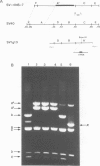
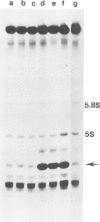
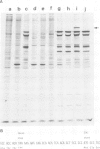
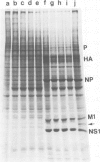
Abstract
Amber, ochre and opal suppressor tRNA genes have been generated by using oligonucleotide directed site-specific mutagenesis to change one or two nucleotides in a human serine tRNA gene. The amber and ochre suppressor (Su+) tRNA genes are efficiently expressed in CV-1 cells when introduced as part of a SV40 recombinant. The expressed amber and ochre Su+ tRNAs are functional as suppressors as demonstrated by readthrough of the amber codon which terminates the NS1 gene of an influenza virus or the ochre codon which terminates the hexon gene of adenovirus, respectively. Interestingly, several attempts to obtain the equivalent virus stock of an SV40 recombinant containing the opal suppressor tRNA gene yielded virus lacking the opal suppressor tRNA gene. This suggests that expression of an efficient opal suppressor derived from a human serine tRNA gene is highly detrimental to either cellular or viral processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Zabielski J., Perricaudet M., Pettersson U. The sequence of the 3' non-coding region of the hexon mRNA discloses a novel adenovirus gene. Nucleic Acids Res. 1981 Jan 10;9(1):1–17. doi: 10.1093/nar/9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L. Context effects: translation of UAG codon by suppressor tRNA is affected by the sequence following UAG in the message. J Mol Biol. 1983 Feb 15;164(1):73–87. doi: 10.1016/0022-2836(83)90088-8. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Changelian P. S., Sharp P. A. Immunoprecipitation with two-dimensional pools as a hybridoma screening technique: production and characterization of monoclonal antibodies against adenovirus 2 proteins. Virology. 1981 Apr 30;110(2):385–401. doi: 10.1016/0042-6822(81)90069-6. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H. UGA suppression by normal tRNA Trp in Escherichia coli: codon context effects. Nucleic Acids Res. 1981 Feb 25;9(4):983–991. doi: 10.1093/nar/9.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
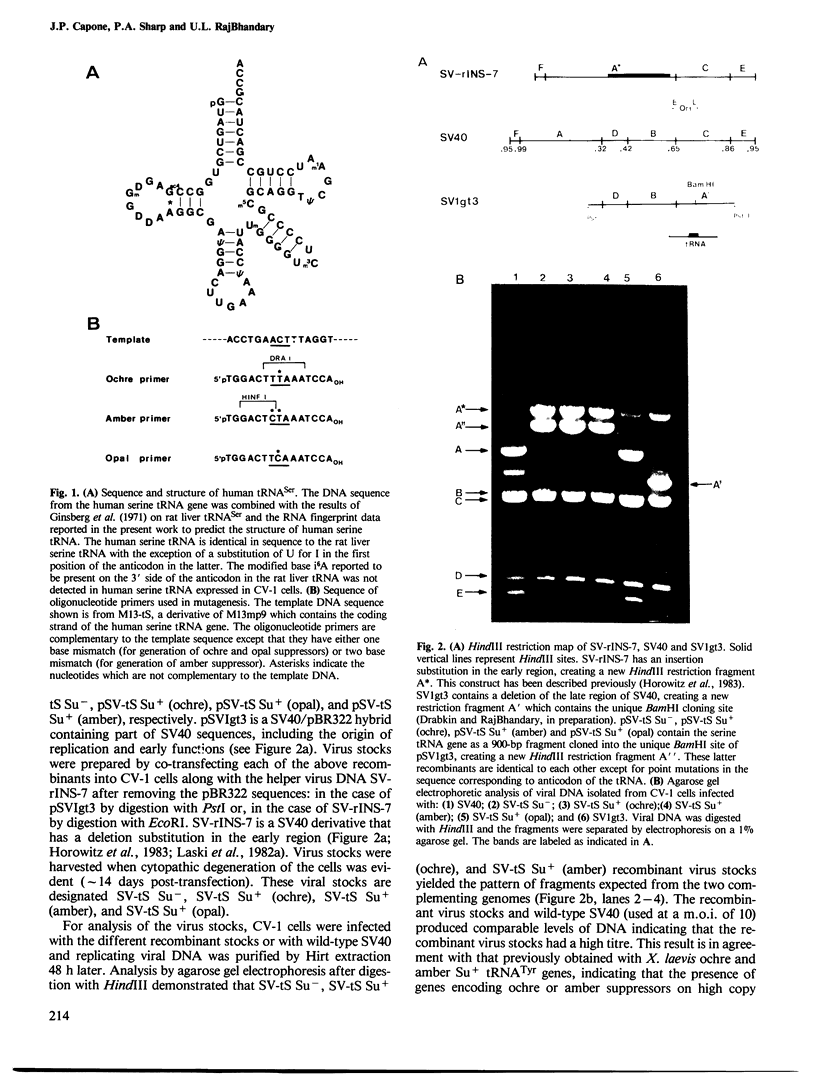
- Ginsberg T., Rogg H., Staehelin M. Nucleotide sequences of rat liver serine-tRNA. 3. The partial enzymatic of serine-tRNA and derivation of its total primary structure. Eur J Biochem. 1971 Jul 29;21(2):249–257. doi: 10.1111/j.1432-1033.1971.tb01463.x. [DOI] [PubMed] [Google Scholar]
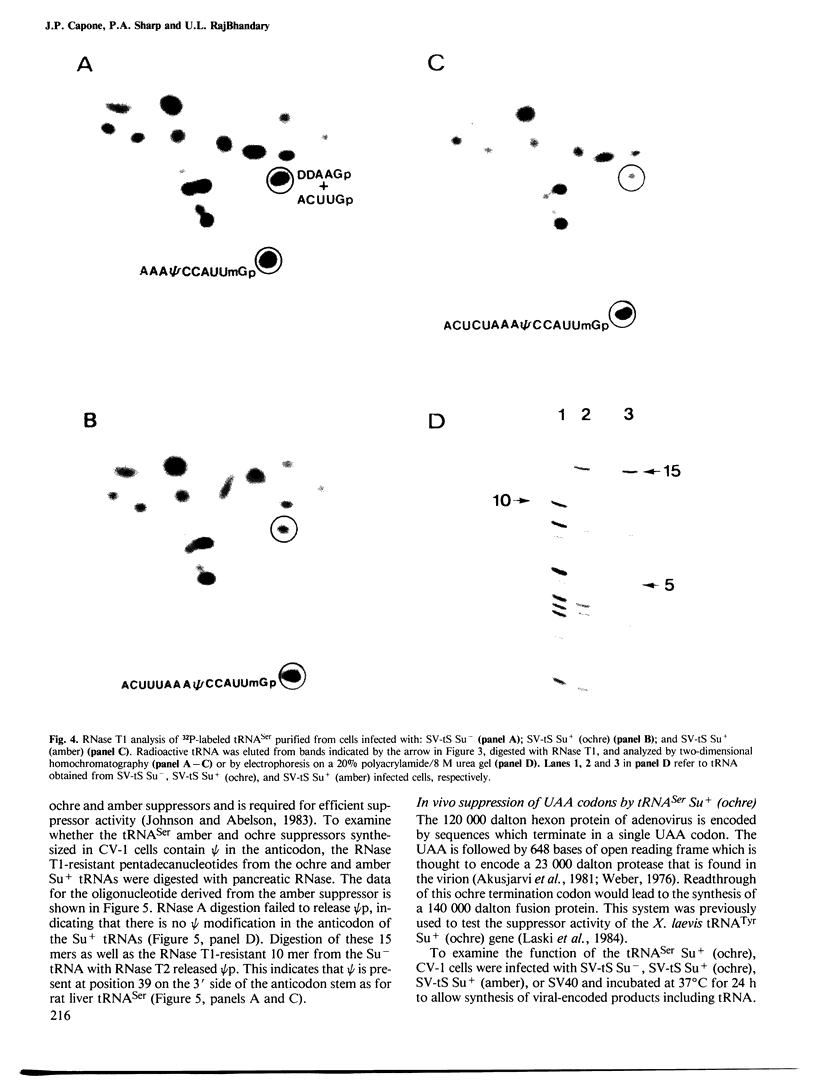
- Horowitz M., Cepko C. L., Sharp P. A. Expression of chimeric genes in the early region of SV40. J Mol Appl Genet. 1983;2(2):147–159. [PubMed] [Google Scholar]
- Hudziak R. M., Laski F. A., RajBhandary U. L., Sharp P. A., Capecchi M. R. Establishment of mammalian cell lines containing multiple nonsense mutations and functional suppressor tRNA genes. Cell. 1982 Nov;31(1):137–146. doi: 10.1016/0092-8674(82)90413-5. [DOI] [PubMed] [Google Scholar]
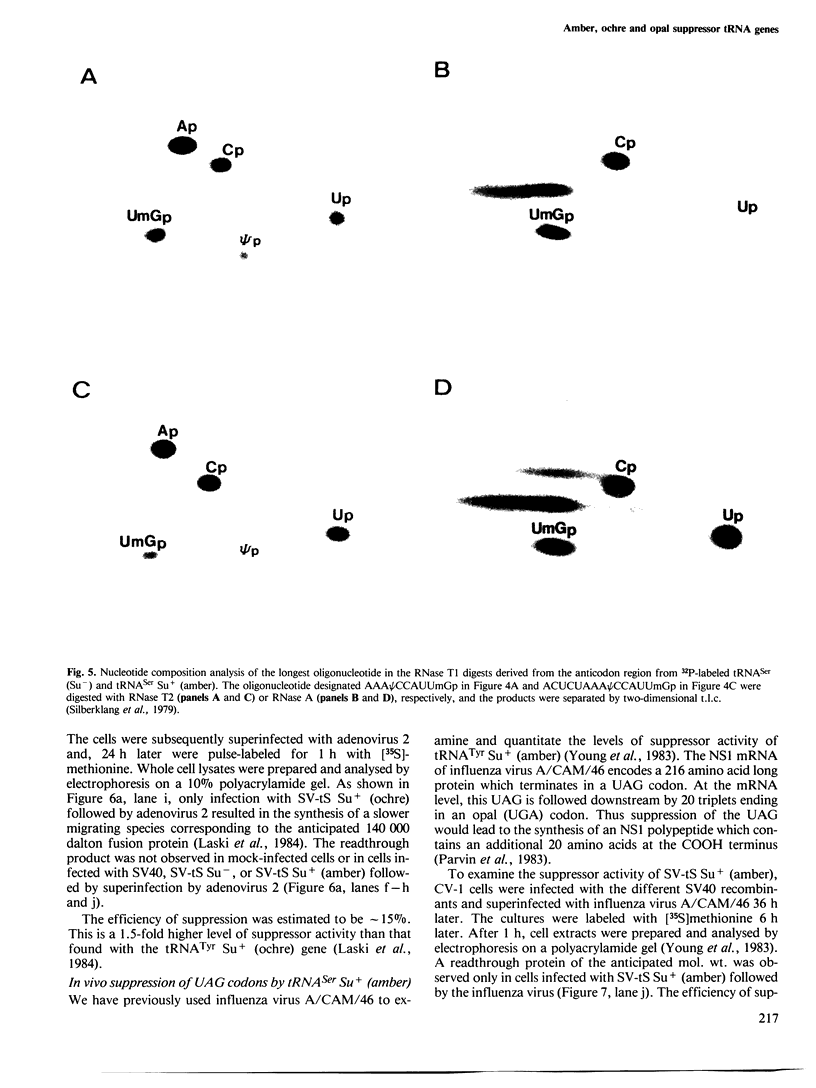
- Johnson P. F., Abelson J. The yeast tRNATyr gene intron is essential for correct modification of its tRNA product. Nature. 1983 Apr 21;302(5910):681–687. doi: 10.1038/302681a0. [DOI] [PubMed] [Google Scholar]
- Kohli J., Grosjean H. Usage of the three termination codons: compilation and analysis of the known eukaryotic and prokaryotic translation termination sequences. Mol Gen Genet. 1981;182(3):430–439. doi: 10.1007/BF00293932. [DOI] [PubMed] [Google Scholar]
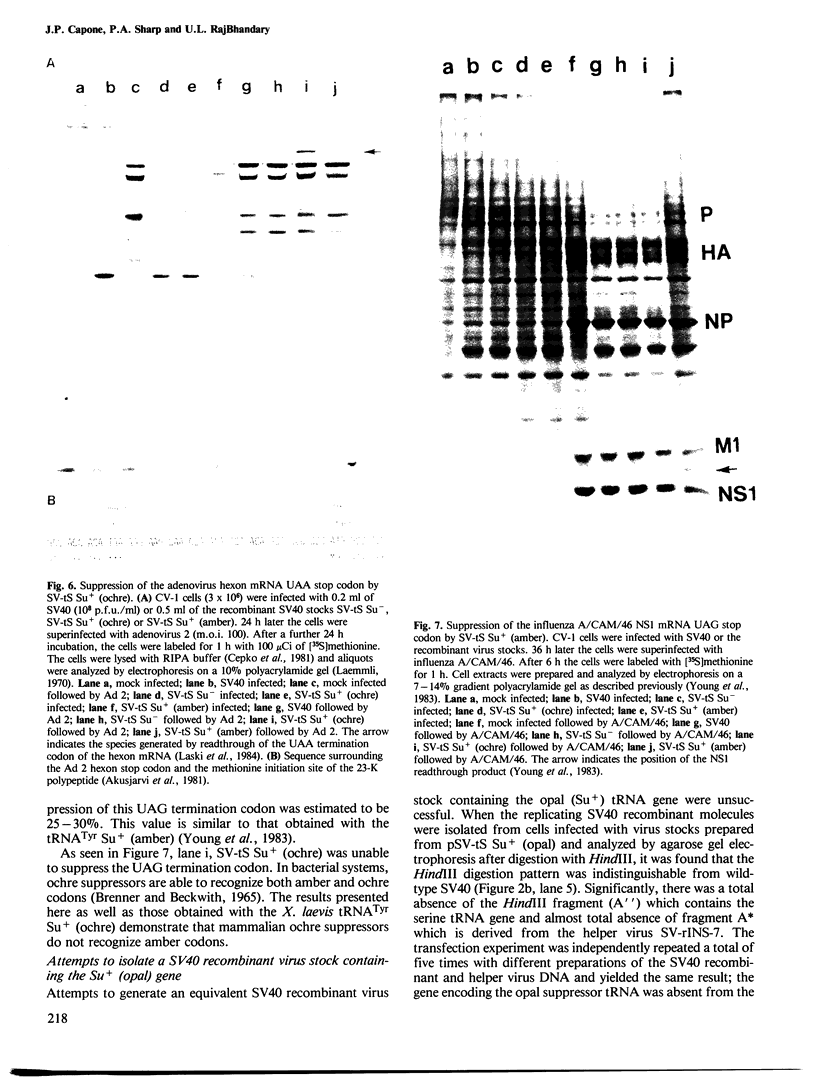
- Kudo I., Leineweber M., RajBhandary U. L. Site-specific mutagenesis on cloned DNAs: generation of a mutant of Escherichia coli tyrosine suppressor tRNA in which the sequence G-T-T-C corresponding to the universal G-T-pseudouracil-C sequence of tRNAs is changed to G-A-T-C. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4753–4757. doi: 10.1073/pnas.78.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Alzner-DeWeerd B., RajBhandary U. L., Sharp P. A. Expression of a X. laevis tRNATyr gene in mammalian cells. Nucleic Acids Res. 1982 Aug 11;10(15):4609–4626. doi: 10.1093/nar/10.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Belagaje R., Hudziak R. M., Capecchi M. R., Norton G. P., Palese P., RajBhandary U. L., Sharp P. A. Synthesis of an ochre suppressor tRNA gene and expression in mammalian cells. EMBO J. 1984 Nov;3(11):2445–2452. doi: 10.1002/j.1460-2075.1984.tb02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Belagaje R., RajBhandary U. L., Sharp P. A. An amber suppressor tRNA gene derived by site-specific mutagenesis: cloning and function in mammalian cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5813–5817. doi: 10.1073/pnas.79.19.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Fire A. Z., RajBhandary U. L., Sharp P. A. Characterization of tRNA precursor splicing in mammalian extracts. J Biol Chem. 1983 Oct 10;258(19):11974–11980. [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Parvin J. D., Young J. F., Palese P. Nonsense mutations affecting the lengths of the NS1 nonstructural proteins of influenza A virus isolates. Virology. 1983 Jul 30;128(2):512–517. doi: 10.1016/0042-6822(83)90280-5. [DOI] [PubMed] [Google Scholar]
- Randerath E., Gopalakrishnan A. S., Gupta R. C., Agrawal H. P., Randerath K. Lack of a specific ribose methylation at guanosine 17 in Morris hepatoma 5123D tRNASer1IGA. Cancer Res. 1981 Jul;41(7):2863–2867. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills N., Gesteland R. F., Karn J., Barnett L., Bolten S., Waterston R. H. The genes sup-7 X and sup-5 III of C. elegans suppress amber nonsense mutations via altered transfer RNA. Cell. 1983 Jun;33(2):575–583. doi: 10.1016/0092-8674(83)90438-5. [DOI] [PubMed] [Google Scholar]
- Young J. F., Capecchi M., Laski F. A., RajBhandary U. L., Sharp P. A., Palese P. Measurement of suppressor transfer RNA activity. Science. 1983 Aug 26;221(4613):873–875. doi: 10.1126/science.6308765. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]