential oils and Beauveria bassiana against Dermanyssus gallinae (Acari: Dermanyssidae): toward new natural acaricides. Vet Parasitol. 2016;229:159–165. doi: 10.1016/j.vetpar.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Gassel M, Wolf C, Noack S, Williams H, Ilg T. The novel isoxazoline ectoparasiticide fluralaner: selective inhibition of arthropod y-aminobutyric acid and L-glutamate-gated chloride channels and insecticide/acaricidal activity. Insect Biochem Mol Biol. 2014;45:111–124. doi: 10.1016/j.ibmb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Casida J. Golden age of RyR and GABA R diamide and isoxazoline insecticides: common genesis, serendipity, surprises, selectivity and safety. Chem Res Toxicol. 2015;28:560–566. doi: 10.1021/tx500520w. [DOI] [PubMed] [Google Scholar]
- 17.Taenzler J, Liebenberg J, Roepke R, Frénais R, Heckeroth A. Efficacy of fluralaner administered either orally or topically for the treatment of naturally acquired Sarcoptes scabiei var. canis infestation in dogs. Parasit Vectors. 2016;9:392. doi: 10.1186/s13071-016-1670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wengenmayer C, Williams H, Zschiesche F, Moritz A, Langenstein J, Roepke RKA, Heckeroth AR. The speed of kill of fluralaner (Bravecto) against Ixodes ricinus ticks on dogs. Parasit Vectors. 2014;7:525. doi: 10.1186/s13071-014-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beugnet F, Liebenberg J, Halos L. Comparative efficacy of two oral treatments for dogs containing either afoxolaner or fluralaner against Rhipicephalus sanguineus sensu lato and Dermancentor reticulatus. Vet Parasitol. 2015;209:142–145. doi: 10.1016/j.vetpar.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Becskei C, De Bock F, Illambas J, Cherni JA, Fourie JJ, Lane M, Mahabir SP, Six RH. Efficacy and safety of a novel oral isoxazoline, sarolaner (Simparica™), for the treatment of sarcoptic mange in dogs. Vet Parasitol. 2016;222:56–61. doi: 10.1016/j.vetpar.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Stone BF, Haydock KP. A method for measuring the acaricide susceptibility of the cattle tick Boophilus microplus (can.) Bull Entomol Res. 1962;53:563–578. doi: 10.1017/S000748530004832X. [DOI] [Google Scholar]
- 22.Rodrigues-Vivas RI, Miller RJ, Ojeda-Chi MM, Rosado-Aguillar JA, Trinidad-Martinez IC, Perez de Leon AA. Acaricide and ivermectin resistance in a field population of Rhipicephalus microplus (Acari: Ixodidae) collected from red deer (Cervus elaphus) in the Mexican tropics. Vet Parasitol. 2014;200:179–188. doi: 10.1016/j.vetpar.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:323–337. doi: 10.2307/1931961. [DOI] [Google Scholar]
- 24.Henderson CF, Tilton W. Tests with acaricides against the brown wheat mite. J Econ Entomol. 1955;48:157–161. doi: 10.1093/jee/48.2.157. [DOI] [Google Scholar]
- 25.Hall RD, Townsend LH, Jr, Turner EC., Jr Laboratory and field tests to compare the effectiveness of organophosphorous, carbamate and synthetic pyrethroid acaricides against northern fowl mites. J Econ Entomol. 1978;71:315–318. doi: 10.1093/jee/71.2.315. [DOI] [PubMed] [Google Scholar]
- 26.Crystal MM, DeMilo AB. Susceptibility of laboratory-reared northern fowl mites, Ornithonyssus sylviarum (Acari: Macronyssidae) to selected acaricides. Exp Appl Acarol. 1988;4:353–358. doi: 10.1007/BF01275166. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher MG, Axtell RC. Susceptibilities of northern fowl mite, Ornithonyssus sylviarum (Acarina: Macronyssidae), and chicken mite (Acarina: Dermanyssidae), to selected acaricides. Exp Appl Acarol. 1991;13:137–142. doi: 10.1007/BF01193664. [DOI] [PubMed] [Google Scholar]
- 28.Abbas RZ, Colwell DD, Iqbal Z, Khan A. Acaricidal drug resistance in poultry red mite (Dermanyssus gallinae) and approaches to its management. World’s Poultry Sci J. 2014;70:113–124. doi: 10.1017/S0043933914000105. [DOI] [Google Scholar]
- 29.Elector® PSP Label. Elanco, Indianapolis, IN. 2011. https://www.elanco.us/labels/Dairy/Elector_PSP.pdf. Accessed 8 Dec 2016.
- 30.Murillo AC, Gerry AC, Gallagher NT, Peterson NG, Mullens BA. Laboratory and field assessment of cyantraniliprole relative to existing fly baits. Pest Manag Sci. 2014;71:752–758. doi: 10.1002/ps.3847. [DOI] [PubMed] [Google Scholar]
- 31.George DR, Shiel RS, Appleby WGC, Know A, Guy JH. In vitro and in vivo acaricidal activity and residual toxicity of spinosad to the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2010;173:307–316. doi: 10.1016/j.vetpar.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Elector Technical Manual. Elanco, Indianapolis. 2012. https://www.salmonella360.com/cms3/assets/fullsize/1045. Accessed 8 Dec 2016.
- 33.Rohdich N, Roepke RKA, Zschiesche E. A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of Bravecto™ (fluralaner) against frontline™ (fipronil) in flea- and tick-infested dogs. Parasit Vectors. 2014;7:83. doi: 10.1186/1756-3305-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams H, Zoller H, Roepke RKA, Zschiesche E, Heckeroth AR. Fluralaner activity against life stages of ticks using Rhipicephalus sanguineus and Ornithodorus moubata in in vitro contact and feeding assays. Parasit Vectors. 2015;8:90. doi: 10.1186/s13071-015-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article. Raw mite mortality data are provided in Additional file 1.


