Abstract
Advancements in accelerometer analytic and visualization techniques allow researchers to more precisely identify and compare critical periods of physical activity (PA) decline by age across the lifespan, and describe how daily PA patterns may vary across age groups. We used accelerometer data from the 2003–2006 cohorts of the National Health and Nutrition Examination Survey (NHANES) (n = 12,529) to quantify total PA as well as PA by intensity across the lifespan using sex-stratified, age specific percentile curves constructed using generalized additive models. We additionally estimated minute-to-minute diurnal PA using smoothed bivariate surfaces. We found that from childhood to adolescence (ages 6–19) across sex, PA is sharply lower by age partially due to a later initiation of morning PA. Total PA levels, at age 19 are comparable to levels at age 60. Contrary to prior evidence, during young adulthood (ages 20–30) total and light intensity PA increases by age and then stabilizes during midlife (ages 31–59) partially due to an earlier initiation of morning PA. We additionally found that males compared to females have an earlier lowering in PA by age at midlife and lower total PA, higher sedentary behavior, and lower light intensity PA in older adulthood; these trends seem to be driven by lower PA in the afternoon compared to females. Our results suggest a reevaluation of how emerging adulthood may affect PA levels and the importance of considering time of day and sex differences when developing PA interventions.
Introduction
While greater physical activity 1 has been linked to a broad range of beneficial health outcomes across the lifespan 2, the majority of Americans do not meet physical activity (PA) guidelines 3,4. One of the most consistently reported risk factors associated with decreased PA is age. Prior research indicates an almost universal decline in PA throughout the lifespan, with critical periods at childhood (ages 6–11 years) and adolescence (ages 11–19 years) 4–6. Emerging adulthood (ages 18–30) has also shown to be associated with a decline in PA 7,8; some evidence suggests that PA may stabilize during midlife 9,10 and then decline again at older ages 11.
Age-related decline in PA is driven by physiological, psychosocial, and environmental factors. For example, the dramatic decline during childhood and adolescence is driven partially by physiology/development (e.g., shift to a later chronotype 12,13) and environment (e.g., decrease in school-based PA 14,15). Declines during emerging adulthood may be driven by psychosocial factors, including life transitions (e.g., completion of mandatory schooling and full time work 7,8), and declines at older ages are driven be chronic disease morbidity 11 and environmental factors related to safety and accessibility 16. These effects may also vary by sex during childhood and adolescence due to differences in motivation, interests 17, and access to sports participation 18; and, during later life due to differences in chronic disease prevalence 19, frailty 20, and fall risk 21.
While age, sex, and many associated physiologic factors are not modifiable, a number of factors that contribute to declines across the lifespan are potentially modifiable, including environmental factors 22,23. The explicit goal of public health researchers in understanding age-related declines and differences in PA across age groups is to identify age groups that are at higher and lower risk. This can lead tofurther investigations of specific factors contributing to PA levels and designing specific interventions targeting those factors and age groups.
Advancements in accelerometer analytic and visualization techniques 24 allow researchers to better understand demographic trends across the lifespan and differences in PA across age groups. Insights gained from these analyses can clarify demographic trends in PA and more clearly identify high-risk groups. Additionally, recently developed methods that move beyond average activity and describe and quantify minute-by-minute daily activity patterns 25,26 may provide critical insights into what may drive PA differences across age groups; this in turn can lead to age-specific, tailored interventions.
This study used the pooled accelerometer data from the 2003–2004 and 2005–2006 cycles of the National Health and Nutrition Examination Survey (NHANES) to identify and compare critical periods of PA decline by age and sex across the lifespan, and describe how daily PA patterns may vary across age groups.
Methods
Study Sample
The NHANES is a cross-sectional, nationally representative survey that assesses demographic, dietary and health-related questions and can be used to better understand differences in health and nutrition across age groups 27. Survey data are made publically available by the National Center for Health Statistics (NCHS). All individuals participating in data collection provided informed consent, and the NCHS Ethics Review Board approved all survey protocols.
Accelerometer data collection and processing
The 2003–2004 and 2004–2005 survey cycles (2003–2006) collected accelerometer data for 7 consecutive days on individuals aged 6 years and older who were ambulatory 4. The Actigraph AM-7164 (Actigraph, Ft. Walton Beach, FL) uni-axial accelerometer was placed on an elastic belt and participants were instructed to wear the device on the right hip at all times other than during any aquatic activity, including swimming and bathing, and at bedtime 4. After completing data collection protocol, participants were instructed to return the device by mail.
The Actigraph accelerometer recorded movement intensity values, or activity counts, at 1-minute epochs; the NCHS and survey collaborators performed initial data review for outliers and unreasonable values.
Similar to previous studies 24,28 a criteria of at least 90 consecutive minutes of zero counts, with allowance for up to two consecutive minutes with up to 100 counts, was used to determine non-wear time; days with more than 14 hours of non-wear time were excluded. The final sample included 12529 individuals.
We explored PA in 5 age groups associated in prior studies (e.g., 15,29) with distinct transitions across the lifespan: children (ages 6–11); adolescents (ages 12–19); young adults (ages 20–30); adults at midlife (ages 31–59); and older adults post retirement (60+).
Measures and Statistical Analysis
Data was analyzed in 2016. Minute level activity counts were log-transformed by applying log(1+activity counts) resulting in the log-transformed activity counts (LAC). This transformation has the advantage that minutes with 0 activity counts are transformed into 0 counts on the natural log scale and the severely skewed nature of the counts data is dramatically reduced 25,30. The total log-transformed activity counts (TLACs) were then obtained by summing LACs over all minutes during the day. Daily TLAC was used as a measure of total volume of daily PA. Prior studies exploring accelerometer measured PA across the lifespan 6,31 have relied on total (non-transformed) activity counts, which result in a highly skewed measure of PA. Appendix Figure A displays the quantile plot and the histogram of daily TLACs for all subjects and indicates that TLAC follows a normal distribution.
The sex-stratified, age-specific percentiles curves of the TLAC were constructed using generalized additive models for location, scale and shape (GAMLSS) implementation 32 of Lambda-Mu-Sigma (LMS) method 33 that was originally introduced for constructing child growth charts. Briefly, the LMS method transforms data for each age using a Box-Cox transformation under the assumption that the parameters of transformation changes smoothly with age; the transformed data is assumed to follow a known distribution (such as normal or t-distribution) and quantiles are fitted to the transformed data and then mapped back to the original scale. The LMS model was fitted using survey weighted penalized likelihood 32. For computational stability, the survey weights were normalized to sum up to the sample size. Among Box-Cox Cole-Green distribution, Box-Cox Power Exponential distribution, and Box-Cox T distribution (BCT), the latter was chosen as optimal based on the global deviance criteria 34. The goodness-of-fit of the optimal-transformation, LMS-BCT, was investigated by testing fit over the age range of 6–84 via Q-statistics that test normality of the residuals through the first four central moments (mean, variance, skewness, and kurtosis) 32. Similarly, the age-specific percentiles curves for the time spent in sedentary PA (SePA), light PA (LiPA), and moderate-to-vigorous PA (MVPA) were constructed using the methods described above. The standard NHANES cut-off points were used to define sedentary PA (AC less than or equal 100), light PA (AC between 101 and 2019) and moderate-to-vigorous PA (AC above 2020) 4. In order to statistically test patterns observed in Figures 1,2, and 3, we split the lifespan (6–84) into 6-year increments (13 age groups) and modeled TLAC, SePA, LiPA, and MVPA as outcomes in regression models with covariates including sex, age, and the interaction of sex and age. The group 17–22 year old females was chosen as the reference group because it contains the PA low indicated in Figure 1. The model is as follows:
where Yi denotes the outcome (TLAC, SePA, LiPA, or MVPA) of the i-th NHANES participant having Genderi and Agei. The estimated models were used to calculate the gender and age-specific fitted values as follows: i) gF and gF + gM estimate the female and male effects in the 17–22 year old reference group, respectively; ii) gF +al and gF + gM + al + gal estimate the female and male effects in the Agel age group, respectively. The model estimates and statistically tests the significance of the sex, age, and sex-by-age effects. The models were fit using the R package “survey” 35 that accounts for the complex design of NHANES.
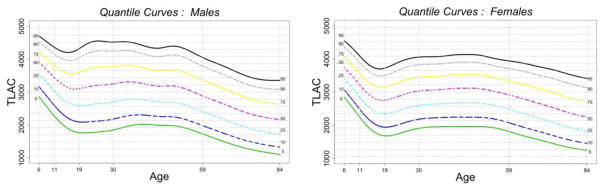
Figure 1.
Percentiles of TLAC over the lifespan (6 years old to 84 years old) for males (left) and females (right).
TLAC = total log-transformed activity counts
Percentiles of TLAC, from 5%-95%, are indicated using different colors (black = 95%, gray = 90%, yellow = 75%, purple = 50%, cyan = 25%, blue = 10%, green = 5%).
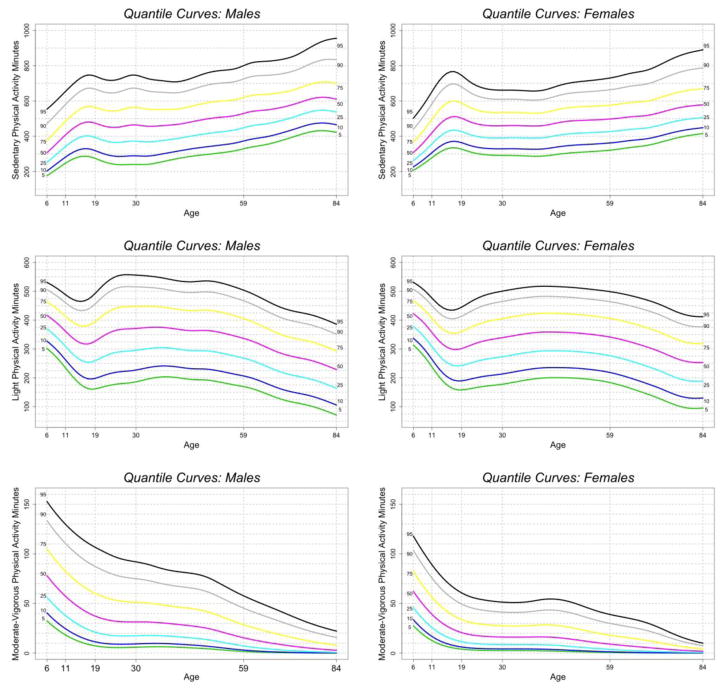
Figure 2.
Percentile Curves of Sedentary, Light, and Moderate-to-Vigorous PA over the lifespan (6 years old to 84 years old) for males (left) and females (right)
Percentiles of SePA, LiPA, and MVPA, from 5%-95%, are indicated using different colors(black = 95%, gray = 90%, yellow = 75%, purple = 50%, cyan = 25%, blue = 10%, green = 5%).
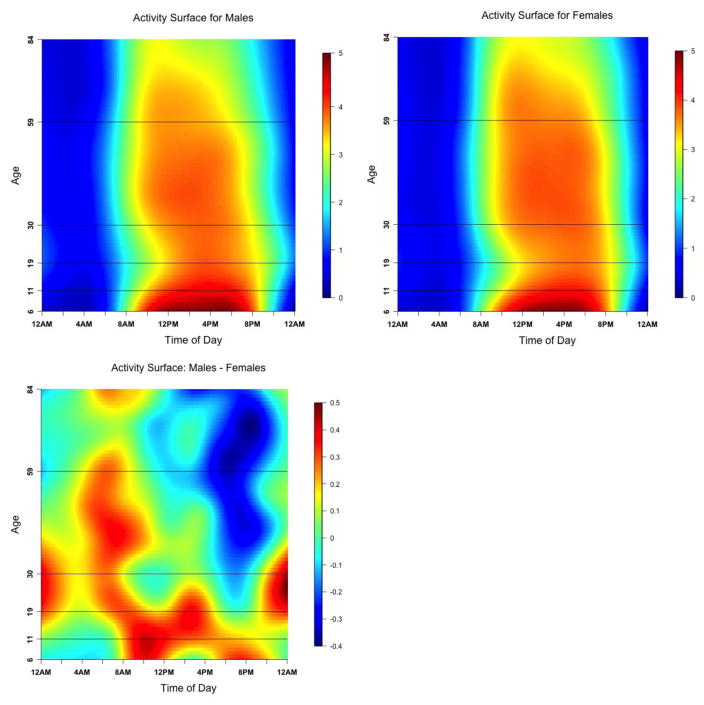
Figure 3.
The diurnal distribution of LAC over the lifespan (6 years old to 84 years old) for males (top left), females (top right) and the difference between males and females (bottom).
LAC = log-transformed activity counts
The diurnal distribution of LAC is represented by a surface with time of day as the horizontal dimension and age as the vertical dimension. Dark blue and dark red colors correspond to lower and higher levels of PA, respectively.
The age-specific changes in diurnal patterns of PA were estimated via average time-of-day by age bivariate surfaces of LAC. Specifically, subject-specific diurnal profiles of ten-minute LACs were survey-weighted and ordered by age and a bivariate spline smoother 26 was applied. The optimal smoothing parameters were determined using leave-one-subject-out cross validation.
Results
Demographic characteristics of the U.S. population representative sample surveyed using NHANES accelerometer data for each of the five age groups and for the entire population is reported in Appendix Table A. Briefly, the sample had 51.3% female, 69.8% were white, average BMI was 26.91(6.97), and the sample size for age groups 6–11, 12–19, 20–30, 31–59, and 60+ were 1713, 3456, 1515, 3428, and 2417 respectively.
Figure 1 shows the LMS-BCT percentile curves: median, 5%, 10%, 25% and 75%, 90%, 95% of TLAC over the lifespan (6 years old to 84 years old) for males and females, separately. Percentiles are represented using different colors (e.g. median in purple). The main patterns that emerge across all percentiles are lower PA by age during childhood through adolescence where PA of individuals at the end of adolescence (age 19) is similar to PA levels of individuals at the end of midlife and beginning of older adulthood (age 60). PA is higher by age over young adulthood (ages 20–30), stabilizes during midlife (ages 31–59), and is lower by age at the end of midlife and beginning of older adulthood (ages 60+). This pattern of PA across the lifespan is robust to exclusion criteria based on number of valid days (see Appendix Figure B) and is generally similar in males and females. It is important to note however that males, compared to females, show an earlier and more precipitous lowering in PA by age from the end of midlife through older adulthood.
The estimated regression coefficients from the model with TLAC as an outcome used to test trends in Figure 1 is reported in columns 1 and 5 of Appendix Table B for females and males respectively; the fitted values are reported in Panel 1 of Appendix Table C. The results confirm trends observed in Figure 1: females in the 17–22 year old reference group have a mean TLAC of 2739 that is significantly lower than TLAC of 6–16 year old and 23–58 year old females; there is no significant difference from TLAC of 59–70 year old females, and mean TLAC is significantly higher than TLAC of 71–84 year old females. A similar pattern is observed for males. The largest sex difference is in the 65–76 year old group where males have significantly lower TLAC than females.
Figure 2 shows the LMS-BCT percentile curves by intensity, including SePA, LiPA, and MVPA for males and females, separately. Similar to Figure 1, percentiles are represented using different colors. The patterns that emerge in SePA (or inactivity) and LiPA complement the patterns in TLAC over the lifespan. SePA (top panel) is higher by age until the end of adolescence (age 19), after which it is lower and then stabilizes by age through young adulthood and half-way through mid-life (~age 45), after which it is higher by age through the end of midlife and older adulthood (ages 60+). LiPA (middle panel) is lower by age through most of childhood and adolescence; LiPA is gradually higher by age near to the end of adolescence (~age 18) through young adulthood and the majority of midlife, until it gradually lowers near the end of midlife (~age 50) through older adulthood (ages 60+). It is important to note that levels of LiPA at the end of adolescence are similar to levels among older adults (~ age 65), and in the higher percentiles (>50%) mid-life levels of LiPA are almost similar to peak levels at early childhood. The main sex differences are similar to that seen in TLAC; generally males show an earlier and more precipitous lowering of LiPA and associated higher SePA at midlife extending to older adulthood.
MVPA (Figure 2 bottom panel) generally is lower by age across the entire lifespan. MVPA is dramatically lower by age during childhood to the end of adolescence (age 19); there is some stabilization during young adulthood and part of midlife, and then gradual lowering by age near the end of midlife (~age 50) through older adulthood (ages 60+). The main sex difference in MVPA is lower MVPA in females compared to males across all age groups; in older ages both males and females have very low levels of MVPA.
The estimated regression coefficients from the three models with SePA, LiPA, and MVPA as outcomes used to test trends in Figure 2 are reported in Appendix Table B: Columns 2 and 6 for SePA, columns 3 and 8 for LiPA, and columns 4 and 8 for MVPA for females and males, respectively. The fitted values are reported in Panels 2, 3, and 4 of Appendix Table C for SEPA, LiPA, and MVPA, respectively. The results for SePA confirm trends observed in Figure 2. Females in the 17–22 year old reference group have a mean SePA of 506 minutes that is significantly higher than SePA of 6–16 year olds and 23–46 year olds, not significantly different from SePA of 47–70 year olds, and significantly lower than SePA of 71–84 year olds. Overall, males tend to be less sedentary than females before approximately 40 years old and then become more sedentary than females after age of 40 years old. The results for LiPA also confirm trends observed in Figure 2. Females in the 17–22 year old reference group have a mean LiPA of approximately 300 minutes that is significantly lower than LiPA of 6–16 year old and 23–64 year olds, not significantly different from LiPA of 65–76 year olds, and significantly higher than LiPA of 77–84 year olds. Overall, males compared to females tend to have comparable LiPA in 6–16 year old group, higher or comparable LiPA in 17–58 years old group, and lower LiPA after the age of 59. Finally, the results for MVPA also confirm Figure 2 indicating a monotonic decline over the lifespan with consistently higher MVPA in males across all age groups.
Figure 3 shows diurnal patterns of PA across the lifespan for males (top left), females (top right) and the sex difference (bottom). An alternative visualization is provided in Appendix Figures C, D, and E that show the changes in diurnal patterns of PA for males, females and difference by sex respectively across all five age groups. Expressed in the ten-minute LAC this surface qualitatively shows a relative allocation of PA over the 24-hour period as well as contrasts the intensity of PA across the age groups. The main pattern that emerges is that the initiation of PA occurs progressively later in the day from childhood to the end of adolescence (6–19). At the beginning of young adulthood, initiation of PA occurs progressively earlier in the day until it stabilizes at the beginning of midlife (age 31). Males compared to females (indicated in bottom of Figure 3 and Appendix Figure E) have higher PA (expressed in LAC) across the entire day in the 6–30 year old group. However after age 30, males only have higher PA before 12pm, and lower PA compare to females after 12 pm. This pattern becomes more pronounced in older ages.
Discussion
Based on data from 2003–2006 NHANES survey, we found that from childhood to adolescence PA is sharply lower by age until age 19, where PA levels are similar to those among 60 year olds. Contrary to prior evidence, age 20 is a turning point; young adulthood represents the first time period in the life course where PA increases by age. This “catch up” period flattens during the midlife, and then begins a gradual lowering at the end of midlife and beginning of older adulthood. Based on analyses of PA across the day (diurnal patterns), we found that lower PA particularly among adolescents may be driven by a later initiation of daily PA. Additionally, a progressively earlier initiation of daily PA may drive higher PA after age 20.
We were additionally able to identify key sex differences across the lifespan. We found that during adolescence and early adulthood, males generally have higher PA than females, particularly, within the MVPA range. These findings are consistent with prior work (e.g.,18,36,37). However, from midlife to older adulthood, males show sharper lowering in PA by age compared with females, and actually have lower total PA measured by TLAC during older adulthood, including lower LiPA and higher SePA. These findings are in contrast with the majority of studies suggesting that men are more active than females at older ages 17,38. A few studies have suggested a reversal in the sex difference at older ages 39 potentially due to increased indoor household activity among older females compared to males 40–42. Our study provides strong evidence of this reversal and additionally suggests that this may be driven by a differential diurnal pattern. After young adulthood (31+) we found that females are increasingly more active than males after 12 noon, which is consistent with prior findings in the Baltimore Longitudinal Study of Aging 25,26. This increase is mainly from lower SePA and higher LiPA. This novel finding suggests that better understanding diurnal patterns may provide important insight into understanding sex differences in physical activity.
The lifespan curves split by intensity complement patterns seen in TLAC curves across sex. For LiPA, which represents the majority of active minutes/day across all age groups, PA levels begin the catch-up period earlier in adolescence than TLAC, and peak at higher levels at midlife. This suggests that perhaps PA in the light intensity, non-exercise range may be a potentially important intervention target, and studies should continue to explore the health benefits of LiPA 43,44. This is particularly important considering the universal decline in MVPA across all ages over the lifespan.
The finding that PA among individuals at the end of adolescence match those among individuals 40 years older provides strong evidence that childhood and adolescence represents a high-risk time period for physical inactivity. Lower PA among older adults may be partially related to physiologic changes, including physical and cognitive impairments 11. However lower PA among children and adolescents are generally not driven by factors related to impaired mobility. It is therefore extremely concerning that younger adults are exhibiting similar patterns of PA to older adults considering that those patterns are potentially preventable through public health interventions.
Our analyses of minute-to-minute daily PA patterns are consistent with prior evidence indicating progressively later wake times during childhood and adolescence, due to both biologic 12,13 and environmental/structural factors 14. Because early life PA may have long-term effects on mortality and morbidity 45,46, time of day specific public health interventions, perhaps focusing on late chronotype adolescents 47 are urgently needed.
Emerging adulthood represent a period of multiple life transitions, including initiation of full-time work, increased household responsibilities, and changes in family structure including marriage and becoming a parent. Employment in particular has been shown to be associated with reduced PA particularly in females 7,48 (see 49 for contrary evidence); conversely some research suggests that male full-time workers may be more active than their non-working counterparts 50. While the majority of evidence suggests that life transitions during emerging adulthood are associated with lower PA (e.g., 7–9,51), it is important to note that the majority of these studies use self-report measures of PA. Additionally, lifespan studies that have used objective measures have relied on non-transformed total activity counts that are highly skewed and may results in spurious results particularly when using statistical models that required distributional assumptions. Our analysis improves on this by utilizing log-transformed activity counts; our result suggest that the emerging adulthood period, beginning at age 20, coincides with a slow but steadily higher PA by age and an earlier initiation of daily physical activity. Considering that young adulthood represents the only time period over the entire lifespan where PA increases, understanding what may be driving this trend is an important aim for future research.
This study has a number of strengths including the use of innovative analytic and visualization methods to better understand PA changes across the lifespan within a large, nationally representative sample. Unlike prior studies exploring PA across age groups, our study utilized TLAC and LAC, which is a more robust approach to analyzing accelerometry data compared to non-transformed PA metrics, including total activity counts. Additionally, the use of weighted quantile curves and weighted bivariate surface plots to visualize and compare minute-to-minute PA across the day by age, are a methodological advance compared to prior work. These plots allow researchers to more precisely identify age trends as well as diurnal changes that may be driving trends.
This study has limitations. First, the study does not use tracking or longitudinal data, and therefore is only able to explore differences in PA across age groups, rather than change in PA across the lifespan. Considering that PA is broadly decreasing due to secular changes in types of occupation and household activities, younger individuals may actually be at higher risk for lower PA than what our study concludes. Second, the use of a hip worn accelerometer excludes certain physical activities, including swimming, cycling and upper body activities, which may be more or less common within specific age ranges.
Conclusion
Using nationally representative data from across the lifespan, we show that the end of adolescence represents a PA low that matches levels among older adults. Beginning at age 20, there is a”catch up” period that lasts until midlife. These trends may be partially driven by a later initiation of daily PA by adolescents, and a progressively early initiation after age 20. We additionally show that males compared to females have an earlier lowering in PA by age that leads to lower total PA measured by TLAC, due to higher SePA and lower LiPA, in older adulthood. These trends may be driven by differences in diurnal patters across sex where females are more active in the afternoon. These results broadly suggest the importance of better understanding and exploring time of the day specific PA interventions that target individuals within their childhood and adolescent years, a re-evaluation of how emerging adulthood may affect PA levels, and an exploration of how afternoon activity may drive sex differences in PA among older adults.
Supplementary Material
Highlights.
Total PA levels in the U.S. at age 19 are comparable to levels at age 60
Young adulthood is the only time in the lifespan when PA increases
Males have lower PA than females at older adulthood
Time of day may drive differences across age groups and sex
Time of day should be considered when developing PA interventions
Acknowledgments
This work was supported by the National Institute of Health (grants 5R01HL123407-02, 5R01AG049872-02, 5R01AG050507-02). VRV is supported by the Intramural Research Program, National Institute on Aging, NIH
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985 Mar-Apr;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 2.Healthy People 2020. Washington, DC: Office of Disease Prevention and Health Promotion. United States Department of Health and Human Services; 2010. [Google Scholar]
- 3.Physical Activity Guidelines Advisory Committee Report. Washington D.C: United States Department of Health and Human services; 2008. [Google Scholar]
- 4.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. 2008 Jan;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 5.Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Medicine and science in sports and exercise. 2000 Sep;32(9):1598–1600. doi: 10.1097/00005768-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Wolff-Hughes DL, Fitzhugh EC, Bassett DR, Churilla JR. Waist-Worn Actigraphy: Population-Referenced Percentiles for Total Activity Counts in U.S. Adults. Journal of physical activity & health. 2015 Apr;12(4):447–453. doi: 10.1123/jpah.2013-0464. [DOI] [PubMed] [Google Scholar]
- 7.Brown WJ, Trost SG. Life transitions and changing physical activity patterns in young women. American journal of preventive medicine. 2003 Aug;25(2):140–143. doi: 10.1016/s0749-3797(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 8.Corder K, Ogilvie D, van Sluijs EM. Invited commentary: Physical activity over the life course--whose behavior changes, when, and why? American journal of epidemiology. 2009 Nov 1;170(9):1078–1081. doi: 10.1093/aje/kwp273. discussion 1082–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Medicine and science in sports and exercise. 2000 Sep;32(9):1601–1609. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Hyde AL, Maher JP, Elavsky S. Enhancing our understanding of physical activity and wellbeing with a lifespan perspective. International Journal of Wellbeing. 2013;3(1):98–115. [Google Scholar]
- 11.DiPietro L. Physical activity in aging: changes in patterns and their relationship to health and function. The journals of gerontology. 2001 Oct;56(Spec No 2):13–22. doi: 10.1093/gerona/56.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- 12.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental neuroscience. 2009;31(4):276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Current biology: CB. 2004 Dec 29;14(24):R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Racette SB, Cade WT, Beckmann LR. School-based physical activity and fitness promotion. Physical therapy. 2010 Sep;90(9):1214–1218. doi: 10.2522/ptj.20100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healthy People 2020. United States Department of Health and Human Services (HHS); 2011. [Google Scholar]
- 16.Moran M, Van Cauwenberg J, Hercky-Linnewiel Cerin E, Deforche B, Pnina P. Understanding the relationships between the physical environment and physical activity in older adults: a systematic review of qualitative studies. International Journal of Behavioral Nutrition and Physical Activity. 2014;11(79) doi: 10.1186/1479-5868-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azevedo MR, Araujo CL, Reichert FF, Siqueira FV, da Silva MC, Hallal PC. Gender differences in leisure-time physical activity. International journal of public health. 2007;52(1):8–15. doi: 10.1007/s00038-006-5062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deaner RO, Geary DC, Puts DA, et al. A sex difference in the predisposition for physical competition: males play sports much more than females even in the contemporary U.S. PloS one. 2012;7(11):e49168. doi: 10.1371/journal.pone.0049168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward B, Schiller J. Prevalence of Multiple Chronic Conditions Among US Adults: Estimates From the National Health Interview Survey, 2010. Preventing chronic disease. 2013;10(120203) doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walston J, Fried LP. Frailty and the older man. The Medical clinics of North America. 1999 Sep;83(5):1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 21.Stahl ST, Albert SM. Gender differences in physical activity patterns among older adults who fall. Preventive medicine. 2015 Feb;71:94–100. doi: 10.1016/j.ypmed.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster C, Hillsdon M. Changing the environment to promote health-enhancing physical activity. Journal of sports sciences. 2004 Aug;22(8):755–769. doi: 10.1080/02640410410001712458. [DOI] [PubMed] [Google Scholar]
- 23.Humpel N, Owen N, Leslie E. Environmental factors associated with adults’ participation in physical activity: a review. American journal of preventive medicine. 2002 Apr;22(3):188–199. doi: 10.1016/s0749-3797(01)00426-3. [DOI] [PubMed] [Google Scholar]
- 24.Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. British journal of sports medicine. 2014 Jul;48(13):1019–1023. doi: 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. The journals of gerontology. 2014 Aug;69(8):973–979. doi: 10.1093/gerona/glt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao L, Huang L, Schrack JA, Ferrucci L, Zipunnikov V, Crainiceanu CM. Quantifying the lifetime circadian rhythm of physical activity: a covariate-dependent functional approach. Biostatistics (Oxford, England) 2015 Apr;16(2):352–367. doi: 10.1093/biostatistics/kxu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Countrol and Prevention. National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. 2014 http://www.cdc.gov/nchs/nhanes.htm.
- 28.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Medicine and science in sports and exercise. 2011 Feb;43(2):357–364. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. National Center for Health Statistics. Vital health Stat. 2013;2(161) [PubMed] [Google Scholar]
- 30.Xiao L, Zipunnikov V, Ruppert D, Crainiceanu C. Fast Covariance Estimation for High-dimensional Functional Data. Statistics and computing. 2016 Jan 1;26(1):409–421. doi: 10.1007/s11222-014-9485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff-Hughes DL, Bassett DR, Fitzhugh EC. Population-referenced percentiles for waist-worn accelerometer-derived total activity counts in U.S. youth: 2003 - 2006 NHANES. PloS one. 2014;9(12):e115915. doi: 10.1371/journal.pone.0115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stasinopoulos D, Rigby R. Generalized additive models for location scale and shape (GAMLSS) in R. Journal of Statistical Software. 2007:1–46. [Google Scholar]
- 33.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Statistics in medicine. 1992 Jul;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 34.Rigby R, Stasinopoulos D. Using the Box-Cox t distribution in GAMLSS to model skewness and kurtosis. Statistical Modelling. 2006;6(3):209–229. [Google Scholar]
- 35.Lumley T. Complex Surveys: A Guide to Analysis Using R. Vol. 565. John Wiley & Sons; 2011. [Google Scholar]
- 36.Rosenfeld CS. Sex-dependent differences in voluntary physical activity. Journal of neuroscience research. 2017 Jan 02;95(1–2):279–290. doi: 10.1002/jnr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butt J, Weinberg RS, Breckon JD, Claytor RP. Adolescent physical activity participation and motivational determinants across gender, age, and race. Journal of physical activity & health. 2011 Nov;8(8):1074–1083. doi: 10.1123/jpah.8.8.1074. [DOI] [PubMed] [Google Scholar]
- 38.Booth ML, Owen N, Bauman A, Clavisi O, Leslie E. Social-cognitive and perceived environment influences associated with physical activity in older Australians. Preventive medicine. 2000 Jul;31(1):15–22. doi: 10.1006/pmed.2000.0661. [DOI] [PubMed] [Google Scholar]
- 39.Martin KR, Koster A, Murphy RA, et al. Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003–04 and 2005–06. Journal of the American Geriatrics Society. 2014 Jul;62(7):1263–1271. doi: 10.1111/jgs.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chipperfield JG, Newall NE, Chuchmach LP, Swift AU, Haynes TL. Differential determinants of men’s and women’s everyday physical activity in later life. The journals of gerontology. 2008 Jul;63(4):S211–S218. doi: 10.1093/geronb/63.4.s211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett KM. Gender and longitudinal changes in physical activities in later life. Age and ageing. 1998 Dec;27(Suppl 3):24–28. doi: 10.1093/ageing/27.suppl_3.24. [DOI] [PubMed] [Google Scholar]
- 42.Kolbe-Alexander TL, Lambert EV, Harkins JB, Ekelund U. Comparison of two methods of measuring physical activity in South African older adults. Journal of aging and physical activity. 2006 Jan;14(1):98–114. doi: 10.1123/japa.14.1.98. [DOI] [PubMed] [Google Scholar]
- 43.Varma VR, Tan EJ, Wang T, et al. Low-Intensity Walking Activity is Associated with Better Health. Journal of Applied Gerontology. 2014;33(7):870–887. doi: 10.1177/0733464813512896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varma VR, Chuang Y, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2014 Dec 7; doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. The New England journal of medicine. 1986 Mar 6;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 46.Harsha DW, Berenson GS. The Benefits of Physical Activity in Childhood. The American Journal of Medical Sciences. 1995;310(Supplement 1):S109–S113. doi: 10.1097/00000441-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Avidan A, Zee P. Handbook of Sleep Medicine. 2. Lippincott Williams & Wilkins Handbook Series; 2011. [Google Scholar]
- 48.Allender S, Hutchinson L, Foster C. Life-change events and participation in physical activity: a systematic review. Health promotion international. 2008 Jun;23(2):160–172. doi: 10.1093/heapro/dan012. [DOI] [PubMed] [Google Scholar]
- 49.Bell S, Lee C. Emerging adulthood and patterns of physical activity among young Australian women. International journal of behavioral medicine. 2005;12(4):227–235. doi: 10.1207/s15327558ijbm1204_3. [DOI] [PubMed] [Google Scholar]
- 50.Van Domelen DR, Koster A, Caserotti P, et al. Employment and physical activity in the U.S. American journal of preventive medicine. 2011 Aug;41(2):136–145. doi: 10.1016/j.amepre.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity (Silver Spring, Md. 2008 Oct;16(10):2205–2211. doi: 10.1038/oby.2008.365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.