Abstract
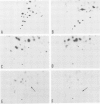
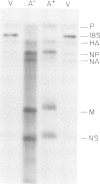
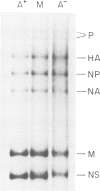
Influenza virus-specific RNA has been synthesized in vitro, using cytoplasmic or microsomal fractions of influenza virus-infected MDCK cells. The RNA polymerase activity was stimulated 5-30 times by priming with ApG. About 20-30% of the product was polyadenylated. Most of the in vitro product was of positive polarity, as shown by hybridization to strand specific probes and by T1 fingerprinting of the poly(A)+ and poly(A)- RNA segments encoding haemagglutinin and nucleoprotein. The size of poly(A)- RNA segments, determined on sequencing gels, was indistinguishable from that of virion RNA, whereas poly(A)+ RNA segments contain poly(A) tails approximately 50 nucleotides long. The size of in vitro synthesized RNA segments was also determined by gel electrophoresis of S1-treated double-stranded RNAs, obtained by hybridization of poly(A)+ or poly(A)- RNA fractions with excess of unlabelled virion RNA. The results of these experiments indicate that poly(A)- RNA contains full-length complementary RNA. This conclusion is further substantiated by the presence of additional oligonucleotides in the T1 fingerprints of in vitro synthesized poly(A)- haemagglutinin or nucleoprotein RNA, selected by hybridization to cloned DNA probes corresponding to the 3' termini of the genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaton A. R., Krug R. M. Selected host cell capped RNA fragments prime influenza viral RNA transcription in vivo. Nucleic Acids Res. 1981 Sep 11;9(17):4423–4436. doi: 10.1093/nar/9.17.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Synthesis of the templates for influenza virion RNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4682–4686. doi: 10.1073/pnas.81.15.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Braam J., Ulmanen I., Krug R. M. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983 Sep;34(2):609–618. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Robertson J. S. Structure of the host-derived sequences present at the 5' ends of influenza virus mRNA. Nucleic Acids Res. 1980 Jun 25;8(12):2591–2603. doi: 10.1093/nar/8.12.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Mahy B. W. RNA-dependent RNA polymerase in nuclei of cells infected with influenza virus. J Virol. 1973 Nov;12(5):951–961. doi: 10.1128/jvi.12.5.951-961.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J. Characterization of influenza virus RNA transcripts synthesized in vitro. J Gen Virol. 1979 Sep;44(3):599–608. doi: 10.1099/0022-1317-44-3-599. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., McCauley J. Characterization of influenza virus RNA complete transcripts. Virology. 1982 Jan 30;116(2):517–522. doi: 10.1016/0042-6822(82)90144-1. [DOI] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981 Nov;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Ho P. P., Walters C. P. Influenza virus-induced ribonucleic acid nucleotidyltransferase and the effect of actinomycin D on its formation. Biochemistry. 1966 Jan;5(1):231–235. doi: 10.1021/bi00865a030. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahy B. W., Bromley P. A. In vitro product of a ribonucleic acid polymerase induced by influenza virus. J Virol. 1970 Sep;6(3):259–268. doi: 10.1128/jvi.6.3.259-268.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D., Kitron N. Influenza virion RNA-dependent RNA polymerase: stimulation by guanosine and related compounds. J Virol. 1975 Apr;15(4):686–695. doi: 10.1128/jvi.15.4.686-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Doerfler W. Transcription of the genome of adenovirus type 12. I. Viral mRNA in abortively infected and transformed cells. J Virol. 1975 Jan;15(1):27–35. doi: 10.1128/jvi.15.1.27-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortín J., Martínez C., del Río L., Dávila M., López-Galíndez C., Villanueva N., Domingo E. Evolution of the nucleotide sequence of influenza virus RNA segment 7 during drift of the H3N2 subtype. Gene. 1983 Aug;23(2):233–239. doi: 10.1016/0378-1119(83)90055-0. [DOI] [PubMed] [Google Scholar]
- Ortín J., Nájera R., López C., Dávila M., Domingo E. Genetic variability of Hong Kong (H3N2) influenza viruses: spontaneous mutations and their location in the viral genome. Gene. 1980 Nov;11(3-4):319–331. doi: 10.1016/0378-1119(80)90072-4. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruck B. J., Brammer K. W., Page M. G., Coombes J. D. The detection and characterization of an induced RNA polymerase in the chorioallantoic membranes of embryonated eggs infected with influenza A2 virus. Virology. 1969 Sep;39(1):31–41. doi: 10.1016/0042-6822(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Ribonucleic acid nucleotidyl transferase induced in chick fibroblasts after infection with an influenza virus. J Gen Virol. 1969 Jan;4(1):125–137. doi: 10.1099/0022-1317-4-1-125. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Synthesis in vitro of RNA complementary to parental viral RNA by RNA polymerase induced by influenza virus. Biochim Biophys Acta. 1969 Apr 22;179(2):389–397. doi: 10.1016/0005-2787(69)90047-1. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Burke D. C. Ribonucleic acid synthesis in chick embryo cells infected with fowl-plague virus. J Virol. 1969 Apr;3(4):429–438. doi: 10.1128/jvi.3.4.429-438.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. RNA-dependent RNA polymerase activity of the influenza virus. Virology. 1971 Sep;45(3):793–796. doi: 10.1016/0042-6822(71)90197-8. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Hay A. J. Replication of the influenza virus genome. Virology. 1982 Apr 15;118(1):96–108. doi: 10.1016/0042-6822(82)90323-3. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Ortín J., Domingo E., Delamarter J., Allet B., Davies J., Bertrand K. P., Wray L. V., Jr, Reznikoff W. S. Plasmid vectors based on Tn10 DNA: gene expression regulated by tetracycline. Plasmid. 1984 Sep;12(2):103–110. doi: 10.1016/0147-619x(84)90056-8. [DOI] [PubMed] [Google Scholar]